Abstract
With 119 species distributed in 19 genera, most species of the subtribe Lychnophorinae are endemic to the Espinhaço Range in central eastern Brazil. This region is characterized especially by the campos rupestres, a grassland mosaic associated with vegetation on rock outcrops, which display a high level of endemism. The present work aims to identify distribution patterns, collection density, species richness and research bias in collections. Ten geographic distribution patterns were identified: Chapada Diamantina, Chapada dos Veadeiros and adjacent mountains, Pico da Aliança, Extension of the Espinhaço Range, Central-South Arc of Minas Gerais, Espinhaço Range and Brasília Arc, Campos Rupestres and Restinga, Chapada Diamantina and Caatinga, Northwest-Southeast Diagonal and East Triangle. Other Angiosperm families present similar distribution, mainly in the Espinhaço Meridional, where higher collecting efforts are present. Species richness is higher in sites with higher collection intensity, however, the northeast of Goiás shows the opposite pattern. Spearman correlation analysis shows a strong correlation between collection density and species richness, with an exponential asymptotic model that is quite significant for the total variation of species richness. The cluster analysis presented five clusters correlated with five distribution patterns in Lychnophorinae.
Key words
biogeography; compositae; endemism; Espinhaço Range
Resumo
Com 119 espécies distribuídas em 19 gêneros, a maioria das espécies da subtribo Lychnophorinae é endêmica da Cadeia do Espinhaço no centro leste do Brasil, área caracterizada especialmente pelos campos rupestres, que são descritos como mosaicos vegetacionais em afloramentos rochosos que apresentam alto grau de endemismo. Este trabalho objetivou identificar padrões de distribuição, densidade de coleta, riqueza de espécies e viés de coletas. Foram identificados dez padrões de distribuição geográfica: Chapada Diamantina, Chapada dos Veadeiros e montanhas adjacentes, Pico da Aliança, Extensão da Cadeia do Espinhaço, Arco Centro-Sul de Minas Gerais, Cadeia do Espinhaço e Arco da Brasília, Disjunção Campos Rupestres e Restinga, Chapada Diamantina e Caatinga, Diagonal Noroeste-Sudeste e Triângulo Leste. Outras famílias de Angiospermas apresentam distribuição semelhante, principalmente no Espinhaço Meridional. A riqueza de espécies é maior em locais com alta intensidade de coleta, porém, o nordeste de Goiás mostra comportamento oposto. A análise de correlação de Spearman demonstrou forte correlação entre densidade de coleta e riqueza de espécies, apresentando modelo exponencial assintótico significativo para a variação total da riqueza de espécies. A análise de agrupamento apresentou cinco grupos correlacionados com cinco padrões de distribuição em Lychnophorinae.
Palavras-chave
biogeografia; compositae; endemismo; Cadeia do Espinhaço
Introduction
The study of species distribution patterns significantly contributes to understanding the dynamics of species establishment, indicating its possible causes and consequences through the knowledge of factors that limit or contribute to their development (Brown & Lomolino 2006Brown JH & Lomolino MV (2006) Biogeografia. 2nd ed. Ed. Funpec, Ribeirão Preto. 691p.). Meticulously describing geographic distribution patterns is one of the essential stages to delimit areas of endemism, which are defined by the exclusive occurrence of two or more species in a specific place (Anderson 1994Anderson S (1994) Area and endemism. The Quarterly Review of Biology 69: 451-471.; Morrone 1994Morrone JJ (1994) On the identification of areas of endemism. Systematic Biology 43: 438-441.; Noguera-Urbano 2016Noguera-Urbano EA (2016) Areas of endemism: travelling through space and the unexplored dimension. Systematics and Biodiversity 14: 131-139.).
Several phytogeographic studies have been continuously developed in Brazil to answer questions about species distribution, evolutionary behavior in relation to biogeographic barriers, location of areas harboring unique species composition, and others that contribute to scientific knowledge (Harley 1995Harley RM (1995) Introduction. In: Stannard BL (ed.) Flora of the Pico das Almas, Chapada Diamantina, Bahia. Royal Botanic Gardens, Kew. Pp. 1-40.; Giulietti et al. 1997Giulietti AM, Pirani JR & Harley RM (1997) Espinhaço range region. Eastern Brazil. In: Davis SD, Heywood VH, Herrera-MacBryde O, Villa-Lobos J & Hamilton AC (eds.) Centres of plant diversity. A guide and strategies for the conservation. Vol. 3. The Americas. WWF/IUCN, Cambridge. Pp. 397-404. ; Rapini et al. 2002Rapini A, Mello-Silva R & Kawasaki ML (2002) Richness and endemism in Asclepiadoideae (Apocynaceae) from the Espinhaço Range of Minas Gerais, Brazil - a conservationist view. Biodiversity and Conservation 11: 1733-1746.; Fiaschi & Pirani 2008Fiaschi P & Pirani JR (2008) Padrões de distribuição geográfica das espécies de Schefflera J.R. Forst. & G. Forst. (Araliaceae) do Brasil extra-amazônico. Revista Brasileira de Botânica 31: 633-644.; Echternacht et al. 2011Echternacht L, Trovo M, Oliveira CT & Pirani JR (2011) Areas of endemism in the Espinhaço Range in Minas Gerais, Brazil. Flora 206: 782-791.; Alves et al. 2014Alves RJV, Silva NG, Oliveira JA & Medeiros D (2014) Circumscribing campo rupestre - megadiverse Brazilian rocky montane savanas. Brazilian Journal of Biology 74: 355-362.; Silveira et al. 2016Silveira FAO, Negreiros D, Barbosa NPU, Buisson E, Carmo FF, Carstensen DW, Conceição AA, Cornelissen TG, Echternacht L, Fernandes GW, Garcia QS, Guerra TJ, Jacobi CM, Lemos-Filho JP, Stradic SL, Morellato LPC, Neves FS, Oliveira RS, Schaefer CE, Viana PL & Lambers H (2016) Ecology and evolution of plant diversity in the endangered campo rupestre: a neglected conservation priority. Plant Soil 403: 129-152.; Magenta et al. 2017Magenta MAG, Loeuille B & Pirani JR (2017) Fitogeografia de Aldama (Asteraceae, Heliantheae) na América do Sul. Rodriguésia 68: 463-480.; Morellato & Silveira 2017Morellato LPC & Silveira FAO (2017) Plant life in campo rupestre: new lessons from an ancient biodiversity hotspot. Flora 238: 1-10.; Zappi et al. 2017Zappi DC, Moro MF, Meagher TR & Lughadha EN (2017) Plant biodiversity drivers in Brazilian Campos Rupestres: insights from phylogenetic structure. Frontiers in Plant Science 8: 2141.). The identification and classification of distribution patterns of several families of Angiosperms can assist in other works involving evolutionary processes, species dispersion, influence of geological formations on biodiversity levels, zoning of endemic habitats, relationships and ecological indicatives, among others (Giulietti & Pirani 1988Giulietti AM & Pirani JR (1988) Patterns of geographical distribution of some plant species from Espinhaço range, Minas Gerais and Bahia, Brazil. In: Vanzolini PE & Heyer WR (eds.) Proceedings of a workshop on Neotropical distribution patterns. Academia Brasileira de Ciências, Rio de Janeiro. Pp. 39-69. ; Rosauer et al. 2009Rosauer D, Laffan SW, Crisp MD, Donnellan SC & Cook LG (2009) Phylogenetic endemism: a new approach for identifying geographical concentrations of evolutionary history. Molecular Ecology 18: 4061-4072.; Lusa et al. 2014Lusa MG, Appezzato-Da-Glória B, Loeuille B, Bartoli G & Ciccarelli D (2014) Functional groups in Lychnophorinae (Asteraceae: Vernonieae) based on morphological and anatomical traits. Australian Journal of Botany 62: 150-163.; Prado et al. 2014Prado JR, Brennand PGG, Godoy LP, Libardi GS, Abreu-Júnior EF, Roth PRO, Chiquito EA & Percequillo AR (2014) Species richness and areas of endemism of oryzomyine rodents (Cricetidae, Sigmodontinae) in South America: an ndm/vndm approach. Journal of Biogeography 42: 540-551.; Rando et al. 2016Rando JG, Zuntini AR, Conceição AS, van den Berg C, Pirani JR & de Queiroz LP (2016) Phylogeny of Chamaecrista ser. Coriaceae (Leguminosae) unveils a lineage recently diversified in Brazilian Campo Rupestre vegetation. International Journal of Plant Sciences 177: 3-17.; Magenta et al. 2017Magenta MAG, Loeuille B & Pirani JR (2017) Fitogeografia de Aldama (Asteraceae, Heliantheae) na América do Sul. Rodriguésia 68: 463-480.; Morellato & Silveira 2017Morellato LPC & Silveira FAO (2017) Plant life in campo rupestre: new lessons from an ancient biodiversity hotspot. Flora 238: 1-10.).
Occurring at elevations above 900 m in the Neotropical region, campos rupestres (highland rock outcrops) are the main phytophysiognomy of the Espinhaço Range (Giulietti & Pirani 1988Giulietti AM & Pirani JR (1988) Patterns of geographical distribution of some plant species from Espinhaço range, Minas Gerais and Bahia, Brazil. In: Vanzolini PE & Heyer WR (eds.) Proceedings of a workshop on Neotropical distribution patterns. Academia Brasileira de Ciências, Rio de Janeiro. Pp. 39-69. ; Echternacht et al. 2011Echternacht L, Trovo M, Oliveira CT & Pirani JR (2011) Areas of endemism in the Espinhaço Range in Minas Gerais, Brazil. Flora 206: 782-791.). The geological origin of the constituent blocks of this region is dated to the Pre-Cambrian, with the soil being characteristically shallow, sandy, acidic and nutrient-poor, contributing to the formation of a phytophysiographic mosaic with high endemism levels, due to the segmentation of the vegetation in different and small populations between rock outcrops, providing specific niches to the species (Giulietti & Pirani 1988Giulietti AM & Pirani JR (1988) Patterns of geographical distribution of some plant species from Espinhaço range, Minas Gerais and Bahia, Brazil. In: Vanzolini PE & Heyer WR (eds.) Proceedings of a workshop on Neotropical distribution patterns. Academia Brasileira de Ciências, Rio de Janeiro. Pp. 39-69. ; Giulietti et al. 1997Giulietti AM, Pirani JR & Harley RM (1997) Espinhaço range region. Eastern Brazil. In: Davis SD, Heywood VH, Herrera-MacBryde O, Villa-Lobos J & Hamilton AC (eds.) Centres of plant diversity. A guide and strategies for the conservation. Vol. 3. The Americas. WWF/IUCN, Cambridge. Pp. 397-404. ; Fernandes 2016Fernandes GW (2016) Ecology and conservation of mountaintop grasslands in Brazil. Springer International Publishing, Cham. 561p.; Schaefer et al. 2016Schaefer CEGR, Corrêa GR, Candido HG, Arruda DM, Nunes JA, Araujo RW, Rodrigues PMS, Fernandes Filho EI, Pereira AFS, Brandão PC & Neri AV (2016) The physical environment of rupestrian grasslands (Campos Rupestres) in Brazil: geological, geomorphological and pedological characteristics, and interplays. In: Fernandes GW (ed.) Ecology and conservation of mountaintop grasslands in Brazil. Springer International Publishing, New York. Pp. 15-53.; Silveira et al. 2016Silveira FAO, Negreiros D, Barbosa NPU, Buisson E, Carmo FF, Carstensen DW, Conceição AA, Cornelissen TG, Echternacht L, Fernandes GW, Garcia QS, Guerra TJ, Jacobi CM, Lemos-Filho JP, Stradic SL, Morellato LPC, Neves FS, Oliveira RS, Schaefer CE, Viana PL & Lambers H (2016) Ecology and evolution of plant diversity in the endangered campo rupestre: a neglected conservation priority. Plant Soil 403: 129-152.; Colli-Silva et al. 2019Colli-Silva M, Vasconcelos TNC & Pirani JR (2019) Outstanding plant endemism levels strongly support the recognition of campo rupestre provinces in mountaintops of eastern South America. Journal of Biogeography 46: 1723-1733.). With approximately 1,100 km in length and 50 to 100 km width, the Espinhaço Range is a mountain chain spanning through the states of Bahia and Minas Gerais. Its southern boundary is in Serra de Cambotas, Minas Gerais, with the northern boundary in the Chapada Diamantina and the watershed that feeds the São Francisco River. A marked topographic feature of the region is the rock outcrop formations, providing a specific habitat for many species (Alves et al. 2014Alves RJV, Silva NG, Oliveira JA & Medeiros D (2014) Circumscribing campo rupestre - megadiverse Brazilian rocky montane savanas. Brazilian Journal of Biology 74: 355-362.; Fernandes 2016Fernandes GW (2016) Ecology and conservation of mountaintop grasslands in Brazil. Springer International Publishing, Cham. 561p.).
In addition to the characteristics highlighted for the Espinhaço Range, the Serra do Cipó plateau features remnants of the Gondwanan (Lower-Middle Cretaceous), Post-Gondwanan (Upper Cretaceous-Paleocene) and South American (Eocene-Oligocene) surfaces, with elevations between 800 and 1,600 m a.s.l. Its lithostratigraphy presents with metasedimentary deposits and neoproterozoic glaciogenics, mountain ridges interspersed by valleys, and mesoproterozoic and paleoproterozoic metasedimentary rocks (Saadi 1995Saadi A (1995) A geomorfologia da Serra do Espinhaço em Minas Gerais e de suas margens. Geonomos 3: 41-63.; Felippe et al. 2012Felippe MF, Silva CA, Souza AH & Magalhães Júnior AP (2012) Caracterização morfométrica dos compartimentos do relevo do Parque Nacional da Serra do Cipó, Serra do Espinhaço Meridional - Minas Gerais. Revista Espinhaço 1: 3-14.).
The Diamantina Plateau comprises the largest topographic volume of the Espinhaço Meridional, with slightly convex relief where the average elevation in the central portion is 1,300 m a.s.l. and 900 m and 1,200 m a.s.l. in its northern and southern limits, respectively. It presents regional staggered levels of Post-Gondwanan (1,200–1,400 m), South American (1,000–1,100 m) and Ciclo Velhas (750–800 m) surfaces, being an important region of the Espinhaço Range folding system, which was caused by geotectonic cycles between 1,800 and 1,300 m.y.a (Saadi 1995Saadi A (1995) A geomorfologia da Serra do Espinhaço em Minas Gerais e de suas margens. Geonomos 3: 41-63.; Schaefer et al. 2002Schaefer CEGR, Ker JC, Gilkes RJ, Campos JC, Costa LM & Saadi A (2002) Pedogenesis on the uplands of the Diamantina Plateau, Minas Gerais, Brazil: a chemical and micropedological study. Geoderma 107: 243-269.).
The relief of Serra do Cabral is marked by two distinct domains, one composed by the elevations and depressions that form the mountain itself and another formed by lower areas in the eastern portion. The lower areas are formed by limestone and pelitic rocks at elevations of 600–700 m a.s.l., the higher areas and flatter portions on the mountain tops are composed of sandstones and pelitic rocks, at elevations of 1,000 m a.s.l. (Miazaki 2016Miazaki AS (2016) Geoprocessamento aplicado nos campos rupestres do Parque Estadual da Serra do Cabral. Ed. Prospectiva, Frutal. 75p.).
The Serra da Canastra region presents elevations between 630 and 1,500 m a.s.l., rainy summers and dry winters, and a diverse relief that ranges from ridges to depressions. It is part of the geomorphological structures of the São Francisco Craton (granite-gneiss rocks from the Archean) and the Brasília Belt (schist and phyllite rocks from the upper Proterozoic) (Couto Junior et al. 2010; Souza & Rodrigues 2014Souza DA & Rodrigues SC (2014) Aspectos morfoestruturais e morfoesculturais da Serra da Canastra e entorno (MG). Revista do Departamento de Geografia-USP 27: 47-66.).
Localized in the Brazilian Central Plateau, the region of Chapada dos Veadeiros varies in elevation from 577 to 1,676 m a.s.l., presents a conserved pediplaned surface and geology composed mainly by the Araí (quartzites, conglomerates, calcareous-pelitic and felsic metavolcanic rocks) and Paranoá (siliciclastic sedimentary rocks, with quartzite and metasiltstones layers) groups (Nascimento 1992Nascimento MAS (1992) Geomorfologia do estado de Goiás. Boletim Goiano de Geografia 12: 1-22.; Carvalho Júnior et al. 2015Carvalho Júnior OA, Guimarães RF, Martins ÉS & Gomes RAT (2015) Chapada dos Veadeiros: the highest landscapes in the Brazilian Central Plateau. In: Vieira B, Salgado A & Santos L (eds.) Landscapes and landforms of Brazil. Vol. 1. World Geomorphological Landscapes, Springer, Dordrecht. Pp. 221-230.).
The subtribe Lychnophorinae (Asteraceae: Vernonieae) is a monophyletic group comprising 119 species in 19 genera and is notable for occurring mainly in campos rupestres, being distributed almost restrictedly in the Cerrado phytogeographical domain (Loeuille et al. 2015Loeuille B, Semir J, Lohmann LG & Pirani JR (2015) A phylogenetic analysis of Lychnophorinae (Asteraceae: Vernonieae) based on molecular and morphological data. Systematic Botany 40: 299-315.; Loeuille et al. 2019Loeuille B, Semir J & Pirani JR (2019) A synopsis of Lychnophorinae (Asteraceae: Vernonieae). Phytotaxa 398: 1-139.). Most species are shrubs and treelets with indumentum composed of 3- to 5-armed trichomes, presenting leaf sheath, syncephaly (second-order capitulum), apical anther appendages with thickened wall, absence of style basal node, sublophate pollen, and with the pappus type being paleaceous, subpaleaceous or setose and pappus duration being persistent, deciduous or caducous (Loeuille et al. 2019Loeuille B, Semir J & Pirani JR (2019) A synopsis of Lychnophorinae (Asteraceae: Vernonieae). Phytotaxa 398: 1-139.).
The present work aims to determine the geographical distribution patterns of all species in subtribe Lychnophorinae, to infer the relationship between collection density and species richness and identify possible bias in our understanding of the distribution patterns of Lychnophorinae.
Material and Methods
In this work, the distribution patterns were hierarchically defined according to the area involved in each pattern. The subpattern is a pattern that is inserted within a distribution pattern. The local pattern refers to a pattern that is inserted in a subpattern. In addition, the patterns outlined here follow the qualitative concept of distribution patterns circumscription (Morrone 2009Morrone JJ (2009) Evolutionary biogeography: an integrative approach with case studies. Columbia University Press, New York. 320p.), involving at least 95% of the records in each pattern.
In geological terms, the Espinhaço Range is structured in two blocks, Chapada Diamantina in Bahia and Serra do Espinhaço, corresponding to the rest of the mountain range, extending from southern Bahia to Minas Gerais (Hasui et al. 2012Hasui Y, Carneiro CDR, Almeida FFM & Bartorelli A (2012) Geologia do Brasil. Beca, São Paulo. 907p.). However, the present work uses as basis for delimitation of the study area the works carried out by Alkmin (2012)Alkmin FF (2012) Serra do Espinhaço e Chapada Diamantina. In: Hasui Y, Carneiro CDR, Almeida FFM & Bartorelli A (eds.) Geologia do Brasil. Beca, São Paulo. Pp. 236-244., Campos et al. (2016)Campos L, Guedes MLS, Acevedo-Rodrigues P & Roque N (2016) Contributions to the floristic and vegetation knowledge of Espinhaço Septentrional, Bahia, Brazil. Brazilian Journal of Botany 40: 427-437. and Saadi (1995)Saadi A (1995) A geomorfologia da Serra do Espinhaço em Minas Gerais e de suas margens. Geonomos 3: 41-63., regarding the geomorphological evolution of Serra do Espinhaço, due to the analyses of substrate morphology, lithological properties and geophysical structure that characterizes this formation. These authors defined the Chapada Diamantina in central-northern Bahia, split from the Serra do Espinhaço by the Paramirim corridor. The Serra do Espinhaço block is subdivided in Espinhaço Meridional, which comprises the central part of Minas Gerais, and Espinhaço Septentrional, that extends from southern Bahia to northern Minas Gerais. In addition, the environmental conditions of the different sectors of the Espinhaço Range differ significantly from each other, directly affecting biological communities and endemism (Echternacht et al. 2011Echternacht L, Trovo M, Oliveira CT & Pirani JR (2011) Areas of endemism in the Espinhaço Range in Minas Gerais, Brazil. Flora 206: 782-791.).
Datasets
Of the 119 species belonging to subtribe Lychnophorinae, 117 were used in the present study (Tab. 1). Lychnophora phylicifolia DC. and Eremanthus brasiliensis (Gardner) MacLeish were not used due to the lack of data (both species are known only by a single collection from the 19th century each).
Species of subtribe Lychnophorinae and their respective distribution patterns. BO = Bolivia; BR = Brazil; AL = Alagoas; BA = Bahia; CE = Ceará; DF = Distrito Federal; ES = Espírito Santo; GO = Goiás; MT = Mato Grosso; MS = Mato Grosso do Sul; MG = Minas Gerais; PA = Pará; PB = Paraíba; PE = Pernambuco; RJ = Rio de Janeiro; RO = Rondônia; SP = São Paulo; SE = Sergipe; TO = Tocantins.
Two datasets were assembled and used to produce maps and perform analyses. A first dataset was built using all records of all species of the subtribe Lychnophorinae obtained from a high-quality, personal taxonomic database previously assembled by the second author, with additional input from the SpeciesLink (2019) and Global Biodiversity Information Facility (GBIF 2019GBIF - The Global Biodiversity Information Facility (2019) Available at <https://www.gbif.org>. Access on 5 November 2019.
https://www.gbif.org...
) databases. The second dataset was built by filtering the records from the first dataset to maintain only unique geographic coordinates, i.e., keeping only the records that were not repeated.
These datasets were reviewed against the reliable records, to flag incorrect coordinates, incorrect names or lack of information (records without coordinates were georeferenced by municipality seat). These errors were appropriately corrected or unreliable and unrecoverable records were eliminated, in order to avoid interferences that could bias the results and impact their reliability (Hijmans et al. 1999Hijmans RJ, Schreuder M, De la Cruz J & Guarino L (1999) Using GIS to check co-ordinates of germoplasm accessions. Genetic Resources and Crop Evolution 46: 291-296.; Colli-Silva et al. 2019Colli-Silva M, Vasconcelos TNC & Pirani JR (2019) Outstanding plant endemism levels strongly support the recognition of campo rupestre provinces in mountaintops of eastern South America. Journal of Biogeography 46: 1723-1733.).
Maps
Individual distribution maps were drawn for each species of subtribe Lychnophorinae using the software Quantum GIS 2.18.14 (QGIS Development Team 2015QGIS Development Team (2015) QGIS Geographic Information System. Open Source Geospatial Foundation Project. Available at <https://www.qgis.org/en/site/>. Access on 7 February 2019.
https://www.qgis.org/en/site/...
). Each map of the distribution patterns found in subtribe Lychnophorinae was constructed using a representative species of the corresponding pattern. The other species of the subtribe that were mapped and not represented in the cartographic maps present in this work have their individual distributions in agreement to their respective distribution pattern, except for inherent peculiarities of each individual species distribution. All individual distributions of Lychnophorinae species were manually overlapped using Quantum GIS 2.18.14 to identify groups of species with the same distributions to circumscribe the distribution patterns, and also to determine whether Lychnophorinae presents exclusive distribution patterns within the group that were not described in literature. The divisions and subdivisions used to characterize all distribution patterns in this work result from the distribution patterns intrinsically found in subtribe Lychnophorinae and were mainly corroborated with the works of Echternacht et al. (2011)Echternacht L, Trovo M, Oliveira CT & Pirani JR (2011) Areas of endemism in the Espinhaço Range in Minas Gerais, Brazil. Flora 206: 782-791. and Rando & Pirani (2011)Rando JG & Pirani JR (2011) Padrões de distribuição geográfica das espécies de Chamaecrista sect. Chamaecrista ser. Coriaceae (Benth.) H.S. Irwin & Barneby, Leguminosae - Caesalpinioideae. Revista Brasileira de Botânica 34: 499-513.. After this step, all the maps for distribution patterns found in subtribe Lychnophorinae were created using Quantum GIS 2.18.14. A separate distribution map was built for Centratherum punctatum, due to its pantropical distribution.
Comparison with different families
This step is hereby included as an explanatory tool for the comparative approach used with other angiosperm families as a way to enrich the discussion in the present work. The angiosperm genera Actinocephalus (Eriocaulaceae), Baccharis (Asteraceae), Chamaecrista (Leguminosae), Declieuxia (Rubiaceae), Encholirium (Bromeliaceae), Lippia (Verbenaceae), Luxemburgia (Ochnaceae), Ossaea (Melastomataceae), Philodendron (Araceae), Pilosocereus (Cactaceae), Schefflera (Araliaceae) and Staurogyne (Acanthaceae) were selected through literature review of available taxonomic treatments (Marquete 1979Marquete NF (1979) Revisão taxonômica do gênero Barjonia Decne. (Asclepiadaceae). Rodriguésia 31: 7-70.; Urbatsch et al. 1986Urbatsch LE, Zlotsky A & Pruski JF (1986) Revision of Calea sect. Lemmatium (Asteraceae: Heliantheae) from Brazil. Systematic Botany 11: 501-514.; Mayo 1988Mayo SJ (1988) Aspectos da evolução e da geografia do gênero Philodendron Schott (Araceae). São Paulo. Acta Botanica Brasilica 1: 27-40.; Marcondes-Ferreira Neto 1988Marcondes-Ferreira Neto W (1988) Aspidosperma Mart., nom. cons. (Apocynaceae): estudos taxonômicos. Ph.D. Thesis. Universidade Estadual de Campinas, Campinas. 453p.; Occhioni 1990Occhioni EML (1990) Considerações taxonômicas no gênero Stryphnodendron Mart. (Leguminosae-Mimosoideae) e distribuição geográfica das espécies. Porto Alegre. Acta Botanica Brasilica 4: 153-158.; Siqueira 1991Siqueira JC (1991) O gênero Gomphrena L. (Amaranthaceae) no Brasil. PhD Thesis. University of Campinas, Campinas. 273p.; Souza 1998Souza MLDR (1998) Revisão taxonômica do gênero Ossaea DC. (Melastomataceae) no Brasil. Tese de Doutorado. Universidade de São Paulo, São Paulo. 317p.; Rapini 2000Rapini A (2000) Sistemática: estudos em Asclepiadoideae (Apocynaceae) da Cadeia do Espinhaço de Minas Gerais. PhD Thesis. Universidade de São Paulo, São Paulo. 283p.; Salimena 2000Salimena FRG (2000) Revisão taxonômica de Lippia L. sect. Rhodolippia Schauer (Verbenaceae). Ph.D. Thesis. Universidade de São Paulo, São Paulo. 216p.; Feres 2001Feres F (2001) O gênero Luxemburgia A. St.-Hil. (Ochnaceae) - revisão taxonômica e estudo cladístico. MSc Thesis. Universidade Estadual de Campinas, Campinas. 158p.; Zanin 2001Zanin A (2001) Revisão de Andropogon L. (Poaceae - Panicoideae - Andropogoneae) no Brasil. Tese de Doutorado. Instituto de Botânica. Universidade de São Paulo, São Paulo. 347p.; Baumgratz 2004Baumgratz JFA (2004) Sinopse de Huberia DC. (Melastomataceae: Merianieae). Revista Brasileira de Botânica 27: 545-561.; Sano 2004Sano PT (2004) Actinocephalus (Körn.) Sano (Paepalanthus sect. Actinocephalus), a new genus of Eriocaulaceae, and other taxonomic and nomenclatural changes involving Paepalanthus Mart. Taxon 53: 99-107.; Forzza 2005Forzza RC (2005) Revisão taxonômica de Encholirium Mart. ex Schult. & Schult. f. (Pitcairnioideae - Bromeliaceae). Boletim de Botânica da Universidade de São Paulo 23: 1-49.; Sakuragui et al. 2005Sakuragui CM, Mayo SJ & Zappi DC (2005) Taxonomic revision of Brazilian species of Philodendron section Macrobelium. Kew Bulletin 60: 465-513.; Scalon 2007Scalon VR (2007) Revisão taxonômica do gênero Stryphnodendron Mart. (Leguminosae - Mimosoideae). PhD Thesis. Universidade de São Paulo, São Paulo. 264p.; Lovo 2009Lovo J (2009) Filogenia e revisão de Pseudotrimezia (Iridaceae). Ph.D. Thesis. Universidade de São Paulo, São Paulo. 102p.; Rapini 2010Rapini A (2010) Revisitando as Asclepiadoideae (Apocynaceae) da Cadeia do Espinhaço. Boletim de Botânica da Universidade de São Paulo 28: 97-123.; Trovó 2010Trovó M (2010) Sistemática de Paepalanthoideae (Eriocaulaceae): filogenia, morfologia e taxonomia de Diphyomene (Ruhland) Trovó. PhD Thesis. Universidade de São Paulo, São Paulo. 259p.; Rando & Pirani 2011Rando JG & Pirani JR (2011) Padrões de distribuição geográfica das espécies de Chamaecrista sect. Chamaecrista ser. Coriaceae (Benth.) H.S. Irwin & Barneby, Leguminosae - Caesalpinioideae. Revista Brasileira de Botânica 34: 499-513.; Echternacht 2012Echternacht L (2012) Sistemática de Comanthera e de Syngonanthus (Eriocaulaceae). Ph.D. thesis. Universidade de São Paulo, São Paulo. 287p.; Costa & Sano 2013Costa FN & Sano PT (2013) New Circumscription of the Endemic Brazilian Genus Actinocephalus (Eriocaulaceae). Novon 22: 281-287.; Liede-Schumann et al. 2014Liede-Schumann S, Nikolaus M, Soares e Silva UCS, Rapini A, Mangelsdorff RD & Meve U (2014) Phylogenetics and biogeography of the genus Metastelma (Apocynaceae Asclepiadoideae-Asclepiadeae: Metastelmatinae). Systematic Botany 39: 594-612.; Roque & Pirani 2014Roque N & Pirani JR (2014) Taxonomic revision of Richterago (Asteraceae, Gochnatieae). Systematic Botany 39: 997-1026.; Echternacht et al. 2015Echternacht L, Sano PT & Dubuisson JY (2015) Taxonomic study of Comanthera subg. Thysanocephalus (Eriocaulaceae). Systematic Botany 40: 136-150.; Heiden & Pirani 2016Heiden G & Pirani JRP (2016) Taxonomy of Baccharis subgen. Tarchonanthoides (Asteraceae: Astereae: Baccharidinae), a group from the southeastern South American grasslands and savannas. Phytotaxa 241: 1-70.; Braz & Monteiro 2017Braz DM & Monteiro R (2017) Taxonomic revision of staurogyne (Nelsonioideae, Acanthaceae) in the Neotropics. Phytotaxa 296: 1-40.) because they are diversified, have endemic species occurring in similar areas to the subtribe and are part of important families present in campos rupestres (Giulietti et al. 1997Giulietti AM, Pirani JR & Harley RM (1997) Espinhaço range region. Eastern Brazil. In: Davis SD, Heywood VH, Herrera-MacBryde O, Villa-Lobos J & Hamilton AC (eds.) Centres of plant diversity. A guide and strategies for the conservation. Vol. 3. The Americas. WWF/IUCN, Cambridge. Pp. 397-404. ; Rapini et al. 2008Rapini A, Ribeiro PL, Lambert S & Pirani JR (2008) A flora dos campos rupestres da Cadeia do Espinhaço. Megadiversidade 4: 16-24.; Borges et al. 2011Borges RF, Carneiro MA & Viana P (2011) Altitudinal distribution and species richness of herbaceous plants in campos rupestres of the Southern Espinhaço Range, Minas Gerais, Brazil. Rodriguésia 62: 139-152.; Echternacht et al. 2011Echternacht L, Trovo M, Oliveira CT & Pirani JR (2011) Areas of endemism in the Espinhaço Range in Minas Gerais, Brazil. Flora 206: 782-791.; Colli-Silva et al. 2019Colli-Silva M, Vasconcelos TNC & Pirani JR (2019) Outstanding plant endemism levels strongly support the recognition of campo rupestre provinces in mountaintops of eastern South America. Journal of Biogeography 46: 1723-1733.). Information contained in the Brasilian Flora Group (BFG 2018BFG - The Brazil Flora Group (2018) Brazilian Flora 2020: innovation and collaboration to meet Target 1 of the Global Strategy for Plant Conservation (GSPC). Rodriguésia 69: 1513-1527.) database was obtained, in order to analyze and identify species with relevant distribution patterns that are shared with the Lychnophorinae species. Records of all species of the selected genera were obtained through the SpeciesLink and GBIF databases and plotted in Quantum GIS 2.18.14. Then, species (Tab. 2) with distribution patterns congruent with those found in subtribe Lychnophorinae were manually selected by overlapping the distributions of each species.
Collection density and species richness
A point density map with all sample records of the subtribe (first dataset), except for Centratherum punctatum, was constructed to analyze whether collection efforts are homogeneous throughout the distribution or focused on certain areas. A species richness map was developed with the unique records (second dataset) to understand which areas present higher species richness. We also analyzed if sampling bias is correlated to the number of species registered in each area, by applying the comparative method between the maps that resulted from collection density and species richness analyses, to identify whether the highlighted areas in both analyses were congruent with each other. The same process was applied to draw collection density (based on the first dataset) and species richness (based on the second dataset) maps encompassing only the species that occur in the Espinhaço Range, i.e., 74% of all species of subtribe Lychnophorinae, aiming to analyze this important endemic area in depth. The analyses were carried out in DIVA GIS 7.5 (Hijmans et al. 2012Hijmans RJ, Guarino L & Mathur P (2012) DIVA-GIS manual version 7.5. University of California. Available at <https://www.diva-gis.org/docs/DIVA-GIS_manual_7.pdf>. Access on 5 November 2019.
https://www.diva-gis.org/docs/DIVA-GIS_m...
) and posteriorly edited in ArcGIS Desktop 10.5 (Esri 2016).
Regression analysis
Regression analyses were carried out in R version 3.5.1 and RStudio version 1.1.463 (RStudio Team 2015RStudio Team (2015) RStudio: integrated development for R. RStudio, Inc., Boston, MA. Available at <http://www.rstudio.com/>. Access on 7 February 2019.
http://www.rstudio.com/...
). Data obtained from the collection density and species richness analyses were tested using the Shapiro-Wilk normality test, Pearson’s (Benesty et al. 2009Benesty J, Chen J, Huang Y & Cohen I (2009) Pearson correlation coefficient. In: Benesty J, Chen J, Huang Y & Cohen I (eds.) Noise reduction in speech processing. Springer Topics in Signal Processing. Vol. 2. Springer, Berlin, Heidelberg. Pp. 1-4.) and Spearman’s (Myers & Sirois 2006Myers L & Sirois MJ (2006) Spearman correlation coefficients, differences between. Encyclopedia of Statistical Sciences. John Wiley & Sons, Hoboken. Pp. 1-2.) correlation tests, generalized linear model (GLM) and generalized additive model (GAM), as well as the non-linear model asymptotic exponential with two and three parameters (Crawley 2015Crawley MJ (2015) Statistics: an introduction using R. 2nd. ed. Imperial College London, Wiley. Pp. 114-149.). These procedures were used to define which model best explains the relationship between collection effort and the number of species found.
Clustering analysis
In the present work, this methodology was applied only for determination of species clusters, serving as corroboration for other results, but it can also be used as an indicator of possible distribution patterns. The previously described first database was used to carry out a clustering analysis using the online platform Infomap Bioregions (Edler et al. 2017Edler D, Guedes T, Zizka A, Rosvall M & Antonelli A (2017) Infomap Bioregions: interactive mapping of biogeographical regions from species distributions. Systematic Biology 66: 197-204.). This analysis uses the Infomap algorithm, originally described by Rosvall & Bergstrom (2008)Rosvall M & Bergstrom CT (2008) Maps of information flow reveal community structure in complex networks, Proceedings of the National Academy of Sciences USA 105: 1118-1123. and based on the method reported by Vilhena & Antonelli (2015)Vilhena D & Antonelli A (2015) A network approach for identifying and delimiting biogeographical regions. Nature communications 6: 6848., and is very reliable in terms of comparative studies. Several variations in the parameters used for clustering were applied to identify the best fit for Lychnophorinae distribution. The minimum and maximum cell size ranged from 0.25º to 1º. The minimum cell capacity was fixed in 10 records and maximum cell capacity varied between 100 and 500 records. The constant number of 10 trials and variations from 1.0 to 2.0 in cluster cost number were applied.
Results
Distribution patterns
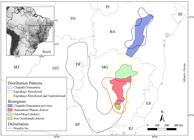
The distribution patterns of subtribe Lychnophorinae (Fig. 1) described in this work demonstrate that 74% of the species are endemic to the Espinhaço Range, mainly in Espinhaço Meridional among different mountains and regions. Ten distribution patterns, five subpatterns and three local patterns (Tab. 1) are defined for the subtribe and described below, with the last classification being a grouping of species that differ from all other distributions.
1. Chapada Diamantina (CD) - 17 spp.: the genus Lychnophorella is endemic to this area.
1.1. Chapada Diamantina and disjunct area (CDA) - three spp.
2. Chapada dos Veadeiros and adjacent mountains (CV) - six spp.
3. Pico da Aliança (PA) - two spp.: this pattern stands out for including only Pico da Aliança, in the state of Minas Gerais, near the border with the state of Espírito Santo.
4. Extension of the Espinhaço Range (EER) - four spp.: species that occur from Chapada Diamantina to Serra do Espinhaço belong to this pattern.
4.1. Serra do Espinhaço (SE) - two spp.
4.2. Espinhaço Septentrional (ES) - five spp.: these are the species with distribution in the phytophysiographic block of the northern Serra do Espinhaço.
4.3. Espinhaço Meridional (EM) - 11 spp.: the genera Lychnocephalus, Minasia and Prestelia belong to this pattern.
4.3.1. Serra do Cabral (SCa) - three spp.
4.3.2. Diamantina Plateau (DP) - 17 spp.
4.3.3. Serra do Cipó (SCi) - eight spp.
4.4. Espinhaço Meridional and Septentrional (EMS) - 12 spp.
5. Central-South Arc of Minas Gerais (CAMG) - four spp.: a semicircle area involving mainly the Diamantina Plateau, Iron Quadrangle and Serra da Canastra region.
6. Espinhaço Range and Brasília Arc (ERB) - three spp.: in this pattern, the species are simultaneously distributed along the Espinhaço Range and in the Brasília Arc, an area composed of an arc of mountainous elevations that extend from the southern portion of the Espinhaço Range, passing through Serra da Canastra and reaching Chapada dos Veadeiros.
7. Campos Rupestres and Restinga (CRR) - two spp.: the species delimited in this pattern have as main characteristic the abundance of occurrence in the Espinhaço Range, mainly Espinhaço Meridional, and a more diffuse occurrence in the restinga in the states of Espírito Santo and Rio de Janeiro.
8. Chapada Diamantina and Caatinga (CDC) - two spp.
9. Northwest-Southeast Diagonal (NSD) - four spp.: the species part of this pattern have unique distributions, forming a diagonal along the central region of Brazil, across the states of Rondônia, Mato Grosso, Goiás and Minas Gerais.
10. East Triangle (ET) - three spp.: this pattern presents a roughly triangular area with the northern and southern limits in the restinga region between Vicente Ferrer, in the state of Pernambuco, and Nova Iguaçu, in the state of Rio de Janeiro, respectively. Its western limit is located at Cavalcante, in Chapada dos Veadeiros, state of Goiás. Bahia is the state with the highest concentration of records, and Chapada Diamantina is the most prominent area.
11. Unique Distribution - eight spp.: this group contains all species with specific distributions that do not fit into other patterns described above (Tab. 1).
With a very wide distribution, Centratherum punctatum is found in several countries along the tropical regions of the globe. The most significant occurrence is in Latin America, where the species is native, with Brazil presenting 54% of the total collection records (507 records), but it also occurs in smaller areas such as Hawaii (USA), Galapagos and Fiji. Australia is another important occurrence center for the species, with 117 records representing 12% of the total collections, having a linear distribution on the northeast coast. Likewise, Central America also has an important concentration of records, especially in Costa Rica. According to Loeuille et al. (2019)Loeuille B, Semir J & Pirani JR (2019) A synopsis of Lychnophorinae (Asteraceae: Vernonieae). Phytotaxa 398: 1-139., the species is native to the American continent and was introduced in other countries, such as the Philippines and Australia, through Portuguese and Spanish trade routes, being used as an ornamental plant in Australia. The exact distribution is not completely known, considering that the presence of the species in Florida (USA), Hawaii (USA) and Taiwan is more recent. This behavior demonstrates a high versatility for dispersion and establishment in the environment that contributes to its propagation, likely being present but not yet collected in other countries.
Distribution similarities with other families
All genera selected for this comparative study have at least one species included in the EM distribution pattern. Due to the high levels of endemism present in this portion of the Espinhaço Range, the area is intensely researched, consequently providing more information about several families (Echternacht et al. 2011Echternacht L, Trovo M, Oliveira CT & Pirani JR (2011) Areas of endemism in the Espinhaço Range in Minas Gerais, Brazil. Flora 206: 782-791.; Bitencourt & Rapini 2013Bitencourt C & Rapini A (2013) Centres of endemism in the Espinhaço Range: identifying cradles and museums of Asclepiadoideae (Apocynaceae). Systematics and Biodiversity 11: 525-536.). The genera Ossaea, Pilosocereus and Staurogyne have each only one species with distribution similar to subtribe Lychnophorinae, whereas Actinocephalus and Chamaecrista have two species each. Encholirium and Schefflera share the same patterns between them in comparison to Lychnophorinae patterns, with both occurring in ES, EM and EMS distribution patterns. Lippia is included in the largest number of patterns, being present in CD, CV, EM, EMS and ERB distribution patterns. The remaining genera present different configurations of distribution patterns: Baccharis is present in CD, EER, EM and NSD distribution patterns, Declieuxia occurs in CV, EER, EM and ERB distribution patterns, Philodendron is present in CD, EM, EMS and ERB distribution patterns and Luxemburgia occurs in CD, ES and EM distribution patterns.
Collection density and species richness analyses
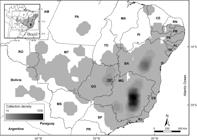
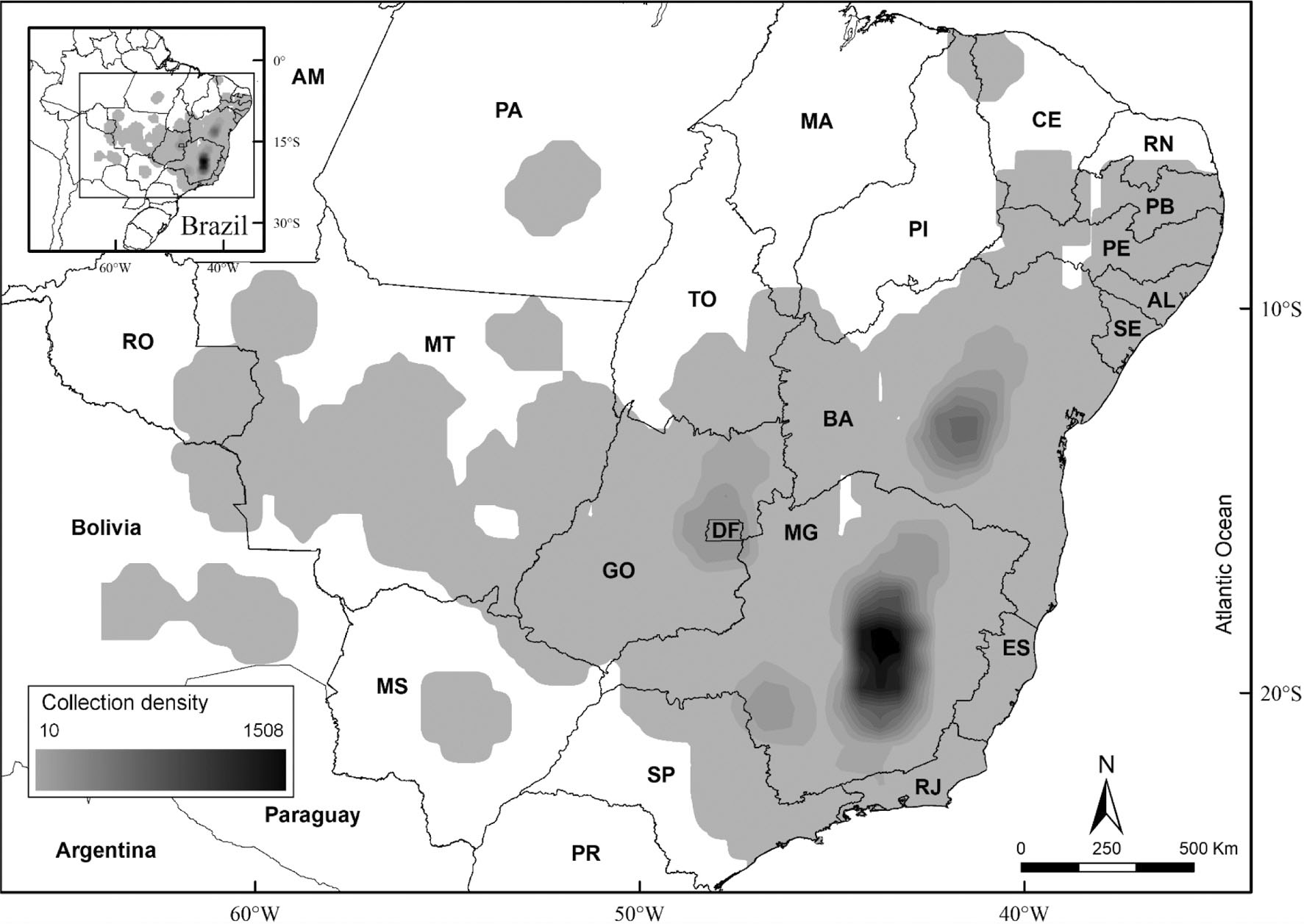
The comparative analysis between collection density (Fig. 2) and species richness (Fig. 3) for Lychnophorinae demonstrates there is a proportional bias in which the areas of greatest collection effort are also the areas with the highest species concentration, but this bias is not equally present throughout the distribution. Similar results showing a directly proportional correlation between species richness and collection density, especially in the Espinhaço Range (Fig. 4), have been previously found and discussed by other researchers (Echternacht et al. 2011Echternacht L, Trovo M, Oliveira CT & Pirani JR (2011) Areas of endemism in the Espinhaço Range in Minas Gerais, Brazil. Flora 206: 782-791.; Bitencourt & Rapini 2013Bitencourt C & Rapini A (2013) Centres of endemism in the Espinhaço Range: identifying cradles and museums of Asclepiadoideae (Apocynaceae). Systematics and Biodiversity 11: 525-536.; Campos et al. 2016Campos L, Guedes MLS, Acevedo-Rodrigues P & Roque N (2016) Contributions to the floristic and vegetation knowledge of Espinhaço Septentrional, Bahia, Brazil. Brazilian Journal of Botany 40: 427-437.).
Collection density analysis map with interpolation representation. Darker areas represent the areas with higher collection density and light grey area represents the area with lower collection density.
Species richness analysis map with interpolation representation. Darker areas represent the areas with higher species richness and light grey area represents the area with lower species richness.
a. Collection density analysis map of the Espinhaço Range with interpolation representation. Darker areas represent the areas with higher collection density and light grey area represents the area with lower collection density. b. Species richness analysis map of the Espinhaço Range with interpolation representation. Darker areas represent the areas with higher species richness and light grey area represents the area with lower species richness.
Most part of the collection records evaluated for Lychnophorinae come from three states: 60% from Minas Gerais, 21% from Bahia and 11% from Goiás and Distrito Federal. The other 13 Brazilian states from the 16 where the subtribe occurs each hold less than 3% of the collections records. Diamantina Plateau and Serra do Cipó are the locations with the highest collection rate and species richness. This correlation between species richness and collection density is also observed in the southern part of Chapada Diamantina and in the Serra da Canastra region. However, the northeast region of Goiás presents a contrasting pattern when comparing collection density and species richness in Chapada dos Veadeiros and further south in the Brasília region. Chapada dos Veadeiros presents greater species richness compared to the southern Brasília region, even though the latter has higher collection density than Chapada dos Veadeiros.
Regression analysis
The Shapiro-Wilk test deemed the data generated by collection density and species richness analyses as positive for a non-normal distribution with p < 2.2-16 for both variables, being corroborated by histograms that do not display the typical bell-shaped normal distribution. Due to the non-normality of the data, the analysis turned to the non-linearity of the distributions, with the application of non-parametric methods. The Spearman’s correlation test showed a strong positive correlation between the variables, with rho = 0.8846 and p < 2.2-16. The model that best represented the relationship between variables was the non-linear exponential asymptotic regression with three parameters (Fig. 5) (Stevens 1951Stevens WL (1951) Asymptotic regression, Biometrics, Vol. 7, n. 3. Pp. 247-267.), presenting a high degree of significance and explaining 82.7% of the total variation in species richness (Crawley 2015Crawley MJ (2015) Statistics: an introduction using R. 2nd. ed. Imperial College London, Wiley. Pp. 114-149.).
Exponential asymptotic nonlinear regression model with three parameters. Histograms of collection density are in the upper part and species richness in the right side.
Clustering analysis
The result generated by Infomap Bioregions that showed higher similarity with some Lychnophorinae distribution patterns in the Espinhaço Range is constituted of cells with minimum and maximum size of 0.5º, minimum and maximum cell capacity of 10 and 500 records, number of trials 10 and number of cluster cost 1.2. Edler et al. (2017)Edler D, Guedes T, Zizka A, Rosvall M & Antonelli A (2017) Infomap Bioregions: interactive mapping of biogeographical regions from species distributions. Systematic Biology 66: 197-204. and Colli-Silva et al. (2019)Colli-Silva M, Vasconcelos TNC & Pirani JR (2019) Outstanding plant endemism levels strongly support the recognition of campo rupestre provinces in mountaintops of eastern South America. Journal of Biogeography 46: 1723-1733. found a good fit between the actual data and the results obtained in their works and, similarly, the Espinhaço Range also presented significant results in the analysis. The analysis delimited five clusters congruent with the CD, ES, SCa, DP and SCi distribution patterns and the Iron Quadrangle region (Fig. 6).
Results of clustering analysis showing compatibility with some distribution patterns of the Lychnophorinae subtribe. Hatched squares represent analysis noise and are not part of the clusters.
The analysis report classifies the species that have higher frequency of occurrence within a given region than in all other regions, thus defined as the most indicative species for each cluster (Edler et al. 2017Edler D, Guedes T, Zizka A, Rosvall M & Antonelli A (2017) Infomap Bioregions: interactive mapping of biogeographical regions from species distributions. Systematic Biology 66: 197-204.; Colli-Silva et al. 2019Colli-Silva M, Vasconcelos TNC & Pirani JR (2019) Outstanding plant endemism levels strongly support the recognition of campo rupestre provinces in mountaintops of eastern South America. Journal of Biogeography 46: 1723-1733.). The species of the distribution patterns that are also present in the clusters corresponding to these patterns score higher in the list of the most indicative species. The clusters with the most indicative species directly associated with the DP, SCa, SCi and CD distribution patterns presented the scores of 4.66, 4.09 (except Minasia cabralensis H.Rob., with 3.63), 3.64 (except Prestelia eriopus Sch.Bip., with 3.53) and 2.24. The same was verified in the cluster representing the ES distribution pattern, although it characterizes the most indicative species Lychnophora ramosissima Gardner and Maschalostachys mellosilvae Loeuille & Roque as almost totally present in this cluster due to uncertainty in region delimitation. This is reinforced by the high score of 12.9 for L. ramosissima and 12.1 for M. mellosilvae, and a score of 21.2 for Anteremanthus piranii Roque & F.A.Santana, the highest indicative score values found among all clusters (Tab. 3). Despite the Iron Quadrangle region also being represented by a cluster in the Espinhaço Range, all the most indicative species do not have exclusive presence in this cluster and the maximum score is 1.00 represented only by Lychnophora pinaster Mart.
Most indicative species of the cluster analysis and their respective scores. The species that belong to the corresponding distribution pattern mentioned in the table title are highlighted in bold.
Discussion
Almost all species of Lychnophorinae are endemic to Brazil, with the exception of Eremanthus mattogrossensis Kuntze and E. rondoniensis MacLeish & H.Schumach., occurring in Brazil and Bolivia, Centratherum cardenasii H.Rob. that occurs only in Bolivia and C. punctatum (Fig. 7), with a large distribution in several tropical countries.
Distribution patterns generally reflect the organization of the biota in a given environment, considering several factors that led that species group to establish in a similar distribution area. Some distribution patterns found in Lychnophorinae are also reported for other Angiosperms (Tab. 2) by Rapini (2002) (Asclepiadoideae), Echternacht et al. (2011)Echternacht L, Trovo M, Oliveira CT & Pirani JR (2011) Areas of endemism in the Espinhaço Range in Minas Gerais, Brazil. Flora 206: 782-791. (Eriocaulaceae) and Rando & Pirani (2011)Rando JG & Pirani JR (2011) Padrões de distribuição geográfica das espécies de Chamaecrista sect. Chamaecrista ser. Coriaceae (Benth.) H.S. Irwin & Barneby, Leguminosae - Caesalpinioideae. Revista Brasileira de Botânica 34: 499-513. (Chamaecrista, Leguminosae). The southern portion of the Espinhaço Range has been more thoroughly sampled and presents very characteristic subdivisions of distribution between mountains and surrounding areas, due to the geography of the region, presenting microendemic zones created by rivers and depressions. The great diversity of the Serra do Cipó was noted by Rapini et al. (2008)Rapini A, Ribeiro PL, Lambert S & Pirani JR (2008) A flora dos campos rupestres da Cadeia do Espinhaço. Megadiversidade 4: 16-24. and the high index of collection in this area make the discovering of new (undescribed) species more likely, thus increasing species richness in these locations (Bitencourt & Rapini 2013Bitencourt C & Rapini A (2013) Centres of endemism in the Espinhaço Range: identifying cradles and museums of Asclepiadoideae (Apocynaceae). Systematics and Biodiversity 11: 525-536.).
Species in the Espinhaço Range have distribution patterns with boundaries congruent with the limits of this mountain range and his subdivisions, which are possibly explained, in this case, by the species occurring in high elevation environments. In the context of the principle of vicariance, where the geographical barriers may be an impediment of distribution for some species and not for others, the CD distribution pattern is noteworthy as species present distinct distributions between the eastern and western parts of the plateau, however, the presence of the Paramirim corridor between Chapada Diamantina and Espinhaço Septentrional does not prevent some species from being found on both sides.
All families selected for comparison of distribution patterns had species with similar distribution patterns to those found in subtribe Lychnophorinae, notably in the Espinhaço Range, where the highest number of records was found. The Chapada Diamantina and Espinhaço Meridional, in the states of Bahia and Minas Gerais, presented the highest number of species used as comparison, containing 11 and 38 species respectively. Families Bromeliaceae and Verbenaceae stand out, with nine members of genus Encholirium distributed in the Espinhaço Meridional and seven representatives of genus Lippia in the Chapada Diamantina, respectively.
The CAMG, CDC, ET, PA and SE distribution patterns are uniquely found in Lychnophorinae and not seen in other species of the genera studied here for comparative distribution analyses. This is likely due to the use of only a small fraction of the angiosperms that occur in the Espinhaço Range in the analyses. Further in-depth studies with other groups could highlight species with similar distributions to these exclusive patterns presented here. Most part of the patterns identified for the subtribe comprehend the meridional portion of the Espinhaço Range, which becomes clear when overlapping the distribution patterns (Fig. 1). The high concentration of species in this region, with a variety of spatial distributions among them, increases the number of distribution patterns and contributes to delimit more specific zones, such as Diamantina Plateau, Serra do Cipó and Serra do Cabral (Echternacht et al. 2011Echternacht L, Trovo M, Oliveira CT & Pirani JR (2011) Areas of endemism in the Espinhaço Range in Minas Gerais, Brazil. Flora 206: 782-791.; Rando & Pirani 2011Rando JG & Pirani JR (2011) Padrões de distribuição geográfica das espécies de Chamaecrista sect. Chamaecrista ser. Coriaceae (Benth.) H.S. Irwin & Barneby, Leguminosae - Caesalpinioideae. Revista Brasileira de Botânica 34: 499-513.).
Collection efforts can influence not only the richness of species in a region, but also the number of distribution patterns that can be found. There is a possible trend where the higher the number of species described for a region, the probability of finding different distribution patterns in that location increases. Similarly, sites with few collection records indicate these areas may harbor a high number of species still to be described and in need of improved research effort. Although these relationships are not always evident or direct, the distribution patterns of Lychnophorinae in the Espinhaço Meridional show cases where a high intensity of collection corresponds to a concentration of species. The lower species richness of the Espinhaço Septentrional is a reflection of lower collection intensity, thus making this area promising for the identification of new, undescribed species. Future studies with detailed statistical approaches may help clarify these patterns.
The contrasting pattern seen in the northeast of Goiás points to an even greater potential of identifying new species in Chapada dos Veadeiros. In the Brasília portion, the potential to find new species appears to be lower when comparing collection density and species richness, especially if compared to Chapada dos Veadeiros. After the analyses, it is evident there is a correlation between the most collected areas and higher species richness throughout the distribution area of the group, as previously observed in other works (e.g., Bitencourt & Rapini 2013Bitencourt C & Rapini A (2013) Centres of endemism in the Espinhaço Range: identifying cradles and museums of Asclepiadoideae (Apocynaceae). Systematics and Biodiversity 11: 525-536.; Campos et al. 2016Campos L, Guedes MLS, Acevedo-Rodrigues P & Roque N (2016) Contributions to the floristic and vegetation knowledge of Espinhaço Septentrional, Bahia, Brazil. Brazilian Journal of Botany 40: 427-437.).
The statistical results and the graphical model corroborate the interpretation offered by collection density and species richness analyses. They demonstrated a strong exponential correlation between the variables, making the proportional increase between them evident and showing that differences in the collection density and species richness values increase gradually. This is explained by the fact that collected areas harbor an unknown number of species and although collection effort increases the amount of new species found, it also decreases when approaching the threshold of undescribed species in the region. In addition, the results corroborate the potential of finding a larger number of new species in poorly studied areas. Echternacht et al. (2011)Echternacht L, Trovo M, Oliveira CT & Pirani JR (2011) Areas of endemism in the Espinhaço Range in Minas Gerais, Brazil. Flora 206: 782-791. and Bitencourt & Rapini (2013)Bitencourt C & Rapini A (2013) Centres of endemism in the Espinhaço Range: identifying cradles and museums of Asclepiadoideae (Apocynaceae). Systematics and Biodiversity 11: 525-536. also discuss the similarity between species richness and collection density by statistical means, however in the first case it shows a high index of Pearson’s correlation of 0.87 and r = 0.886 and p = 0 in the second case.
Although the clustering analysis did not find congruence with all the distribution patterns described for Lychnophorinae, the previously mentioned congruence of the clustering analysis results with some distribution patterns shows this methodology as a strong tool in delimiting distribution patterns in a given area, including the previously described subpatterns and local patterns. The clustering analysis is additionally corroborated by the list of most indicative species in each cluster and their respective score value of presence in that pattern (Edler et al. 2017Edler D, Guedes T, Zizka A, Rosvall M & Antonelli A (2017) Infomap Bioregions: interactive mapping of biogeographical regions from species distributions. Systematic Biology 66: 197-204.; Colli-Silva et al. 2019Colli-Silva M, Vasconcelos TNC & Pirani JR (2019) Outstanding plant endemism levels strongly support the recognition of campo rupestre provinces in mountaintops of eastern South America. Journal of Biogeography 46: 1723-1733.). Except for the Iron Quadrangle region, which does not present the necessary components to be recognized as a distribution pattern for Lychnophorinae, due to lack of exclusive species for the area and low score, all other clusters in the Espinhaço Range showed visible circumscription with high score for the most indicative species, reinforcing the endemic characteristic of this area and agreeing with previous works (Echternacht et al. 2011Echternacht L, Trovo M, Oliveira CT & Pirani JR (2011) Areas of endemism in the Espinhaço Range in Minas Gerais, Brazil. Flora 206: 782-791.; Rando & Pirani 2011Rando JG & Pirani JR (2011) Padrões de distribuição geográfica das espécies de Chamaecrista sect. Chamaecrista ser. Coriaceae (Benth.) H.S. Irwin & Barneby, Leguminosae - Caesalpinioideae. Revista Brasileira de Botânica 34: 499-513.; Bitencourt & Rapini 2013Bitencourt C & Rapini A (2013) Centres of endemism in the Espinhaço Range: identifying cradles and museums of Asclepiadoideae (Apocynaceae). Systematics and Biodiversity 11: 525-536.; Campos et al. 2016Campos L, Guedes MLS, Acevedo-Rodrigues P & Roque N (2016) Contributions to the floristic and vegetation knowledge of Espinhaço Septentrional, Bahia, Brazil. Brazilian Journal of Botany 40: 427-437.) on distribution patterns and the high endemism levels and species richness present in the campos rupestres, markedly in the Espinhaço Range.
The Chapada Diamantina and Southern Espinhaço provinces proposed by Colli-Silva et al. (2019)Colli-Silva M, Vasconcelos TNC & Pirani JR (2019) Outstanding plant endemism levels strongly support the recognition of campo rupestre provinces in mountaintops of eastern South America. Journal of Biogeography 46: 1723-1733. are aligned with the CD and EMS distribution patterns in the present work, as well as the Diamantina Plateau and Iron Quadrangle districts (Colli-Silva et al. 2019Colli-Silva M, Vasconcelos TNC & Pirani JR (2019) Outstanding plant endemism levels strongly support the recognition of campo rupestre provinces in mountaintops of eastern South America. Journal of Biogeography 46: 1723-1733.), which correspond to the EM distribution pattern (Fig. 8). Thus, the complementarity between the distribution patterns shown here and the bioregions presented by Colli-Silva et al. (2019)Colli-Silva M, Vasconcelos TNC & Pirani JR (2019) Outstanding plant endemism levels strongly support the recognition of campo rupestre provinces in mountaintops of eastern South America. Journal of Biogeography 46: 1723-1733. highlights the different floristic composition, species concentration and the endemic characteristic of the areas along the Espinhaço Range.
Congruence among the bioregions described by Colli-Silva et al. (2019)Colli-Silva M, Vasconcelos TNC & Pirani JR (2019) Outstanding plant endemism levels strongly support the recognition of campo rupestre provinces in mountaintops of eastern South America. Journal of Biogeography 46: 1723-1733. and some Lychnophorinae distribution patterns.
The analyses we chose to carry out complement each other, composing a congruent set of results, thus providing reliable tools for future plant biogeography studies. The distribution patterns found in Lychnophorinae, the diversity of taxa in each pattern, including species from other families, and the clusters delimited by the clustering analysis together constitute a strong evidence of the endemic patterns present in mountain environments. The results from collection density, species richness, and regression analyses also are aligned, pointing to cores of species richness where higher collection density is present, putting the research bias in evidence and showing that botanical studies are needed is lesser known areas. These analyses and the overlap of distribution patterns are aligned, showing Diamantina Plateau and Serra do Cipó as important areas for diversity of species and highlighting the importance of the biogeographic and taxonomic researches in the Espinhaço Meridional. These findings agree with Colli-Silva et al. (2019)Colli-Silva M, Vasconcelos TNC & Pirani JR (2019) Outstanding plant endemism levels strongly support the recognition of campo rupestre provinces in mountaintops of eastern South America. Journal of Biogeography 46: 1723-1733., making distribution patterns and bioregions essential tools for the understanding of the botanical biogeography of the Espinhaço Range.
Acknowledgments
We thank Universidade Federal de Pernambuco and the Laboratório de Morfo-Taxonomia Vegetal at the Centro de Biociências, for logistic support; the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), for the scientific initiation scholarship; Andreza Luciana de Araújo Barbosa Oliveira, for helping with the organization of Centratherum punctatum data; Anselmo Nogueira, for the support in the development of the statistical model; Carolina Moriani Siniscalchi, Gleison Soares de Oliveira and co-workers of the Asteraceae group at UFPE, who assisted with valuable suggestions.
References
- Alkmin FF (2012) Serra do Espinhaço e Chapada Diamantina. In: Hasui Y, Carneiro CDR, Almeida FFM & Bartorelli A (eds.) Geologia do Brasil. Beca, São Paulo. Pp. 236-244.
- Alves RJV, Silva NG, Oliveira JA & Medeiros D (2014) Circumscribing campo rupestre - megadiverse Brazilian rocky montane savanas. Brazilian Journal of Biology 74: 355-362.
- Anderson S (1994) Area and endemism. The Quarterly Review of Biology 69: 451-471.
- Baumgratz JFA (2004) Sinopse de Huberia DC. (Melastomataceae: Merianieae). Revista Brasileira de Botânica 27: 545-561.
- Benesty J, Chen J, Huang Y & Cohen I (2009) Pearson correlation coefficient. In: Benesty J, Chen J, Huang Y & Cohen I (eds.) Noise reduction in speech processing. Springer Topics in Signal Processing. Vol. 2. Springer, Berlin, Heidelberg. Pp. 1-4.
- BFG - The Brazil Flora Group (2018) Brazilian Flora 2020: innovation and collaboration to meet Target 1 of the Global Strategy for Plant Conservation (GSPC). Rodriguésia 69: 1513-1527.
- Bitencourt C & Rapini A (2013) Centres of endemism in the Espinhaço Range: identifying cradles and museums of Asclepiadoideae (Apocynaceae). Systematics and Biodiversity 11: 525-536.
- Borges RF, Carneiro MA & Viana P (2011) Altitudinal distribution and species richness of herbaceous plants in campos rupestres of the Southern Espinhaço Range, Minas Gerais, Brazil. Rodriguésia 62: 139-152.
- Braz DM & Monteiro R (2017) Taxonomic revision of staurogyne (Nelsonioideae, Acanthaceae) in the Neotropics. Phytotaxa 296: 1-40.
- Brown JH & Lomolino MV (2006) Biogeografia. 2nd ed. Ed. Funpec, Ribeirão Preto. 691p.
- Campos L, Guedes MLS, Acevedo-Rodrigues P & Roque N (2016) Contributions to the floristic and vegetation knowledge of Espinhaço Septentrional, Bahia, Brazil. Brazilian Journal of Botany 40: 427-437.
- Carvalho Júnior OA, Guimarães RF, Martins ÉS & Gomes RAT (2015) Chapada dos Veadeiros: the highest landscapes in the Brazilian Central Plateau. In: Vieira B, Salgado A & Santos L (eds.) Landscapes and landforms of Brazil. Vol. 1. World Geomorphological Landscapes, Springer, Dordrecht. Pp. 221-230.
- Colli-Silva M, Vasconcelos TNC & Pirani JR (2019) Outstanding plant endemism levels strongly support the recognition of campo rupestre provinces in mountaintops of eastern South America. Journal of Biogeography 46: 1723-1733.
- Costa FN & Sano PT (2013) New Circumscription of the Endemic Brazilian Genus Actinocephalus (Eriocaulaceae). Novon 22: 281-287.
- Crawley MJ (2015) Statistics: an introduction using R. 2nd. ed. Imperial College London, Wiley. Pp. 114-149.
- Echternacht L, Sano PT & Dubuisson JY (2015) Taxonomic study of Comanthera subg. Thysanocephalus (Eriocaulaceae). Systematic Botany 40: 136-150.
- Echternacht L (2012) Sistemática de Comanthera e de Syngonanthus (Eriocaulaceae). Ph.D. thesis. Universidade de São Paulo, São Paulo. 287p.
- Echternacht L, Trovo M, Oliveira CT & Pirani JR (2011) Areas of endemism in the Espinhaço Range in Minas Gerais, Brazil. Flora 206: 782-791.
- Edler D, Guedes T, Zizka A, Rosvall M & Antonelli A (2017) Infomap Bioregions: interactive mapping of biogeographical regions from species distributions. Systematic Biology 66: 197-204.
- Esri Inc (2016) ArcGIS Desktop 10.5 software. Esri Inc., Redlands. Available at <https://desktop.arcgis.com/en/>. Access on 05 August 2021.
» https://desktop.arcgis.com/en/ - Felippe MF, Silva CA, Souza AH & Magalhães Júnior AP (2012) Caracterização morfométrica dos compartimentos do relevo do Parque Nacional da Serra do Cipó, Serra do Espinhaço Meridional - Minas Gerais. Revista Espinhaço 1: 3-14.
- Feres F (2001) O gênero Luxemburgia A. St.-Hil. (Ochnaceae) - revisão taxonômica e estudo cladístico. MSc Thesis. Universidade Estadual de Campinas, Campinas. 158p.
- Fernandes GW (2016) Ecology and conservation of mountaintop grasslands in Brazil. Springer International Publishing, Cham. 561p.
- Fiaschi P & Pirani JR (2008) Padrões de distribuição geográfica das espécies de Schefflera J.R. Forst. & G. Forst. (Araliaceae) do Brasil extra-amazônico. Revista Brasileira de Botânica 31: 633-644.
- Forzza RC (2005) Revisão taxonômica de Encholirium Mart. ex Schult. & Schult. f. (Pitcairnioideae - Bromeliaceae). Boletim de Botânica da Universidade de São Paulo 23: 1-49.
- GBIF - The Global Biodiversity Information Facility (2019) Available at <https://www.gbif.org>. Access on 5 November 2019.
» https://www.gbif.org - Giulietti AM & Pirani JR (1988) Patterns of geographical distribution of some plant species from Espinhaço range, Minas Gerais and Bahia, Brazil. In: Vanzolini PE & Heyer WR (eds.) Proceedings of a workshop on Neotropical distribution patterns. Academia Brasileira de Ciências, Rio de Janeiro. Pp. 39-69.
- Giulietti AM, Pirani JR & Harley RM (1997) Espinhaço range region. Eastern Brazil. In: Davis SD, Heywood VH, Herrera-MacBryde O, Villa-Lobos J & Hamilton AC (eds.) Centres of plant diversity. A guide and strategies for the conservation. Vol. 3. The Americas. WWF/IUCN, Cambridge. Pp. 397-404.
- Harley RM (1995) Introduction. In: Stannard BL (ed.) Flora of the Pico das Almas, Chapada Diamantina, Bahia. Royal Botanic Gardens, Kew. Pp. 1-40.
- Hasui Y, Carneiro CDR, Almeida FFM & Bartorelli A (2012) Geologia do Brasil. Beca, São Paulo. 907p.
- Heiden G & Pirani JRP (2016) Taxonomy of Baccharis subgen. Tarchonanthoides (Asteraceae: Astereae: Baccharidinae), a group from the southeastern South American grasslands and savannas. Phytotaxa 241: 1-70.
- Hijmans RJ, Guarino L & Mathur P (2012) DIVA-GIS manual version 7.5. University of California. Available at <https://www.diva-gis.org/docs/DIVA-GIS_manual_7.pdf>. Access on 5 November 2019.
» https://www.diva-gis.org/docs/DIVA-GIS_manual_7.pdf - Hijmans RJ, Schreuder M, De la Cruz J & Guarino L (1999) Using GIS to check co-ordinates of germoplasm accessions. Genetic Resources and Crop Evolution 46: 291-296.
- Liede-Schumann S, Nikolaus M, Soares e Silva UCS, Rapini A, Mangelsdorff RD & Meve U (2014) Phylogenetics and biogeography of the genus Metastelma (Apocynaceae Asclepiadoideae-Asclepiadeae: Metastelmatinae). Systematic Botany 39: 594-612.
- Loeuille B, Semir J & Pirani JR (2019) A synopsis of Lychnophorinae (Asteraceae: Vernonieae). Phytotaxa 398: 1-139.
- Loeuille B, Semir J, Lohmann LG & Pirani JR (2015) A phylogenetic analysis of Lychnophorinae (Asteraceae: Vernonieae) based on molecular and morphological data. Systematic Botany 40: 299-315.
- Lovo J (2009) Filogenia e revisão de Pseudotrimezia (Iridaceae). Ph.D. Thesis. Universidade de São Paulo, São Paulo. 102p.
- Lusa MG, Appezzato-Da-Glória B, Loeuille B, Bartoli G & Ciccarelli D (2014) Functional groups in Lychnophorinae (Asteraceae: Vernonieae) based on morphological and anatomical traits. Australian Journal of Botany 62: 150-163.
- Magenta MAG, Loeuille B & Pirani JR (2017) Fitogeografia de Aldama (Asteraceae, Heliantheae) na América do Sul. Rodriguésia 68: 463-480.
- Marcondes-Ferreira Neto W (1988) Aspidosperma Mart., nom. cons. (Apocynaceae): estudos taxonômicos. Ph.D. Thesis. Universidade Estadual de Campinas, Campinas. 453p.
- Marquete NF (1979) Revisão taxonômica do gênero Barjonia Decne. (Asclepiadaceae). Rodriguésia 31: 7-70.
- Mayo SJ (1988) Aspectos da evolução e da geografia do gênero Philodendron Schott (Araceae). São Paulo. Acta Botanica Brasilica 1: 27-40.
- Miazaki AS (2016) Geoprocessamento aplicado nos campos rupestres do Parque Estadual da Serra do Cabral. Ed. Prospectiva, Frutal. 75p.
- Morellato LPC & Silveira FAO (2017) Plant life in campo rupestre: new lessons from an ancient biodiversity hotspot. Flora 238: 1-10.
- Morrone JJ (1994) On the identification of areas of endemism. Systematic Biology 43: 438-441.
- Morrone JJ (2009) Evolutionary biogeography: an integrative approach with case studies. Columbia University Press, New York. 320p.
- Myers L & Sirois MJ (2006) Spearman correlation coefficients, differences between. Encyclopedia of Statistical Sciences. John Wiley & Sons, Hoboken. Pp. 1-2.
- Nascimento MAS (1992) Geomorfologia do estado de Goiás. Boletim Goiano de Geografia 12: 1-22.
- Noguera-Urbano EA (2016) Areas of endemism: travelling through space and the unexplored dimension. Systematics and Biodiversity 14: 131-139.
- Occhioni EML (1990) Considerações taxonômicas no gênero Stryphnodendron Mart. (Leguminosae-Mimosoideae) e distribuição geográfica das espécies. Porto Alegre. Acta Botanica Brasilica 4: 153-158.
- Prado JR, Brennand PGG, Godoy LP, Libardi GS, Abreu-Júnior EF, Roth PRO, Chiquito EA & Percequillo AR (2014) Species richness and areas of endemism of oryzomyine rodents (Cricetidae, Sigmodontinae) in South America: an ndm/vndm approach. Journal of Biogeography 42: 540-551.
- QGIS Development Team (2015) QGIS Geographic Information System. Open Source Geospatial Foundation Project. Available at <https://www.qgis.org/en/site/>. Access on 7 February 2019.
» https://www.qgis.org/en/site/ - Rando JG & Pirani JR (2011) Padrões de distribuição geográfica das espécies de Chamaecrista sect. Chamaecrista ser. Coriaceae (Benth.) H.S. Irwin & Barneby, Leguminosae - Caesalpinioideae. Revista Brasileira de Botânica 34: 499-513.
- Rando JG, Zuntini AR, Conceição AS, van den Berg C, Pirani JR & de Queiroz LP (2016) Phylogeny of Chamaecrista ser. Coriaceae (Leguminosae) unveils a lineage recently diversified in Brazilian Campo Rupestre vegetation. International Journal of Plant Sciences 177: 3-17.
- Rapini A (2010) Revisitando as Asclepiadoideae (Apocynaceae) da Cadeia do Espinhaço. Boletim de Botânica da Universidade de São Paulo 28: 97-123.
- Rapini A, Ribeiro PL, Lambert S & Pirani JR (2008) A flora dos campos rupestres da Cadeia do Espinhaço. Megadiversidade 4: 16-24.
- Rapini A, Mello-Silva R & Kawasaki ML (2002) Richness and endemism in Asclepiadoideae (Apocynaceae) from the Espinhaço Range of Minas Gerais, Brazil - a conservationist view. Biodiversity and Conservation 11: 1733-1746.
- Rapini A (2000) Sistemática: estudos em Asclepiadoideae (Apocynaceae) da Cadeia do Espinhaço de Minas Gerais. PhD Thesis. Universidade de São Paulo, São Paulo. 283p.
- Roque N & Pirani JR (2014) Taxonomic revision of Richterago (Asteraceae, Gochnatieae). Systematic Botany 39: 997-1026.
- Rosauer D, Laffan SW, Crisp MD, Donnellan SC & Cook LG (2009) Phylogenetic endemism: a new approach for identifying geographical concentrations of evolutionary history. Molecular Ecology 18: 4061-4072.
- Rosvall M & Bergstrom CT (2008) Maps of information flow reveal community structure in complex networks, Proceedings of the National Academy of Sciences USA 105: 1118-1123.
- RStudio Team (2015) RStudio: integrated development for R. RStudio, Inc., Boston, MA. Available at <http://www.rstudio.com/>. Access on 7 February 2019.
» http://www.rstudio.com/ - Saadi A (1995) A geomorfologia da Serra do Espinhaço em Minas Gerais e de suas margens. Geonomos 3: 41-63.
- Sakuragui CM, Mayo SJ & Zappi DC (2005) Taxonomic revision of Brazilian species of Philodendron section Macrobelium Kew Bulletin 60: 465-513.
- Salimena FRG (2000) Revisão taxonômica de Lippia L. sect. Rhodolippia Schauer (Verbenaceae). Ph.D. Thesis. Universidade de São Paulo, São Paulo. 216p.
- Sano PT (2004) Actinocephalus (Körn.) Sano (Paepalanthus sect. Actinocephalus), a new genus of Eriocaulaceae, and other taxonomic and nomenclatural changes involving Paepalanthus Mart. Taxon 53: 99-107.
- Scalon VR (2007) Revisão taxonômica do gênero Stryphnodendron Mart. (Leguminosae - Mimosoideae). PhD Thesis. Universidade de São Paulo, São Paulo. 264p.
- Schaefer CEGR, Corrêa GR, Candido HG, Arruda DM, Nunes JA, Araujo RW, Rodrigues PMS, Fernandes Filho EI, Pereira AFS, Brandão PC & Neri AV (2016) The physical environment of rupestrian grasslands (Campos Rupestres) in Brazil: geological, geomorphological and pedological characteristics, and interplays. In: Fernandes GW (ed.) Ecology and conservation of mountaintop grasslands in Brazil. Springer International Publishing, New York. Pp. 15-53.
- Schaefer CEGR, Ker JC, Gilkes RJ, Campos JC, Costa LM & Saadi A (2002) Pedogenesis on the uplands of the Diamantina Plateau, Minas Gerais, Brazil: a chemical and micropedological study. Geoderma 107: 243-269.
- Silveira FAO, Negreiros D, Barbosa NPU, Buisson E, Carmo FF, Carstensen DW, Conceição AA, Cornelissen TG, Echternacht L, Fernandes GW, Garcia QS, Guerra TJ, Jacobi CM, Lemos-Filho JP, Stradic SL, Morellato LPC, Neves FS, Oliveira RS, Schaefer CE, Viana PL & Lambers H (2016) Ecology and evolution of plant diversity in the endangered campo rupestre: a neglected conservation priority. Plant Soil 403: 129-152.
- Siqueira JC (1991) O gênero Gomphrena L. (Amaranthaceae) no Brasil. PhD Thesis. University of Campinas, Campinas. 273p.
- Souza MLDR (1998) Revisão taxonômica do gênero Ossaea DC. (Melastomataceae) no Brasil. Tese de Doutorado. Universidade de São Paulo, São Paulo. 317p.
- Souza DA & Rodrigues SC (2014) Aspectos morfoestruturais e morfoesculturais da Serra da Canastra e entorno (MG). Revista do Departamento de Geografia-USP 27: 47-66.
- SpeciesLink network (2019) Available at <http://www.splink.org.br>. Access on 5 November 2019.
» http://www.splink.org.br - Stevens WL (1951) Asymptotic regression, Biometrics, Vol. 7, n. 3. Pp. 247-267.
- Trovó M (2010) Sistemática de Paepalanthoideae (Eriocaulaceae): filogenia, morfologia e taxonomia de Diphyomene (Ruhland) Trovó. PhD Thesis. Universidade de São Paulo, São Paulo. 259p.
- Urbatsch LE, Zlotsky A & Pruski JF (1986) Revision of Calea sect. Lemmatium (Asteraceae: Heliantheae) from Brazil. Systematic Botany 11: 501-514.
- Vilhena D & Antonelli A (2015) A network approach for identifying and delimiting biogeographical regions. Nature communications 6: 6848.
- Zanin A (2001) Revisão de Andropogon L. (Poaceae - Panicoideae - Andropogoneae) no Brasil. Tese de Doutorado. Instituto de Botânica. Universidade de São Paulo, São Paulo. 347p.
- Zappi DC, Moro MF, Meagher TR & Lughadha EN (2017) Plant biodiversity drivers in Brazilian Campos Rupestres: insights from phylogenetic structure. Frontiers in Plant Science 8: 2141.
Edited by
Publication Dates
-
Publication in this collection
20 Sept 2021 -
Date of issue
2021
History
-
Received
08 Nov 2019 -
Accepted
30 Aug 2020