Abstract
Restingas are extreme environments present in the Atlantic Rainforest biome. These ecosystems show peculiar characteristics, like sandy soil, high salinity, and high solar exposure, which brings scientific interest to their native species. Stachytarpheta schottiana is an endemic Brazilian species found in Jurubatiba Restinga, and just like other species of the genus Stachytarpheta, is used in folk medicine. In this paper, we describe, for the first time, 28 secondary metabolites from S. schottiana polar extract, among them iridoids, flavonoids, lignans and phenylethanoids, with the use of LC-HRMS/MS dereplication and molecular networking methodologies. Many of these compounds have not been described yet for the genus Stachytarpheta, like astragalin, taxifolin, lamiide and the lignans secondary metabolites class. Also, in this paper, High Speed Counter-Current Chromatography (HSCCC) isolation and Nuclear Magnetic Resonance (NMR) of two compounds were used to correct a misidentification in the dereplication procedure and to create seeds for molecular networking. Some of the suggested known compounds found in this work have had their biological activities described in the literature, such as the hepatoprotective activity of verbascoside, that matches those already related for the genus Stachytarpheta and for the folk use of Stachytarpheta schottiana itself.
Key words
dereplication; Jurubatiba restinga; LC-MS/MS; molecular networking; Stachytarpheta schottiana
Resumo
As restingas são ambientes com características extremas, que fazem parte do bioma da Mata Atlântica. Estes ambientes apresentam características peculiares, como solo arenoso e altas salinidade e exposição solar, o que torna as espécies presentes interessantes para a ciência. A espécie Stachytarpheta schottiana é endêmica do Brasil, presente em restingas e, assim como outras espécies do gênero, apresenta uso na medicina popular. Neste trabalho são descritos pela primeira vez 28 metabólitos secundários da espécie S. schottiana, como iridoides, lignanas, flavonoides e feniletanoides, através do uso de metodologias de desreplicação e molecular networking por LC-HRMS/MS. Destas substâncias, muitas sequer foram descritas para o gênero, como astragalina, taxifolina, lamiídeo e toda a classe de lignanas. Ainda neste trabalho, a Cromatografia Contracorrente de Alta Velocidade (High Speed Countercurrent Chromatography - HSCCC) e a Ressonância Magnética Nuclear (RMN) foram utilizadas para corrigir a identificação equivocada de duas substâncias, bem como para a criação de seeds que seriam posteriormente utilizadas nos molecular networkings. Algumas das substâncias conhecidas sugeridas neste trabalho apresentam atividades biológicas já descritas na literatura que coincidem com o uso popular descrito para a espécie e também para o gênero, como o efeito hepatoprotetor do verbascosídeo.
Palavras-chave
desreplicação; restinga de Jurubatiba; LC-HRMS; molecular networking; Stachytarpheta schottiana
Introduction
Restinga environments are part of the Atlantic Rainforest biome, characterized by large sandy plain areas near the sea (Scarano 2002Scarano FR (2002) Structure, function and floristic relationships of plant communities in stressful habitats marginal to the Brazilian Atlantic rainforest. Annals of Botany 90: 517-524. <https://doi.org/10.1093/aob/mcf189>). These characteristics make the restingas extreme environments and bring scientific interest to the species living there. However, these are extremely fragile ecosystems submitted to intense degradation processes and, despite being one of the most threatened ecosystems in Brazil, only a few areas are protected by the government, like Parque Nacional da Restinga de Jurubatiba (PARNA Jurubatiba) (Luz et al. 2011Luz JL, Mangolin R, Lustosa Esbérard CE & Bergallo HG (2011) Morcegos (Chiroptera) capturados em lagoas do Parque Nacional da Restinga de Jurubatiba, Rio de Janeiro, Brasil. Biota Neotropica 11: 161-168. <https://doi.org/10.1590/S1676-06032011000400016>). The PARNA Jurubatiba is the first Brazilian national park composed exclusively by the restinga ecosystem, which makes it vital to the ecosystem preservation. Also, the ecosystem comprised of this park possesses plant species that are used in Brazilian folk medicine, which can be the starting point to researches of biological interest (Boscolo & Senna Valle 2008Boscolo OH & Senna Valle L (2008) Plantas de uso medicinal em Quissamã, Rio de Janeiro, Brasil. Iheringia - Serie Botanica 63: 263-272. <https://doi.org/10.1002/jbm.a.32263>.).
The genus Stachytarpheta (Verbenaceae) is consisted of about 40 species, native from America, and shows a wide array of biological activities, such as antioxidant, anti-inflammatory (Schapoval et al. 1998Schapoval EES, Winter De Vargas MR, Chaves CG, Bridi R, Zuanazzi JA & Henriques AT (1998) Antiinflammatory and antinociceptive activities of extracts and isolated compounds from Stachytarpheta cayennensis. Journal of Ethnopharmacology 60: 53-59. <https://doi.org/10.1016/S0378-8741(97)00136-0>), gastroprotective (Penido et al. 2006Penido C, Costa KA, Futuro DO, Paiva SR, Kaplan MAC, Figueiredo MR & Henriques MGMO (2006) Anti-inflammatory and anti-ulcerogenic properties of Stachytarpheta cayennensis (L.C. Rich) Vahl. Journal of Ethnopharmacology 104: 225-233. <https://doi.org/10.1016/j.jep.2005.09.006>), antibacterial (Awah et al. 2010Awah FM, Uzoegwu PN, Oyugi JO, Rutherford J, Ifeonu P, Yao XJ, Fowke KR & Eze MO (2010) Free radical scavenging activity and immunomodulatory effect of Stachytarpheta angustifolia leaf extract. Food Chemistry 119: 1409-1416. <https://doi.org/10.1016/j.foodchem.2009.09.020>) and anti-hypertensive (Okokon et al. 2008Okokon J, Ettebong E & Antia B (2008) In vivo antimalarial activity of ethanolic leaf extract of Stachytarpheta cayennensis. Indian Journal of Pharmacology 40: 111-113. <https://doi.org/10.4103/0253-7613.42303>).
Stachytarpheta schottiana Schauer species, popularly known as “gervão-da-praia”, is a small shrub endemic in Brazil and can be found in the restingas of Espírito Santo and Rio de Janeiro states, like the PARNA Jurubatiba (BFG 2018BFG - The Brazilian Flora Group (2018) Brazilian Flora 2020: innovation and collaboration to meet target 1 of the Global Strategy for Plant Conservation (GSPC). Rodriguésia 69: 1513-1527.). Stachytarpheta schottiana is used in folk medicine as a vermifuge and hepatoprotective plant, by the population living near PARNA Jurubatiba (Boscolo & Senna Valle 2008Boscolo OH & Senna Valle L (2008) Plantas de uso medicinal em Quissamã, Rio de Janeiro, Brasil. Iheringia - Serie Botanica 63: 263-272. <https://doi.org/10.1002/jbm.a.32263>.) but the phytochemical and pharmacological profiles of S. schottiana have not yet been addressed, and further studies are required to it.
Classical methods of structural elucidation are usually costly and time-consuming. Therefore, dereplication methodologies using state-of-art techniques have been introduced to address this problem. This methodology uses separation techniques, as Liquid or Gas Chromatography coupled to detectors such as Mass Spectrometry (MS) or Nuclear Magnetic Resonance (NMR) to gather information of compounds in complex mixtures. The collected information is then compared with existing databases, such as Mass Bank (Horai et al. 2010Horai H, Arita M, Kanaya S, Nihei Y, Ikeda T, Suwa K, Ojima Y, Tanaka K, Tanaka S, Aoshima K, Oda Y, Kakazu Y, Kusano M, Tohge T, Matsuda F, Sawada Y, Hirai MY, Nakanishi H, Ikeda K, Akimoto N, Maoka T, Takahashi H, Ara T, Sakurai N, Suzuki H, Shibata D, Neumann S, Iida T, Tanaka K, Funatsu K, Matsuura F, Soga T, Taguchi R, Saito K & Nishioka T (2010) MassBank: a public repository for sharing mass spectral data for life sciences. Journal of Mass Spectrometry 45: 703-714. <https://doi.org/10.1002/jms.1777>) or PubChem (Kim et al. 2019Kim S, Chen J, Cheng T, Gindulyte A, He J, He S, Li Q, Shoemaker BA, Thiessen PA, Yu B, Zaslavsky L, Zhang J & Bolton EE (2019) PubChem 2019 update: improved access to chemical data. Nucleic Acids Research 47: D1102-D1109. <https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6324075/>), to allow the annotation of the compounds present in the sample (Gaudêncio & Pereira 2015Gaudêncio SP & Pereira F (2015) Dereplication: racing to speed up the natural products discovery process. Natural Products Reports 32: 779-810. <https://doi.org/10.1039/C4NP00134F>).
The aim of this work was to investigate the chemical profile of the methanolic fraction of S. schottiana aerial parts by LC-HRMS/MS dereplication and molecular networking using the Global Natural Products Social Molecular Networking (GNPS) platform (Wang et al. 2016Wang M, Carver JJ, Phelan VV, Sanchez LM, Garg N, Peng Y, Nguyen D-TTDD, Watrous J, Kapono CA, Luzzatto-Knaan T, Porto C, Bouslimani A, Melnik AV, Meehan MJ, Liu W-TT, Crüsemann M, Boudreau PD, Esquenazi E, Sandoval-Calderón M, Kersten RD, Pace LA, Quinn RA, Duncan KR, Hsu C-CC, Floros DJ, Gavilan RG, Kleigrewe K, Northen T, Dutton RJ, Parrot D, Carlson EE, Aigle B, Michelsen CF, Jelsbak L, Sohlenkamp C, Pevzner P, Edlund A, McLean J, Piel J, Murphy BT, Gerwick L, Liaw C-CC, Yang Y-LL, Humpf H-UU, Maansson M, Keyzers RA, Sims AC, Johnson AR, Sidebottom AM, Sedio BE, Klitgaard A, Larson CB, P CAB, Torres-Mendoza D, Gonzalez DJ, Silva DB, Marques LM, Demarque DP, Pociute E, O’Neill EC, Briand E, Helfrich EJNN, Granatosky EA, Glukhov E, Ryffel F, Houson H, Mohimani H, Kharbush JJ, Zeng Y, Vorholt JA, Kurita KL, Charusanti P, McPhail KL, Nielsen KF, Vuong L, Elfeki M, Traxler MF, Engene N, Koyama N, Vining OB, Baric R, Silva RR, Mascuch SJ, Tomasi S, Jenkins S, Macherla V, Hoffman T, Agarwal V, Williams PG, Dai J, Neupane R, Gurr J, Rodríguez AMCC, Lamsa A, Zhang C, Dorrestein K, Duggan BM, Almaliti J, Allard P-MM, Phapale P, Nothias L-FF, Alexandrov T, Litaudon M, Wolfender J-LL, Kyle JE, Metz TO, Peryea T, Nguyen D-TTDD, VanLeer D, Shinn P, Jadhav A, Müller R, Waters KM, Shi W, Liu X, Zhang L, Knight R, Jensen PR, Palsson BO, Pogliano K, Linington RG, Gutiérrez M, Lopes NP, Gerwick WH, Moore BS, Dorrestein PC, Bandeira N, Boya CAP, Torres-Mendoza D, Gonzalez DJ, Silva DB, Marques LM, Demarque DP, Pociute E, O’Neill EC, Briand E, Helfrich EJNN, Granatosky EA, Glukhov E, Ryffel F, Houson H, Mohimani H, Kharbush JJ, Zeng Y, Vorholt JA, Kurita KL, Charusanti P, McPhail KL, Nielsen KF, Vuong L, Elfeki M, Traxler MF, Engene N, Koyama N, Vining OB, Baric R, Silva RR, Mascuch SJ, Tomasi S, Jenkins S, Macherla V, Hoffman T, Agarwal V, Williams PG, Dai J, Neupane R, Gurr J, Rodríguez AMCC, Lamsa A, Zhang C, Dorrestein K, Duggan BM, Almaliti J, Allard P-MM, Phapale P, Nothias L-FF, Alexandrov T, Litaudon M, Wolfender J-LL, Kyle JE, Metz TO, Peryea T, Nguyen D-TTDD, VanLeer D, Shinn P, Jadhav A, Müller R, Waters KM, Shi W, Liu X, Zhang L, Knight R, Jensen PR, Palsson BO, Pogliano K, Linington RG, Gutiérrez M, Lopes NP, Gerwick WH, Moore BS, Dorrestein PC & Bandeira N (2016) Sharing and community curation of mass spectrometry data with Global Natural Products Social Molecular Networking. Nature Biotechnology 34: 828-837. <https://doi.org/10.1038/nbt.3597>), in order to provide the first phytochemical report for the S. schottiana species.
Material and Methods
The Stachytarpheta schottiana collection was authorized under SISBIO/ICMBio No 62.455-11, and the work was authorized by SISGEN/MMA No: AAA989F. S. schottiana was collected in Parque Nacional da Restinga de Jurubatiba (PARNA Jurubatiba), in Carapebus, Rio de Janeiro state, Brazil, (coordinates: 22°16’13”S, 41°38’54’’W), in June, 2018. The botanical identification was carried out by Dr. Tatiana U.P. Konno, and a voucher specimen was deposited under RFA No 40922 in NUPEM, UFRJ Macaé.
The dried aerial parts powder was macerated three times with ethanol 96° GL (1:4 w/v) for 72 h (Casa Wolff, Rio de Janeiro, Brazil). About 50 mg of the dried crude extract was washed three times with 2 mL of n-hexane 95% to remove lipophyllic compounds (TEDIA, Fairfield, USA), and the supernatant was removed. The precipitate was weighed, dissolved in methanol and filtered with 0.45 μm PTFE Unichro syringe filters (Cobbeter, Hangzhou, China) to achieve a 0.5 mg/mL final solution. Then, a sample of 3 µL was injected and analyzed by LC-HRMS/MS.
The LC-HRMS/MS analyses were performed with a Thermo Scientific Dionex Ultimate 3000 liquid chromatography system chromatograph coupled to a Thermo ScientificTM Q ExactiveTM Plus high-resolution mass spectrometer (Waltham, MA, USA). Liquid chromatography analyses were performed using an Ascentis C18 Express (100 × 4.6 mm; 2.7 µm) column (with a guard column) (Supelco, Bellefonte, PA, USA) with ammonium formate (0.1% w/v) (mobile phase A): acetonitrile/formic acid (0.1% w/v) (mobile phase B) at a flow rate of 0.5 mL/min in gradient elution mode as follows: B -15% (0–1 min); B 15-95% (1–16 min); B 95% (16–21 min); B 95-15% (21–22 min) and B 15% (22–30 min). The oven temperature was kept at 40 °C. Source ionization parameters were: spray voltage 3.9 kV; capillary temperature 300 °C; S-Lens level 50, sheath gas 50, auxiliary gas 15. Samples were analyzed in the scan mass range of m/z 150 to 1000 at resolution of 35000 (positive and negative full scan) followed by data-dependent MS2 (ddMS2 Top3 experiments) using a resolution of 17500 and normalized collision energy (NCE) stepped 35-50%.
Files obtained by the LC-HRMS/MS analyses were processed in MzMine software, v 3.51 (Pluskal et al. 2010Pluskal T, Castillo S, Villar-Briones A & Oresic M (2010) MZmine 2: modular framework for processing, visualizing, and analyzing mass spectrometry-based molecular profile data. BMC Bioinformatics 11: 395. <https://doi.org/10.1186/1471-2105-11-395>), to generate the peak lists that were to be used in the dereplication. Dereplication procedure was performed in the GNPS platform, using the following parameters: parent mass and MS/MS fragment tolerance of 0.02 Da and minimum cosine of 0.7 and 6 matched peaks to library match. Molecular Networking was performed using the same parameters described for dereplication, and tentative identification of compounds was performed using molecular masses, fragmentation patterns, network correlations and literature data. The molecular networking data was then analyzed in Cytoscape (Shannon et al. 2003Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schiwikowski B & Ideker T (2003) Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Research 13: 2498-2504. <https://doi.org/10.1101/gr.1239303>).
A two-step High-Speed Counter-Current Chromatography (HSCCC) separation of two of the major compounds was performed in an AECS QuikPrep Quattro HSCCC (Bridgend, UK), equipped with two bobbins containing two coils of 112 mL each. 1 g of crude ethanolic extract was dissolved in 10 mL of a biphasic solvent system (EbuWat, 1:0.05:1) and injected after hydrodynamic equilibrium was reached (Vm = 43 mL, SF = 81%, 850 rpm, two-coils volume). 480 mL of the mobile phase was pumped at 2 mL/min under rotation, and 240 mL of stationary phase were used to extrusion without rotation.
In the second step, two samples containing higher concentrations of the desired compounds were injected in two separate analyses (Vm = 17 mL, SF = 85%, 850 rpm, one-coil volume), using 240 mL of mobile phase under rotation and 120 mL of stationary phase for extrusion. Fractions obtained in both steps were first analyzed by thin-layer chromatography (TLC) (Silicycle, Québec, Canada) and HPLC-DAD (Shimadzu, Kyoto, Japan) were used to evaluate the separation. The chromatographic conditions were the same used in the LC-HRMS analysis.
After the isolation, the purified compounds were dissolved in CD3OD (Cambridge Isotope Laboratories, Andover, MA, USA), and submitted to 1D (1H and 13C) and 2D (COSY, HSQC, HMBC) NMR analysis using a 500 MHz Varian spectrometer (Palo Alto, CA, USA).
Results and Discussion
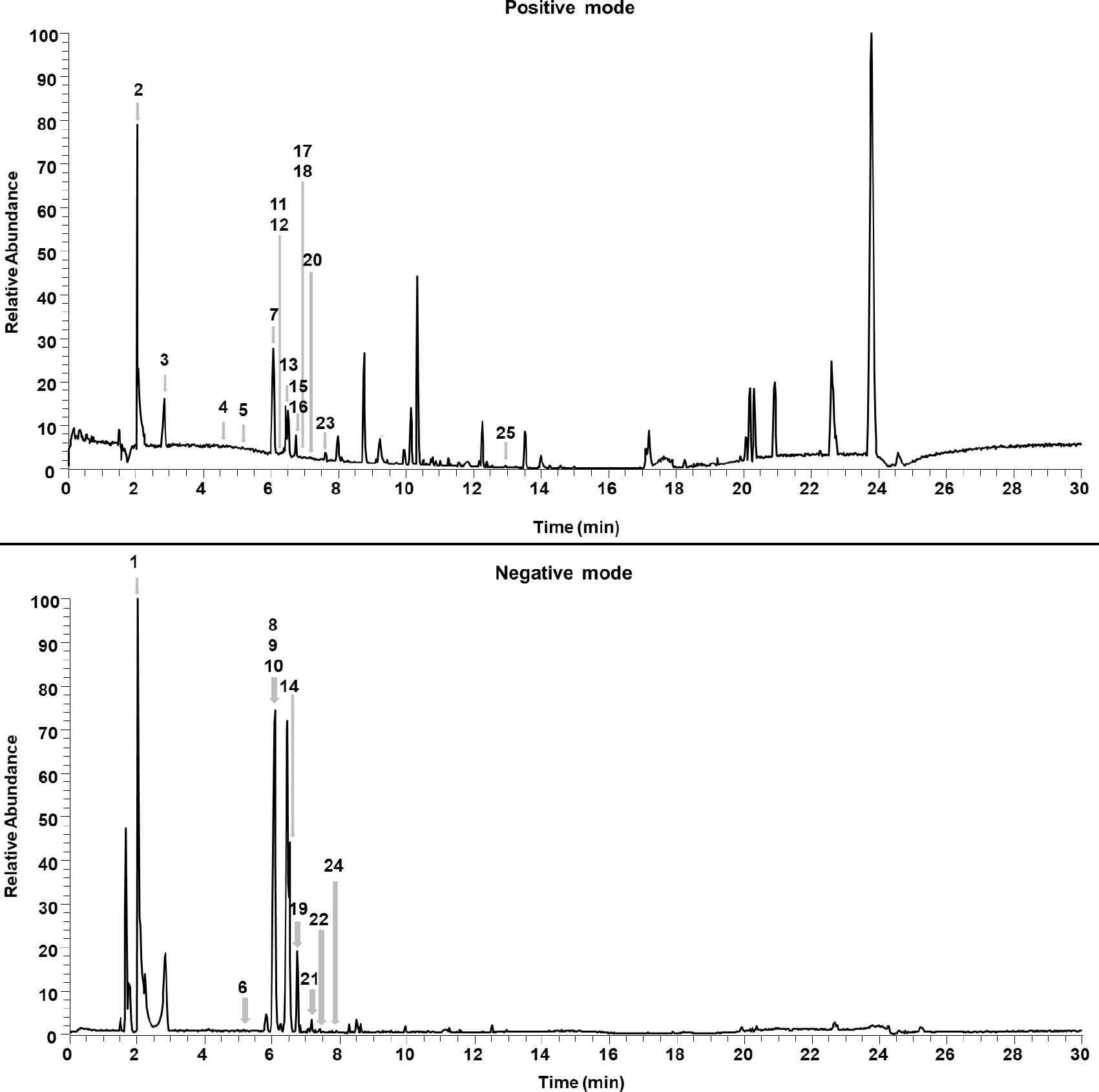
The MS/MS dereplication process returned a total of 38 library matches in both modes (28 positive and 10 negative), with 25 suggested compounds pointed as secondary metabolites, such as iridoids, flavonoids, lignans and phenylethanoids (Tab. 1; Fig. 1). The presence of some of these classes of metabolites, except for lignans, had already been described for the genus Stachytarpheta (Penido et al. 2006Penido C, Costa KA, Futuro DO, Paiva SR, Kaplan MAC, Figueiredo MR & Henriques MGMO (2006) Anti-inflammatory and anti-ulcerogenic properties of Stachytarpheta cayennensis (L.C. Rich) Vahl. Journal of Ethnopharmacology 104: 225-233. <https://doi.org/10.1016/j.jep.2005.09.006>; Kumar et al. 2012Kumar HNK, Preethi SD, Chandana E & Chauhan JB (2012) Phytochemical screening and antibacterial activity of Stachytarpheta indica. International Journal of Pharmaceutical Sciences adn Research 21: 1684-1687. <https://doi.org/http://dx.doi.org/10.13040/IJPSR.0975-8232.3(6).1684-87>). Since there are no previous descriptions of S. schottiana phytochemical composition, all suggested compounds are new to the species and many compounds are described for the first time for the genus.
Base Peak Chromatograms (BPC) of Stachytarpheta schottiana polar extract, in positive (top) and negative (bottom) modes. The numbered peaks are the suggested secondary metabolites (Tab. 1). The unmarked peaks are not secondary metabolites or are peaks without a library match.
Secondary metabolites suggested by LC-HRMS/MS dereplication, molecular networking and NMR elucidation.
The iridoid lamiide (compound 2 in Tab. 1) can be identified by the sodium adduct formed in ionization at [M + Na]+ (m/z 445.132), which is recognized by the platform. The identified lignans (compounds 6, 17 and 18 in Tab. 1) showed fragments representing the removal of their sugar moieties (loss of 162 Da) and the ionization of the remaining structure in the case of (7’R)-(+)-lyoniresinol 9’-glucoside (m/z 419) and acanthoside B (m/z 401).
Seven flavonoids were identified by dereplication and are highlighted in sequence: isoquercitrin, a flavonol constituted of quercetin, that is seen in the fragmentation as the m/z 303 signal, and a 3-glucosyl moiety; peltatoside, an isoquercetin with a xylanopyranosyl moiety (fragments m/z 465 and 303); astragalin, kaempferol, a flavonol with a 3-glucosyl moiety attached (fragment m/z 287 denotes the protonated aglycone); rhamnetin 3-O-neohesperidoside, a rhamnetin aglycone attached with a xylopyranosyl-glucopyranoside moiety; 6’’-O-L-arabinopyranosylastragalin is an astragalin flavonol with an arabinopyranosyl moiety (132 Da mass difference); taxifolin, a di-hydroflavonol aglycone that is a reduced form of quercetin (which explains the 2 Da mass increase); 6-O-methylscutellarin, a flavone derived from scutellarin and 3,3’-dimethyl-7-O-glucuronic acid quercetin, a dimethylated-glucuronyl derivative of quercetin (m/z 329 denotes the aglycone).
The phenylethanoids suggested by dereplication showed similar fragmentation patterns, which are related to the bond break between the moieties and the central sugar. In decaffeoyl-verbascoside, for example, m/z 135 and 315 represent the aglycone fragment and the remaining molecular fragment, respectively. Similarly, benzyl gentibioside showed the m/z 163 and 325 fragments, related to the caffeoyl and the remaining structural fragments.
Dereplication showed two phenylethanoid isomers as two of the major compounds (Fig. 1 - negative mode) in the methanolic extract of S. schottiana, which have a molecular mass of 624.205 Da. They were identified in GNPS as isoverbascoside (t R = 6.08 min) and forsythoside A (t R = 6.42 min), composed by an aglycone (phenethyl alcohol derivatives), a phenylpropanoid (caffeoyl), and two sugar moieties (glucosyl and rhamnosyl). These moieties can be observed in the MS/MS fragments (m/z 463 and 163 fragments in the positive ionization and m/z 461 and 161 in negative). The observed fragmentations are related to the ones described in the literature (Marchetti et al. 2019Marchetti L, Pellati F, Graziosi R, Brighenti V, Pinetti D & Bertelli D (2019) Identification and determination of bioactive phenylpropanoid glycosides of Aloysia polystachya (Griseb. et Moldenke) by HPLC-MS. Journal of Pharmaceutical and Biomedical Analysis 166: 364-370. <https://doi.org/10.1016/j.jpba.2019.01.033>) and the fact that these compounds have the same m/z and different retention (t R) times (Fig. 1) indicates that they are structural or geometric isomers.
In order to isolate these compounds, for usage as seeds to the molecular networking procedure and, in addition, for elucidation by NMR, the HSCCC technique was employed. After NMR analyses the compound previously identified as isoverbascoside by dereplication consisted, in fact, of verbascoside, shown in Figure S1 and Table S1 (Supplemental File 1, available on supplementary material <https://doi.org/10.6084/m9.figshare.16688791.v1>). The other compound had been identified as forsythoside A by dereplication, but NMR analysis identified it as isoverbascoside (Fig. S2 and Tab. S2, Supplemental File 1, available on supplementary material <https://doi.org/10.6084/m9.figshare.16688791.v1>).
Verbascoside and isoverbascoside present different chemical shifts in 1H NMR to the hydrogen H-4’ and H-6’, respectively. Verbascoside shows δH 4.94 ppm to H-4’, δH 3.56 ppm to H-6’a and δH 3.63ppm to H-6’b (Fig. S1, available on supplementary material <https://doi.org/10.6084/m9.figshare.16688791.v1>), while isoverbascoside shows δH 3.36 ppm to H-4’, δH 4.48 ppm to H-6’a and δH 4.38 ppm to H-6’b (Fig. S2, available on supplementary material <https://doi.org/10.6084/m9.figshare.16688791.v1>). The deshield shifts of H-4’ to verbascoside and H-6’ to isoverbascoside indicated that the caffeoyl moieties were attached to C-4’ and C-6’ from glucose, respectively. All chemical shifts and scalar couplings values matches those described in the literature for both compounds (Kawada et al. 2002Kawada T, Asano R, Makino K & Sakuno T (2002) Synthesis of isoacteoside, a dihydroxyphenylethyl glycoside. Journal of Wood Science 48: 512-515. <https://doi.org/10.1007/BF00766648>; Caufin et al. 2014Caufin S, Navarra C, Riva S & Danieli B (2014) Enzymatic acylation as an efficient tool for an easy access to specific acyl derivatives of the natural antioxidants verbascoside, teupolioside and echinacoside. Journal of Molecular Catalysis B Enzymatic 104: 42-47. <https://doi.org/10.1016/j.molcatb.2014.02.019>).
Other observed phenylethanoids showed similar fragmentation patterns, but with variations. Phlinoside C and cassifolioside isomers showed mass increments that suggests the addition of a sugar moiety (132 Da). Leucosceptoside A and martynoside showed very similar fragmentation patterns to verbascoside and isoverbascoside, but with a fragment indicating the presence of a ferulic moiety instead of a caffeoyl one (m/z 175) in both cases, and a fragment indicating a methoxylated aglycone in martynoside (m/z 475). GNPS dereplication suggested identifying the compounds 19 and 20 as plantainoside C, but the isomer leucosceptoside A was already described for the genus Stachytarpheta (Froelich et al. 2008Froelich S, Gupta MP, Siems K& Jenett-Siems K (2008) Phenylethanoid glycosides from Stachytarpheta cayennensis (Rich.) Vahl, Verbenaceae, a traditional antimalarial medicinal plant. Revista Brasileira de Farmacognosia 18: 517-520. <https://doi.org/10.1590/S0102-695X2008000400003>.).
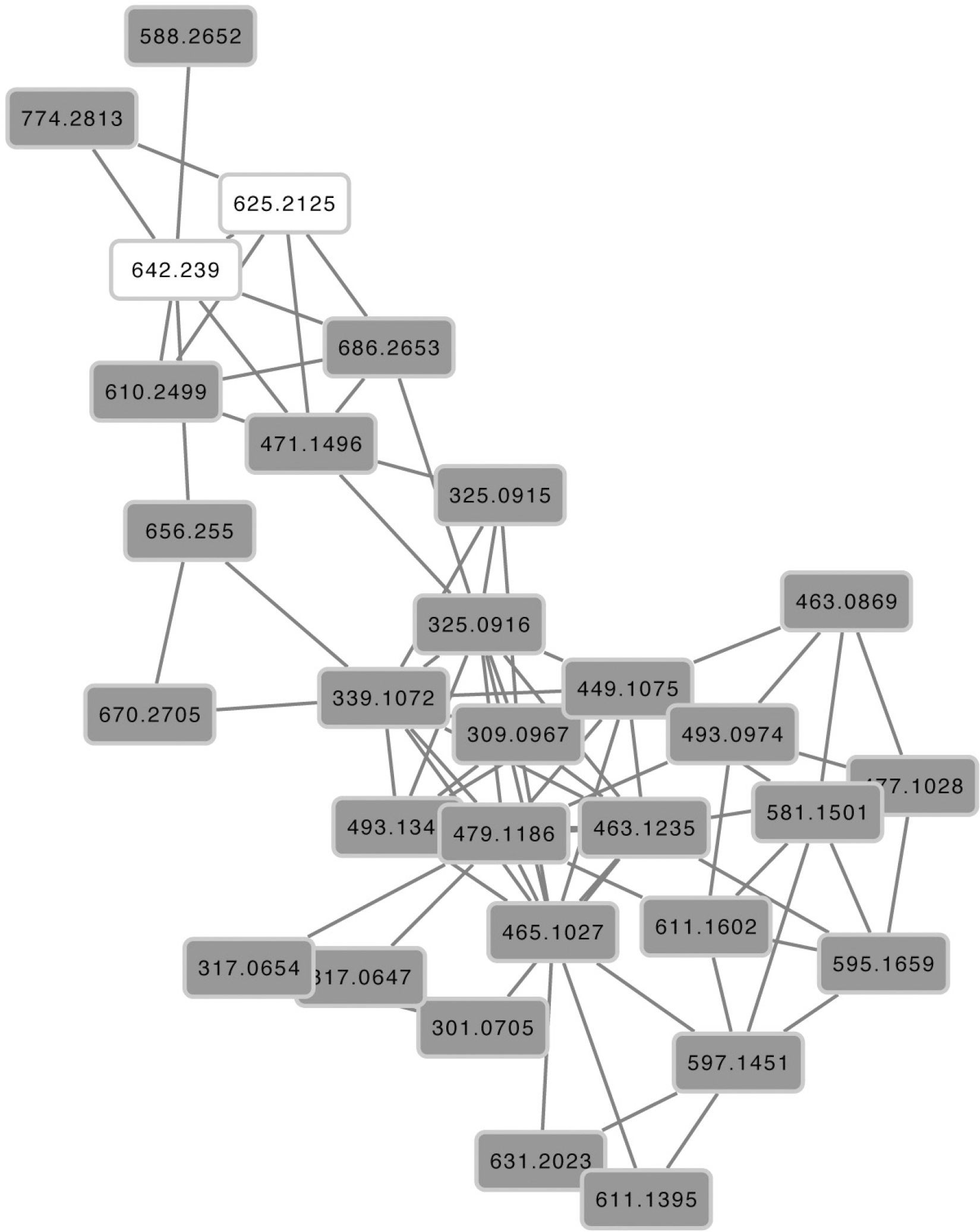
It is worth of note that dereplication has not allowed the identification of some of the compounds present in the methanolic extract. So, Molecular Networking in positive ionization mode was used for the tentative dereplication of some compounds, using their chemical similarity with suggested compounds used as seeds, fragmentation patterns and the available literature information. Three compounds were then suggested by this method. The first was a phenylethanoid which mass [M + H]+ (m/z 610.249) was directly connected to verbascoside (m/z 625.212, Fig. 2) and which showed the same fragments (m/z 163 and 325). Therefore, based on this information, a search was performed in the literature and available databases, leading to the suggestion that it is a jionoside C similar compound (Shen et al. 2016Shen L, Chen H, Zhu Q, Wang Y, Wang S, Qian J, Wang Y & Qu H (2016) Identification of bioactive ingredients with immuno-enhancement and anti-oxidative effects from Fufang-Ejiao-Syrup by LC-MSncombined with bioassays. Journal of Pharmaceutical and Biomedical Analysis 117: 363-371. <https://doi.org/10.1016/j.jpba.2015.09.024>). The second compound was another phenylethanoid, with a precursor mass [M + H]+ m/z 471.149, which was also connected to verbascoside in the molecular network (Fig. 2) and had the fragments m/z 163 and 325. This information led the search to the suggestion that the compound is similar to tangshenoside V, a smaller phenylethanoid (Shen et al. 2016Shen L, Chen H, Zhu Q, Wang Y, Wang S, Qian J, Wang Y & Qu H (2016) Identification of bioactive ingredients with immuno-enhancement and anti-oxidative effects from Fufang-Ejiao-Syrup by LC-MSncombined with bioassays. Journal of Pharmaceutical and Biomedical Analysis 117: 363-371. <https://doi.org/10.1016/j.jpba.2015.09.024>). The third compound has a precursor mass [M + H]+ (m/z 611.139) and was connected in the molecular network to peltatoside [M + H]+ m/z 597.145 and isoquercitrin [M + H]+ m/z 465.102 (Fig. 2), suggesting that the compound is a flavonoid. It had the fragments m/z 303 and 465, and this information led to the suggestion of rutin, a quercetin-3-O-rutinoside (Fu et al. 2020Fu C, Yu P, Wang M & Qiu F (2020) Phytochemical analysis and geographic assessment of flavonoids, coumarins and sesquiterpenes in Artemisia annua L. based on HPLC-DAD quantification and LC-ESI-QTOF-MS/MS confirmation. Food Chemistry 312: 126070. <https://doi.org/10.1016/j.foodchem.2019.126070>).
LC-HRMS positive mode molecular networking of the Stachytarpheta schottiana polar extract, where the compounds’ precursor masses are correlated by similarities between their MS/MS fragmentation patterns. Seed nodes = grey contour and white filling; other nodes = grey contour and dark grey filling.
The chemical profiling of S. schottiana showed some compounds already described in the literature for the genus Stachytarpheta, such as the iridoid lamiide (Viccini et al. 2008Viccini LF, Silva PS, Almeida MV, Saraiva MF, Peixoto PHP, Salimena FRG, Diniz R, Rodrigues BL, Scowen I, Edwards HGM & Oliveira LFC (2008) Ipolamiide and fulvoipolamiide from Stachytarpheta glabra (Verbenaceae): a structural and spectroscopic characterization. Journal of Molecular Structure 875: 27-31. <https://doi.org/10.1016/j.molstruc.2007.03.056>) and the phenylethanoids verbascoside, isoverbascoside, leucosceptoside A and martynoside (Leitão et al. 2005Leitão GG, Souza PA, Moraes AA & Brown L (2005) Step-gradient CCC separation of phenylpropanoid and iridoid glycosides from roots of Stachytarpheta cayennensis (Rich.) Vahl. Journal of Liquid Chromatography and Related Technologies 28: 2053-2060. <https://doi.org/10.1081/JLC-200063672>; Froelich et al. 2008Froelich S, Gupta MP, Siems K& Jenett-Siems K (2008) Phenylethanoid glycosides from Stachytarpheta cayennensis (Rich.) Vahl, Verbenaceae, a traditional antimalarial medicinal plant. Revista Brasileira de Farmacognosia 18: 517-520. <https://doi.org/10.1590/S0102-695X2008000400003>.). However, the flavonoids isoquercitrin, peltatoside and astragalin; and the phenylethanoids, phlinoside B and hebeoside were not described for the genus. In addition, the class of lignans is described for the first time for the Stachytarpheta genus, represented by acanthoside B, (+)/(−)-lyoniresinol 9’-O-glucoside, and dehydrodiconiferyl alcohol 4-β-D-glucoside.
The presence of several phenolic compounds of the shikimate pathway can be correlated with the harsh environmental conditions that the specimen was exposed. The specimen was collected in the restinga habitat (Zaluar & Scarano 2000Zaluar HLT & Scarano FR (2000) Facilitação em restingas de moitas: um século de buscas por espécies focais. In: Esteves FD & Lacerda LD (eds.) Ecologia de Restingas e Lagoas Costeiras. NUPEM/UFRJ, Rio de Janeiro. Pp. 3-23.; Scarano 2002Scarano FR (2002) Structure, function and floristic relationships of plant communities in stressful habitats marginal to the Brazilian Atlantic rainforest. Annals of Botany 90: 517-524. <https://doi.org/10.1093/aob/mcf189>) in winter, dry season. High solar exposure and drought stress may be responsible for the production of phenylethanoids and flavonoids in this species (Quan et al. 2016Quan NT, Anh LH, Khang DT, Tuyen PT, Toan NP, Minh TN, Minh LT, Bach DT, Ha PTT, Elzaarely AA, Khanh TD, Trung KH & Xuan TD (2016) Involvement of secondary metabolites in response to drought stress of rice (Oryza sativa L.). Agriculture 6: 1-14. <https://doi.org/10.3390/agriculture6020023>; Falahi et al. 2018Falahi H, Sharifi M, Maivan HZ & Chashmi NA (2018) Phenylethanoid glycosides accumulation in roots of Scrophularia striata as a response to water stress. Environmental and Experimental Botany 147: 13-21. <https://doi.org/10.1016/j.envexpbot.2017.11.003>; Yang et al. 2020Yang L, Yang L, Yang X, Zhang T, Lan Y, Zhao Y, Han M & Yang L (2020) Drought stress induces biosynthesis of flavonoids in leaves and saikosaponins in roots of Bupleurum chinense DC. Phytochemistry 177: 112434. <https://doi.org/10.1016/j.phytochem.2020.112434>).
Some of the compounds suggested in this work show a wide variety of biological activities described in the literature, such as the anti-inflammatory activity of lamiide (Delaporte et al. 2002Delaporte RH, Sánchez GM, Cuellar AC, Giuliani A & Palazzo de Mello JC (2002) Anti-inflammatory activity and lipid peroxidation inhibition of iridoid lamiide isolated from Bouchea fluminensis (Vell.) Mold. (Verbenaceae). Journal of Ethnopharmacology 82: 127-130. <https://doi.org/10.1016/S0378-8741(02)00181-2>.) and anti-oxidant and anti-inflammatory activities of the flavonoids isoquercitrin, astragalin and taxifolin (Yang et al. 2016Yang C, Wang Z, Mi Y, Gao M, Lv J, Meng Y, Yang B & Kuang H (2016) UHPLC-MS/MS determination, pharmacokinectic, and bioavailability study of taxifolin in rat plasma after oral administration of its nanodispersion. Molecules 21: 494-505. <https://doi.org/10.3390/molecules21040494>; Han et al. 2017Han S, Hanh Nguyen TT, Hur J, Kim NM, Kim SB, Hwang KH, Moon YH, Kang C, Chung B, Kim YM, Kim TS, Park JS & Kim D (2017) Synthesis and characterization of novel astragalin galactosides using β-galactosidase from Bacillus circulans. Enzyme and Microbial Technology 103: 59-67. <https://doi.org/10.1016/j.enzmictec.2017.05.003>; Hobbs et al. 2018Hobbs CA, Koyanagi M, Swartz C, Davis J, Kasamoto S, Maronpot R, Recio L & Hayashi S mo (2018) Comprehensive evaluation of the flavonol anti-oxidants, alpha-glycosyl isoquercitrin and isoquercitrin, for genotoxic potential. Food and Chemical Toxicology 113: 218-227. <https://doi.org/10.1016/j.fct.2017.12.059>). The phenylethanoids verbascoside and isoverbascoside have described anti-hepatotoxic, anti-inflammatory, anti-nociceptive and antioxidant activities (Jiménez & Riguera 1994Jiménez C & Riguera R (1994) Phenylethanoid glycosides in plants: structure and biological activity. Natural Products Reports 11: 591-606. <https://doi.org/10.1039/NP9941100591>; Jin et al. 2004Jin K, Woo E, Yung C & Weon D (2004) Protective effect of acteoside on carbon tetrachloride-induced hepatotoxicity. Life Sciences 74: 1051-1064. <https://doi.org/10.1016/j.lfs.2003.07.020>). Also, the suggested lignans have biological activitied already described, such as the ability to alleviate hepatic steatosis and reduce High Glucose (HG)-induced Reactive Oxygen Species (ROS) in vitro of both isomers (+)/(−)-lyoniresinol 9’-O-glucoside (Shi et al. 2019Shi X, Li Z, Cai W, Liu Y, Li S, Ai M, Sun J, Hou B, Ni L & Qiu L (2019) Chemical constituents from Albiziae cortex and their ability to ameliorate steatosis and promote proliferation and anti-oxidation in vitro. Molecules 24: 4041. <https://doi.org/10.3390/molecules24224041>), the neuroprotective activity of acanthoside B (Karthivashan et al. 2019Karthivashan G, Kweon M-H, Park S-Y, Kim J-S, Kim D-H, Ganesan P & Choi D-K (2019) Cognitive-enhancing and ameliorative effects of acanthoside B in a scopolamine-induced amnesic mouse model through regulation of oxidative/inflammatory/cholinergic systems and activation of the TrkB/CREB/BDNF pathway. Food and Chemical Toxicology 129: 444-457. <https://doi.org/10.1016/j.fct.2019.04.062>) and in vitro anti-inflammatory and antioxidant potential of dehydrodiconiferyl alcohol 4-β-D-glucoside (Shan et al. 2018Shan S, Luo J, Xu D, Niu X, Xu D, Zhang P & Kong L (2018) Elucidation of micromolecular phenylpropanoid and lignan glycosides as the main antioxidants of Ginkgo seeds. Industrial Crops and Products 112: 830-838. <https://doi.org/10.1016/j.indcrop.2017.12.013>; Yang et al. 2019Yang S, Sun F, Ruan J, Yan J, Huang P, Wang J, Han L, Zhang Y & Wang T (2019) Anti-inflammatory constituents from Cortex Dictamni. Fitoterapia 134: 465-473. <https://doi.org/10.1016/j.fitote.2019.03.026>) . The activities described for the compounds pointed by this work matches the effects related to the folk use of S. schottiana as a hepatoprotective plant, as cited by Boscolo & Senna Valle (2008)Boscolo OH & Senna Valle L (2008) Plantas de uso medicinal em Quissamã, Rio de Janeiro, Brasil. Iheringia - Serie Botanica 63: 263-272. <https://doi.org/10.1002/jbm.a.32263>., suggesting this species as a potential candidate for studies focused in hepatic diseases.
The polar extract of S. schottiana was submitted to LC-HRMS/MS dereplication and molecular networking using the GNPS platform, which allowed the identification of 28 natural products, 25 by dereplication and 3 by molecular networking. Some of the dereplication results suggested compounds in the extract have described biological activities, that match the folk use of the plant as a hepatoprotective by the population that lives in the area near the PARNA Jurubatiba, as cited by Boscolo & Senna Valle (2008)Boscolo OH & Senna Valle L (2008) Plantas de uso medicinal em Quissamã, Rio de Janeiro, Brasil. Iheringia - Serie Botanica 63: 263-272. <https://doi.org/10.1002/jbm.a.32263>.. This work provided research of an endemic plant species in a preserved environment, corroborating one basic objective seek in the preservation plan (ICMBio 2007ICMBio (2007) Plano de manejo do Parque Nacional da Restinga de Jurubatiba. Ministério do Meio Ambiente, Brasilia. 670p.). Dereplication allowed the fast identification of a series of known compounds in a never described species, which can allow the focus in unknown compounds if present.
Acknowledgements
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001. The authors also thank to Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ), for Project grant (E26/010.001617/2016); and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). The authors would like to thanks Dr. Nelilma C. Romeiro, for constructive suggestions and grammar revision.
References
- Awah FM, Uzoegwu PN, Oyugi JO, Rutherford J, Ifeonu P, Yao XJ, Fowke KR & Eze MO (2010) Free radical scavenging activity and immunomodulatory effect of Stachytarpheta angustifolia leaf extract. Food Chemistry 119: 1409-1416. <https://doi.org/10.1016/j.foodchem.2009.09.020>
- BFG - The Brazilian Flora Group (2018) Brazilian Flora 2020: innovation and collaboration to meet target 1 of the Global Strategy for Plant Conservation (GSPC). Rodriguésia 69: 1513-1527.
- Boscolo OH & Senna Valle L (2008) Plantas de uso medicinal em Quissamã, Rio de Janeiro, Brasil. Iheringia - Serie Botanica 63: 263-272. <https://doi.org/10.1002/jbm.a.32263>.
- Caufin S, Navarra C, Riva S & Danieli B (2014) Enzymatic acylation as an efficient tool for an easy access to specific acyl derivatives of the natural antioxidants verbascoside, teupolioside and echinacoside. Journal of Molecular Catalysis B Enzymatic 104: 42-47. <https://doi.org/10.1016/j.molcatb.2014.02.019>
- Delaporte RH, Sánchez GM, Cuellar AC, Giuliani A & Palazzo de Mello JC (2002) Anti-inflammatory activity and lipid peroxidation inhibition of iridoid lamiide isolated from Bouchea fluminensis (Vell.) Mold. (Verbenaceae). Journal of Ethnopharmacology 82: 127-130. <https://doi.org/10.1016/S0378-8741(02)00181-2>.
- Falahi H, Sharifi M, Maivan HZ & Chashmi NA (2018) Phenylethanoid glycosides accumulation in roots of Scrophularia striata as a response to water stress. Environmental and Experimental Botany 147: 13-21. <https://doi.org/10.1016/j.envexpbot.2017.11.003>
- Froelich S, Gupta MP, Siems K& Jenett-Siems K (2008) Phenylethanoid glycosides from Stachytarpheta cayennensis (Rich.) Vahl, Verbenaceae, a traditional antimalarial medicinal plant. Revista Brasileira de Farmacognosia 18: 517-520. <https://doi.org/10.1590/S0102-695X2008000400003>.
- Fu C, Yu P, Wang M & Qiu F (2020) Phytochemical analysis and geographic assessment of flavonoids, coumarins and sesquiterpenes in Artemisia annua L. based on HPLC-DAD quantification and LC-ESI-QTOF-MS/MS confirmation. Food Chemistry 312: 126070. <https://doi.org/10.1016/j.foodchem.2019.126070>
- Gaudêncio SP & Pereira F (2015) Dereplication: racing to speed up the natural products discovery process. Natural Products Reports 32: 779-810. <https://doi.org/10.1039/C4NP00134F>
- Han S, Hanh Nguyen TT, Hur J, Kim NM, Kim SB, Hwang KH, Moon YH, Kang C, Chung B, Kim YM, Kim TS, Park JS & Kim D (2017) Synthesis and characterization of novel astragalin galactosides using β-galactosidase from Bacillus circulans. Enzyme and Microbial Technology 103: 59-67. <https://doi.org/10.1016/j.enzmictec.2017.05.003>
- Hobbs CA, Koyanagi M, Swartz C, Davis J, Kasamoto S, Maronpot R, Recio L & Hayashi S mo (2018) Comprehensive evaluation of the flavonol anti-oxidants, alpha-glycosyl isoquercitrin and isoquercitrin, for genotoxic potential. Food and Chemical Toxicology 113: 218-227. <https://doi.org/10.1016/j.fct.2017.12.059>
- Horai H, Arita M, Kanaya S, Nihei Y, Ikeda T, Suwa K, Ojima Y, Tanaka K, Tanaka S, Aoshima K, Oda Y, Kakazu Y, Kusano M, Tohge T, Matsuda F, Sawada Y, Hirai MY, Nakanishi H, Ikeda K, Akimoto N, Maoka T, Takahashi H, Ara T, Sakurai N, Suzuki H, Shibata D, Neumann S, Iida T, Tanaka K, Funatsu K, Matsuura F, Soga T, Taguchi R, Saito K & Nishioka T (2010) MassBank: a public repository for sharing mass spectral data for life sciences. Journal of Mass Spectrometry 45: 703-714. <https://doi.org/10.1002/jms.1777>
- ICMBio (2007) Plano de manejo do Parque Nacional da Restinga de Jurubatiba. Ministério do Meio Ambiente, Brasilia. 670p.
- Jiménez C & Riguera R (1994) Phenylethanoid glycosides in plants: structure and biological activity. Natural Products Reports 11: 591-606. <https://doi.org/10.1039/NP9941100591>
- Jin K, Woo E, Yung C & Weon D (2004) Protective effect of acteoside on carbon tetrachloride-induced hepatotoxicity. Life Sciences 74: 1051-1064. <https://doi.org/10.1016/j.lfs.2003.07.020>
- Karthivashan G, Kweon M-H, Park S-Y, Kim J-S, Kim D-H, Ganesan P & Choi D-K (2019) Cognitive-enhancing and ameliorative effects of acanthoside B in a scopolamine-induced amnesic mouse model through regulation of oxidative/inflammatory/cholinergic systems and activation of the TrkB/CREB/BDNF pathway. Food and Chemical Toxicology 129: 444-457. <https://doi.org/10.1016/j.fct.2019.04.062>
- Kawada T, Asano R, Makino K & Sakuno T (2002) Synthesis of isoacteoside, a dihydroxyphenylethyl glycoside. Journal of Wood Science 48: 512-515. <https://doi.org/10.1007/BF00766648>
- Kim S, Chen J, Cheng T, Gindulyte A, He J, He S, Li Q, Shoemaker BA, Thiessen PA, Yu B, Zaslavsky L, Zhang J & Bolton EE (2019) PubChem 2019 update: improved access to chemical data. Nucleic Acids Research 47: D1102-D1109. <https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6324075/>
- Kumar HNK, Preethi SD, Chandana E & Chauhan JB (2012) Phytochemical screening and antibacterial activity of Stachytarpheta indica International Journal of Pharmaceutical Sciences adn Research 21: 1684-1687. <https://doi.org/http://dx.doi.org/10.13040/IJPSR.0975-8232.3(6).1684-87>
- Leitão GG, Souza PA, Moraes AA & Brown L (2005) Step-gradient CCC separation of phenylpropanoid and iridoid glycosides from roots of Stachytarpheta cayennensis (Rich.) Vahl. Journal of Liquid Chromatography and Related Technologies 28: 2053-2060. <https://doi.org/10.1081/JLC-200063672>
- Luz JL, Mangolin R, Lustosa Esbérard CE & Bergallo HG (2011) Morcegos (Chiroptera) capturados em lagoas do Parque Nacional da Restinga de Jurubatiba, Rio de Janeiro, Brasil. Biota Neotropica 11: 161-168. <https://doi.org/10.1590/S1676-06032011000400016>
- Marchetti L, Pellati F, Graziosi R, Brighenti V, Pinetti D & Bertelli D (2019) Identification and determination of bioactive phenylpropanoid glycosides of Aloysia polystachya (Griseb. et Moldenke) by HPLC-MS. Journal of Pharmaceutical and Biomedical Analysis 166: 364-370. <https://doi.org/10.1016/j.jpba.2019.01.033>
- Okokon J, Ettebong E & Antia B (2008) In vivo antimalarial activity of ethanolic leaf extract of Stachytarpheta cayennensis Indian Journal of Pharmacology 40: 111-113. <https://doi.org/10.4103/0253-7613.42303>
- Penido C, Costa KA, Futuro DO, Paiva SR, Kaplan MAC, Figueiredo MR & Henriques MGMO (2006) Anti-inflammatory and anti-ulcerogenic properties of Stachytarpheta cayennensis (L.C. Rich) Vahl. Journal of Ethnopharmacology 104: 225-233. <https://doi.org/10.1016/j.jep.2005.09.006>
- Pluskal T, Castillo S, Villar-Briones A & Oresic M (2010) MZmine 2: modular framework for processing, visualizing, and analyzing mass spectrometry-based molecular profile data. BMC Bioinformatics 11: 395. <https://doi.org/10.1186/1471-2105-11-395>
- Quan NT, Anh LH, Khang DT, Tuyen PT, Toan NP, Minh TN, Minh LT, Bach DT, Ha PTT, Elzaarely AA, Khanh TD, Trung KH & Xuan TD (2016) Involvement of secondary metabolites in response to drought stress of rice (Oryza sativa L.). Agriculture 6: 1-14. <https://doi.org/10.3390/agriculture6020023>
- Scarano FR (2002) Structure, function and floristic relationships of plant communities in stressful habitats marginal to the Brazilian Atlantic rainforest. Annals of Botany 90: 517-524. <https://doi.org/10.1093/aob/mcf189>
- Schapoval EES, Winter De Vargas MR, Chaves CG, Bridi R, Zuanazzi JA & Henriques AT (1998) Antiinflammatory and antinociceptive activities of extracts and isolated compounds from Stachytarpheta cayennensis Journal of Ethnopharmacology 60: 53-59. <https://doi.org/10.1016/S0378-8741(97)00136-0>
- Shan S, Luo J, Xu D, Niu X, Xu D, Zhang P & Kong L (2018) Elucidation of micromolecular phenylpropanoid and lignan glycosides as the main antioxidants of Ginkgo seeds. Industrial Crops and Products 112: 830-838. <https://doi.org/10.1016/j.indcrop.2017.12.013>
- Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schiwikowski B & Ideker T (2003) Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Research 13: 2498-2504. <https://doi.org/10.1101/gr.1239303>
- Shen L, Chen H, Zhu Q, Wang Y, Wang S, Qian J, Wang Y & Qu H (2016) Identification of bioactive ingredients with immuno-enhancement and anti-oxidative effects from Fufang-Ejiao-Syrup by LC-MSncombined with bioassays. Journal of Pharmaceutical and Biomedical Analysis 117: 363-371. <https://doi.org/10.1016/j.jpba.2015.09.024>
- Shi X, Li Z, Cai W, Liu Y, Li S, Ai M, Sun J, Hou B, Ni L & Qiu L (2019) Chemical constituents from Albiziae cortex and their ability to ameliorate steatosis and promote proliferation and anti-oxidation in vitro Molecules 24: 4041. <https://doi.org/10.3390/molecules24224041>
- Viccini LF, Silva PS, Almeida MV, Saraiva MF, Peixoto PHP, Salimena FRG, Diniz R, Rodrigues BL, Scowen I, Edwards HGM & Oliveira LFC (2008) Ipolamiide and fulvoipolamiide from Stachytarpheta glabra (Verbenaceae): a structural and spectroscopic characterization. Journal of Molecular Structure 875: 27-31. <https://doi.org/10.1016/j.molstruc.2007.03.056>
- Wang M, Carver JJ, Phelan VV, Sanchez LM, Garg N, Peng Y, Nguyen D-TTDD, Watrous J, Kapono CA, Luzzatto-Knaan T, Porto C, Bouslimani A, Melnik AV, Meehan MJ, Liu W-TT, Crüsemann M, Boudreau PD, Esquenazi E, Sandoval-Calderón M, Kersten RD, Pace LA, Quinn RA, Duncan KR, Hsu C-CC, Floros DJ, Gavilan RG, Kleigrewe K, Northen T, Dutton RJ, Parrot D, Carlson EE, Aigle B, Michelsen CF, Jelsbak L, Sohlenkamp C, Pevzner P, Edlund A, McLean J, Piel J, Murphy BT, Gerwick L, Liaw C-CC, Yang Y-LL, Humpf H-UU, Maansson M, Keyzers RA, Sims AC, Johnson AR, Sidebottom AM, Sedio BE, Klitgaard A, Larson CB, P CAB, Torres-Mendoza D, Gonzalez DJ, Silva DB, Marques LM, Demarque DP, Pociute E, O’Neill EC, Briand E, Helfrich EJNN, Granatosky EA, Glukhov E, Ryffel F, Houson H, Mohimani H, Kharbush JJ, Zeng Y, Vorholt JA, Kurita KL, Charusanti P, McPhail KL, Nielsen KF, Vuong L, Elfeki M, Traxler MF, Engene N, Koyama N, Vining OB, Baric R, Silva RR, Mascuch SJ, Tomasi S, Jenkins S, Macherla V, Hoffman T, Agarwal V, Williams PG, Dai J, Neupane R, Gurr J, Rodríguez AMCC, Lamsa A, Zhang C, Dorrestein K, Duggan BM, Almaliti J, Allard P-MM, Phapale P, Nothias L-FF, Alexandrov T, Litaudon M, Wolfender J-LL, Kyle JE, Metz TO, Peryea T, Nguyen D-TTDD, VanLeer D, Shinn P, Jadhav A, Müller R, Waters KM, Shi W, Liu X, Zhang L, Knight R, Jensen PR, Palsson BO, Pogliano K, Linington RG, Gutiérrez M, Lopes NP, Gerwick WH, Moore BS, Dorrestein PC, Bandeira N, Boya CAP, Torres-Mendoza D, Gonzalez DJ, Silva DB, Marques LM, Demarque DP, Pociute E, O’Neill EC, Briand E, Helfrich EJNN, Granatosky EA, Glukhov E, Ryffel F, Houson H, Mohimani H, Kharbush JJ, Zeng Y, Vorholt JA, Kurita KL, Charusanti P, McPhail KL, Nielsen KF, Vuong L, Elfeki M, Traxler MF, Engene N, Koyama N, Vining OB, Baric R, Silva RR, Mascuch SJ, Tomasi S, Jenkins S, Macherla V, Hoffman T, Agarwal V, Williams PG, Dai J, Neupane R, Gurr J, Rodríguez AMCC, Lamsa A, Zhang C, Dorrestein K, Duggan BM, Almaliti J, Allard P-MM, Phapale P, Nothias L-FF, Alexandrov T, Litaudon M, Wolfender J-LL, Kyle JE, Metz TO, Peryea T, Nguyen D-TTDD, VanLeer D, Shinn P, Jadhav A, Müller R, Waters KM, Shi W, Liu X, Zhang L, Knight R, Jensen PR, Palsson BO, Pogliano K, Linington RG, Gutiérrez M, Lopes NP, Gerwick WH, Moore BS, Dorrestein PC & Bandeira N (2016) Sharing and community curation of mass spectrometry data with Global Natural Products Social Molecular Networking. Nature Biotechnology 34: 828-837. <https://doi.org/10.1038/nbt.3597>
- Yang C, Wang Z, Mi Y, Gao M, Lv J, Meng Y, Yang B & Kuang H (2016) UHPLC-MS/MS determination, pharmacokinectic, and bioavailability study of taxifolin in rat plasma after oral administration of its nanodispersion. Molecules 21: 494-505. <https://doi.org/10.3390/molecules21040494>
- Yang L, Yang L, Yang X, Zhang T, Lan Y, Zhao Y, Han M & Yang L (2020) Drought stress induces biosynthesis of flavonoids in leaves and saikosaponins in roots of Bupleurum chinense DC. Phytochemistry 177: 112434. <https://doi.org/10.1016/j.phytochem.2020.112434>
- Yang S, Sun F, Ruan J, Yan J, Huang P, Wang J, Han L, Zhang Y & Wang T (2019) Anti-inflammatory constituents from Cortex Dictamni. Fitoterapia 134: 465-473. <https://doi.org/10.1016/j.fitote.2019.03.026>
- Zaluar HLT & Scarano FR (2000) Facilitação em restingas de moitas: um século de buscas por espécies focais. In: Esteves FD & Lacerda LD (eds.) Ecologia de Restingas e Lagoas Costeiras. NUPEM/UFRJ, Rio de Janeiro. Pp. 3-23.
Supplementary Material
See supplementary material at <https://doi.org/10.6084/m9.figshare.16688791.v1 >
Edited by
Publication Dates
-
Publication in this collection
22 Oct 2021 -
Date of issue
2021
History
-
Received
14 May 2020 -
Accepted
28 July 2020