Abstract
We conducted an inventory of the fern, lycophyte and non-palm monocotyledon ground-herbs of terra firme riparian forests in the lower Tapajós region of the Brazilian Amazon. Eight 1.5 × 250 m plots, totaling 0.3 hectares, were surveyed along the watersheds of the Cupari and Curuá-Una rivers, located at the Tapajós National Forest, Pará, Brazil. To characterize the ground-herb community, we calculated species richness, abundance and Fisher’s alpha for each plot. To analyze turnover, we compared composition among plots by pairwise Bray-Curtis distance. In total, we sampled 3,130 individuals, 58 species, 27 genera and 20 families of riparian ground-herbs. Marantaceae (14 spp) was the richest family and Poaceae the most abundant family (738 individuals). The fern Triplophyllum glabrum (Tectariaceae) was the most frequent species, observed in 87.5 % of plots. Ground-herbs communities in the studied area have high species turnover, making it necessary to invest time and resources to adequately characterize and manage riparian habitats. The ground-herb community composition observed in the riparian zone here resembles that of other non-riparian forested sites in the Amazon with the plant families Marantaceae, Pteridaceae and Poaceae generally being the most commonly represented in the Amazonian ground-herb stratum. We highlight the importance of herb inventories, especially in conservation units.
Key words
ferns; lycophytes; monocotyledons; streams; Tapajós National Forest
Resumo
Realizamos um inventário de herbáceas ripárias em uma floresta de terra firme da Amazônia brasileira localizada na região do baixo Rio Tapajós, para samambaias, licófitas e monocotiledôneas não-palmeiras. Oito parcelas de 1,5 × 250 m, totalizando 0,3 hectares, foram amostradas ao longo das bacias hidrográficas dos Rios Cupari e Curuá-Una, localizados na Floresta Nacional do Tapajós, Pará, Brasil. Para caracterizar a comunidade herbácea, calculamos a riqueza, a abundância e o alfa de Fisher para cada parcela. Para analisar a dissimilaridade, comparamos a composição de espécies entre parcelas através da distância Bray-Curtis par-a-par. No total, foram amostrados 3.130 indivíduos, 58 espécies, 27 gêneros e 20 famílias de herbáceas ripárias terrestres. Marantaceae (14 spp) é a família com a maior riqueza e Poaceae a mais abundante (738 indivíduos). A samambaia Triplophyllum glabrum (Tectariaceae) é a espécie mais comum entre as parcelas, observada em 87,5 % delas. As comunidades de herbáceas terrestres têm alta substituição de espécies na área estudada, mostrando a necessidade de investimento de tempo e de recursos para caracterizar e manejar habitats ripários. A composição das comunidades de herbáceas terrestres ripárias observada aqui se assemelha a de sítios de florestas de terra firme não ripárias na Amazônia, sendo as famílias Marantaceae, Pteridaceae e Poaceae geralmente as mais representativas do estrato herbáceo amazônico. Destacamos aqui a importância de inventários de herbáceas terrestres, particularmente em unidades de conservação.
Palavras-chave
samambaias; licófitas; monocotiledôneas; riachos; Floresta Nacional do Tapajós
Introduction
Ground-herbs are an important component of the vegetation that make up terra firme riparian forests. Riparian zones are the interface between riverine aquatic and terrestrial environments, and they are considered vulnerable to human actions and climate change (Capon et al. 2013Capon SJ, Chambers LE, Mac Nally R, Naiman RJ, Davies P, Marshall N, Pittock J, Reid M, Capon T, Douglas M, Catford J, Baldwin DS, Stewardson M, Roberts J, Parsons M & Williams SE (2013) Riparian ecosystems in the 21th century: hotspot for climate change adaptation? Ecosystems 16: 359-381.). The structural, functional and ecosystemic characteristics of riparian zones are notable for their maintenance of rich biodiversity and their ecosystem services (Naiman et al. 1993Naiman RJ, Decamps H & Pollock M (1993) The role of riparian corridors in maintaining regional biodiversity. Ecological Applications 3: 209-212.; Pokrovsky 2016Pokrovsky OS (2016) Riparian zones: characteristics, management, practices and ecological impacts. Nova Science Publishers, New York. 383p.). It is therefore essential they are well inventoried to better understand their regional uniqueness for contemporary comparison, and as a baseline for future assessments under change scenarios. In Amazonia, the vegetation of the riparian zone is both non-forested and forested, with the forested regions comprised of flooded forests (e.g., várzeas, igapós) and non-flooded (terra firme) (Martins 2007Martins SB (2007) Recuperação de Matas Ciliares. 2a ed. Editora Aprenda Fácil, Viçosa. 255p.; Naiman et al. 2005Naiman RJ, Decamps H & McClain ME (2005) Riparia. Ecology, conservation and management of streamside communities. Elsevier Academic Press, London. 488p.).
Herbaceous plants in forest formations typically include herbs, sub-shrubs and seedlings (Gilliam et al. 1995Gilliam FS, Turrill NL & Adams MB (1995) Herbaceous-layer and overstory species in clear-cut and mature Central Appalachian Hardwood Forests. Ecological Applications 5: 947-955.; Zickel 1995Zickel CS (1995) Fitossociologia e dinâmica do estrato herbáceo de dois fragmentos florestais do estado de São Paulo. Tese de Doutorado. Universidade Estadual de Campinas, Campinas. 125p.; Costa 2004Costa FRC (2004) Structure and composition of the ground-herb community in a terra-firme Central Amazonian forest. Acta Amazonica 34: 53-59.). In this study we focus on herbaceous species that spend their entire life cycle on the forest floor (Cestaro et al. 1986Cestaro LA, Waechter JL & Baptista LRM (1986) Fitossociologia do estrato herbáceo da Mata de Araucária da Estação Ecológica de Aracuri, Esmeralda, RS. Hoehnea 13: 59-72.; Costa 2004Costa FRC (2004) Structure and composition of the ground-herb community in a terra-firme Central Amazonian forest. Acta Amazonica 34: 53-59.). In tropical forests, herbs correspond from 8% up to 29% of the total species (Gentry & Dodson 1987Gentry AH & Dodson C (1987) Contribution of nontrees to species richness of a Tropical rainforest. Biotropica 19: 149-156.; Gentry & Emmons 1987Gentry AH & Emmons LH (1987) Geographical variation in fertility, phenology, and composition of the understory of neotropical forests. Biotropica 19: 216-22. ; Spicer et al. 2020Spicer ME, Mellor H & Carson WP (2020) Seeing beyond the trees: a comparison of tropical and temperate plant growth forms and their vertical distribution. Ecology 101: e02974.), and in some hyperdiverse regions previously inventoried, such as the Ecuadorian Amazon, they reach about 100 species per hectare (Poulsen & Balslev 1991Poulsen AD & Balslev H (1991) Abundance and cover of ground herbs in an Amazonian rainforest. Journal of Vegetation Science 2: 315-322.). In Central Amazonia, the diversity within this forest component is dominated by non-palm monocotyledons, ferns and lycophytes (Drucker et al. 2008Drucker D, Costa F & Magnusson W (2008) How wide is the riparian zone of small streams in tropical forests? A test with terrestrial herbs. Journal of Tropical Ecology 24: 65-74.; Moulatlet et al. 2014Moulatlet GM, Costa FRC, Rennó CD, Emilio T & Schietti J (2014) Local hydrological conditions explain floristic composition in lowland amazonian forests. Biotropica 46: 395-403.).
Amazon terra firme streams can hold a very diverse terrestrial herb community along their riverscapes (Drucker et al. 2008Drucker D, Costa F & Magnusson W (2008) How wide is the riparian zone of small streams in tropical forests? A test with terrestrial herbs. Journal of Tropical Ecology 24: 65-74.). Studying herbs along streams is crucial for understanding any Amazon diversity pattern, since it is estimated that small streams represent two-thirds of the total length in a river network (Leopold et al. 1964Leopold LB, Wolman, MG & Miller JP (1964) Fluvial processes in geomorphology. W.H. Freeman and Company, San Francisco. 506p.) and an area of one million square kilometers in the Amazon basin (Junk 1993Junk WJ (1993) Wetlands of Tropical South America. In: Whigham DF, Dykyjová D & Hejny S (eds.) Wetlands of the world: inventory, ecology and management. Dr. W. Junk Publ., Dordrecht. Pp. 679-739.). Riparian forests associated with streams can vary significantly through the landscape due to topographic and hydrological local changes, creating dynamic mosaics in terms of herbs community composition (Schietti et al. 2013Schietti J, Emilio T, Rennó CD, Drucker DP, Costa FRC, Nogueira A, Baccaro FB, Figueiredo F, Castilho CV, Kinupp V, Guillaumet J-L, Garcia ARM, Lima AP & Magnusson WE (2013) Vertical distance from drainage drives floristic composition changes in an Amazonian rainforest. Plant Ecology & Diversity 7: 241-253.), where no site is a replicate of another.
Amazonian riparian forests are protected by Brazilian Forest Code (Law 12.651/25/2012) aiming to maintain hydrological, edaphic and ecological services provided from biological processes of species that inhabit this aquatic-terrestrial interface. Unfortunately, riparian zones have become linear remnants throughout the Amazon basin due to its disordered human colonization history (Fearnside 2003Fearnside PM (2003) A floresta amazônica nas mudanças globais. Editora Inpa, Manaus. 134p.). Recently, the Amazon has been experiencing rising deforestation rates and devastation from river dam constructions, which alter stream and river connectivity in several ways, affecting both upstream and downstream freshwater ecosystems (Macedo & Castello 2015Macedo M & Castello L (2015) State of the Amazon: freshwater connectivity and ecosystem health. WWF Living Amazon Initiative, Brasília. 136p.).
In the Amazon Rainforest, knowledge on plant diversity distribution is extremely biased around very few relatively well-collected areas, while modelled diversity is uniformly high across most of the basin (Hopkins 2007Hopkins MJG (2007) Modelling the known and unknown plant biodiversity of the Amazon Basin. Journal of Biogeography 34: 1400-1411.), indicating that plant inventories are currently at insufficient density to represent Amazonian diversity patterns. Recent surveys targeting the ground-herb communities highlight the floristic and ecological importance of these growth forms in forest ecosystems of the region (e.g., Costa 2004Costa FRC (2004) Structure and composition of the ground-herb community in a terra-firme Central Amazonian forest. Acta Amazonica 34: 53-59.; Drucker et al. 2008Drucker D, Costa F & Magnusson W (2008) How wide is the riparian zone of small streams in tropical forests? A test with terrestrial herbs. Journal of Tropical Ecology 24: 65-74.; Zuquim et al. 2012Zuquim G, Tuomisto H, Costa FRC, Prado J, Magnusson WE, Pimentel T, Braga-Neto R & Figueiredo FO (2012) Broad scale distribution of ferns and lycophytes along environmental gradients in Central and Northern Amazonia, Brazil. Biotropica 44: 752-762. doi: 10.1111/j.1744-7429.2012.00880.x; Moulatlet et al. 2014Moulatlet GM, Costa FRC, Rennó CD, Emilio T & Schietti J (2014) Local hydrological conditions explain floristic composition in lowland amazonian forests. Biotropica 46: 395-403.). Moreover, it is important to recognize riparian specimens within this group, both because of its ecological role and habitat conservation implications. Here, we present an inventory of riparian ground-herb of the poorly known lower Tapajós region.
Material and Methods
Study area
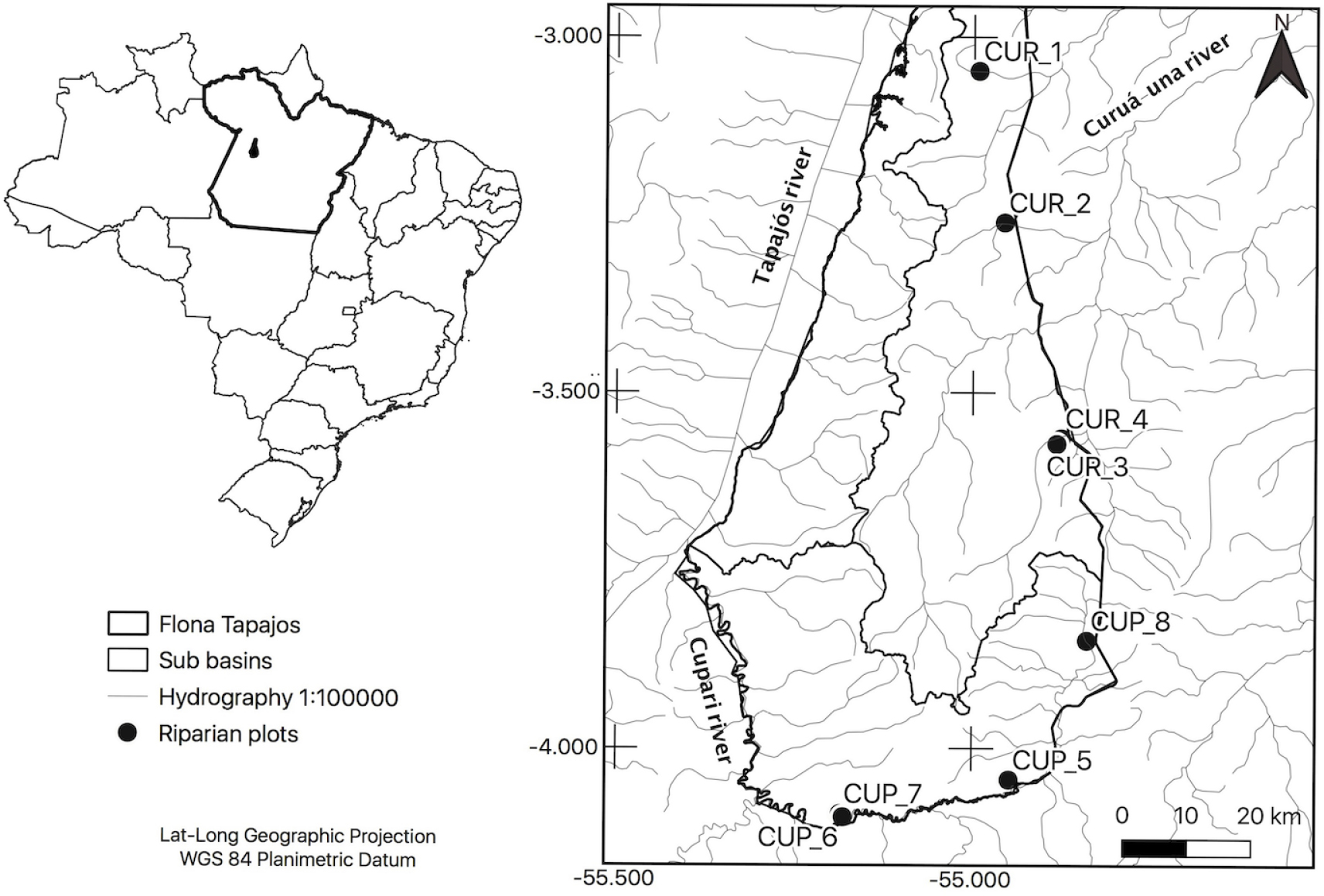
This study was conducted in the Brazilian national sustainable use protected area Floresta Nacional do Tapajós (FLONA Tapajós hereafter), located on the right bank of the Tapajós River, Brazilian Amazon (4.022310-3.744208 degrees South, 54.921920-55.392877 degrees West, <https://www.icmbio.gov.br/flonatapajos/mapas-e-limites.html>). FLONA Tapajós covers 527,319 hectares (Ibama 2004Ibama (2004) Floresta Nacional do Tapajós, Plano de Manejo. Ministério do meio ambiente, Brasília. 580p. ) and is mostly vegetated by Dense Ombrophilous Forest (Veloso et al. 1991Veloso HP, Rangel Filho ALR & Lima JCA (1991) Classificação da vegetação brasileira, adaptada a um sistema universal. IBGE, Rio de Janeiro. 123p.) over Dystrophic Yellow Latosols, deep and low cation exchange soils (Hernandez et al. 1993Hernandez Filho P, Shimabukuro YE, Lee DCL, Santos Filho CP & Almeida RR (1993) Relatório Final do Projeto de Inventário Florestal Na Floresta Nacional do Tapajós. INPE, São José dos Campos. 126p.). Three distinct drainages occur within FLONA Tapajós: the Tapajós, Curuá-Una and Cupari River basins (Silva-Oliveira et al. 2016Silva-Oliveira C, Canto ALC & Ribeiro FRV (2016) Stream ichthyofauna of the Tapajós National Forest, Pará, Brazil. ZooKeys 580: 125-144., Fig. 1). The Cupari River is the right tributary of the lower Tapajós River, and its main tributaries are the Braço Leste and Braço Oeste rivers (Riós-Villamizar et al. 2014Ríos-Villamizar EA, Piedade MTF, Costa JG, Adeney JM & Junk WJ (2014) Chemistry of different Amazonian water types for river classification: a preliminary review. WIT Transactions on Ecology and The Environment 178: 17-28. ), with part of its basin located in the FLONA Tapajós area. The Curuá-Una River is a tributary of the Amazon River and has several protected headwaters in FLONA Tapajós (Fig. 1). Plots were implemented in the Curuá-Una and Cupari basins within the protected area with the view to maintain these plots for long term studies. Riparian plots were randomly selected considering at least 1 km minimum distance. Sampling size was balanced between the two drainages in our study site exclusively to improve ecological and spatial heterogeneity.
Sampling and data analysis
Plant sampling occurred in May, November and December 2018 and February and May 2019. For community assessment, eight riparian plots were installed in permanent streams of terra firme forest, four plots in the Curuá-Una River basin and four plots in the Cupari River basin. Ground-herbs were collected according to the Protocol for Surveys of Ferns and Lycophytes in PPBio RAPELD Modules (available at <https://ppbio.inpa.gov.br/>), in 1.5 × 250 m plots, with the longer axis parallel and adjacent to stream’s left or right margin. The assemblages surveyed here were classified as a set of vascular ground-herb species, including only those species that germinate and spend their entire life cycle on the forest floor (Poulsen 1996Poulsen AD (1996) Species richness and density of ground herbs within a plot of lowland rainforest in north-west Borneo. Journal of Tropical Ecology 12: 177-190.), belonging to the lineages of ferns, lycophytes and monocotyledons. Palms (Arecaceae Bercht. & J. Presl) were excluded because even the more herbaceous life-forms tend to occupy shrub or even tree forest strata at some point in their life cycle. In each riparian plot, all individuals taller than 5 cm and rooted within the plots were counted and identified at the lowest possible taxonomic level. Voucher specimens for every individual were collected. We followed Costa et al. (2005)Costa FRC, Magnusson WE & Luizão RC (2005) Mesoscale distribution patterns of Amazonian understory herbs in relation to topography, soil and watersheds. Journal of Ecology 93: 863-878. to distinguish and count individuals, considering groups of stems or leaves/fronds that were at least 20 cm apart. For species that occur in high density in a small area (e.g., Adiantum and Selaginella), forming large mats, impeding distinction between individuals, we counted each stem as an individual, following Moulatlet et al. (2014)Moulatlet GM, Costa FRC, Rennó CD, Emilio T & Schietti J (2014) Local hydrological conditions explain floristic composition in lowland amazonian forests. Biotropica 46: 395-403.. All fertile botanical material was deposited at the HSTM herbarium, Universidade Federal do Oeste do Pará (barcode numbers 13,332–13,375 and 13,983–13,993).
The identification of species was based on the PPBio / INPA Identification Guides (<https://ppbio.inpa.gov.br/guias>) for Marantaceae (Costa et al. 2008Costa FRC, Espinelli FP & Figueiredo FOG (2008) Guia de marantáceas da Reserva Ducke e da Reserva Biológica do Uatumã - Guide to the Marantaceae of the Reserva Ducke and Reserva Biológica do Uatumã. Editora Inpa, Manaus. 154p. ), Zingiberales (Costa et al. 2011Costa FRC, Espinelli FP & Figueiredo FOG (2011) Guia de Zingiberales dos sítios PPBio na Amazônia Ocidental Brasileira. Átema Design Editorial, Manaus. 284p.) and Ferns and Lycophytes (Zuquim et al. 2008Zuquim G, Costa FRC, Prado J & Tuomisto H (2008) Guia de identificação das samambaias e licófitas da REBIO Uatumã, Amazônia Central. Áttema Design Editorial, Manaus. 321p. ). Identification keys were also used (Kramer 1957Kramer KU (1957) A revision of the genus Lindsaea in the new world with notes on allied genera. Acta Botanica Neerlandica 6: 97-290. ; Alston et al. 1981Alston AHG, Jermy AC & Rankin JM (1981) The genus Selaginella in tropical South America. Bulletin of the British Museum (Natural history) Botany Series 4: 233-330. ; Tryon & Stolze 1989aTryon RM & Stolze RG (1989a) Pteridophyta of Peru: pt.1. 1. Ophioglossaceae - 12. Cyatheaceae. Fieldiana 20: 1-145. , 1989bTryon RM & Stolze RG (1989b) Pteridophyta of Peru: pt.2. 13. Pteridaceae - 15. Dennstaedtiaceae. Fieldiana 22: 1-128. ; Tuomisto & Groot 1995Tuomisto H & Groot AT (1995) Identification of the juveniles of some ferns from western Amazonia. American Fern Journal 85: 1-28. ; Steyermark et al. 1995Steyermark JA, Berry PE & Holst BK (1995) Flora of the Venezuelan Guayana. Vol. 2. Pteridophytes and Spermatophytes (Acanthaceae - Araceae). Missouri Botanical Garden Press, St Louis. 681p. ; Windisch 1996Windisch PG (1996) Pteridófitas do estado do Mato Grosso: Hymenophyllaceae. Bradea 6: 1-22. ; Mori et al. 1997Mori SA, Cremers G, Gracie C, Degranville J-J, Hoff M & Mitchell JD (1997) Guide to the vascular plants of central French Guiana. Vol. 1. Pteridophytes, gymnosperms and monocotyledons. Memoirs of the New York Botanical Garden 76: 1-422.; Prado & Moran 2008Prado J & Moran RC (2008) Revision of the neotropical species of Triplophyllum (Tectariaceae). Brittonia 60: 103-130.) as was consultation with specialists and identified material already deposited in herbaria.
For each plot, we calculated species richness, overall abundance and Fisher’s alpha (Magurran 2004Magurran AE (2004) Measuring biological diversity. Blackwell Publishing, Malden. 256p.). Species composition of each plot was compared by pairwise Bray-Curtis distances. Species accumulation curves were constructed to verify sampling effort. All estimates were calculated with the vegan package version 2.5 (Oksanen et al. 2019Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Stevens MHH, Szoecs E & Wagner H (2019) Vegan: community ecology package. R package version 2.5-6. Available at <https://CRAN.R-project.org/package=vegan>. Access on 15 December 2019.
https://CRAN.R-project.org/package=vegan...
) in R (R Development Core Team 2020).
Results
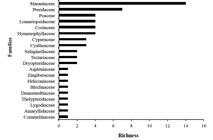
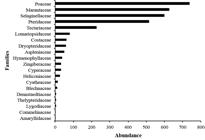
A total of 3,130 individuals and 58 ground-herb species belonging to 27 genera and 20 families were recorded in riparian plots in the lower Tapajós River region (Tab. 1). The richest families were: Marantaceae (14 spp; 24.14%), Pteridaceae (7 spp; 12.07%), Poaceae (4 spp; 6.9%), Lomariopsidaceae (4 spp; 6.9%), Costaceae (4 spp; 6.9%) and Hymenophyllaceae (4 spp; 6.9%) that together comprise 63.79% (37 spp) of all species recorded. The remaining families had one to three species, comprising 36.21% of the total richness (Fig. 2). Considering the abundance of species, the families Poaceae (738 indiv.), Marantaceae (627), Selaginellaceae (599) and Pteridaceae (516) corresponded to 79.23% of the total number of individuals (Fig. 3).
Species richness for each riparian ground-herb family in the lower Tapajós region, terra firme forests, Brazilian Amazon.
Species abundance for each riparian ground-herb family in the lower Tapajós region, terra firme forests, Brazilian Amazon.
Riparian ground-herbs of lower Tapajós region, terra firme forests, Brazilian Amazon. Species from eight 1.5 × 250 m plots. Abun = abundance (total individual number per species); F% = species frequency (number of plots in which the species occurred).
The most frequent species was the fern Triplophyllum glabrum (Tectariaceae), distributed in seven of the eight plots (88%). Three other species were distributed in six plots (75%): Selaginella conduplicata, Pariana sp. and Ischnosiphon puberulus. The most abundant species were Selaginella conduplicata (574), Pariana sp. (497), Adiantum argutum (386), Triplophyllum glabrum (214), Piresia goeldi (123) and Ischnosiphon martianus (111), which together account for 60.86% of the total inventoried individuals (Tab. 1).
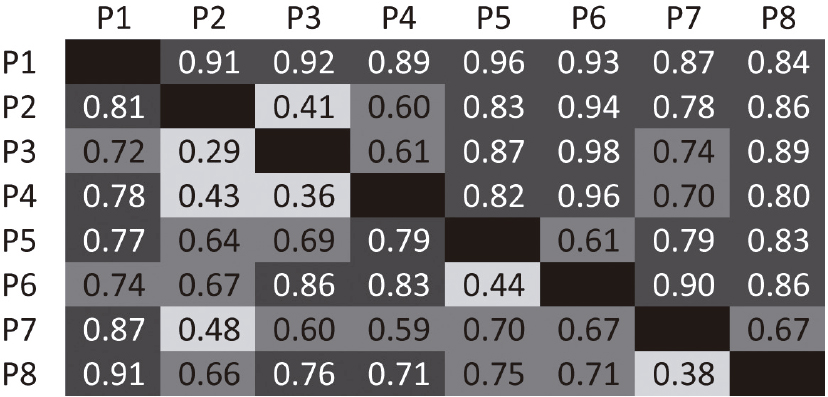
Riparian ground-herb floristic dissimilarity matrix reveals high complementarity among plots, for both quantitative and qualitative data inputs using Bray Curtis distance (Fig. 4). The species accumulation curve shows a steady and gradual rise in the number of species per additional plot and a tendency to asymptote species number for our data based on richness estimators (Fig. 5).
Riparian ground-herb dissimilarity matrix (Bray Curtis distances) from eight plots in the lower Tapajós region, terra firme forests, Brazilian Amazon. Darker cells indicate higher dissimilarity. Lower diagonal cells are Bray Curtis distances calculated from quantitative data, while upper diagonal cells are binary Bray Curtis distances, which considers species presence/absence only.
Species accumulation curves from eight riparian ground-herb community plots in the lower Tapajós region, terra firme forests, Brazilian Amazon. Solid line for exact and dashed line for Jackknife 1 estimator.
Discussion
Riparian ground-herbs are an important component of terra firme forests in the lower Tapajós River region, given their large species representativeness. We identified 58 species in 8 plots (summing 0.3 ha). The richest families in this inventory, i.e. Marantaceae, Pteridaceae, Poaceae, Lomariopsidaceae, Costaceae and Hymenophyllaceae, are also the richest in environments including non-riparian forests of Central and Eastern Amazon (Costa 2004Costa FRC (2004) Structure and composition of the ground-herb community in a terra-firme Central Amazonian forest. Acta Amazonica 34: 53-59.; Zuquim et al. 2008Zuquim G, Costa FRC, Prado J & Tuomisto H (2008) Guia de identificação das samambaias e licófitas da REBIO Uatumã, Amazônia Central. Áttema Design Editorial, Manaus. 321p. ; Costa & Pietrobom 2010Costa JM & Pietrobom MR (2010) Samambaias e licófitas do Parque Estadual do Gunma, município de Santa Bárbara do Pará, estado do Pará, Brasil. Rodriguésia 61: 223-232.; Pallos et al. 2016Pallos J, Góes-Neto LAA, Costa JM, Souza FS & Pietrobom MR (2016) Licófitas e samambaias da Serra do Itauajuri, município de Monte Alegre, Pará, Brasil. Rodriguésia 67: 997-1009. <https://dx.doi.org/10.1590/2175-7860201667410>).
Marantaceae is an important component of this inventory, having 14 species and three genera, and represented in 627 individuals. This family is characteristic of neotropical forests understory (Gentry & Emmons 1987Gentry AH & Emmons LH (1987) Geographical variation in fertility, phenology, and composition of the understory of neotropical forests. Biotropica 19: 216-22. ; Kennedy 2000Kennedy H (2000) Diversification in pollination mechanisms in the Marantaceae. In: Wilson KL & Morrison DA (eds.) Monocots: systematics and evolution. Publishing Collingwood, Sydney. Pp. 335-344.). Ischnosiphon is the richest genus of this family in our study site. Studies conducted in riparian zones of Central Amazon, in permanent plots of the Adolpho Ducke Forest Reserve north of Manaus and in BR-319 in the Purus-Madeira interfluve, also found Marantaceae as a component of relative importance for ground-herb diversity, reaching the highest species richness values in both areas (Costa 2004Costa FRC (2004) Structure and composition of the ground-herb community in a terra-firme Central Amazonian forest. Acta Amazonica 34: 53-59.; Drucker et al. 2008Drucker D, Costa F & Magnusson W (2008) How wide is the riparian zone of small streams in tropical forests? A test with terrestrial herbs. Journal of Tropical Ecology 24: 65-74.; Moulatlet et al. 2014Moulatlet GM, Costa FRC, Rennó CD, Emilio T & Schietti J (2014) Local hydrological conditions explain floristic composition in lowland amazonian forests. Biotropica 46: 395-403.).
Our study demonstrates the importance of herbs that constitute large population densities in the riparian vegetation of terra firme forests. The families Poaceae (738 ind. and 4 spp), Selaginellaceae (599 ind. and 2 spp) and Pteridaceae (516 ind. and 7spp), are three of the most abundant and represent 59.2% of the individuals and 22.4% species of ground-herbs studied here. The Poaceae occurred in almost all plots, often forming dense clusters. Its species produce large quantities of flowers and seeds and the family was also the one with the highest density of 1 ha in Ecuador (Poulsen & Balslev 1991Poulsen AD & Balslev H (1991) Abundance and cover of ground herbs in an Amazonian rainforest. Journal of Vegetation Science 2: 315-322.). Pteridaceae and Selaginellaceae are families that are related to wet soils, as the banks of rivers and streams (Paixão et al. 2013Paixão EC, Noronha JC, Cunha CN & Arruda R (2013) More than light: distance-dependent variation on riparian fern community in Southern Amazonia. Brazilian Journal of Botany 36: 25-30. <http://doi.org/10.1007/s40415-013-0003-8>), which could be the reason for its high density in riparian areas. The genera Adiantum and Selaginella, the only representatives of these families in the present study, are among the most representative fern genera in the Brazilian Amazon and were find as most abundant groups in other inventories (e.g., Zuquim et al. 2009Zuquim G, Costa FRC, Prado J & Braga-Neto R (2009) Distribution of pteridophyte communities along environmental gradients in Central Amazonia, Brazil. Biodiversity and Conservation 18: 151-166., 2014; Pansonato et al. 2013Pansonato MP, Costa FRC, Castilho CV, Carvalho FA & Zuquim G (2013) Spatial scale or amplitude of predictors as determinants of the relative importance of environmental factors to plant community structure. Biotropica 45: 299-307.).
Local diversity (Fisher’s alpha, Tab. 2) is highly variable among plots, probably as a result of local environmental characteristics. Soil type and light conditions, for example, can vary broadly, since our samples were distributed along headwaters of two watersheds and comprise an area with a radius of about 115 km and different geological formation (Tuomisto et al. 2019Tuomisto H, Van doninck J, Ruokolainen K, Moulatlet GM, Figueiredo FOG, Sirén A, Cárdenas G, Lehtonen S & Zuquim G (2019) Discovering floristic and geoecological gradients across Amazonia. Journal of Biogeography 46: 1734-1748.). Additionally, we found great complementarity among the riparian herb communities, observed here by means of pairwise dissimilarity (Fig. 4) and the estimated number of species (Fig. 5). This high community species turnover has been already documented for Central Amazonia (Schietti et al. 2013Schietti J, Emilio T, Rennó CD, Drucker DP, Costa FRC, Nogueira A, Baccaro FB, Figueiredo F, Castilho CV, Kinupp V, Guillaumet J-L, Garcia ARM, Lima AP & Magnusson WE (2013) Vertical distance from drainage drives floristic composition changes in an Amazonian rainforest. Plant Ecology & Diversity 7: 241-253.), where waterlogged areas accumulate more community dissimilarity than upper and dryer areas into the same watershed. This finding highlights the relevance of including riparian environments in botanical and ecological inventories, in such a threatened habitat.
Riparian ground-herb richness, abundance and Fisher’s alpha of the lower Tapajós region, terra firme forests, Brazilian Amazon.
We found 58 species of understory ground-herbs in just 0.3 ha at lowland riparian forests located in the Tapajós National Forest. For instance, Drucker et al. (2008)Drucker D, Costa F & Magnusson W (2008) How wide is the riparian zone of small streams in tropical forests? A test with terrestrial herbs. Journal of Tropical Ecology 24: 65-74. found 75 species in 40 plots (2 × 100 m, 0.8 ha) at Reserva Florestal Adolpho Ducke, north of Manaus, where most species (61) were recorded within the riparian zone, of which 29 species did not occur elsewhere; and Moulatlet et al. (2014)Moulatlet GM, Costa FRC, Rennó CD, Emilio T & Schietti J (2014) Local hydrological conditions explain floristic composition in lowland amazonian forests. Biotropica 46: 395-403. reported 148 species in 88 plots (2 × 250 m, 4.4 ha) at BR-319 highway sites in a flat regional landscape. These results reiterate the importance of standardized inventories by area (such as RAPELD plots, Magnusson et al. 2005Magnusson WE, Lima AP, Luizão R, Luizão F, Costa FRC, Castilho CV & Kinupp VF (2005) RAPELD: a modification of the Gentry method for biodiversity surveys in long-term ecological research sites. Biota Neotropica 5: 19-24.), particularly in conservation units, for the advance of scientific knowledge and for subsidizing biodiversity monitoring and conservation practices.
Acknowledgements
This study was funded by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (Capes) - Finance Code 001, and by PELD do Oeste do Pará - POPA (Conselho Nacional de Desenvolvimento Científico e Tecnológico Pesquisa: CNPq/Capes/FAPs/BC-Fundo Newton PELD nº 15/2016, nº processo 441443/2016-8). Scholarships were provided to the following authors by the following agencies: DBR, Fundação Amazônia de Amparo a Estudos e Pesquisas do Estado do Pará (Fapespa); MO, Capes; AS, CNPq Pibic. We are thankful to A.R. Field, for English review; and to A.C.S. and J.M.O., for field assistance. We are also thankful for PROCAD-AM/CAPES, Nº 88887.200472/2018-00 support.
References
- Alston AHG, Jermy AC & Rankin JM (1981) The genus Selaginella in tropical South America. Bulletin of the British Museum (Natural history) Botany Series 4: 233-330.
- APG IV - Angiosperm Phylogeny Group (2016) An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Botanical Journal of the Linnean Society 181: 1-20.
- Capon SJ, Chambers LE, Mac Nally R, Naiman RJ, Davies P, Marshall N, Pittock J, Reid M, Capon T, Douglas M, Catford J, Baldwin DS, Stewardson M, Roberts J, Parsons M & Williams SE (2013) Riparian ecosystems in the 21th century: hotspot for climate change adaptation? Ecosystems 16: 359-381.
- Cestaro LA, Waechter JL & Baptista LRM (1986) Fitossociologia do estrato herbáceo da Mata de Araucária da Estação Ecológica de Aracuri, Esmeralda, RS. Hoehnea 13: 59-72.
- Costa FRC (2004) Structure and composition of the ground-herb community in a terra-firme Central Amazonian forest. Acta Amazonica 34: 53-59.
- Costa FRC, Magnusson WE & Luizão RC (2005) Mesoscale distribution patterns of Amazonian understory herbs in relation to topography, soil and watersheds. Journal of Ecology 93: 863-878.
- Costa FRC, Espinelli FP & Figueiredo FOG (2008) Guia de marantáceas da Reserva Ducke e da Reserva Biológica do Uatumã - Guide to the Marantaceae of the Reserva Ducke and Reserva Biológica do Uatumã. Editora Inpa, Manaus. 154p.
- Costa FRC, Espinelli FP & Figueiredo FOG (2011) Guia de Zingiberales dos sítios PPBio na Amazônia Ocidental Brasileira. Átema Design Editorial, Manaus. 284p.
- Costa JM & Pietrobom MR (2010) Samambaias e licófitas do Parque Estadual do Gunma, município de Santa Bárbara do Pará, estado do Pará, Brasil. Rodriguésia 61: 223-232.
- Drucker D, Costa F & Magnusson W (2008) How wide is the riparian zone of small streams in tropical forests? A test with terrestrial herbs. Journal of Tropical Ecology 24: 65-74.
- Fearnside PM (2003) A floresta amazônica nas mudanças globais. Editora Inpa, Manaus. 134p.
- Gentry AH & Dodson C (1987) Contribution of nontrees to species richness of a Tropical rainforest. Biotropica 19: 149-156.
- Gentry AH & Emmons LH (1987) Geographical variation in fertility, phenology, and composition of the understory of neotropical forests. Biotropica 19: 216-22.
- Gilliam FS, Turrill NL & Adams MB (1995) Herbaceous-layer and overstory species in clear-cut and mature Central Appalachian Hardwood Forests. Ecological Applications 5: 947-955.
- Hernandez Filho P, Shimabukuro YE, Lee DCL, Santos Filho CP & Almeida RR (1993) Relatório Final do Projeto de Inventário Florestal Na Floresta Nacional do Tapajós. INPE, São José dos Campos. 126p.
- Hopkins MJG (2007) Modelling the known and unknown plant biodiversity of the Amazon Basin. Journal of Biogeography 34: 1400-1411.
- Ibama (2004) Floresta Nacional do Tapajós, Plano de Manejo. Ministério do meio ambiente, Brasília. 580p.
- Junk WJ (1993) Wetlands of Tropical South America. In: Whigham DF, Dykyjová D & Hejny S (eds.) Wetlands of the world: inventory, ecology and management. Dr. W. Junk Publ., Dordrecht. Pp. 679-739.
- Kennedy H (2000) Diversification in pollination mechanisms in the Marantaceae. In: Wilson KL & Morrison DA (eds.) Monocots: systematics and evolution. Publishing Collingwood, Sydney. Pp. 335-344.
- Kramer KU (1957) A revision of the genus Lindsaea in the new world with notes on allied genera. Acta Botanica Neerlandica 6: 97-290.
- Leopold LB, Wolman, MG & Miller JP (1964) Fluvial processes in geomorphology. W.H. Freeman and Company, San Francisco. 506p.
- Macedo M & Castello L (2015) State of the Amazon: freshwater connectivity and ecosystem health. WWF Living Amazon Initiative, Brasília. 136p.
- Magnusson WE, Lima AP, Luizão R, Luizão F, Costa FRC, Castilho CV & Kinupp VF (2005) RAPELD: a modification of the Gentry method for biodiversity surveys in long-term ecological research sites. Biota Neotropica 5: 19-24.
- Magurran AE (2004) Measuring biological diversity. Blackwell Publishing, Malden. 256p.
- Martins SB (2007) Recuperação de Matas Ciliares. 2a ed. Editora Aprenda Fácil, Viçosa. 255p.
- Mori SA, Cremers G, Gracie C, Degranville J-J, Hoff M & Mitchell JD (1997) Guide to the vascular plants of central French Guiana. Vol. 1. Pteridophytes, gymnosperms and monocotyledons. Memoirs of the New York Botanical Garden 76: 1-422.
- Moulatlet GM, Costa FRC, Rennó CD, Emilio T & Schietti J (2014) Local hydrological conditions explain floristic composition in lowland amazonian forests. Biotropica 46: 395-403.
- Naiman RJ, Decamps H & McClain ME (2005) Riparia. Ecology, conservation and management of streamside communities. Elsevier Academic Press, London. 488p.
- Naiman RJ, Decamps H & Pollock M (1993) The role of riparian corridors in maintaining regional biodiversity. Ecological Applications 3: 209-212.
- Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Stevens MHH, Szoecs E & Wagner H (2019) Vegan: community ecology package. R package version 2.5-6. Available at <https://CRAN.R-project.org/package=vegan>. Access on 15 December 2019.
» https://CRAN.R-project.org/package=vegan - Paixão EC, Noronha JC, Cunha CN & Arruda R (2013) More than light: distance-dependent variation on riparian fern community in Southern Amazonia. Brazilian Journal of Botany 36: 25-30. <http://doi.org/10.1007/s40415-013-0003-8>
- Pallos J, Góes-Neto LAA, Costa JM, Souza FS & Pietrobom MR (2016) Licófitas e samambaias da Serra do Itauajuri, município de Monte Alegre, Pará, Brasil. Rodriguésia 67: 997-1009. <https://dx.doi.org/10.1590/2175-7860201667410>
- Pansonato MP, Costa FRC, Castilho CV, Carvalho FA & Zuquim G (2013) Spatial scale or amplitude of predictors as determinants of the relative importance of environmental factors to plant community structure. Biotropica 45: 299-307.
- Pokrovsky OS (2016) Riparian zones: characteristics, management, practices and ecological impacts. Nova Science Publishers, New York. 383p.
- Poulsen AD (1996) Species richness and density of ground herbs within a plot of lowland rainforest in north-west Borneo. Journal of Tropical Ecology 12: 177-190.
- Poulsen AD & Balslev H (1991) Abundance and cover of ground herbs in an Amazonian rainforest. Journal of Vegetation Science 2: 315-322.
- Prado J & Moran RC (2008) Revision of the neotropical species of Triplophyllum (Tectariaceae). Brittonia 60: 103-130.
- PPG - Pteridophyte Phylogeny Group (2016) A community-derived classification for extant lycophytes and ferns. Journal of Systematics and Evolution 54: 563-603.
- R Core Team (2020). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available at <https://www.R-project.org/> . Access on 15 December 2019.
» https://www.R-project.org/ - Ríos-Villamizar EA, Piedade MTF, Costa JG, Adeney JM & Junk WJ (2014) Chemistry of different Amazonian water types for river classification: a preliminary review. WIT Transactions on Ecology and The Environment 178: 17-28.
- Schietti J, Emilio T, Rennó CD, Drucker DP, Costa FRC, Nogueira A, Baccaro FB, Figueiredo F, Castilho CV, Kinupp V, Guillaumet J-L, Garcia ARM, Lima AP & Magnusson WE (2013) Vertical distance from drainage drives floristic composition changes in an Amazonian rainforest. Plant Ecology & Diversity 7: 241-253.
- Silva-Oliveira C, Canto ALC & Ribeiro FRV (2016) Stream ichthyofauna of the Tapajós National Forest, Pará, Brazil. ZooKeys 580: 125-144.
- Spicer ME, Mellor H & Carson WP (2020) Seeing beyond the trees: a comparison of tropical and temperate plant growth forms and their vertical distribution. Ecology 101: e02974.
- Steyermark JA, Berry PE & Holst BK (1995) Flora of the Venezuelan Guayana. Vol. 2. Pteridophytes and Spermatophytes (Acanthaceae - Araceae). Missouri Botanical Garden Press, St Louis. 681p.
- Tryon RM & Stolze RG (1989a) Pteridophyta of Peru: pt.1. 1. Ophioglossaceae - 12. Cyatheaceae. Fieldiana 20: 1-145.
- Tryon RM & Stolze RG (1989b) Pteridophyta of Peru: pt.2. 13. Pteridaceae - 15. Dennstaedtiaceae. Fieldiana 22: 1-128.
- Tuomisto H & Groot AT (1995) Identification of the juveniles of some ferns from western Amazonia. American Fern Journal 85: 1-28.
- Tuomisto H, Van doninck J, Ruokolainen K, Moulatlet GM, Figueiredo FOG, Sirén A, Cárdenas G, Lehtonen S & Zuquim G (2019) Discovering floristic and geoecological gradients across Amazonia. Journal of Biogeography 46: 1734-1748.
- Veloso HP, Rangel Filho ALR & Lima JCA (1991) Classificação da vegetação brasileira, adaptada a um sistema universal. IBGE, Rio de Janeiro. 123p.
- Windisch PG (1996) Pteridófitas do estado do Mato Grosso: Hymenophyllaceae. Bradea 6: 1-22.
- Zickel CS (1995) Fitossociologia e dinâmica do estrato herbáceo de dois fragmentos florestais do estado de São Paulo. Tese de Doutorado. Universidade Estadual de Campinas, Campinas. 125p.
- Zuquim G, Costa FRC, Prado J & Tuomisto H (2008) Guia de identificação das samambaias e licófitas da REBIO Uatumã, Amazônia Central. Áttema Design Editorial, Manaus. 321p.
- Zuquim G, Costa FRC, Prado J & Braga-Neto R (2009) Distribution of pteridophyte communities along environmental gradients in Central Amazonia, Brazil. Biodiversity and Conservation 18: 151-166.
- Zuquim G, Tuomisto H, Costa FRC, Prado J, Magnusson WE, Pimentel T, Braga-Neto R & Figueiredo FO (2012) Broad scale distribution of ferns and lycophytes along environmental gradients in Central and Northern Amazonia, Brazil. Biotropica 44: 752-762. doi: 10.1111/j.1744-7429.2012.00880.x
- Zuquim G, Tuomisto H, Jones MM, Prado J, Figueiredo FOG, Moulatlet GM, Costa FRC, Quesada CA & Emilio T (2014) Predicting environmental gradients with fern species composition in Brazilian Amazonia. Journal of Vegetation Science 25: 1195-1207.
Edited by
Publication Dates
-
Publication in this collection
22 Oct 2021 -
Date of issue
2021
History
-
Received
13 Jan 2020 -
Accepted
29 Aug 2020