Abstract
We evaluated the diversity of Myrtaceae in Chapada Diamantina National Park (CDNP) and neighboring municipalities (NM), identifying the areas with the highest richness and those with the lowest sampling efforts, collecting specimens in situ, and analyzing herbarium collections. The geographic data and maps include collection efforts (CE), species richness (SR), and estimated richness (J1). Ninety-seven species and nine genera were recorded for NM, with 82 species and nine genera occurring in CDNP. The CE and SR were similar in NM and CDNP, and the quadrants with the highest values were in the municipalities of Lençóis and Palmeiras. The J1 was also similar in NM and CDNP. Fifteen species found in NM do not occur in CDNP, and some are considered endangered or are restricted to non-protected areas, especially caatinga areas. Some species showed distributions limited to only one or two vegetation types, although many areas have been only superficially surveyed. Endemic species are subject to anthropic pressure, and some are currently considered endangered or vulnerable. The expansion of the limits of the CDNP to encompass areas of caatinga vegetation will improve the conservation status of the family.
Key words
caatinga; campo rupestre; cerrado; Inland Atlantic Forest; Myrteae
Resumo
Avaliamos a diversidade de Myrtaceae no Parque Nacional da Chapada Diamantina (CDNP) e nos municípios vizinhos (NM), identificando as áreas com maior riqueza e aquelas com menor esforço amostral, coletando amostras in situ e analisando as coleções de herbário. Os dados e mapas geográficos incluem esforços de coleta (CE), riqueza de espécies (SR) e riqueza estimada (J1). Noventa e sete espécies e nove gêneros foram registrados para NM, com 82 espécies e nove gêneros ocorrendo no CDNP. A CE e SR foram semelhantes no NM e CDNP, e os quadrantes com os maiores valores foram nos municípios de Lençóis e Palmeiras. O J1 também foi semelhante no NM e CDNP. Quinze espécies encontradas no NM não ocorrem no CDNP, e algumas são consideradas ameaçadas ou restritas a áreas não protegidas, principalmente as áreas de caatinga. Algumas espécies apresentaram distribuições limitadas a apenas um ou dois tipos de vegetação, embora muitas áreas tenham sido apenas superficialmente pesquisadas. As espécies endêmicas estão sujeitas a pressão antrópica e algumas são atualmente consideradas ameaçadas ou vulneráveis. A expansão dos limites do CDNP para abranger áreas da vegetação da caatinga melhorará o status de conservação da família.
Palavras-chave
caatinga; campo rupestre; cerrado; Floresta Atlântica; Myrteae
Introduction
Information concerning the diversity and distribution of plant species is essential for studies of phytogeography, ecology, and conservation (CBD 2010CBD - Convention on Biological Diversity (2010) Global Biodiversity Outlook 3. Progress Press, Montréal. 94p. ), although data is often scarce in tropical regions - which can hinder effective conservation planning (Antonelli et al. 2015Antonelli A, Zizka A, Silvestro D, Scharn R, Cascales-Miñana B & Bacon CD (2015) An engine for global plant diversity: highest evolutionary turnover and emigration in the American tropics. Frontiers in Genetics 6: 130. DOI: 10.3389/fgene.2015.00130.
https://doi.org/10.3389/fgene.2015.00130...
; Maldonado et al. 2015Maldonado C, Molina CI, Zizka A, Persson C, Taylor CM, Albán J, Chiquillo E, Rønsted N & Antonelli A (2015) Estimating species diversity and distribution in the era of Big Data: to what extent can we trust public databases? Global Ecology and Biogeography 24: 973-984. DOI: <https://doi.org/10.1111/geb.12326>). Biodiversity conservation efforts must be efficient and ensure that species are adequately protected, and protection sites that are truly representative of the regional biota must be selected (Margules & Pressey 2000Margules CR & Pressey RL (2000). Systematic conservation planning. Nature 405: 243. DOI: <https://doi.org/10.1038/35012251>
https://doi.org/10.1038/35012251...
).
Brazil is the world’s most diverse country floristically, with more than 30,000 species of angiosperms (BFG 2018BFG - The Brazil Flora Group (2018) Brazilian Flora 2020: innovation and collaboration to meet Target 1 of the Global Strategy for Plant Conservation (GSPC). Rodriguésia 69: 1513-1527. DOI: 10.1590/2175-7860201869402.
https://doi.org/10.1590/2175-78602018694...
). One of its diversity hotspots is the Espinhaço Range, which, by itself, comprises almost 15% of the Brazilian vascular flora (Silveira et al. 2016Silveira FAO, Negreiros D, Barbosa NPU, Buisson E, Carmo FF, Carstensen DW, Conceição AA, Cornelissen TG, Echternacht L, Fernandes GW, Garcia QS, Guerra TJ, Jacobi CM, Lemos-Filho JP, Le Stradic S, Morellato LPC, Neves FS, Oliveira RS, Schaefer CE, Viana PL & Lambers H (2016) Ecology and evolution of plant diversity in the endangered campo rupestre: a neglected conservation priority. Plant Soil 403: 129-152. DOI: 10.1007/s11104-015-2637-8.
https://doi.org/10.1007/s11104-015-2637-...
). The Espinhaço Range extends into Bahia state as the Chapada Diamantina mountain range, in the center of the semiarid region, and it harbors a mosaic of campo rupestre, cerrado, Atlantic forest, and caatinga vegetations (Funch et al. 2009Funch RR, Harley RM & Funch LS (2009) Mapping and evaluation of the state of conservation of the vegetation in and surrounding the Chapada Diamantina National Park, NE Brazil. Biota Neotropica 9: 21-30. DOI: <http://dx.doi.org/10.1590/S1676-06032009000200001>
https://doi.org/<http://dx.doi.org/10.15...
; Beserra et al. 2007Beserra MML, Ferreira LM, Gonçalves CN, Casella PLC & Ferreira JG (2007) Plano de Manejo do Parque Nacional da Chapada Diamantina. Instituto Chico Mendes, Brasília. 657p.). The largest legal protection area outside the Amazon is the Chapada Diamantina National Park (CDNP) in the Serra do Sincorá Range within the Chapada Diamantina (Funch et al. 2009Funch RR, Harley RM & Funch LS (2009) Mapping and evaluation of the state of conservation of the vegetation in and surrounding the Chapada Diamantina National Park, NE Brazil. Biota Neotropica 9: 21-30. DOI: <http://dx.doi.org/10.1590/S1676-06032009000200001>
https://doi.org/<http://dx.doi.org/10.15...
). The CDNP was originally designed to protect the natural landscape, and no previous evaluation was made in terms of the preservation of the regional flora and vegetation - and caatinga (dryland) vegetation was not included within its boundaries (Funch & Harley 2007Funch RR & Harley RM (2007) Reconfiguring the boundaries of the Chapada Diamantina National Park (Brazil) using ecological criteria in the context of a human-dominated landscape. Landscape and Urban Planning 83: 355-362. DOI: 10.1016/j.landurbplan.2007.06.003
https://doi.org/10.1016/j.landurbplan.20...
).
Only infrequent floristic studies have since been carried out there, providing information on the richness and heterogeneous distribution of Myrtaceae in the CDNP and surroundings regions (Harley & Simmons 1986; Barroso & Funch 1998Barroso GM & Funch LS (1998) Myrtaceae. In: Guedes MLS & Orge MDR (eds.) Checklist das espécies vasculares do Morro do Pai Inácio (Palmeiras) e Serra da Chapadinha (Lençóis), Chapada Diamantina, Bahia - Brasil. Instituto de Biologia da Universidade Federal da Bahia, Salvador. 46p.). Currently, of the 29 genera and 1193 species of Myrtaceae reported for Brazil (Proença et al. 2020Proença CEB, Amorim BS, Antonicelli MC , Bünger M, Burton GP, Caldas DKD, Costa IR, Faria JEQ, Fernandes T, Gaem PH, Giaretta A, Lima DF, Lourenço ARL, Lucas EJ, Mazine FF, Meireles LD, Oliveira MIU, Pizzardo RC, Rosa PO, Santana KC, Santos LLD, Santos MF, Souza MC, Souza MAD, Stadnik A, Staggemeier VG, Tuler AC, Valdemarin KS, Vasconcelos TNC, Vieira FCS, Walter BMT, Sobral M (2020) Myrtaceae. In: Flora do Brasil 2020. Jardim Botânico do Rio de Janeiro. Disponível em <http://floradobrasil.jbrj.gov.br/reflora/floradobrasil/FB171>. Acesso em 27 September 2020.), 17 genera and 338 species have been recorded for Bahia state, representing more than 80% of the family’s richness in the northeastern region of the country (Silva et al. 2013Silva BCMN, Silva MP & Silva SBME (2013) Atlas Escolar Bahia - espaço geo-histórico e cultural. Grafset, João Pessoa. 200p.; Proença et al. 2020Proença CEB, Amorim BS, Antonicelli MC , Bünger M, Burton GP, Caldas DKD, Costa IR, Faria JEQ, Fernandes T, Gaem PH, Giaretta A, Lima DF, Lourenço ARL, Lucas EJ, Mazine FF, Meireles LD, Oliveira MIU, Pizzardo RC, Rosa PO, Santana KC, Santos LLD, Santos MF, Souza MC, Souza MAD, Stadnik A, Staggemeier VG, Tuler AC, Valdemarin KS, Vasconcelos TNC, Vieira FCS, Walter BMT, Sobral M (2020) Myrtaceae. In: Flora do Brasil 2020. Jardim Botânico do Rio de Janeiro. Disponível em <http://floradobrasil.jbrj.gov.br/reflora/floradobrasil/FB171>. Acesso em 27 September 2020.). A recent floristic analysis of Myrtaceae in the Espinhaço Range identified 15 genera and 199 species (Bünger et al. 2014Bünger MO, Stehman JR & Oliveira-Filho AT (2014) Myrtaceae throughout the Espinhaço Mountain Range of Central Eastern Brazil: floristic relationships and geoclimatic controls. Acta Botanica Brasilica 28: 109-119. DOI: <http://dx.doi.org/10.1590/S0102-33062014000100011>.
https://doi.org/<http://dx.doi.org/10.15...
). In the Serra Geral de Licínio de Almeida (SGLA), part of Espinhaço Range, Myrtaceae ranks 4th among plant families in terms of species richness (43 spp.) (Campo et al. 2016Campo L, Guedes MLS, Acevedo-Rodriguez P & Roque N (2016) Contributions to the floristic and vegetation knowledge of Espinhaço Septentrional, Bahia, Brazil. Brazilian Journal of Botany 40: 427-437. DOI: 10.1007/s40415-016-0347-y.
https://doi.org/10.1007/s40415-016-0347-...
; Stadnik et al. 2018Stadnik A, Oliveira MIU & Roque N (2018) Myrtaceae na Serra Geral de Licínio de Almeida, Bahia, Brasil. Rodriguésia 69: 515-552. DOI: 10.1590/2175-7860201869220.
https://doi.org/10.1590/2175-78602018692...
).
Numerous studies have highlighted the importance of Myrtaceae in the CDNP in the Serra do Sincorá (Conceição & Giulietti 2002Conceição AA & Giulietti AM (2002) Composição florística e aspectos estruturais de campo rupestre em dois platôs do Morro do Pai Inácio, Chapada Diamantina, Bahia, Brasil. Hoehnea 29: 37-48.; Funch et al. 2008Funch LS, Rodal MJN & Funch RR (2008) Floristic aspects of the Chapada Diamantina, Bahia, Brazil. In: Thomas WW (ed.) The Atlantic Coastal Forests of Northeastern Brazil. Memoirs of the New York Botanical Garden, New York. Pp. 193-220.; 2009; Ribeiro-Filho et al. 2009Ribeiro-Filho AA, Funch LS & Rodal MJN (2009) Composição florística da floresta ciliar do Rio Mandassaia, Parque Nacional da Chapada Diamantina, Bahia, Brasil. Rodriguésia 60: 265-276. DOI: <http://dx.doi.org/10.1590/2175-7860200960203>
https://doi.org/10.1590/2175-78602009602...
; Oliveira & Funch 2010; Couto-Santos et al. 2011Couto-Santos APL, Funch LS & Conceição AA (2011) Composição florística e fisionomia de floresta estacional semidecídua submontana na Chapada Diamantina, Bahia, Brasil. Rodriguésia 62: 391-405. DOI: <http://dx.doi.org/10.1590/2175-7860201162213>.
https://doi.org/<http://dx.doi.org/10.15...
), although few surveys have specifically focused on the family. Based on analyses of regional herbarium collections, Oliveira & Funch (2010) estimated the richness of Myrtaceae in the CDNP to be 67 species distributed among ten genera, although that study was undertaken 10 years ago, and many names now require taxonomic revision.
Herbaria are essential tools for the development of biodiversity and conservation studies, as they are sources of information concerning the distribution of plant species and their areas of occurrence (Giaretta et al. 2015Giaretta A, Menezes LFT & Peixoto AL (2015) Diversity of Myrtaceae in the southeastern Atlantic Forest of Brazil as a tool for conservation. Brazilian Journal of Botany 38: 175-185. DOI: <https://doi.org/10.1007/s40415-014-0121-y>
https://doi.org/<https://doi.org/10.1007...
). That information is critical to assessing regional diversity and making decisions regarding the creation and management of conservation areas (Silva 2005Silva M (2005) The Brazilian Protected Areas Program. Conservation Biology 19: 608-611. DOI: 10.1111/j.1523-1739.2005.00707.x.
https://doi.org/10.1111/j.1523-1739.2005...
; Ramirez-Villagas et al. 2012; Giaretta et al. 2015Giaretta A, Menezes LFT & Peixoto AL (2015) Diversity of Myrtaceae in the southeastern Atlantic Forest of Brazil as a tool for conservation. Brazilian Journal of Botany 38: 175-185. DOI: <https://doi.org/10.1007/s40415-014-0121-y>
https://doi.org/<https://doi.org/10.1007...
). The current study therefore sought to: 1) inventory the diversity of Myrtaceae in the CDNP and surroundings areas; 2) identify the areas of greatest species richness; 3) determine areas that have been poorly sampled and will require greater collection efforts. That information will be used to determine whether the CDNP fully includes the diversity of Myrtaceae in the Serra do Sincorá, or if significant richness occurs outside the protection offered by the National Park.
Materials & Methods
Study site
The Chapada Diamantina National Park (CDNP) was created in 1985 (Beserra et al. 2007Beserra MML, Ferreira LM, Gonçalves CN, Casella PLC & Ferreira JG (2007) Plano de Manejo do Parque Nacional da Chapada Diamantina. Instituto Chico Mendes, Brasília. 657p.), and occupies an area of 1,524 km² within the Serra do Sincorá Range, within the boundaries of six municipalities (Andaraí, Ibicoara, Itaetê, Lençóis, Mucugê, and Palmeiras) (Funch & Harley 2007Funch RR & Harley RM (2007) Reconfiguring the boundaries of the Chapada Diamantina National Park (Brazil) using ecological criteria in the context of a human-dominated landscape. Landscape and Urban Planning 83: 355-362. DOI: 10.1016/j.landurbplan.2007.06.003
https://doi.org/10.1016/j.landurbplan.20...
; Funch et al. 2009Funch RR, Harley RM & Funch LS (2009) Mapping and evaluation of the state of conservation of the vegetation in and surrounding the Chapada Diamantina National Park, NE Brazil. Biota Neotropica 9: 21-30. DOI: <http://dx.doi.org/10.1590/S1676-06032009000200001>
https://doi.org/<http://dx.doi.org/10.15...
) (Fig. 1). Park elevations generally lie above 500 m a.s.l., with peaks exceeding 1,700 m. Due to its geographic position, the park is influenced by four air masses (Continental Equatorial, Tropical Continental, Tropical Atlantic, and Polar) (Beserra et al. 2007Beserra MML, Ferreira LM, Gonçalves CN, Casella PLC & Ferreira JG (2007) Plano de Manejo do Parque Nacional da Chapada Diamantina. Instituto Chico Mendes, Brasília. 657p.), which, in association with its high altitudes, promotes a Tropical Semi-humid climate (Alvares et al. 2013Alvares CA, Stape JL, Sentelhas PC, Gonçalves JL & Sparovek G (2013) Köppen’s climate classification map for Brazil. Meteorologische Zeitschrift 22: 711-728.). The surrounding region, however, represents the most extensive nucleus of the semiarid (caatinga) biome in the neotropics (Beserra et al. 2007Beserra MML, Ferreira LM, Gonçalves CN, Casella PLC & Ferreira JG (2007) Plano de Manejo do Parque Nacional da Chapada Diamantina. Instituto Chico Mendes, Brasília. 657p.), with a marked rainy season (November to March) and a strong dry season (July to November), with frequent and irregular periods of prolonged drought (Funch et al. 2009Funch RR, Harley RM & Funch LS (2009) Mapping and evaluation of the state of conservation of the vegetation in and surrounding the Chapada Diamantina National Park, NE Brazil. Biota Neotropica 9: 21-30. DOI: <http://dx.doi.org/10.1590/S1676-06032009000200001>
https://doi.org/<http://dx.doi.org/10.15...
). Elevations influence rainfall rates, which vary throughout the year, with an annual average of 1,192 mm (Seixas 2004Seixas BS (2004) Água: usos características e potencialidades. Superintendência de Recursos Hídricos, Salvador. 353p.). Much like the rains, temperatures are also influenced by the landscape relief, with average annual temperatures ranging between 18 and 22 ºC; daily temperatures rarely exceed 35 ºC (Funch et al. 2009Funch RR, Harley RM & Funch LS (2009) Mapping and evaluation of the state of conservation of the vegetation in and surrounding the Chapada Diamantina National Park, NE Brazil. Biota Neotropica 9: 21-30. DOI: <http://dx.doi.org/10.1590/S1676-06032009000200001>
https://doi.org/<http://dx.doi.org/10.15...
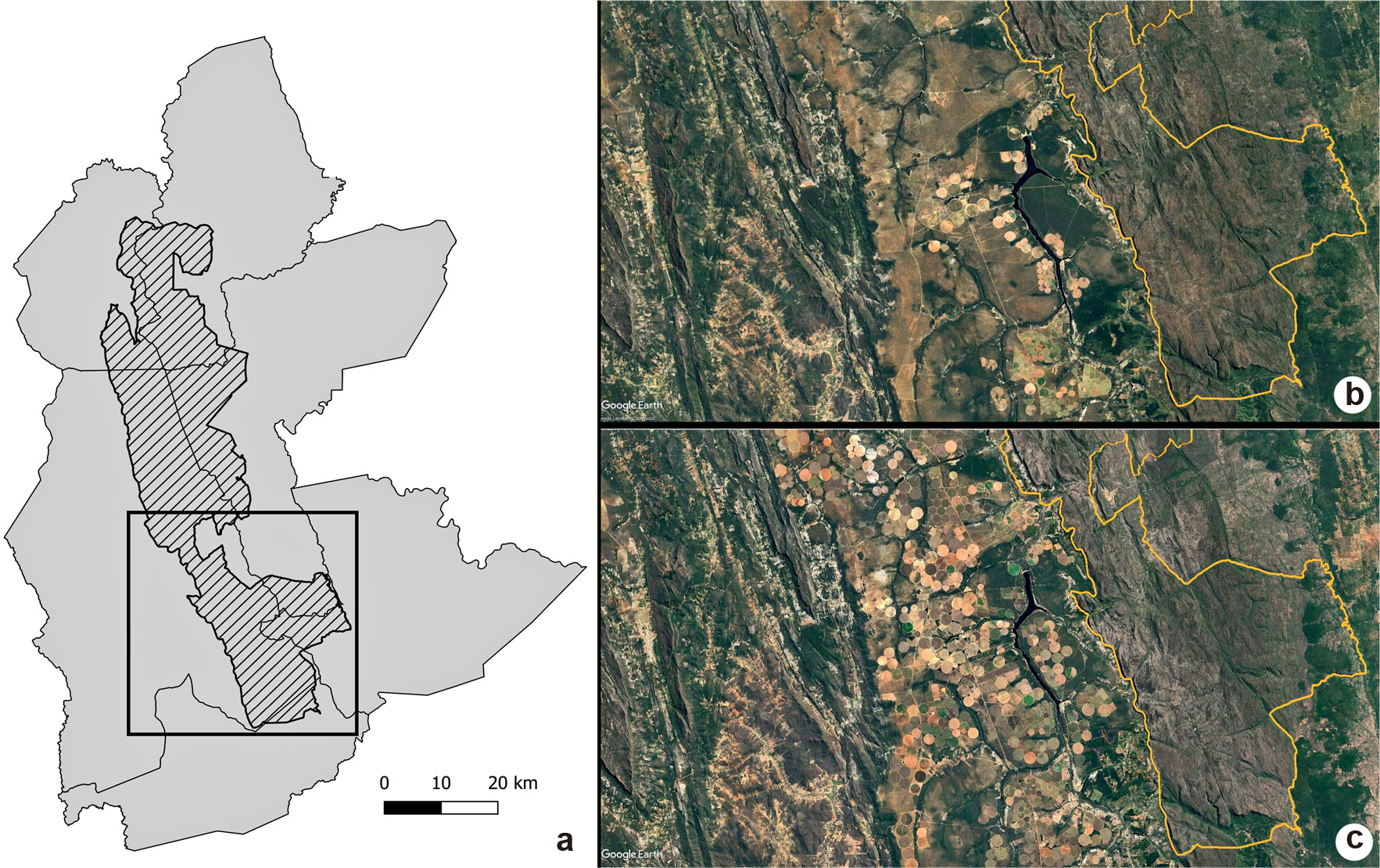
). The region surrounding the CDNP is susceptible to anthropic impacts, which have caused drastic changes over the years (Fig. 2).
Location of the Chapada Diamantina National Park and the six municipalities surrounding it: Andaraí, Ibicoara, Itaetê, Lençóis, Mucugê, and Palmeiras, in Bahia, BA, Brazil. An = Andaraí; Ib = Ibicoara; It = Itaetê; Le = Lençóis; Mu = Mucugê; Pa = Palmeiras.
a-c. The advance of central pivot agriculture in the municipality of Mucugê between 2000 and 2016 – a. detailed view of the Chapada Diamantina National Park (CDNP - hatched), with cultivated land areas (rectangles) represented in b & c; b. image of the cultivated land area in 2000; c. image of the cultivated land area in 2016.
Caatinga is the only vegetation present outside the park boundaries to the west (Fig. 3a) - a region of small trees and shrubs bearing small and usually deciduous leaves, with many terrestrial cacti and bromeliads (Funch et al. 2009Funch RR, Harley RM & Funch LS (2009) Mapping and evaluation of the state of conservation of the vegetation in and surrounding the Chapada Diamantina National Park, NE Brazil. Biota Neotropica 9: 21-30. DOI: <http://dx.doi.org/10.1590/S1676-06032009000200001>
https://doi.org/<http://dx.doi.org/10.15...
), but the CDNP harbors only the following vegetation types: cerrado (neotropical savanna) with discontinuous areas of arboreal elements and continuous areas dominated by herbaceous vegetation and small shrubs (Fig. 3b); campo rupestre, characterized by herbs, sub-shrubs, shrubs, and small trees, and generally occurring at altitudes above 900 m on poor soils, with low pH and exposed to high insolation (Fig. 3c); forest formations, ranging from sub-montane to montane, and from semi-deciduous to deciduous or evergreen (Fig. 3d).
a-d. Vegetation types found in the Chapada Diamantina National Park (CDNP) and NM, in northeastern Brazil – a. Caatinga; b. Cerrado; c. Campo Rupestre; d. Forest.
Data collection
Excursions were carried out in the CDNP and in the six neighboring municipalities (NM) surrounding the CDNP (Andaraí, Ibicoara, Itaête, Lençóis, Mucugê, and Palmeiras) at distances of up to 34 km from the CDNP limits to the east and west, 28 km to the north, and 17 km to the south to collect botanical material. The collections were carried out on a monthly basis from December/2018 to October/2019, following the techniques described by Peixoto & Maia (2013)Peixoto AL & Maia LC (2013) Manual de procedimentos para herbários. Editora Universitária UFPE, Recife. 97p. through random walks, prioritizing poorly sampled areas and the acquisition of fertile material.
The collected specimens were deposited at the Bahia state University of Feira de Santana Herbarium (HUEFS) (acronym according to Thiers, continuously updatedThiers B [continuously updated] Index Herbariorum: a global directory of public herbaria and associated staff. New York Botanical Garden’s Virtual Herbarium. Available at <http://sweetgum.nybg.org/science/ih/>. Access on 15 January 2020.
http://sweetgum.nybg.org/science/ih/...
). Regional herbaria were also visited to study their collections of Myrtaceae from the CDNP and neighboring municipalities. The identification of botanical material was performed with the help of specialized bibliographies, comparisons with herbarium collections and nomenclatural types available on specific websites [such as CRIA (2020)CRIA - Centro de Referência em Informação Ambiental (2020) speciesLink: simple search. Available at <http://www.splink.org.br/>. Access on 15 January 2020.
http://www.splink.org.br/...
], and consultations with specialists.
The collections of the following herbaria were analyzed through online sites (CRIA 2020CRIA - Centro de Referência em Informação Ambiental (2020) speciesLink: simple search. Available at <http://www.splink.org.br/>. Access on 15 January 2020.
http://www.splink.org.br/...
): ALCB, ASE, ASU-PLANTS, BHCB, CEPEC, EAC, ESA, FLOR, HEPH, HPL, HST, HTSA, HUEFS, HUEG, HUESB, HUFSJ, HUNEB, HURB, HVASF, ICN, INPA, IPA, JOI, MBNM, NYBG_BR, PEUFR, SERUNM, SP, SPF, UB, UEC, UFP, UPCB, and US. In cases where there was no access to herbarium samples, priority was given to identifications made by family specialists. Accepted names were updated based on the World Checklist of Selected Plant Families (WCSP 2020WCSP - World Checklis of Selected Plant Families (2020) World Checklist of Myrtaceae. Facilitated by the Royal Botanic Gardens, Kew. Available at <http://wcsp.science.kew.org/>. Access on 20 Febuary 2020.
http://wcsp.science.kew.org/...
). The conservation statuses of the endemic species were verified and species considered at risk of extinction were indicated in the species list. For those species which this information wasn’t available, it was determined using the IUCN criteria (IUCNSPC 2019IUCNSPC - International Union for Conservation of Nature Standards and Petitions Committee (2019) Guidelines for using the IUCN Red List categories and criteria. Version 14. Available at <https://www.iucnredlist.org/resources/redlistguidelines>. Access on 4 December 2019.
https://www.iucnredlist.org/resources/re...
).
The analyses of the herbarium collections included noting the geographic and vegetation data on the exsiccatae label. In cases where the coordinates differed from the described locations, they were corrected whenever possible with approximate coordinates, using the Google Maps® tool; otherwise, they were removed from the analysis. A polygon that delimited the CDNP area was used (Diva-Gis v. 7.5 software; Hajimans et al. 2012) to verify which collections occurred inside ou outside the park boundaries. That polygon is available at <https://www.icmbio.gov.br/portal/unidadesdeconservacao/biomas-brasileiros/caatinga/unidades-de-conservacao-caatinga/2129-parna-da-chapada-da-diamantina>). When information concerning the vegetation type was not present, or was not clear, the geographic position of the collection was compared with the vegetation distribution map of the Chapada Diamantina, provided by Dr. Roy Funch. This map was published in Funch (2006) and Funch et al. (2009)Funch RR, Harley RM & Funch LS (2009) Mapping and evaluation of the state of conservation of the vegetation in and surrounding the Chapada Diamantina National Park, NE Brazil. Biota Neotropica 9: 21-30. DOI: <http://dx.doi.org/10.1590/S1676-06032009000200001>
https://doi.org/<http://dx.doi.org/10.15...
.
Geographical analyses
Geographical analyses and species distribution maps were made using Diva-GIS v. 7.5 software (Hijmans et al. 2012Hijmans RJ, Guarino L & Mathur P (2012) DIVA-GIS version 7.5 manual. Available at <http://www.diva-gis.org/docs/DIVA-GIS_manual_7.pdf>. Access on 15 May 2020.
http://www.diva-gis.org/docs/DIVA-GIS_ma...
), using 0.08° × 0.08° quadrants. That level of resolution was chosen to minimize mapping errors by grid projections. The Jackknife 1 non-parametric species richness estimator (J1) was used to identify well-sampled cells (Hortal et al. 2006Hortal J, Borges PAV & Gaspar C (2006) Evaluating the performance of species richness estimators: sensitivity to sample grain size. Journal of Animal Ecology 75: 274-287. DOI: <https://doi.org/10.1111/j.1365-2656.2006.01048.x>
https://doi.org/<https://doi.org/10.1111...
). Cultivated specimens and duplicate herbarium specimens were not included in the analyses. Records with identifications only to the genus or family level were not used in the preparation of the Species Richness (SR) maps (which present the numbers of species in the quadrants), but only in the Collection-Effort (CE) maps (which present the numbers of collections in each quadrant). The quadrants in red and orange were considered well-sampled, and with high species richness, while the others were considered poorly-sampled/low species richness. The colorless areas of the maps represent areas lacking data.
Results
The search for specimen records resulted in 2,341 records. After the analyses, a total of 1,426 specimens and 174 names remained (Tab. 1). After confirming species` identifications, the final listing was composed of 97 species and nine genera (Tab. S1, available on supplementary material <https://doi.org/10.6084/m9.figshare.16682662.v1>). Among the records obtained from the different herbaria, 1,215 were identified to the species level, 158 to the genus level, and 53 to the family level (Tab. 1). Figure 4 illustrates the collection sites of the 1,426 identified specimens, distributed among 234 quadrants. Figure 5 demonstrates a correlation between collection effort and species richness (n = 234; R2 = 0.7675), indicating that the higher the numbers of collections, the higher the numbers of species, approaching a saturation point at approximately 120 collection records. Thus, quadrants with 120 or more collection records were considered well-sampled.
a-b. Distribution of Myrtaceae specimen records and Collection Effort in 0.08º × 0.08º quadrants – a. Chapada Diamantina National Park (CDNP), represented by the hatched area; b. the six municipalities surrounding the CDNP: Andaraí, Ibicoara, Itaête, Lençóis, Mucugê e Palmeiras (NM), in northeastern Brazil. The quadrants in red and orange represent well-sampled / highest richness areas.
Correlation between collection efforts and the numbers of Myrtaceae species in the six municipalities surrounding the Chapada Diamantina National Park (CDNP), in northeastern Brazil. Natural algorithm regression conducted using DIVA-GIS 7.5 software (n = 234; R² = 0.7675).
Identification of the taxonomic levels, as well as numbers of accepted names and synonyms of herbaria specimens collected in the Chapada Diamantina National Park (CDNP), Bahia, Brazil, and in the six municipalities surrounding it.
The genera with the greatest representivity cataloged in NM were: Myrcia DC. ex Guill. with 42 species (43% of the total) and Eugenia P.Micheli ex L. with 33 (34%), followed by Psidium L. with 11 (11%), Myrciaria O.Berg with six (6%), and Campomanesia Ruiz & Pav. with two (2%) (Figs. 6; 7). The other genera were represented by only a single species: Algrizea macrochlamys (DC.) Proença & NicLugh., Blepharocalyx salicifolius (Kunth) O.Berg, and Siphoneugena kiaerskoviana (Burret) Kausel.
a-f. Myrtaceae species that occur in Serra do Sincorá, Bahia, Brazil – a. immature fruits of Myrcia neoregeliana; b. ripe (red) and immature (green) fruits of Eugenia pistaciifolia; c. immature fruits of Psidium grandifolium; d. inflorescence with flowers in anthesis and buds of Blepharocalyx salicifolius; e. inflorescence with buds of Myrciaria floribunda; f. inflorescence of Myrcia densa with flowers in anthesis and already withered.
a-f. Myrtaceae species that occur in the Serra do Sincorá, Bahia state, Brazil – a. inflorescence of Eugenia anisomischa with flowers in anthesis; b. flowers of Eugenia hirta in anthesis and buds; c. flowers of Eugenia splendens in anthesis and buds; d. inflorescence of Myrcia sylvatica with buds; e. inflorescence of Myrcia venulosa with buds and withered flowers; f. inflorescence of Myrcia reticulosa with flowers in anthesis.
In terms of the distributions of the species records for the six municipalities (NM), 841 records (82 species) also occurred within the boundaries of the CDNP (Fig. 4). Thus, 87.6% of the total richness found is protected within the National Park. The richest genera within the CDNP were Myrcia and Eugenia, with 38 (45%) and 28 (34%) species respectively. Psidium was the third richest, with 10 species (9%), followed by Myrciaria with six (6%), and Campomanesia with two (2%).
The collection-effort map was similar to the species richness map for both NM and CDNP (Figs. 4; 8; 9). The observed species richness was also similar between NM and CDNP (Figs. 8; 9). Two quadrants with high richness were observed in NM: in Palmeiras, corresponding to the Morro do Pai Inácio Mountain region, and near the town of Mucugê. The quadrants with the second highest richness in NM were: in the Marimbus wetland region in Andaraí, and near the town of Lençóis (Fig. 8). Of the 234 quadrants evaluated in NM by Jackknife 1 (J1), three stood out as having the greatest estimated richness (the red and orange quadrants) (Fig. 9): one quadrant in Palmeiras, corresponding to the Morro do Pai Inácio Mountain, another in Andaraí, close to Mucugê, and corresponding to “Marimbus” wetlands, and another in the central region of the municipality of Mucugê near Andaraí; and the quadrant nearest the southern limit of the municipality of Lençóis demonstrated the second-highest estimated richness. Similar results were found for the CDNP scale map (Fig. 9), except for a quadrant in the municipality of Lençóis, where J1 estimated greater richness for the quadrant near to the central region of the municipality and close to the red quadrant in Palmeiras.
a-b. Observed Myrtaceae species according to the Jackknife 1 estimator, in 0.08º × 0.08º quadrants in the Chapada Diamantina National Park (CDNP) and in the six surrounding municipalities: Andaraí, Ibicoara, Itaête, Lençóis, Mucugê, and Palmeiras (NM), in northeastern Brazil – a. richness; b. estimated richness. The quadrants in red and orange represents the areas well-sampled / highest richness.
a-b. Observed Myrtaceae species according to the Jackknife 1 estimator, in 0.08º × 0.08º in quadrants in the Chapada Diamantina National Park (CDNP), in northeastern Brazil, represented by the hatched area – a. richness; b. estimated richness. The quadrants in red and orange represents the areas well-sampled / highest richness.
Some species are restricted to a single vegetation type, while others occur in two or more vegetation types (Tab. S1, available on supplementary material <https://doi.org/10.6084/m9.figshare.16682662.v1>; Fig. 10). Twenty species were recorded in caatinga dryland vegetation (21%), three of which are restricted to that vegetation type; 55 species were recorded in areas of campo rupestre (57%), with 14 being limited to that vegetation type; 47 were recorded in cerrado (48%), with 11 restricted to that vegetation; and 60 were found in forest formations (62%), with 16 occurring only in forests (Fig. 10).
Venn diagram, showing the number of Myrtaceae species shared between vegetation types. Ca = caatinga; Ce = cerrado; Cr = campo rupestre; and F = forest.
Our results showed that 15 species did not occur within the boundaries of the CDNP (Tab. S1, available on supplementary material <https://doi.org/10.6084/m9.figshare.16682662.v1>), which corresponds to approximately 15% of the total species in NM. It should be noted that those 15 species were each encountered in only a single vegetation type: Eugenia bimarginata DC. (Mucugê and Palmeiras), Myrcia almasensis NicLugh. (Mucugê), and M. eximia DC. (Andaraí) in forest areas; E. neosilvestris Sobral (Palmeiras), E. verticillata (Vell.) Angely (Palmeiras), and M. salzmannii O.Berg (Palmeiras) in caatinga; E. capparidifolia DC. (Mucugê) and M. multipunctata Mazine (Mucugê) in campo rupestre; E. rotula Sobral (Mucugê), M. glauca Cambess. (Mucugê), M. nobilis O.Berg (Palmeiras), Myrciaria cuspidata O.Berg (Itaetê), Psidium ganevii Landrum & Funch (Mucugê and Palmeiras), P. grandifolium Mart. ex DC. (Andaraí, Mucugê and Palmeiras), and P. laruotteanum Cambess. (Palmeiras) in cerrado.
Discussion
Myrtaceae is one of the largest plant families of the Brazilian flora (Forzza et al. 2010Forzza RC, Baumgratz JFA, Bicudo CEM, Carvalho Jr. AA, Costa DP, Hopkins M, Leitman PM, Lohmann LG, Maia LC, Marinelli G, Menezes M, Morim MP, Coelho MAN, Peixoto AL, Pirani JR, Prado J, Queiroz LP, Souza VC, Stehmann JR, Sylvestre LS, Walter BMT & Zappi D (2010) Catálogo de plantas e fungos do Brasil. Vol. 1. Andrea Jakobsson Estúdio, Rio de Janeiro. 871p.) and is often among the largest collections in herbaria in Bahia state, especially in the HUEFS and ALCB herbaria, which hold the largest collections from the current study area. Although the family shows complicated taxonomic circumscriptions (Wilson 2011Wilson PG (2011) Myrtaceae. In: Kubitzki K (ed.) The families and genera of vascular plants: Sapindales, Cucurbitales, Myrtaceae. Springer, Berlin. Pp. 212-271.), specialists are continuously examining those collections; the efforts made throughout this study to update those collections make them a reliable source of data.
The cataloged diversity showed that Myrcia (42 species), Eugenia (33), and Psidium (11) were the most diverse genera, and indicated a greater diversity of Myrtaceae in NM than was found by Stadnik et al. (2018)Stadnik A, Oliveira MIU & Roque N (2018) Myrtaceae na Serra Geral de Licínio de Almeida, Bahia, Brasil. Rodriguésia 69: 515-552. DOI: 10.1590/2175-7860201869220.
https://doi.org/10.1590/2175-78602018692...
in the Serra Geral de Licínio de Almeida (SGLA), which is also part of the Chapada Diamantina Range. Those authors listed 43 species and eight genera, with Psidium being the richest (14 species), followed by Eugenia (10) and Myrcia (8). The greater diversity seen in NM may reflect more varied climatic factors, as there are significant differences between the Tropical Semi-humid climate of the Serra do Sincorá and the neighboring Semiarid climate of the surrounding area, while the SGLA is subordinated to a generally uniform Semiarid climate (Beserra et al. 2007Beserra MML, Ferreira LM, Gonçalves CN, Casella PLC & Ferreira JG (2007) Plano de Manejo do Parque Nacional da Chapada Diamantina. Instituto Chico Mendes, Brasília. 657p.; Lucas & Lisboa 2007Lucas EL & Lisboa PC (2007) Secretaria de Desenvolvimento e Integração Regional (SEDIR). Programa de Desenvolvimento Regional Sustentável. PDRS Serra Geral, Salvador. 316p.).
Our data were similar to those presented by Bünger et al. (2014)Bünger MO, Stehman JR & Oliveira-Filho AT (2014) Myrtaceae throughout the Espinhaço Mountain Range of Central Eastern Brazil: floristic relationships and geoclimatic controls. Acta Botanica Brasilica 28: 109-119. DOI: <http://dx.doi.org/10.1590/S0102-33062014000100011>.
https://doi.org/<http://dx.doi.org/10.15...
, who listed 199 species of Myrtaceae for the Espinhaço Range, with the genera Myrcia, Eugenia, and Psidium being the most species rich. Therefore, the study area comprises 48.7% of the estimated richness of the family in the Espinhaço Range - which demonstrates the importance of both the CDNP and NM for biodiversity conservation.
Among the species encountered, eleven are endemic to Bahia (Tab. S1, available on supplementary material <https://doi.org/10.6084/m9.figshare.16682662.v1>), and six are restricted to the Chapada Diamantina (Sobral et al. 2015Sobral M, Faria JEQ, Ibrahim, MU, Lucas EJ, Rigueira D, Stadnik A & Villadroel D (2015) Thirteen new Myrtaceae from Bahia, Brazil. Phytotaxa 224: 201. DOI: 10.11646/phytotaxa.224.3.1.
https://doi.org/10.11646/phytotaxa.224.3...
, 2018; Proença et al. 2020Proença CEB, Amorim BS, Antonicelli MC , Bünger M, Burton GP, Caldas DKD, Costa IR, Faria JEQ, Fernandes T, Gaem PH, Giaretta A, Lima DF, Lourenço ARL, Lucas EJ, Mazine FF, Meireles LD, Oliveira MIU, Pizzardo RC, Rosa PO, Santana KC, Santos LLD, Santos MF, Souza MC, Souza MAD, Stadnik A, Staggemeier VG, Tuler AC, Valdemarin KS, Vasconcelos TNC, Vieira FCS, Walter BMT, Sobral M (2020) Myrtaceae. In: Flora do Brasil 2020. Jardim Botânico do Rio de Janeiro. Disponível em <http://floradobrasil.jbrj.gov.br/reflora/floradobrasil/FB171>. Acesso em 27 September 2020.). Also, according to the list of endangered and endemic species prepared by the Bahia state government (SEMA 2017SEMA - Secretaria do Meio Ambiente (2017) Governo publica a Lista das Espécies da Flora Ameaçadas de Extinção do Estado da Bahia, Secretaria do Meio Ambiente. Available at <http://www.meioambiente.ba.gov.br/2017/08/11254/Governo-publica-a-Lista-das-Especies-da-Flora-Ameacadas-de-Extincao-do-Estado-da-Bahia.html>. Access on 20 December 2019.
http://www.meioambiente.ba.gov.br/2017/0...
), seven species recorded in this study are considered at some risk of extinction (Tab. S1, available on supplementary material <https://doi.org/10.6084/m9.figshare.16682662.v1>), complicating the conservation of the family, as some of those species do not occur within the boundries of the CDNP. Although not present on this list (SEMA 2017SEMA - Secretaria do Meio Ambiente (2017) Governo publica a Lista das Espécies da Flora Ameaçadas de Extinção do Estado da Bahia, Secretaria do Meio Ambiente. Available at <http://www.meioambiente.ba.gov.br/2017/08/11254/Governo-publica-a-Lista-das-Especies-da-Flora-Ameacadas-de-Extincao-do-Estado-da-Bahia.html>. Access on 20 December 2019.
http://www.meioambiente.ba.gov.br/2017/0...
), Eugenia anisomischa Sobral & K. Coutinho was considered by Sobral et al. (2018)Sobral M, Faria JEQ & Coutinho K (2018) Five new Brazilian species of Eugenia (Myrtaceae). Phytotaxa 347: 59-70. DOI: 10.11646/Phytotaxa.347.1.3.
https://doi.org/10.11646/Phytotaxa.347.1...
as Endangered (EN), due to its limited distribution and low numbers of collections [abbreviation according to IUCN (2019)IUCN - International Union for Conservation of Nature (2019) The IUCN Red List of Threatened Species. Version 2019-3. Available at <http://www.iucnredlist.org>. Access on 10 December 2019.
http://www.iucnredlist.org...
]. During the present study, however, more collections of E. anisomischa were made (although limited to the northern sector of Serra do Sincorá, in campo rupestre and forest vegetation) - suggesting that the species faces a similar level of threat as Myrcia mucugensis Sobral or M. pseudovenulosa Stadnik & Sobral (SEMA 2017SEMA - Secretaria do Meio Ambiente (2017) Governo publica a Lista das Espécies da Flora Ameaçadas de Extinção do Estado da Bahia, Secretaria do Meio Ambiente. Available at <http://www.meioambiente.ba.gov.br/2017/08/11254/Governo-publica-a-Lista-das-Especies-da-Flora-Ameacadas-de-Extincao-do-Estado-da-Bahia.html>. Access on 20 December 2019.
http://www.meioambiente.ba.gov.br/2017/0...
).
Richness estimations
The data obtained from the geographical analyses and the species list indicate that approximately 15% of all of the species are not protected within the boundaries of the CDNP, and are therefore susceptible to more severe anthropic impacts. Some of them are known only from herbarium specimens (e.g., Myrcia salzmannii), while others, such as Psidium ganevii and Myrcia almasensis, are already considered vulnerable or endangered, thus increasing the possibility of local extinction (Knapp 2002Knapp S (2002) Assessing patterns of plant endemismo in Neotropical Uplands. Botanical Review 68: 22-37. DOI: <https://www.jstor.org/stable/4354409>).
The results of the CE and SR maps, as well as their correlations, reflect the fact that although collection efforts in the region has been ongoing for decades (Harley & Simmons 1986Harley RM & Simmons NA (1986) Florula of Mucuge. Chapada Diamantina - Bahia, Brazil. Royal Botanic Gardens, Kew. 228p.; Barroso & Funch 1998Barroso GM & Funch LS (1998) Myrtaceae. In: Guedes MLS & Orge MDR (eds.) Checklist das espécies vasculares do Morro do Pai Inácio (Palmeiras) e Serra da Chapadinha (Lençóis), Chapada Diamantina, Bahia - Brasil. Instituto de Biologia da Universidade Federal da Bahia, Salvador. 46p.; Conceição & Giulietti 2002Conceição AA & Giulietti AM (2002) Composição florística e aspectos estruturais de campo rupestre em dois platôs do Morro do Pai Inácio, Chapada Diamantina, Bahia, Brasil. Hoehnea 29: 37-48.; Couto-Santos et al. 2011Couto-Santos APL, Funch LS & Conceição AA (2011) Composição florística e fisionomia de floresta estacional semidecídua submontana na Chapada Diamantina, Bahia, Brasil. Rodriguésia 62: 391-405. DOI: <http://dx.doi.org/10.1590/2175-7860201162213>.
https://doi.org/<http://dx.doi.org/10.15...
), they have mostly been concentrated close to tourist areas and urban centers, leaving many areas requiring better coverage. Something similar occurred with Myrtaceae in Espírito Santo, with Giaretta et al. (2015)Giaretta A, Menezes LFT & Peixoto AL (2015) Diversity of Myrtaceae in the southeastern Atlantic Forest of Brazil as a tool for conservation. Brazilian Journal of Botany 38: 175-185. DOI: <https://doi.org/10.1007/s40415-014-0121-y>
https://doi.org/<https://doi.org/10.1007...
reporting that most collections were from locations close to urban centers or education and research institutions - reflecting a “museum effect” (Ponder et al. 2001Ponder WF, Carter GA, Flemons P & Chapman RR (2001) Evaluation of museum collection data for use in biodiversity assessment. Conservation Biology 15: 648-657. DOI: 10.1046/j.1523-1739.2001.015003648.x
https://doi.org/10.1046/j.1523-1739.2001...
).
The species richness test showed that with more intensive CE, the quadrants could be found to hold more species than those observed in the SR map, while the Jackknife 1 richness estimator revealed that this value could be even higher. It therefore appears that much data concerning richness, as well as the occurrence of new taxa, remains to be collected. Also, large sections of the CDNP, as well as the surrounding area, remain poorly sampled (the green, brown, yellow, and colorless quadrants), with several localities totally lacking any collection data, especially in the eastern region. Thus, it will be necessary to increase collection activities to better assess the real diversity of Myrtaceae in the region. That view is supported by the recent discovery of new endemic species, such as Myrcia pseudovenulosa (Sobral et al. 2015Sobral M, Faria JEQ, Ibrahim, MU, Lucas EJ, Rigueira D, Stadnik A & Villadroel D (2015) Thirteen new Myrtaceae from Bahia, Brazil. Phytotaxa 224: 201. DOI: 10.11646/phytotaxa.224.3.1.
https://doi.org/10.11646/phytotaxa.224.3...
) and Eugenia anisomischa (Sobral et al. 2018Sobral M, Faria JEQ & Coutinho K (2018) Five new Brazilian species of Eugenia (Myrtaceae). Phytotaxa 347: 59-70. DOI: 10.11646/Phytotaxa.347.1.3.
https://doi.org/10.11646/Phytotaxa.347.1...
).
Diversity in the vegetation types
Increasing collection efforts in areas with low sampling histories would therefore allow a more accurate assessment of the diversity of this family. Those collection efforts would also produce more data on the area of occurrence of many taxa, reducing possible biases in geographic analyses (Hortal et al. 2006Hortal J, Borges PAV & Gaspar C (2006) Evaluating the performance of species richness estimators: sensitivity to sample grain size. Journal of Animal Ecology 75: 274-287. DOI: <https://doi.org/10.1111/j.1365-2656.2006.01048.x>
https://doi.org/<https://doi.org/10.1111...
).
The largest number of species (60 spp.) were found in forest environments, corroborating the fact that Myrtaceae is an extremely important component of that vegetation type (Forzza et al. 2010Forzza RC, Baumgratz JFA, Bicudo CEM, Carvalho Jr. AA, Costa DP, Hopkins M, Leitman PM, Lohmann LG, Maia LC, Marinelli G, Menezes M, Morim MP, Coelho MAN, Peixoto AL, Pirani JR, Prado J, Queiroz LP, Souza VC, Stehmann JR, Sylvestre LS, Walter BMT & Zappi D (2010) Catálogo de plantas e fungos do Brasil. Vol. 1. Andrea Jakobsson Estúdio, Rio de Janeiro. 871p.). In campos rupestres fields, the second-largest vegetation type in terms of the floristics of this study, the unique enviromental conditions and the heterogeneity observed in that vegetation (Conceição & Giulietti 2002Conceição AA & Giulietti AM (2002) Composição florística e aspectos estruturais de campo rupestre em dois platôs do Morro do Pai Inácio, Chapada Diamantina, Bahia, Brasil. Hoehnea 29: 37-48.), favored plant diversity (55 spp.). Although Myrtaceae is not among the 10 richest families in the cerrado biome (Forzza et al. 2010Forzza RC, Baumgratz JFA, Bicudo CEM, Carvalho Jr. AA, Costa DP, Hopkins M, Leitman PM, Lohmann LG, Maia LC, Marinelli G, Menezes M, Morim MP, Coelho MAN, Peixoto AL, Pirani JR, Prado J, Queiroz LP, Souza VC, Stehmann JR, Sylvestre LS, Walter BMT & Zappi D (2010) Catálogo de plantas e fungos do Brasil. Vol. 1. Andrea Jakobsson Estúdio, Rio de Janeiro. 871p.), it was the third richest vegetation in and near the CDNP, with about 48% of all species occurring in cerrado sites (47 spp.).
Caatinga vegetation contained the fewest representatives of the family, but nonetheless held approximately 21% of the total number of species (20 spp.). Although the caatinga shares most of its species with other environments, three exclusive taxa were found there: Eugenia verticillata (Vell.) Angely, E. neosilvestris Sobral, and Myrcia salzmannii. The first two represent new occurrences for Bahia, while the third has its distribution limited to the states of Bahia and Alagoas (Proença et al. 2020Proença CEB, Amorim BS, Antonicelli MC , Bünger M, Burton GP, Caldas DKD, Costa IR, Faria JEQ, Fernandes T, Gaem PH, Giaretta A, Lima DF, Lourenço ARL, Lucas EJ, Mazine FF, Meireles LD, Oliveira MIU, Pizzardo RC, Rosa PO, Santana KC, Santos LLD, Santos MF, Souza MC, Souza MAD, Stadnik A, Staggemeier VG, Tuler AC, Valdemarin KS, Vasconcelos TNC, Vieira FCS, Walter BMT, Sobral M (2020) Myrtaceae. In: Flora do Brasil 2020. Jardim Botânico do Rio de Janeiro. Disponível em <http://floradobrasil.jbrj.gov.br/reflora/floradobrasil/FB171>. Acesso em 27 September 2020.). Additionally, species such as Psidium appendiculatum Kiaersk., P. schenckianum Kiaersk., and P. ganevii are common in that phytophysiognomy.
The rainfall patterns and low water availability in caatinga vegetation (Funch et al. 2009Funch RR, Harley RM & Funch LS (2009) Mapping and evaluation of the state of conservation of the vegetation in and surrounding the Chapada Diamantina National Park, NE Brazil. Biota Neotropica 9: 21-30. DOI: <http://dx.doi.org/10.1590/S1676-06032009000200001>
https://doi.org/<http://dx.doi.org/10.15...
) influences its floristic composition and many plant functional traits (Neves et al. 2016, 2017). While high diversity and plant endemism can be found within that vegetation (Queiroz et al. 2017Queiroz LP, Cardoso D, Fernandes MF & Moro MF (2017) Diversity and evolution of flowering plants of the Caatinga domain In: Silva JMC, Leal IR & Tabarelli M (eds.) Caatinga: the largest tropical dry forest region in South America. Springer, Cham. Pp. 23-63. DOI: <https://doi.org/10.1007/978-3-319-68339-3_2>), the low collection intensity in many areas, as demonstrated by the CE map, could underestimate the real diversity, as mentioned above - thus reinforcing the importance of the inclusion of caatinga vegetation within the CDNP for the conservation of Myrtaceae, as well as other families.
Funch et al. (2007, 2009) noted that no areas of caatinga were included within the CDNP, and indicated that re-delimiting the park boundaries would be necessary for the inclusion of that vegetation. The non-inclusion of the caatinga vegetation could have an impact on Myrtaceae conservation in the region (as well as that of other families), considering growing anthropic activities in the surrounding area.
The limited distributions of many Myrtaceae species, along with the fact that the CDNP does not include any areas of caatinga and only limited portions of cerrado (both subject to constant agriculture and grazing expansion), puts the conservation of those species at risk. Furthermore, the loss of representatives of that family may have consequences for ecosystem structures, since Myrtaceae is an important source of resources for animals, as their flowers are visited by bees, wasps, flies, and in some rare cases by birds, and their fruits provide food resources for birds and other animals (Piso 2002; Gressler et al. 2006Gressler E, Pizo MA & Morellato PC (2006) Polinização e dispersão de sementes em Myrtaceae do Brasil. Revista Brasileira de Botânica 29: 509-503. DOI: <http://dx.doi.org/10.1590/S0100-84042006000400002>
https://doi.org/<http://dx.doi.org/10.15...
; Staggemeier et al. 2015Staggemeier VG, Diniz-Filho JAF, Zipparro VB, Gressler E, Castro ERD & Mazine F (2015) Clade-specific responses regulate phenological patterns in Neotropical Myrtaceae. Perspectives in Plant Ecology, Evolution and Systematics 17: 476-490. , 2016Staggemeier VG, Cazetta E & Morellato LPC (2016) Hyperdominance in fruit production in the Brazilian Atlantic rain forest: the functional role of plants in sustaining frugivores. Biotropica 0: 1-12.).
The CDNP comprises approximately 41% of the Myrtaceae species richness of the Espinhaço Range - with most areas in that range having the greatest richness being located in the CDNP. Due to irregular and infrequent collection efforts, however, some locations within, and outside the CDNP lack floristic data. Additionally, a considerable number of species remain unprotected outside the park boundaries, including endemic species and plants classified as threatened, especially in the caatinga.
Although the caatinga region was considered to comprise only relatively low Myrtaceae species richness, our data indicated the possibility of encountering significantly greater diversity than has previously been recorded. Similar analyses undertaken with Leguminosae an important family on floristic lists of both the caatinga (Queiroz et al. 2017Queiroz LP, Cardoso D, Fernandes MF & Moro MF (2017) Diversity and evolution of flowering plants of the Caatinga domain In: Silva JMC, Leal IR & Tabarelli M (eds.) Caatinga: the largest tropical dry forest region in South America. Springer, Cham. Pp. 23-63. DOI: <https://doi.org/10.1007/978-3-319-68339-3_2>) and the CDNP, favor the idea of including areas of caatinga within redimentioned park boundaries (Funch et al. 2007). Thus, the implementation of public policies aimed at the conservation of the regional vegetation are essential - as well as the expansion of the park area to include caatinga vegetation.
Acknowledgements
The authors would like to thank the Fundação de Amparo à Pesquisa do Estado da Bahia (FAPESB 2515/2018); the Programa de Pós-graduação em Botânica da UEFS support; to R. Funch, for help with the field work; and M. Sobral, J.O. Melo, A. Stadinik and Leslie Landrum, for helping in the identification of species.
References
- Alvares CA, Stape JL, Sentelhas PC, Gonçalves JL & Sparovek G (2013) Köppen’s climate classification map for Brazil. Meteorologische Zeitschrift 22: 711-728.
- Antonelli A, Zizka A, Silvestro D, Scharn R, Cascales-Miñana B & Bacon CD (2015) An engine for global plant diversity: highest evolutionary turnover and emigration in the American tropics. Frontiers in Genetics 6: 130. DOI: 10.3389/fgene.2015.00130.
» https://doi.org/10.3389/fgene.2015.00130 - Barroso GM & Funch LS (1998) Myrtaceae. In: Guedes MLS & Orge MDR (eds.) Checklist das espécies vasculares do Morro do Pai Inácio (Palmeiras) e Serra da Chapadinha (Lençóis), Chapada Diamantina, Bahia - Brasil. Instituto de Biologia da Universidade Federal da Bahia, Salvador. 46p.
- Beserra MML, Ferreira LM, Gonçalves CN, Casella PLC & Ferreira JG (2007) Plano de Manejo do Parque Nacional da Chapada Diamantina. Instituto Chico Mendes, Brasília. 657p.
- BFG - The Brazil Flora Group (2018) Brazilian Flora 2020: innovation and collaboration to meet Target 1 of the Global Strategy for Plant Conservation (GSPC). Rodriguésia 69: 1513-1527. DOI: 10.1590/2175-7860201869402.
» https://doi.org/10.1590/2175-7860201869402. - Bünger MO, Stehman JR & Oliveira-Filho AT (2014) Myrtaceae throughout the Espinhaço Mountain Range of Central Eastern Brazil: floristic relationships and geoclimatic controls. Acta Botanica Brasilica 28: 109-119. DOI: <http://dx.doi.org/10.1590/S0102-33062014000100011>.
» https://doi.org/<http://dx.doi.org/10.1590/S0102-33062014000100011>. - Campo L, Guedes MLS, Acevedo-Rodriguez P & Roque N (2016) Contributions to the floristic and vegetation knowledge of Espinhaço Septentrional, Bahia, Brazil. Brazilian Journal of Botany 40: 427-437. DOI: 10.1007/s40415-016-0347-y.
» https://doi.org/10.1007/s40415-016-0347-y. - CBD - Convention on Biological Diversity (2010) Global Biodiversity Outlook 3. Progress Press, Montréal. 94p.
- Conceição AA & Giulietti AM (2002) Composição florística e aspectos estruturais de campo rupestre em dois platôs do Morro do Pai Inácio, Chapada Diamantina, Bahia, Brasil. Hoehnea 29: 37-48.
- Couto-Santos APL, Funch LS & Conceição AA (2011) Composição florística e fisionomia de floresta estacional semidecídua submontana na Chapada Diamantina, Bahia, Brasil. Rodriguésia 62: 391-405. DOI: <http://dx.doi.org/10.1590/2175-7860201162213>.
» https://doi.org/<http://dx.doi.org/10.1590/2175-7860201162213>. - CRIA - Centro de Referência em Informação Ambiental (2020) speciesLink: simple search. Available at <http://www.splink.org.br/>. Access on 15 January 2020.
» http://www.splink.org.br/ - Forzza RC, Baumgratz JFA, Bicudo CEM, Carvalho Jr. AA, Costa DP, Hopkins M, Leitman PM, Lohmann LG, Maia LC, Marinelli G, Menezes M, Morim MP, Coelho MAN, Peixoto AL, Pirani JR, Prado J, Queiroz LP, Souza VC, Stehmann JR, Sylvestre LS, Walter BMT & Zappi D (2010) Catálogo de plantas e fungos do Brasil. Vol. 1. Andrea Jakobsson Estúdio, Rio de Janeiro. 871p.
- Funch LS, Rodal MJN & Funch RR (2008) Floristic aspects of the Chapada Diamantina, Bahia, Brazil. In: Thomas WW (ed.) The Atlantic Coastal Forests of Northeastern Brazil. Memoirs of the New York Botanical Garden, New York. Pp. 193-220.
- Funch RR & Harley RM (2007) Reconfiguring the boundaries of the Chapada Diamantina National Park (Brazil) using ecological criteria in the context of a human-dominated landscape. Landscape and Urban Planning 83: 355-362. DOI: 10.1016/j.landurbplan.2007.06.003
» https://doi.org/10.1016/j.landurbplan.2007.06.003 - Funch RR, Harley RM & Funch LS (2009) Mapping and evaluation of the state of conservation of the vegetation in and surrounding the Chapada Diamantina National Park, NE Brazil. Biota Neotropica 9: 21-30. DOI: <http://dx.doi.org/10.1590/S1676-06032009000200001>
» https://doi.org/<http://dx.doi.org/10.1590/S1676-06032009000200001> - Giaretta A, Menezes LFT & Peixoto AL (2015) Diversity of Myrtaceae in the southeastern Atlantic Forest of Brazil as a tool for conservation. Brazilian Journal of Botany 38: 175-185. DOI: <https://doi.org/10.1007/s40415-014-0121-y>
» https://doi.org/<https://doi.org/10.1007/s40415-014-0121-y> - Gressler E, Pizo MA & Morellato PC (2006) Polinização e dispersão de sementes em Myrtaceae do Brasil. Revista Brasileira de Botânica 29: 509-503. DOI: <http://dx.doi.org/10.1590/S0100-84042006000400002>
» https://doi.org/<http://dx.doi.org/10.1590/S0100-84042006000400002> - Harley RM & Simmons NA (1986) Florula of Mucuge. Chapada Diamantina - Bahia, Brazil. Royal Botanic Gardens, Kew. 228p.
- Hijmans RJ, Guarino L & Mathur P (2012) DIVA-GIS version 7.5 manual. Available at <http://www.diva-gis.org/docs/DIVA-GIS_manual_7.pdf>. Access on 15 May 2020.
» http://www.diva-gis.org/docs/DIVA-GIS_manual_7.pdf - Hortal J, Borges PAV & Gaspar C (2006) Evaluating the performance of species richness estimators: sensitivity to sample grain size. Journal of Animal Ecology 75: 274-287. DOI: <https://doi.org/10.1111/j.1365-2656.2006.01048.x>
» https://doi.org/<https://doi.org/10.1111/j.1365-2656.2006.01048.x> - IUCN - International Union for Conservation of Nature (2019) The IUCN Red List of Threatened Species. Version 2019-3. Available at <http://www.iucnredlist.org>. Access on 10 December 2019.
» http://www.iucnredlist.org - IUCNSPC - International Union for Conservation of Nature Standards and Petitions Committee (2019) Guidelines for using the IUCN Red List categories and criteria. Version 14. Available at <https://www.iucnredlist.org/resources/redlistguidelines>. Access on 4 December 2019.
» https://www.iucnredlist.org/resources/redlistguidelines - Knapp S (2002) Assessing patterns of plant endemismo in Neotropical Uplands. Botanical Review 68: 22-37. DOI: <https://www.jstor.org/stable/4354409>
- Lucas EL & Lisboa PC (2007) Secretaria de Desenvolvimento e Integração Regional (SEDIR). Programa de Desenvolvimento Regional Sustentável. PDRS Serra Geral, Salvador. 316p.
- Maldonado C, Molina CI, Zizka A, Persson C, Taylor CM, Albán J, Chiquillo E, Rønsted N & Antonelli A (2015) Estimating species diversity and distribution in the era of Big Data: to what extent can we trust public databases? Global Ecology and Biogeography 24: 973-984. DOI: <https://doi.org/10.1111/geb.12326>
- Margules CR & Pressey RL (2000). Systematic conservation planning. Nature 405: 243. DOI: <https://doi.org/10.1038/35012251>
» https://doi.org/10.1038/35012251 - Oliveira MIU & Funch LS (dados não publicados) Estudos florísticos em Myrtaceae no Parque Nacional da Chapada Diamantina (PNCD). In: Anais do X Congresso Latino-americano de Botânica, La Serena.
- Peixoto AL & Maia LC (2013) Manual de procedimentos para herbários. Editora Universitária UFPE, Recife. 97p.
- Ponder WF, Carter GA, Flemons P & Chapman RR (2001) Evaluation of museum collection data for use in biodiversity assessment. Conservation Biology 15: 648-657. DOI: 10.1046/j.1523-1739.2001.015003648.x
» https://doi.org/10.1046/j.1523-1739.2001.015003648.x - Proença CEB, Amorim BS, Antonicelli MC , Bünger M, Burton GP, Caldas DKD, Costa IR, Faria JEQ, Fernandes T, Gaem PH, Giaretta A, Lima DF, Lourenço ARL, Lucas EJ, Mazine FF, Meireles LD, Oliveira MIU, Pizzardo RC, Rosa PO, Santana KC, Santos LLD, Santos MF, Souza MC, Souza MAD, Stadnik A, Staggemeier VG, Tuler AC, Valdemarin KS, Vasconcelos TNC, Vieira FCS, Walter BMT, Sobral M (2020) Myrtaceae. In: Flora do Brasil 2020. Jardim Botânico do Rio de Janeiro. Disponível em <http://floradobrasil.jbrj.gov.br/reflora/floradobrasil/FB171>. Acesso em 27 September 2020.
- Queiroz LP, Cardoso D, Fernandes MF & Moro MF (2017) Diversity and evolution of flowering plants of the Caatinga domain In: Silva JMC, Leal IR & Tabarelli M (eds.) Caatinga: the largest tropical dry forest region in South America. Springer, Cham. Pp. 23-63. DOI: <https://doi.org/10.1007/978-3-319-68339-3_2>
- Ramirez-Villegas J, Jarvis A & Touval J (2012) Analysis of threats to South America flora and its implications for conservation. Journal of Nature Conservation 20: 337-348. DOI: <https://doi.org/10.1016/j.jnc.2012.07.006>
» https://doi.org/10.1016/j.jnc.2012.07.006 - Ribeiro-Filho AA, Funch LS & Rodal MJN (2009) Composição florística da floresta ciliar do Rio Mandassaia, Parque Nacional da Chapada Diamantina, Bahia, Brasil. Rodriguésia 60: 265-276. DOI: <http://dx.doi.org/10.1590/2175-7860200960203>
» https://doi.org/10.1590/2175-7860200960203 - Seixas BS (2004) Água: usos características e potencialidades. Superintendência de Recursos Hídricos, Salvador. 353p.
- SEMA - Secretaria do Meio Ambiente (2017) Governo publica a Lista das Espécies da Flora Ameaçadas de Extinção do Estado da Bahia, Secretaria do Meio Ambiente. Available at <http://www.meioambiente.ba.gov.br/2017/08/11254/Governo-publica-a-Lista-das-Especies-da-Flora-Ameacadas-de-Extincao-do-Estado-da-Bahia.html>. Access on 20 December 2019.
» http://www.meioambiente.ba.gov.br/2017/08/11254/Governo-publica-a-Lista-das-Especies-da-Flora-Ameacadas-de-Extincao-do-Estado-da-Bahia.html - Silva BCMN, Silva MP & Silva SBME (2013) Atlas Escolar Bahia - espaço geo-histórico e cultural. Grafset, João Pessoa. 200p.
- Silva M (2005) The Brazilian Protected Areas Program. Conservation Biology 19: 608-611. DOI: 10.1111/j.1523-1739.2005.00707.x.
» https://doi.org/10.1111/j.1523-1739.2005.00707.x. - Silveira FAO, Negreiros D, Barbosa NPU, Buisson E, Carmo FF, Carstensen DW, Conceição AA, Cornelissen TG, Echternacht L, Fernandes GW, Garcia QS, Guerra TJ, Jacobi CM, Lemos-Filho JP, Le Stradic S, Morellato LPC, Neves FS, Oliveira RS, Schaefer CE, Viana PL & Lambers H (2016) Ecology and evolution of plant diversity in the endangered campo rupestre: a neglected conservation priority. Plant Soil 403: 129-152. DOI: 10.1007/s11104-015-2637-8.
» https://doi.org/10.1007/s11104-015-2637-8. - Sobral M, Faria JEQ & Coutinho K (2018) Five new Brazilian species of Eugenia (Myrtaceae). Phytotaxa 347: 59-70. DOI: 10.11646/Phytotaxa.347.1.3.
» https://doi.org/10.11646/Phytotaxa.347.1.3. - Sobral M, Faria JEQ, Ibrahim, MU, Lucas EJ, Rigueira D, Stadnik A & Villadroel D (2015) Thirteen new Myrtaceae from Bahia, Brazil. Phytotaxa 224: 201. DOI: 10.11646/phytotaxa.224.3.1.
» https://doi.org/10.11646/phytotaxa.224.3.1. - Stadnik A, Oliveira MIU & Roque N (2018) Myrtaceae na Serra Geral de Licínio de Almeida, Bahia, Brasil. Rodriguésia 69: 515-552. DOI: 10.1590/2175-7860201869220.
» https://doi.org/10.1590/2175-7860201869220. - Staggemeier VG, Cazetta E & Morellato LPC (2016) Hyperdominance in fruit production in the Brazilian Atlantic rain forest: the functional role of plants in sustaining frugivores. Biotropica 0: 1-12.
- Staggemeier VG, Diniz-Filho JAF, Zipparro VB, Gressler E, Castro ERD & Mazine F (2015) Clade-specific responses regulate phenological patterns in Neotropical Myrtaceae. Perspectives in Plant Ecology, Evolution and Systematics 17: 476-490.
- Thiers B [continuously updated] Index Herbariorum: a global directory of public herbaria and associated staff. New York Botanical Garden’s Virtual Herbarium. Available at <http://sweetgum.nybg.org/science/ih/>. Access on 15 January 2020.
» http://sweetgum.nybg.org/science/ih/ - WCSP - World Checklis of Selected Plant Families (2020) World Checklist of Myrtaceae. Facilitated by the Royal Botanic Gardens, Kew. Available at <http://wcsp.science.kew.org/>. Access on 20 Febuary 2020.
» http://wcsp.science.kew.org/ - Wilson PG (2011) Myrtaceae. In: Kubitzki K (ed.) The families and genera of vascular plants: Sapindales, Cucurbitales, Myrtaceae. Springer, Berlin. Pp. 212-271.
Supplementary Material
See supplementary material at <https://doi.org/10.6084/m9.figshare.16682662.v1 >
Edited by
Publication Dates
-
Publication in this collection
22 Oct 2021 -
Date of issue
2021
History
-
Received
27 Feb 2020 -
Accepted
04 Aug 2020