Abstract
We present results related to the taxonomic revision of Psittacanthus (Loranthaceae) for “Flora do Brasil 2020”. Three new records were found: Psittacanthus kempffii to Rondônia, P. lasianthus to Roraima, and P. truncatus to Mato Grosso. Twelve new synonyms are proposed here: P. carnosus, P. crassipes and P. pustullosus (synonyms for P. acinarius), P. duckei (for P. biternatus), P. baguensis (for P. crassifolius, P. salvadorensis (for P. excrenulatus), P. bergii (for P. grandifolius, P. brachypodus, P. leptanthus and P. redactus (for P. lamprophyllus), P. acevedoi and P. rugostylus (for P. plagiophyllus). Three lectotypes are designated: for P. lasianthus, P. rugostylus and P. biternatus, which makes the neotype previously designated for P. biternatus superfluous. The type-specimen of P. formosus (synonym of P. robustus) was recently found. We propose an identification key for the 33 Brazilian species, with illustrations of the main characters used.
Key words
Amazon; distribution; mistletoes; Santalales; taxonomy
Resumo
Apresentamos os resultados relacionados à revisão taxonômica de Psittacanthus (Loranthaceae) para o projeto “Flora do Brasil 2020”. Três novos registros foram encontrados: P. kempffii para Rondônia, P. lasianthus para Roraima e P. truncatus para o Mato Grosso. Doze novos sinônimos são aqui propostos: P. carnosus, P. crassipes e P. pustullosus (sinônimos para P. acinarius), P. duckei (para P. biternatus), P. baguensis (para P. crassifolius), P. salvadorensis (para P. excrenulatus), P. bergii (para P. grandifolius), P. brachypodus, P. leptanthus e P. redactus (para P. lamprophyllus), P. acevedoi e P. rugostylus (para P. plagiophyllus). São designados três lectótipos, para P. lasianthus, P. rugostylus e P. biternatus, que torna supérfluo o neótipo designado anteriormente para P. biternatus. O tipo de P. formosus (sinônimo de P. robustus) foi localizado. Propomos uma chave de identificação para as 33 espécies brasileiras, com ilustrações das principais características utilizadas.
Palavras-chave
Amazônia; distribuição; passarinho; Santalales; taxonomia
Psittacanthus Mart. (Loranthaceae) belongs to the Neotropical subtribe Psittacanthinae Engl. (Vidal-Russel & Nickrent 2008Vidal-Russell R & Nickrent DL (2008) Evolutionary relationships in the showy mistletoe family (Loranthaceae). American Journal of Botany 95: 1015-1029.; Nickrent et al. 2010Nickrent DL, Malécot V, Vidal-Russell R & Der JP (2010) A revised classification of Santalales. Taxon 59: 538-558.), with a distribution range from Mexico to northern Argentina and southern Brazil (Kuijt 2009Kuijt J (2009) Monograph of Psittacanthus (Loranthaceae). Systematic Botany Monographs 86: 1-361.; Dettke & Waechter 2014Dettke GA & Waechter JL (2014) Estudo taxonômico das ervas-de-passarinho da Região Sul do Brasil: I. Loranthaceae e Santalaceae. Rodriguésia 65: 939-953.). The basic inflorescence units are diads and triads, and the flower bracts are fused into a subfloral cupule. The flowers exhibit great structural variability - they are very attractive, large, and with a tubular corolla (Kuijt 2009Kuijt J (2009) Monograph of Psittacanthus (Loranthaceae). Systematic Botany Monographs 86: 1-361.). Psittacanthus is morphologically circumscribed by the following characters: large flowers; haustorial connection with the host at one point without epicortical roots and secondary haustoria; large fruits without endosperm, and dorsifixed versatile anthers (Kuijt 2009Kuijt J (2009) Monograph of Psittacanthus (Loranthaceae). Systematic Botany Monographs 86: 1-361.). The anther morphology separates Psittacanthus from its closest genus, Aetanthus (Eichler) Engl., which has basifixed and non-versatile anthers (Eichler 1868Eichler AW (1868) Loranthaceae. In: Martius CFP (ed.) Flora brasiliensis. Fleicher, Leipzig. Vol. 5, pars 2, pp. 1-136, t.1-44.; Kuijt 2014aKuijt J (2014a) A monograph of the genus Aetanthus (Loranthaceae). Plant Diversity and Evolution 131: 1-51.).
Kuijt (2009)Kuijt J (2009) Monograph of Psittacanthus (Loranthaceae). Systematic Botany Monographs 86: 1-361. proposed the most comprehensive revision for Psittacanthus with 119 species, being 51 new to science. After that, some new species were described and new synonyms proposed. Dettke & Waechter (2014)Dettke GA & Waechter JL (2014) Estudo taxonômico das ervas-de-passarinho da Região Sul do Brasil: I. Loranthaceae e Santalaceae. Rodriguésia 65: 939-953. proposed the synonymization of Psittacanthus hatschbachii Kuijt in P. dichroos (Mart.) Mart. Kuijt (2014b)Kuijt J (2014b) Five new species, one new name, and transfers in Neotropical mistletoes (Loranthaceae), Miscellaneous Notes, 61-68. Novon 23: 176-186. described Psittacanthus longiflorus Kuijt from Peru, and Roldán-Palacios & Alzate-Guarin (2014)Roldán-Palacios FJ & Alzate-Guarin F (2014) Psittacanthus longerectus (Loranthaceae), a new showy species from Colombia. Actualidades Biológicas 36: 119-122. described Psittacanthus longerectus Roldán & Alzate from Colombia. Recently, Psittacanthus corderoi F.González et al. was described, also from Colombia (González et al. 2016González F, Roldán FJ & Pabón-Mora N (2016) Psittacanthus corderoi, a new species of Loranthaceae from the Colombian Amazonia. Caldasia 38: 250-256.). Thus, to date, 121 species of Psittacanthus are recognized.
The first revision of Psittacanthus for Brazil included 26 species and was carried out by Eichler (1868)Eichler AW (1868) Loranthaceae. In: Martius CFP (ed.) Flora brasiliensis. Fleicher, Leipzig. Vol. 5, pars 2, pp. 1-136, t.1-44. as part of the extensive Flora Brasiliensis. Later, Rizzini (1956)Rizzini CT (1956) Pars specialis prodromi monographiae Loranthacearum Brasiliae terrarumque finitimarum. Rodriguésia 30/31: 87-234. described seven new species and two new varieties, while Kuijt (2009)Kuijt J (2009) Monograph of Psittacanthus (Loranthaceae). Systematic Botany Monographs 86: 1-361. reported 42 species for Brazil. Species of Psittacanthus occur in all of the Brazilian phytogeographic domains, except in the subtropical Pampa (Arruda et al. 2012Arruda R, Fadini RF, Carvalho LN, Del-Claro K, Mourão FA, Jacobi CM, Teodoro GS, van den Berg E, Caires CS & Dettke GA (2012) Ecology of neotropical mistletoes: an important canopy dwelling component of Brazilian ecosystems. Acta Botanica Brasilica 26: 264-274.).
When preparing a revisional study of Psittacanthus for the project “Flora do Brasil 2020”, we have documented the presence of three species not previously recorded in Brazil, Psittacanthus kempffii Kuijt, P. lasianthus Sandwith and P. truncatus Kuijt. In addition, twelve new synonyms, three lectotypifications, the location of a type specimen and an illustrated identification key for the Brazilian species are here proposed and presented.
Considering the new synonyms, the genus Psittacanthus now includes 110 species, 33 of which are confirmed to occur in Brazil. Full descriptions and comments on distribution are available in Dettke & Caires (2020)Dettke GA & Caires CS (2020) Psittacanthus. In: Flora do Brasil 2020 under construction. Jardim Botânico do Rio de Janeiro. Available at <http://floradobrasil.jbrj.gov.br/reflora/floradobrasil/FB8698>. Access on 21 January 2020.
http://floradobrasil.jbrj.gov.br/reflora...
.
New records
1. Psittacanthus kempffii Kuijt, Syst. Bot. Monogr. 86: 186, Fig. 86. 2009. Type: BOLIVIA. SANTA CRUZ: Velasco, National Park Noel Kempff Mercado, Huanchaca 1, 13º53’21’’S 60º48’46’’W, 22.II.1997, fl., A. Soto et al. 301 (holotype: UC barcode 1957462! (ex-LEA); isotype: MO!).
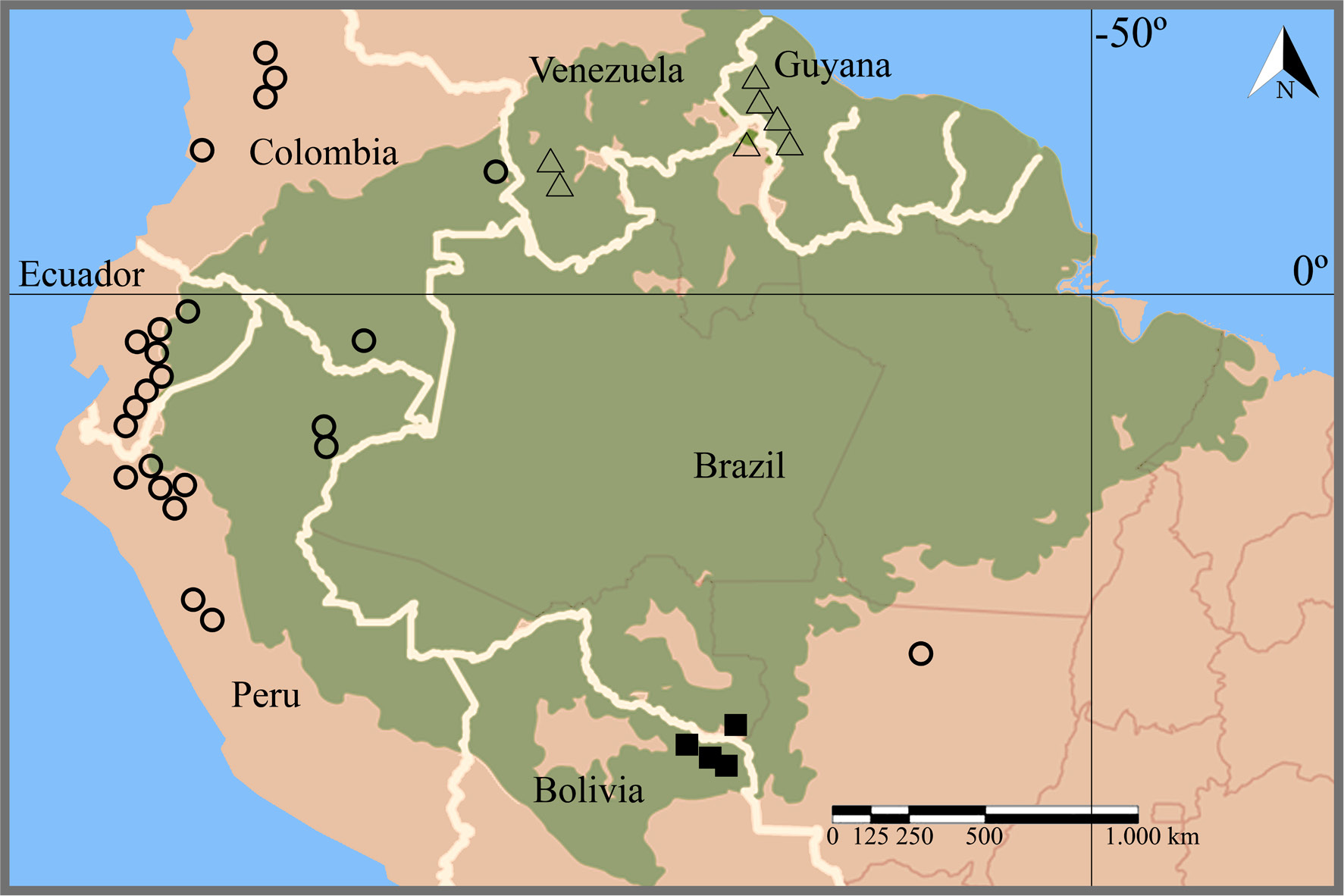
Psittacanthus kempffii was described for east Bolivia, where several collections are recorded for the Parque Nacional de Noel Kempff Mercado (Kuijt 2009Kuijt J (2009) Monograph of Psittacanthus (Loranthaceae). Systematic Botany Monographs 86: 1-361.). It presents a dichotomic growth pattern, ovate leaves with pinnate obscure venation, flowers arranged in dyads, basal ligules absent and stamens arranged at the same height (isomorphic). It is recorded for the first time for adjacent Brazil (Fig. 1), in the riparian vegetation of Rio Guaporé, where it is found sparsely, with less than five individuals seen.
Updated occurrence of Psittacanthus kempffii (black square), Psittacanthus lasianthus (triangle) and Psittacanthus truncatus (circle) on South America. Extra Brazilian points are based on Kuijt (2009)Kuijt J (2009) Monograph of Psittacanthus (Loranthaceae). Systematic Botany Monographs 86: 1-361. and the green area represents the Amazon rainforest.
Examined material: BRAZIL. RONDÔNIA: Cabixi, Fazenda Félix de Lima, near to Porto Félix, Rio Guaporé, 13º40’55’’S 60º44’24’’W, 21.VI.2014, fl., fr., M.G. Caxambu et al. 5421 (HCF, UFMT).
2. Psittacanthus lasianthus Sandwith, Bull. Misc. Inform. Kew 1939(1): 18. 1939. Type: GUYANA. Kaieteur Savannah, Potaro River, 5.IX.1937, fl., N.Y. Sandwith 1366 (lectotype, hic designatus: K barcode 000651845!; isolectotypes: B!, K!, NY!, S!, U!).
Psittacanthus lasianthus (Figs. 2a-b) occurs in Guyana, Venezuela (Kuijt 2009Kuijt J (2009) Monograph of Psittacanthus (Loranthaceae). Systematic Botany Monographs 86: 1-361.), and is recorded here for the first time in Brazil, for the state of Roraima, at elevations of up to 1,300 m.a.s.l. (Fig. 1). This is the only species of Psittacanthus with a dense indumentum on the petals, a characteristic that allows it to be readily recognized. The leaves are obovate to circular (5‒6 × 3‒3.5 cm), with inconspicuous venation; the flowers (4 cm long) are arranged in triads, the petals are vivid reddish with a yellow tip, and, internally, the ligules and the nectary are finely pubescent.
New records of Psittacanthus in Brazil – a-b. Psittacanthus lasianthus. c-f. Psittacanthus truncatus. Photos: a,b. M. Nadruz; c-f. M. Engels. Scale bars: a,b,d = 2 cm; c = 10 cm; e = 5 cm; f = 1 cm.
Two herbarium sheets of the specimen Sandwith 1366 were found in K herbarium, without indication and/or evidence of which one was selected by the author as the holotype and, therefore, we here designate a lectotype, according to Code, Art. 9.11 (Turland et al. 2018Turland NJ, Wiersema JH, Barrie FR, Greuter W, Hawksworth DL, Herendeen PS, Knapp S, Kusber WH, Li DZ, Marhold K, May TW, McNeill J, Monro AM, Prado J, Price MJ & Smith GF (eds.) (2018) International Code of Nomenclature for algae, fungi, and plants (Shenzhen Code) adopted by the Nineteenth International Botanical Congress Shenzhen, China, July 2017. Regnum Vegetabile 159. Koeltz Botanical Books, Glashütten. 254p.).
Examined material: BRAZIL. RORAIMA: Uiramutã, Monte Caburaí, 05º10’21’’N 60º12’57’’W, 1,322 m, 6.XI.2014, fl., M. Nadruz et al. 2862 (RB); Parque Nacional Monte Roraima, Monte Caburaí, 05º10’21’’N 60º12’57’’W, 1,310 m, 8.XI.2014, fl., im.fr., M. Nadruz et al. 2897 (RB).
3. Psittacanthus truncatus Kuijt, in G. Harling & B. Sparre, Fl. Ecuador 24: 174. 1986. Type: ECUADOR. Zamora-Chinchipe: Zamora, rain forest, 1,000 m, 14/19.VII.1959, fl., G. Harling 5921 (holotype: GB barcode 0047984!; isotype: S).
Psittacanthus truncatus (Figs. 2c-f) occurs in Colombia, Ecuador, Peru (Kuijt 2009Kuijt J (2009) Monograph of Psittacanthus (Loranthaceae). Systematic Botany Monographs 86: 1-361.), and is now here recorded in Brazil for the first time, in the state of Mato Grosso (Fig. 1). The record for Brazil is about 2,000‒2,300 km away from the nearest known populations in Peru, suggesting that the species is poorly collected and may have a wider distribution than previously assumed. Psittacanthus truncatus can be easily distinguished from the remaining species of Psittacanthus for its dichotomic ramifications and long internodes (up to 15 cm long); large leaves (12‒15 × 8‒10 cm), ovate to elliptical with abaxially evident pinnate venation; flowers arranged in triads; straight flower buds (4.5‒5 cm long) truncate at the apex; entirely red corolla; and stamens arranged at the same height (isomorphic).
Examined material: BRAZIL. MATO GROSSO: Itaúba, lote G de supressão, 281 m, 11º05’32”S, 55º18’18”W, 5.II.2015, fl., A.Z. Bronholi et al. (HERBAM 14431, MBM 413457a,b).
New synonyms
Psittacanthus acinarius (Mart.) Mart., Flora 13(1): 108. 1830. Loranthus acinarius Mart. in Schultes & Schultes f., Syst. Veg. 7(1): 130. 1829. Type: BRAZIL. PIAUÍ: in silvis, C.F.P. von Martius s.n. (holotype: M = F Neg 19053!).
Psittacanthus carnosus Kuijt, Syst. Bot. Monogr. 86: 110, Fig. 43. 2009. Type: BRAZIL. RONDÔNIA: Porto Velho, UHE de Samuel, Rio Jamari, 18.I.1989-11.II.1989, fl., U.N. Maciel & C.S. Rosário 1555 (holotype: F (mounted in two sheets, F 2208342! and F 2208348!), isotype: MO), syn. nov.
Psittacanthus crassipes Kuijt, Syst. Bot. Monogr. 86: 137, Fig. 59. 2009. Type: VENEZUELA. AMAZONAS: Rio Orinoco, just below mouth of Rio Atabapo, 6.VIII.1959, fl., J.J. Wurdack & L.S. Adderley 43774 (holotype: US barcode 879633!; isotypes: F!, IAN!, NY, RB!), syn. nov.
Psittacanthus pustullosus Rizzini, Rodriguésia 30/31: 139, Tab. XVIII-4. 1956. Type: BRAZIL. AMAZONAS: Mamiá, Solimões, 20.I.1924, J.G. Kuhlmann 1174 (holotype: RB 37342 (mounted in three sheets, barcodes 00540637!, 00545559! and 00545560!)), syn. nov.
Psittacanthus acinarius is widely distributed in South America. In Brazil, it occurs in the Cerrado and Amazon phytogeographic domains and has great morphological variability throughout its distribution. This variability can be observed in the shape of the leaves (falcate with an asymmetric blade, or lanceolate to ovate - which is the typical form), inflorescences (from racemes to umbels), flowers (with a subfloral cupule covering half or more of the calyculus), and corollas (usually green occasionally with red or purple spots). In general, P. acinarius can be recognized by the cylindrical stems, the terminal or subterminal position of the inflorescences, always arranged in triads, the robust and fleshy aspect of peduncles and flowers, the presence of a dilated subfloral cupule, and flowers with dominant green coloration (Fig. 3a).
a. Psittacanthus acinarius – flowers. b-c. P. acinarius (as P. pustullosus) – b. flower buds with ‘pustules’. c. flower buds without ‘pustules’. d. P. biternatus (as P. duckei) – triads (white arrow). e-f. P. excrenulatus – e. flowering branch; f. flowers. g. P. grandifolius (as P. bergii) – flower buds with a rounded apex (black arrow). h-k. P. lamprophyllus – h-i. flowers. j-k. phyllotaxy, showing the nodes (black arrows). l. P. plagiophyllus – inflorescence. Photos: a. C.S. Caires; b-c. J.G. Kuhlmann 1174 (RB); d. Farney & Batista 2103 (NY); e. Zé Junior; f. J.R. Fabricante; g. C.C. Berg P18408 (RB); h. M. Silveira; i. D.C. Daly; j. B.A. Krukoff 4709 (NY); k. R. Spruce 1632 (NY); l. R. Fadini. Scale bars: a,g,j,k = 2 cm; b,c,d,f = 1 cm; e,h,i,l = 3 cm.
Since we acknowledge the wide morphological variation of P. acinarius, three new synonyms are proposed: P. carnosus Kuijt, P. crassipes Kuijt, and P. pustullosus Rizzini.
Psittacanthus carnosus was described based on a single collection, quite fragmented and with few flowers available. Kuijt (2009)Kuijt J (2009) Monograph of Psittacanthus (Loranthaceae). Systematic Botany Monographs 86: 1-361. considered P. carnosus as a new species based on the occurrence of moniliform anthers, umbellate inflorescences, and elongated subfloral cupules, covering more than half of the ovary, which are characteristics that overlap in P. acinarius. Two sheets of Maciel & Rosário 1555 were found in F herbarium with labels indicating that the holotype is mounted in two sheets (“sheet 1 of 2”, and “sheet 2 of 2”) in accordance with Art. 8.3 (Turland et al. 2018Turland NJ, Wiersema JH, Barrie FR, Greuter W, Hawksworth DL, Herendeen PS, Knapp S, Kusber WH, Li DZ, Marhold K, May TW, McNeill J, Monro AM, Prado J, Price MJ & Smith GF (eds.) (2018) International Code of Nomenclature for algae, fungi, and plants (Shenzhen Code) adopted by the Nineteenth International Botanical Congress Shenzhen, China, July 2017. Regnum Vegetabile 159. Koeltz Botanical Books, Glashütten. 254p.).
Psittacanthus crassipes was described based on three collections from Venezuela and Brazil. According to Kuijt (2009)Kuijt J (2009) Monograph of Psittacanthus (Loranthaceae). Systematic Botany Monographs 86: 1-361., it differs from P. acinarius by the obovate shape of the anthers, with trichomes on the back and, especially, by the structure (umbel of four triads) and axillary position of the inflorescence. However, the analysis of the isotypes at IAN and RB, and the paratype (Stevenson 825) at NY revealed the occurrence of pseudo-terminal inflorescences, which is common in P. acinarius. These characteristics were not considered by us as sufficient to separate it from P. acinarius, and we consider it a synonym.
Psittacanthus pustullosus has leaf, inflorescence and flower characteristics that overlap with P. acinarius. However, it can be distinguished by the presence of ‘pustules’ outside the corolla (Fig. 3b). As already pointed out by Kuijt (2009)Kuijt J (2009) Monograph of Psittacanthus (Loranthaceae). Systematic Botany Monographs 86: 1-361., these ‘pustules’ probably have a fungal origin and glabrous corollas can be found in the same sample (e.g., RB holotype, barcode 00545560) (Fig. 3c). There are three sheets of Kuhlmann 1174 in the RB herbarium, with a single original label in common, and will not be physically separated (R.C. Forzza, personal communication), so they could be treated as a holotype mounted in three sheets (Turland et al. 2018Turland NJ, Wiersema JH, Barrie FR, Greuter W, Hawksworth DL, Herendeen PS, Knapp S, Kusber WH, Li DZ, Marhold K, May TW, McNeill J, Monro AM, Prado J, Price MJ & Smith GF (eds.) (2018) International Code of Nomenclature for algae, fungi, and plants (Shenzhen Code) adopted by the Nineteenth International Botanical Congress Shenzhen, China, July 2017. Regnum Vegetabile 159. Koeltz Botanical Books, Glashütten. 254p., Art. 8.3).
Examined material: BRAZIL. AMAZONAS: Rio Xié, 5 km from confluence with Rio Negro, 00º55’N, 66ºW, 25.X.1987, fl., D.W. Stevenson 825 (NY - paratype of Psittacanthus crassipes).
Psittacanthus biternatus (Hoffmanns.) G. Don, Gen. Hist. 3: 415-416. 1834. Psittacanthus biternatus (Hoffmanns.) Blume, in Schultes & Schultes, Syst. Veg. (ed. 15 bis) 7(2): 1730. 1830, nom. illeg. Loranthus biternatus Hoffmanns., in Schultes & Schultes f., Syst. Veg. 7(1): 124. 1829. Type: BRAZIL. PARÁ: F.W. Sieber s.n. (holotype: B, destroyed = F Neg 11823!; lectotype, hic designatus: BR, barcode 0000005216239!).
Psittacanthus duckei Rizzini, Rodriguésia 30/31: 139. 1956. Type: BRAZIL. PARÁ: region of campos do Ariramba, highlands near the Cachoeira Terminus, 1.VII.1912, fl. and fr., A. Ducke s.n. (holotype: MG barcode 11875!; isotype: IAN 52443!), syn. nov.
Psittacanthus duckei was also based on a fragmented type material from ‘Campos do Ariramba’, a set of fragmented grasslands in the Amazon rainforest of Pará State (Fig 4a). In the taxonomic treatment of Psittacanthus, Kuijt (2009)Kuijt J (2009) Monograph of Psittacanthus (Loranthaceae). Systematic Botany Monographs 86: 1-361. treated P. duckei as a doubtful species. However, our analysis of the protologue (Rizzini 1956Rizzini CT (1956) Pars specialis prodromi monographiae Loranthacearum Brasiliae terrarumque finitimarum. Rodriguésia 30/31: 87-234.), the type specimen, and a few more collections from the same locality (Egler 1960Egler WA (1960) Contribuições ao conhecimento dos campos da Amazônia. I - Os campos do Ariramba. Boletim do Museu Paraense Emilio Goeldi, Botânica 4: 1-43.) provided us with a better understanding of the species. As a result, P. duckei is considered here a synonym for P. biternatus. It presents leaves with rounded base and apex, axillary inflorescences arranged in triads (Fig 4b), straight flower buds with crenulated petal on the margins and pubescent ligule, petals red with orange or yellow apex, and dimorphic stamens. All these characters overlap with P. biternatus, and no significant and/or consistent difference can be noted between these two species.
Type locality of Psittacanthus duckei (= P. biternatus). a. grasslands vegetation of ‘Campos do Ariramba’, Pará state. b. flowering branches of P. biternatus. Photos: a. R.G. Barbosa-Silva; b. D. Zappi. Scale bar: b = 2 cm.
The only characteristic in disagreement with the protologue is the arrangement of the flowers in the inflorescences. Rizzini (1956)Rizzini CT (1956) Pars specialis prodromi monographiae Loranthacearum Brasiliae terrarumque finitimarum. Rodriguésia 30/31: 87-234. describes inflorescences arranged in dyads (Flores per binationes in umbellas biradiatas). However, as the holotype is entirely fragmented, it was not possible to visualize this character. The analysis of other samples from ‘Campos do Ariramba’ revealed plants with inflorescences arranged in triads (e.g., Farney & Batista 2103, NY, Fig. 3d). Thus, we believe that this is the typical inflorescence configuration for this species.
Kuijt (2009)Kuijt J (2009) Monograph of Psittacanthus (Loranthaceae). Systematic Botany Monographs 86: 1-361. pointed out that the holotype of P. biternatus is ‘unknown’. This specimen corresponds to a collection of F.W. Sieber for J.C. von Hoffmannsegg and served as a basis for the illustration of the species in Flora Brasiliensis (Eichler 1868Eichler AW (1868) Loranthaceae. In: Martius CFP (ed.) Flora brasiliensis. Fleicher, Leipzig. Vol. 5, pars 2, pp. 1-136, t.1-44., Tab. IX-5). We have located the negative image (F Neg 11823) but the specimen is not kept in the B collection, and it was probably lost during the Second World War (R. Vogel, personal communication). A duplicate of this collection was found in the BR herbarium and is here designated as the lectotype, thus rendering superfluous the neotypification proposed by Kuijt (2009)Kuijt J (2009) Monograph of Psittacanthus (Loranthaceae). Systematic Botany Monographs 86: 1-361..
Examined material: BRAZIL. PARÁ: Taboleta, campos de Ariramba, 30.V.1957, fr., P.B. Cavalcante 149 (RB); region of Ariramba, between campo Mutum and campo da Taboleta, 30.V.1957, fr., G.A. Black et al. (IAN 96219); Oriximiná, Rio Trombetas, campos do Ariramba, between rios Jaramacarú e Ariramba, 100 m, 8.VI.1980, fr., G. Martinelli et al. 6866 (INPA, RB); campos do Ariramba, grasslands near to Rio Ariramba, on Byrsonima, 5.XII.1987, fl., C. Farney & E.F. Batista 2103 (NY, RB, US); Óbidos, campos do Ariramba, Tabuleta, 18 km of Rio Jaramacaru, 01º10’S, 55º35’W, 6.XII.1987, fl., C.A. Cid Ferreira 9787 (NY, RB).
Psittacanthus crassifolius (Mart.) Mart., Flora 13(1): 108. 1830. Psittacanthus crassifolius (Mart.) G. Don, Gen. Hist. 3: 417. 1834, nom. illeg. Loranthus crassifolius Mart. in Schultes & Schultes f., Syst. Veg. 7(1): 123. 1829. Type: BRAZIL. AMAZONAS: Alto Amazonas, in sylvis Yapurensibus ad montem Arara-Coara, C.F.P. von Martius s.n. (holotype: M = F Neg 19056!).
Psittacanthus baguensis Kuijt, Syst. Bot. Monogr. 86: 83, Fig. 29. 2009. Type: PERU. LORETO: Maynas, Iquitos, Allpahuayo, estación IIAP, 04º10’S, 73º30’W, 15.X.1993, fl., R. Vásquez et al. 18365 (holotype: UC (ex-LEA) barcode 1956961!; isotypes: F!, MO), syn. nov.
The description of P. crassifolius is based on a fragmented specimen with few flowers. However, the analysis of the protologue, as well as the description and illustrations presented in Flora Brasiliensis (Eichler 1868Eichler AW (1868) Loranthaceae. In: Martius CFP (ed.) Flora brasiliensis. Fleicher, Leipzig. Vol. 5, pars 2, pp. 1-136, t.1-44., Fig. 9-III), allowed a better characterization of this species, allowing us to include P. baguensis in the synonymy of P. crassifolius.
The shared characters include large and coriaceous leaves (10‒18 × 4‒8 cm), blades ovate or obovate with pinnate venation, obscure ribs with only the midrib conspicuous towards the apex, flowers arranged in triads, peduncle, pedicels and bracts covered with a brown furfuraceous indument (Eichler 1868Eichler AW (1868) Loranthaceae. In: Martius CFP (ed.) Flora brasiliensis. Fleicher, Leipzig. Vol. 5, pars 2, pp. 1-136, t.1-44.: ‘cum pedunculis et bractea obsolete rubro-furfuraceae’, p. 29), straight and long flower buds (7‒8 cm long), with red petals and red trichomes adjacent to the anthers, and isomorphic stamens with septate anthers. Kuijt (2009)Kuijt J (2009) Monograph of Psittacanthus (Loranthaceae). Systematic Botany Monographs 86: 1-361. cited G. Klug 119 as voucher for P. crassifolius for Peru, and simultaneously as a ‘provisional’ paratype of P. baguensis. Unfortunately, there is no comment, in any of the descriptions, on the similarity between these species. Psittacanthus crassifolius occurs in riverside forest areas of Brazil, Colombia and Peru, (Kuijt 2009Kuijt J (2009) Monograph of Psittacanthus (Loranthaceae). Systematic Botany Monographs 86: 1-361.). In Brazil, it is known only from the state of Amazonas.
Examined material: BRAZIL. AMAZONAS: Humaitá, between Rio Livramento and Rio Ipixuma, 7.XI.1934, fl., B.A. Krukoff 7229 (F); Humaitá, between Rio Ipixuna and Rio Itaparana, on Sapotaceae, 24.XI.1966, fl., G.T. Prance et al. 3261 (RB, US). COLOMBIA. AMAZONAS: near Rio Caqueta, Araracuara, 6.IX.1959, fl., B. Maguire et al. 44139 (F). PERU. LORETO: Mishuyacu, near Iquitos, X.1929, fl., G. Klug 119 (F - paratype of P. baguensis).
Psittacanthus excrenulatus Rizzini, Rev. Fac. Agron. Maracay 8(3): 93. 1975. Type: BRAZIL. BAHIA: Maraú, on Theaceae, 18.I.1967, fl., R.P. Belém & R.S. Pinheiro 3177 (holotype: RB; isotypes: CEPEC!, IAN!, NY!(2x), UB!).
Psittacanthus salvadorensis Kuijt, Syst. Bot. Monogr. 86: 291, Fig. 144. 2009. Type: BRAZIL. BAHIA: Salvador, Bairro Itapuã, vicinity of airport, 23.V.1981, fl., S.A. Mori et al. 14089 (holotype: UC (ex-LEA) barcode 1958059!; isotypes: CEPEC!, MO, NY!), syn. nov.
Psittacanthus salvadorensis was based on a single collection from the municipality of Salvador, Bahia state. The analysis of vegetative and reproductive characters allowed us to consider it a synonym of P. excrenulatus, especially for the obovate leaves without conspicuous nervation, and flowers arranged in triads with red petals and dimorphic stamens (Figs. 3e-f).
Psittacanthus excrenulatus is morphologically close to P. dichroos, especially in the vegetative attributes. They can be differentiated by the erect branches (vs. pending in P. dichroos), the shorter flowers with 2.5‒3 cm long (vs. 3.5‒4 cm long), the entirely red petals (vs. bicolor), and the mature black ovoid fruits (vs. orange oblong fruits).
Examined material: BRAZIL. BAHIA: Salvador, ca. 30 km N from the city center, road to the airport, surroundings of Itapuã, 23.V.1981, fl. and fr., A.M. Carvalho et al. 720 (CEPEC); BA-033 from Itapuã to Aeroporto 2 de Julho, 12º55’S, 38º21’W, 27.I.1983, fl., T. Plowman 12766 (CEPEC); bairro Stella Maris, condomínio Petromar, on Byrsonima crassifolia (Malpighiaceae), 23.II.1998, fl., J. Costa & C.B. Nascimento 152 (MBM).
Psittacanthus grandifolius (Mart.) Mart., Flora 13(1): 108. 1830. Loranthus grandifolius Mart., Syst. Veg. (ed. 15 bis) 7(1): 124. 1829. Psittacanthus grandifolius (Mart.) G. Don, Gen. Hist. 3: 415. 1834 [grandiflorus], nom. illeg. Type: BRAZIL. AMAZONAS: in silvis Yapurensibus ad cataractas Cupatenses, C.F.P. von Martius s.n. (holotype: M = F Neg 19059!).
Psittacanthus bergii Kuijt, Syst. Bot. Monogr. 86: 85, Fig. 31. 2009. Type: BRAZIL. MATO GROSSO: Rio Aripuanã, bay near Igarapezinho, 10º12’S, 59º21’W, fl., C.C. Berg et al. P18408 (holotype: UC (ex-LEA); isotypes: INPA!, UC, RB!), syn. nov.
Kuijt (2009)Kuijt J (2009) Monograph of Psittacanthus (Loranthaceae). Systematic Botany Monographs 86: 1-361. recognized the morphological similarity of P. bergii, P. grandifolius and P. peronopetalus, but preferred to describe P. bergii as a new species, based on differences in flower buds, anthers, leaves, and inflorescences. The present evidence suggests that the morphology of leaves, inflorescences, and flowers of P. bergii overlap with P. grandifolius and that these characters are insufficient to distinguish them. Therefore, we propose to include P. bergii in the synonymy of P. grandifolius. Both P. grandifolius and P. bergii have large ovate leaves with only the midrib conspicuous, axillary inflorescences arranged in two triads, flower buds with rounded apices (Fig. 3g), and petals with papillate ligules.
Psittacanthus grandifolius differs from P. peronopetalus in the rounded and dilated apex of the flower buds (vs. straight or recurved petal apex, normally suberified in P. perenopetalus), the shorter anther connective horn (vs. longer), and the inflorescence composed of two triads (vs. four triads).
Examined material: BRAZIL: MATO GROSSO, Rio Aripuanã, above Andurina Falls, 10º12’S, 59º21’W, 19.X.1973, fl., C.C. Berg et al. P18649 (INPA, NY, RB - paratype of Psittacanthus bergii).
Psittacanthus lamprophyllus Eichler in Martius, Fl. bras. 5(2): 28, Fig. 9-I. 1868. Solenocalyx lamprophyllus (Eichler) Tiegh., Bull. Soc. Bot. France 42: 360. 1895. Type: BRAZIL. AMAZONAS: Manaqueri, ad oram meridionalem flum. Amazonum, ad ostium flum. Solimões, VI.1851, fl., R. Spruce 1632 (lectotype: M, designated by Kuijt (1994) = F Neg 19060!; isolectotypes: K!(2x), M(2x), NY!, P!(2x), TCD!).
Psittacanthus brachypodus Kuijt, Syst. Bot. Monogr. 86: 97, Fig. 36. 2009. Type: BRAZIL. PARÁ: Altamira, gleba São Millitão da Reserva Genética, 00º47’S, 52º42’W, 17.VI.1987, fl., M.J. Pires & N.T. Silva 1685 (holotype: UC barcode 1957016! (ex-LEA); isotypes: INPA!, NY!), syn. nov.
Psittacanthus leptanthus A.C. Sm., Phytologia 1: 113. 1935. Type: BRAZIL. AMAZONAS: basin of Rio Jurua, near mouth of Rio Embira (tributary of Rio Tarauaca), 07º30’S, 70º15’W, 10.VI.1933, fl., B.A. Krukoff 4709 (holotype: NY barcode 285234!; isotypes: A!, BM!, F!, K!, LP!, MICH!, MO!, S!, U!, UC!, US!), syn. nov.
Psittacanthus redactus Rizzini, Rodriguésia 30/31: 145. 1956. Type: BRAZIL. AMAPÁ: Oiapoque, campo de aviação, 7.X.1949, fl., G.A. Black 49-8445 (holotype: IAN!; isotype: RB!), syn. nov.
Psittacanthus lamprophyllus was described in Flora brasiliensis (Eichler 1868Eichler AW (1868) Loranthaceae. In: Martius CFP (ed.) Flora brasiliensis. Fleicher, Leipzig. Vol. 5, pars 2, pp. 1-136, t.1-44.). This species can be recognized by the ovate or elliptical leaves, pinnate venation with conspicuous midrib reaching the apex of the blades, and inconspicuous secondary ribs. The leaves are rarely palmatinerved, in which only the midrib is conspicuous towards the apex. However, intermediate forms can also be found. The inflorescences are axillary with dyads, the flower buds are long (5.5‒8 cm long) and delicate, not dilated and the apex is acute. The petals can be entirely red or dark-pink, or bicolor with a red base and a yellow apex (Figs. 3h-i). When revising the type-specimens of the Brazilian species, we realized that three other names also correspond to P. lamprophyllus. Thus, we propose here the synonymization of P. brachypodus Kuijt, P. leptanthus A.C. Sm., and P. redactus Rizzini in P. lamprophyllus.
Psittacanthus brachypodus was based only on the type specimen from the state of Pará. On the occasion, Kuijt (2009)Kuijt J (2009) Monograph of Psittacanthus (Loranthaceae). Systematic Botany Monographs 86: 1-361. merely pointed out its similarity to P. aequatorius Kuijt, described from Ecuador. However, P. brachypodus differs from P. aequatorius by the presence of shorter flowers with 7.5 cm long (vs. 10 cm long in P. aequatorius) and by the monopodial/percurrent branching (vs. sympodial/dichotomous branching). All the characteristics of stems, leaves, inflorescences, and flowers described for P. brachypodus also apply to P. lamprophyllus.
Smith (1935)Smith AC (1935) Plantae Krukovianae, IV. Phytologia 1: 113-126. recognized the similarity of P. leptanthus and P. siphon Eichler, a name accepted by Kuijt (2009)Kuijt J (2009) Monograph of Psittacanthus (Loranthaceae). Systematic Botany Monographs 86: 1-361. as a synonym of P. lamprophyllus. According to Smith (1935)Smith AC (1935) Plantae Krukovianae, IV. Phytologia 1: 113-126., P. leptanthus differs from P. siphon in having wider leaves and less branched inflorescences. Kuijt (2009)Kuijt J (2009) Monograph of Psittacanthus (Loranthaceae). Systematic Botany Monographs 86: 1-361. recognized the similarity between P. lamprophyllus and P. leptanthus but maintained them as distinct species due to the occurrence of less branched inflorescences and irregularly alternate leaves in P. leptanthus. The phyllotaxy in P. lamprophyllus is normally of the opposite type but, with a secondary growth of internodes, an uneven displacement of the nodes may occur. This character was observed in the type material of P. leptanthus (Fig. 3j), but it is also weakly visible in some specimens of P. lamprophyllus (Fig. 3k), indicating that this is a variable feature. The degree of branching of the inflorescence, as well as the color of the flowers, are also variable characters in P. lamprophyllus.
Psittacanthus redactus Rizzini was described for the state of Amapá. Rizzini (1956)Rizzini CT (1956) Pars specialis prodromi monographiae Loranthacearum Brasiliae terrarumque finitimarum. Rodriguésia 30/31: 87-234. distinguished this species from P. lamprophyllus by the less branched inflorescence and the cuneate base of the leaves. However, the base of the leaves in P. lamprophyllus is variable: obtuse/rounded, cuneate or decurrent. For this reason, we did not consider this a strong character for species separation.
Kuijt (2009)Kuijt J (2009) Monograph of Psittacanthus (Loranthaceae). Systematic Botany Monographs 86: 1-361. also denoted the morphological proximity of P. redactus and P. lamprophyllus for their similar inflorescences and flowers and included P. rufescens Rizzini in the synonymy for P. redactus, a decision we find appropriate. However, he maintained P. lamprophyllus and P. redactus as distinct species since P. redactus has thinner leaves with rounded apices and a dull adaxial surface (Kuijt 2009Kuijt J (2009) Monograph of Psittacanthus (Loranthaceae). Systematic Botany Monographs 86: 1-361.). As with the leaf base, we did not consider the leaf consistency and apex shape sufficient to distinguish the two species, as large variation could be observed in the examined specimens.
Considering the new synonyms, the distribution of Psittacanthus lamprophyllus has expanded to French Guiana, Peru, and Brazil. In Brazil, it occurs in the North (Acre, Amapá, Amazonas, Pará, and Rondônia) and Midwest (Mato Grosso), in the Amazonian gallery or lowland forests.
Examined material: BRAZIL. ACRE: Bujari, Riozinho do Andirá, right margin, 20.IV.1996, fl., A.R.S. Oliveira et al. 731 (NY); AMAPÁ: Rio Araguari, road from Porto Platón to Macapá, 19.IX.1961, fl., J.M. Pires et al. 51059 (MO, NY, RB, US - type of Psittacanthus rufescens); Oiapoque, campo de aviação, 26.IV.1960, fl., W.A. Egler 1445 (NY, RB); AMAZONAS: Manaus, ca. 80 km NNE of Manaus, Fazenda Esteio, 02º26’S, 59º48’W, 25.VI.1992, fl., M. Nee 42881 (MBM). Manaus, Rio Negro, right margin, Igarapé da Cachoeira, 02º41’18”S, 60º17’46”W, 3.VII.1999, fl., L.G. Lohmann 311 (SPF, UNIP); Rio Negro, prope Barra, 1855, fl., R. Spruce 3839 (P - holotype of Psittacanthus siphon). PARÁ: Almeirim, Monte Dourado, road to Pedral, 24.XI.1978, fl., M.R. Santos 391 (INPA, NY); RONDÔNIA: Rio Madeira, 4 km to Rio Jaciparaná, 28.VI.1968, fl., G.T. Prance et al. 53308 (NY).
Psittacanthus plagiophyllus Eichler in Martius, Fl. bras. 5(2): 37, Fig. 10-II. 1868. Type: BRAZIL. PARÁ: Santarém, R. Spruce 136 (lectotype, designated by Kuijt, 1994: M = F Neg 19061!; isolectotype: K).
Psittacanthus acevedoi Kuijt, Syst. Bot. Monogr. 86: 57, Fig. 15. 2009. Type: BRAZIL. AMAZONAS: along Rio Cuiuni, 5 km N of boat, boat at 00º46’07”S, 62º13’15”W, 13.VIII.1996, fl., P. Acevedo-Rodríguez et al. 8274 (holotype: UC barcode 1956882! (ex-LEA); isotypes: INPA!, MO, NY!, US!), syn. nov.
Psittacanthus rugostylus Kuijt, Syst. Bot. Monogr. 86: 288, Fig. 141. 2009. Type: BRAZIL. PARÁ: Salinópolis, praia do Atalaia, dunas, 11.XI.1976, fl., M.G. Silva 2819 (lectotype, hic designatus: NY barcode 02219481!; isolectotypes: MO, NY!, RB!), syn. nov.
Psittacanthus acevedoi was based on two collections made along tributaries of the Rio Negro, in the state of Amazonas. Kuijt (2009)Kuijt J (2009) Monograph of Psittacanthus (Loranthaceae). Systematic Botany Monographs 86: 1-361. emphasized the dichotomy of the branches in P. acevedoi as a result of the abortion of the apical meristem. The analysis of the type specimens revealed plants with a predominance of monopodial ramifications, and few dichotomies. This and other morphological characters of leaves, inflorescences, and flowers agree with P. plagiophyllus. Therefore, we propose here the synonymization of P. acevedoi under P. plagiophyllus.
Kuijt (2009)Kuijt J (2009) Monograph of Psittacanthus (Loranthaceae). Systematic Botany Monographs 86: 1-361. described P. rugostylus based on a slender and elongated inflorescence with 4-rayed umbels, presence of a rugulose style, and basal ligules. We accepted P. rugostylus as a synonym of P. plagiophyllus considering the overlap in morphological characteristics of leaves, inflorescences, and flowers. The leaves of both taxa have a pinnate venation and overlapping dimensions (5‒12 × 2‒5 cm). The inflorescences have 2‒4-rayed umbels of triads, concentrated at the apex of the branches, in lateral and terminal positions. The flowers of P. rugostylus are 3.0‒3.5 cm long, pumpkin color (in the lectotype) or red in the lower 1/3 and yellow in the upper 2/3; and the inflorescence branches are purplish (according to the label of the paratype). The same flower size and color pattern are found in P. plagiophyllus (Fig. 3l).
The rugulose base of the style was also observed in specimens of P. plagiophyllus, as described by Kuijt (2009)Kuijt J (2009) Monograph of Psittacanthus (Loranthaceae). Systematic Botany Monographs 86: 1-361.. The presence of ligules in P. rugostylus and its absence in P. acevedoi and P. plagiophyllus calls for further investigation.
Psittacanthus rugostylus was observed parasiting ‘muruci’ Byrsonima crassifolia (L.) Kunth (Malpighiaceae) (for the lectotype) and ‘cashew tree’ Anacardium occidentale L. (Anacardiaceae) (for the paratype). Both host species can be found in the distribution area of P. plagiophyllus and a patch of Amazonian savanna on the right margin of the Tapajós River. Psittacanthus plagiophyllus was found parasiting only A. occidentale (Fadini 2011Fadini RF (2011) Non-overlap of hosts used by three congeneric and sympatric loranthaceous mistletoe species in an Amazonian savanna: host generalization to extreme specialization. Acta Botanica Brasilica 25: 337-345.).
Two sheets from the type specimen (Silva 2819) were found in the NY herbarium, without a clear indication of which one was selected by the author of the species as a holotype. Therefore, we here designate a lectotype (Turland et al. 2018Turland NJ, Wiersema JH, Barrie FR, Greuter W, Hawksworth DL, Herendeen PS, Knapp S, Kusber WH, Li DZ, Marhold K, May TW, McNeill J, Monro AM, Prado J, Price MJ & Smith GF (eds.) (2018) International Code of Nomenclature for algae, fungi, and plants (Shenzhen Code) adopted by the Nineteenth International Botanical Congress Shenzhen, China, July 2017. Regnum Vegetabile 159. Koeltz Botanical Books, Glashütten. 254p., Art. 40.2).
Examined material: BRAZIL. AMAZONAS: Alto Rio Negro, 00º28’46”S, 63º33’15”W, 15.VIII.1996, fl. and fr., P. Acevedo-Rodríguez et al. 8328 (NY, US - paratype of Psittacanthus acevedoi); PARÁ: Marapanim, village Camara ca. 11 km NW Marudá, 00º37’S, 47º41’W, on Anacardium occidentale, 3.IV.1980, fl. and im.fr., G. Davidse et al. 17801 (NY, US - paratype of P. rugostylus); Salinópolis, beach dunes of Atalaia, on cashew tree, 9.III.1989, fl., L. Carreira et al. 1085 (INPA). PIAUÍ: Oeiras, on cashew tree, IV.1839, fl., G. Gardner 2182 (K, P - syntype of P. plagiophyllus).
Types found
Psittacanthus formosus (Cham. & Schltdl.) G. Don, Gen. Hist. 3: 416. 1834. Loranthus formosus Cham. & Schltdl., Linnaea 3: 211. 1828, nom. illeg. hom., non Loranthus formosus Blume, Bijdr. Fl. Ned. Ind. 13: 664. 1826. Type: BRAZIL. Brasilia tropica, 1814-1831, F. Sellow s.n. (holotype: HAL barcode 98467!). = Psittacanthus robustus (Mart.) Mart.
Identification key for the Brazilian species of Psittacanthus (Loranthaceae)
-
1. Leaves sessile (Fig. 5a) ................... Psittacanthus cordatus (Hoffmanns.) G. Don
-
1’. Leaves petiolate ................... 2
-
2. Phyllotaxy in whorls (Fig. 5b) ................... 3
-
3. Leaves pinnate, apex rounded (Fig. 5b); flowers arranged in triads ......................................................... Psittacanthus nodosissimus Rizzini
-
3’. Leaves palmate, apex acuminate; flowers arranged in dyads ................... Psittacanthus ovatus Kuijt
-
-
2’. Phyllotaxy predominantly opposite, rare sub-alternate ................... 4
-
4. Flowers sessile (Fig. 5c) and/or in cymes subtended by foliate bracts (Fig. 5d) ......................................................... Psittacanthus cucullaris (Lam.) G. Don
-
4’. Flowers pedunculated, cymes subtended by squamiform bracts (Fig. 5e) ................... 5
-
5. Outer margin of petals strongly dentate, translucent teeth (Fig. 5f) ................... Psittacanthus dentatus Kuijt
-
5’. Outer margin of petals slightly dentate or smooth, opaque teeth ................... 6
-
6. External surface of petals coated by a dense indumentum (Fig. 2b) ......................................................... Psittacanthus lasianthus Sandwith
-
6’. External surface of petals glabrous, or rarely papillate ................... 7
-
7. Peduncle and pedicels with a furfuraceous surface (Fig. 5g) ................... 8
-
8. Flowers arranged in triads; flower buds straight; petals entirely red; stamens isomorphic, anthers septate ................... Psittacanthus crassifolius (Mart.) Mart.
-
8’. Flowers arranged in dyads; flower buds curved; petals bicolor, red at the base and yellow at the apex; stamens dimorphic, anthers non-septate ......................................................... Psittacanthus cinctus (Mart.) Mart.
-
-
7’. Peduncle and pedicels with a glabrous surface ................... 9
-
9. Petals entirely yellow ................... 10
-
10. Stems quadrangular; flower buds straight, 8‒10 cm long (Fig. 5h) ......................................................... Psittacanthus robustus (Mart.) Mart.
-
10’. Stems circular; flower buds strongly curved, 3‒3.5 cm long (Fig. 5i) ......................................................... Psittacanthus eucalyptifolius (Kunth) G. Don
-
-
9’. Petals bicolor (red with yellow or orange apex) or entirely red ................... 11
-
11. Flowers arranged in dyads (Fig. 5j) ................... 12
-
12. All stamens of the same height (isomorphic) (Fig. 5k) ................... 13
-
13. Leaves acute at the apex; petals entirely red with ligule; apex of the petals truncate with external projections (Fig. 5l) ......................................................... Psittacanthus peculiaris A.C. Sm.
-
13’. Leaves obtuse at the apex; petals bicolor (red/orange at the base and yellow at the apex) without ligule; apex of the petals acute or rounded without external projections ................... 14
-
14. Inflorescences concentrated at the apex of the branches (subterminal); flower buds long (8‒12 cm long) ...................................... Psittacanthus clusiifolius Willd. ex Eichler
-
14’. Inflorescences distributed along the branches (axillary); flower buds short (3.5‒6 cm long) ................... 15
-
15. Internodes short (1‒2 cm long); leaves lanceolate, elliptical or obovate, acute at the base, large (18‒21 × 10‒12 cm); flower buds ca. 6 cm long ......................................................... Psittacanthus brachynema Eichler
-
15’. Internodes long (4‒8 cm long); leaves ovate, obtuse at the base, small (6‒8 × 4‒4.5 cm); flower buds ca. 3.5 cm long ................... Psittacanthus kempffii Kuijt
-
-
-
-
12’. Stamens of two different heights (dimorphic) (Fig. 6a) ................... 16
-
16. Internodes short (1‒1.5 cm long); leaves spatulate (rare obovate or oblanceolate), up to 2 cm wide ................... Psittacanthus irwinii Rizzini
-
16’. Internodes long (2.5‒15 cm long); leaves not spatulate, more than 2 cm wide ................... 17
-
17. Leaves small (4.5‒6 × 2‒3.5 cm) (Fig. 5j); flower buds with a rounded apex; anthers small (ca. 0.5 mm long) ......................................................... Psittacanthus montis-neblinae Rizzini
-
17’. Leaves large (7‒15 × 3‒7 cm); flower buds with an acute or tapered apex; anthers large (3‒4.5 mm long)...................18
-
18. Young stems cylindrical, circular in cross-section; flower buds long (5.5‒8 cm long), pedicels robust, short (ca. 0.5 cm long) (Figs. 3h,i); anthers 3.5‒4.5 mm long ......................................................... Psittacanthus lamprophyllus Eichler
-
18’. Young stems flattened, ellipsoid in cross-section; flower buds short (4‒4.5 cm long), pedicels delicate, long (1‒1.2 cm long) (Fig. 6b); anthers ca. 3 mm long ................... Psittacanthus tenellus Kuijt
-
-
-
-
-
11’. Flowers arranged in triads (Fig. 5e) ................... 19
-
19. Flowers with an inflated central region (Fig. 6c), 0.7‒1 cm wide ......................................................... Psittacanthus amazonicus (Ule) Kuijt
-
19’. Flowers with a non-inflated central region, less than 0.4 cm wide ................... 20
-
20. All stamens of the same height (isomorphic) ................... 21
-
21. Flower bud strongly curved, rounded at the apex; corolla green, stained with red ......................................................... Psittacanthus geniculatus Kuijt
-
21’. Flower bud straight, truncated at the apex; corolla entirely red (Fig. 2d) ......................................................... Psittacanthus truncatus Kuijt
-
-
20’. Stamens of two and/or three different heights (dimorphic and/or trimorphic) ................... 22
-
22. Flowers longer than 4.5 cm long ................... 23
-
23. Leaves small (3.5‒5 × 1.5‒2 cm); peduncles, pedicels, and flowers delicate, elongated in length; flower buds with a tapered apex (Fig. 6d) ...................................... Psittacanthus elegans Kuijt
-
23’. Leaves large (more than 8 × 2.5 cm); peduncles, pedicels, and flowers robust; flower buds with a dilated, acute or a rounded apex ................... 24
-
24. Inflorescences terminal or subterminal; peduncles and pedicels fleshy; subfloral cupule involving part of the ovary (Fig. 3a), sometimes exceeding the middle portion of it; flower buds with an acute apex; corolla green (sometimes with reddish or vinaceous stains); ligule absent ......................................................... Psittacanthus acinarius (Mart.) Mart.
-
24’. Inflorescences axillary; peduncles and pedicels not fleshy; subfloral cupule only at the base of the ovary; flower buds with a rounded apex (Fig. 3g); corolla red with yellowish or purplish apices; ligule present ......................................................... Psittacanthus grandifolius (Mart.) Mart.
-
-
22’. Flowers less than 4 cm long ................... 25
-
25. Inflorescence terminal or axillary-subterminal, concentrated at the apex of the branches ................... 26
-
26. Leaves generally ovate; acute at the apex; ligule present ......................................................... Psittacanthus peronopetalus Eichler
-
26’. Leaves obovate, rounded, oblong or falcate; obtuse, rounded at the apex, eventually retuse; ligule absent ................... 27
-
27’. Leaves rounded, oblong or falcate; umbels highly branched, terminal and subterminal; peduncle and subfloral cupule not fleshy; corolla bicolor (red or orange at the base and yellow at the apex) (Fig. 3l) ......................................................... Psittacanthus plagiophyllus Eichler
-
27. Leaves obovate; umbels terminal with few branches; peduncle and subfloral cupule fleshy; corolla red (Fig. 6e) ...................................... Psittacanthus brasiliensis (Desr.) G. Don
-
-
-
25’. Inflorescences axillary, scattered along the branches or in the older parts of the plant ................... 28
-
28. Leaves obtuse at the base ................... 29
-
29. Internodes short (< 2 cm long); flower buds 3‒3.5 cm long, apex dilated and globular (Fig. 6f); ligule smooth; stamens trimorphic ......................................................... Psittacanthus bolbocephalus Kuijt
-
29’. Internodes long (> 4 cm long); flower buds 4 cm long, apex not dilated (Fig. 6g); ligule pubescent or papillate; stamens dimorphic ......................................................... Psittacanthus biternatus (Hoffmanns.) G. Don
-
-
28’. Leaves acute at the base ................... 31
-
31. Leaves elliptical or oblong, apex acute; cotyledons 12‒14 ......................................................... Psittacanthus pluricotyledonarius Rizzini
-
31’. Leaves mostly obovate, apex obtuse, rounded or retuse; cotyledons 2 ................... 32
-
32. Plants blackened in sicco (Fig. 6h); flower buds dilated at the apex; anthers ca. 2 mm long ......................................................... Psittacanthus atrolineatus Kuijt
-
32’. Plants brownish in sicco; flower buds not dilated, acute apex; anthers 3‒3.5 mm long ................... 33
-
33. Plants with pendant branches; flower buds 3.5‒4 cm long; corolla bicolor (red or orange at the base and yellow at the apex) (Fig. 6i); fruit oblong, orange when ripe ......................................................... Psittacanthus dichroos (Mart.) Mart.
-
33’. Plants with erect branches; flower buds 2.5‒3 cm long; corolla entirely red (Figs. 3e,f); fruit ovoid, black when ripe ................... Psittacanthus excrenulatus Rizzini
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
a. Psittacanthus cordatus – flowering branches with sessile leaves. b. P. nodosissimus – branches innovations, showing a whorled phyllotaxy (arrows). c-d. P. cucullaris – c. inflorescences showing sessile flowers and squamiform bracts (arrows); d. inflorescences showing foliate bracts (arrows). e. P. robustus – flowers with cymes subtended by squamiform bracts (arrows). f. P. dentatus – flowers showing petals strongly toothed (arrows). g. P. cinctus – infructescence with peduncle and pedicels with furfuraceous surface. h. P. robustus – flowering branches. i. P. eucalyptifolius – flowering branches. j. P. montis-neblinae – flowering branches showing flowers organized in dyads (arrows). k. P. clusiifolius – flower bud apex with the petals removed, showing all stamens at the same height (isomorphic) delimited by horizontal lines (arrow on stigma). l. P. peculiaris – flower bud apex with apex of the petals truncated and with external projections. Photos: a,c. C.S. Caires; b. R. Fadini; d. D.C. Daly; e,h. G.A. Dettke; f. M. Engels; g. F. Farroñay; i. L.O.A. Teixeira; j. M. Nadruz; k. C.A.C. Ferreira 9256 (MBM); l. M. Rimachi 5118 (MBM). Scale bars: a,b,c,d,g,i,j = 2 cm; e,f = 1 cm; h = 4 cm; k,l = 2 mm.
a. P. cordatus – flower bud apex with the petals removed, showing stamens at two different heights (dimorphic) delimited by horizontal lines (arrow on stigma). b. P. tenellus – inflorescences with delicate and long pedicels. c. P. amazonicus – flowers with inflated central region. d. P. elegans – flower buds with tapered apex (arrow). e. P. brasiliensis – terminal inflorescence; f. P. bolbocephalus – flowers with apex dilated and globular. g. P. biternatus – flowering branch. h. P. atrolineatus – plants blackened in sicco. i. P. dichroos – flowering branch. Photos: a. G. Hatschbach et al. 67568 (MBM); b. C.N. Fraga; c. D.C. Daly 7462 (NY); d. C.A.C. Ferreira 4031 (NY); e. M. Blanco; f. G. Hatschbach et al. 63179 (MBM); g. C.S. Caires; h. M.G. Silva & A. Pinheiro 4144 (NY); i. J.R. Fabricante. Scale bars: a = 2 mm; b,c,d,e,f,g,h,i = 2 cm.
Acknowledgements
The authors are grateful to several colleagues for sending images of specimens: Claudio Nicoletti de Fraga, Daniela Zappi, Douglas Charles de Burgh Daly, Francisco Farroñay, Juliano Ricardo Fabricante, Luiz Otavio Adão Teixeira, Marcos Silveira, Marcus Nadruz, Mario Blanco, Mathias Engels, Rafael Gomes Barbosa-Silva, Rodrigo Fadini, and Zé Junior. We thank also Dr. Robert Vogt, curator of B Herbarium, for providing information about the holotype of Psittacanthus biternatus and Dra. Rafaela Campostrini Forzza, curator of RB Herbarium, for providing information about types of Rizzini’s names. This is the publication 28 in the Parasitic Plants Research Group technical series.
References
- Arruda R, Fadini RF, Carvalho LN, Del-Claro K, Mourão FA, Jacobi CM, Teodoro GS, van den Berg E, Caires CS & Dettke GA (2012) Ecology of neotropical mistletoes: an important canopy dwelling component of Brazilian ecosystems. Acta Botanica Brasilica 26: 264-274.
- Dettke GA & Waechter JL (2014) Estudo taxonômico das ervas-de-passarinho da Região Sul do Brasil: I. Loranthaceae e Santalaceae. Rodriguésia 65: 939-953.
- Dettke GA & Caires CS (2020) Psittacanthus In: Flora do Brasil 2020 under construction. Jardim Botânico do Rio de Janeiro. Available at <http://floradobrasil.jbrj.gov.br/reflora/floradobrasil/FB8698>. Access on 21 January 2020.
» http://floradobrasil.jbrj.gov.br/reflora/floradobrasil/FB8698 - Egler WA (1960) Contribuições ao conhecimento dos campos da Amazônia. I - Os campos do Ariramba. Boletim do Museu Paraense Emilio Goeldi, Botânica 4: 1-43.
- Eichler AW (1868) Loranthaceae. In: Martius CFP (ed.) Flora brasiliensis. Fleicher, Leipzig. Vol. 5, pars 2, pp. 1-136, t.1-44.
- Fadini RF (2011) Non-overlap of hosts used by three congeneric and sympatric loranthaceous mistletoe species in an Amazonian savanna: host generalization to extreme specialization. Acta Botanica Brasilica 25: 337-345.
- González F, Roldán FJ & Pabón-Mora N (2016) Psittacanthus corderoi, a new species of Loranthaceae from the Colombian Amazonia. Caldasia 38: 250-256.
- Kuijt J (2009) Monograph of Psittacanthus (Loranthaceae). Systematic Botany Monographs 86: 1-361.
- Kuijt J (2014a) A monograph of the genus Aetanthus (Loranthaceae). Plant Diversity and Evolution 131: 1-51.
- Kuijt J (2014b) Five new species, one new name, and transfers in Neotropical mistletoes (Loranthaceae), Miscellaneous Notes, 61-68. Novon 23: 176-186.
- Nickrent DL, Malécot V, Vidal-Russell R & Der JP (2010) A revised classification of Santalales. Taxon 59: 538-558.
- Rizzini CT (1956) Pars specialis prodromi monographiae Loranthacearum Brasiliae terrarumque finitimarum. Rodriguésia 30/31: 87-234.
- Roldán-Palacios FJ & Alzate-Guarin F (2014) Psittacanthus longerectus (Loranthaceae), a new showy species from Colombia. Actualidades Biológicas 36: 119-122.
- Smith AC (1935) Plantae Krukovianae, IV. Phytologia 1: 113-126.
- Turland NJ, Wiersema JH, Barrie FR, Greuter W, Hawksworth DL, Herendeen PS, Knapp S, Kusber WH, Li DZ, Marhold K, May TW, McNeill J, Monro AM, Prado J, Price MJ & Smith GF (eds.) (2018) International Code of Nomenclature for algae, fungi, and plants (Shenzhen Code) adopted by the Nineteenth International Botanical Congress Shenzhen, China, July 2017. Regnum Vegetabile 159. Koeltz Botanical Books, Glashütten. 254p.
- Vidal-Russell R & Nickrent DL (2008) Evolutionary relationships in the showy mistletoe family (Loranthaceae). American Journal of Botany 95: 1015-1029.
Edited by
Publication Dates
-
Publication in this collection
03 Dec 2021 -
Date of issue
2021
History
-
Received
22 Apr 2020 -
Accepted
29 Dec 2020