Abstract
Hemitrichia leiocarpa was collected in the state of Pernambuco, northeastern Brazil, in 1968, and five decades passed before its second collection in the country. The species was rediscovered in the Pedra Talhada Biological Reserve, municipality of Quebrangulo, state of Alagoas, 225 km from the first location. A description of specimens that sporulated in moist chamber culture prepared with ground litter and deposited in the Myxomycetes collection of the UFP Herbarium is provided. Detailed and updated information on the worldwide geographical distribution of the species is provided, as well as information on substrates and microhabitats based on bibliographic sources and herbarium catalogues. The species can be considered near threatened (NT) based on IUCN criteria and its inclusion in the Brazilian Red List of Threatened Species is recommended.
Key words
Atlantic Forest; chorology; endangered species; red list; Trichiaceae
Resumo
Hemitrichia leiocarpa foi coletada no estado de Pernambuco, nordeste do Brasil, em 1968 e cinco décadas passaram até ser novamente coletada no país. A espécie foi redescoberta na Reserva Biológica de Pedra Talhada, município de Quebrangulo, estado de Alagoas, distante 225 km da primeira localidade. Apresenta-se a descrição dos espécimes, esporulados em câmaras úmidas montadas com folhedo de solo e depositados na coleção de Myxomycetes do Herbário UFP. São fornecidas informações detalhadas e atualizadas sobre sua distribuição geográfica mundial, juntamente com substratos e microhabitats, com base em fontes bibliográficas e catálogos de herbários. Esta espécie pode ser considerada como quase ameaçada (NT) com base nos critérios da IUCN e sua inclusão na Lista Vermelha do Brasil é recomendada.
Palavras-chave
Floresta Atlântica; corologia; espécies ameaçadas; lista vermelha; Trichiaceae
The genus Hemitrichia was described by J. T. Rostafinski in 1873 to gather species of the order Calonemeae, tribe Trichiaceae, with stalked or sessile sporocarps, an elastic capillitium formed a by a net of branched threads, with two to six spirals and minutely verrucous or reticulated spores (Lister 1925Lister A (1925) A monograph of the Mycetozoa. British Museum of Natural History, London. 30p.; Lado 2005Lado C (2005-2020) An online nomenclatural information system of Eumycetozoa. Available at <https://eumycetozoa.com/>. Access on 5 October 2020.
https://eumycetozoa.com/...
–2020). In the classification proposed by Leontyev et al. (2019)Leontyev DV, Schnittler M, Stephenson SL, Novozhilov YK & Shchepin ON (2019) Towards a phylogenetic classification of the Myxomycetes. Phytotaxa 399: 209-238., the genus is included in the class Myxomycetes, subclass Lucisporomycetidae, in the same order (Trichiales) and family (Trichiaceae) presented in classical monographs, such as those by Martin & Alexopoulos (1969)Martin GW & Alexopoulos CJ (1969) The Myxomycetes. University of Iowa Press, Iowa City. 561p. and Farr (1976)Farr ML (1976) Myxomycetes. Flora Neotropica. Monograph 16. New York Botanical Garden, New York. 304p..
Thirty species are currently accepted. They are distributed in the two Hemispheres and easily detected in the field by the number of sporocarps produced by each plasmodium as well as their dimensions and color, in tints of red or yellow in the majority (90%). They differ from the color pattern of H. leiocarpa (Cooke) Lister, H. pseudoleiocarpa Illana, G. Moreno, Lizárraga & A. Castillo, and H. montana (Morgan) T. Macbr., whose sporangia vary from whitish to light gray or ochraceous gray (Poulain et al. 2011Poulain M, Meyer M & Bozonnet J (2011) Les Myxomycètes. Fédération Mycologique et Botanique Dauphiné-Savoie, Sévrier. 1119p.; Lado 2005Lado C (2005-2020) An online nomenclatural information system of Eumycetozoa. Available at <https://eumycetozoa.com/>. Access on 5 October 2020.
https://eumycetozoa.com/...
–2020).
In the literature review for the Neotropics, Lado & Basanta (2008)Lado C & Basanta DW (2008) Review of Neotropical Myxomycetes (1828-2008). Anales del Jardín Botánico de Madrid 65: 211-254. listed 13 species of Hemitrichia, with records in Mexico, 14 countries of Central America and 10 of South America, with emphasis on H. calyculata (Speg.) ML Farr and H. serpula (Scop.) Rostaf. ex Lister due to its wide distribution. In Brazil, eight species are known, distributed in the North (2), Northeast (7), Midwest (4), Southeast (3) and South (4) regions, in different phytogeographic domains (Cavalcanti et al. 2020Cavalcanti LH, Bezerra ACC & Agra LAN (2020) Myxomycetes. In: Lista de Espécies da Flora do Brasil. Instituto de Pesquisas Jardim Botânico do Rio de Janeiro. Available at <http://floradobrasil.jbrj.gov.br/>. Access on 9 October 2020.
http://floradobrasil.jbrj.gov.br/...
). Some species, such as H. clavata (Pers.) Rostaf., H. minor G. Lister and H. pardina (Minakata) Ing, are mainly found in areas of the Atlantic Forest, one of the most threatened biodiversity hotspots in the world, while others, such as H. calyculata and H. serpula, are very common, present in natural environments as well as in urbanized and cultivated areas. Three species have only one record in Brazil. These are Hemitrichia insignis Torrend, only known from the type collection, obtained 85 years ago in Poções (BA); H. leiocarpa, collected by one of the authors in 1968, in the urban area of Recife, Pernambuco; and H. spinifera M. L. Farr, registered in 2001 in Brasília (DF), in riparian forest (Cavalcanti 1976Cavalcanti LH (1976) Mixomicetos novos para Pernambuco II. Memórias do Instituto de Biociências. Universidade Federal de Pernambuco, Série Botânica 4: 1-19., 2002Cavalcanti LH (2002) Biodiversidade e distribuição de mixomicetos em ambientes naturais e antropogênicos no Brasil: espécies ocorrentes nas Regiões Norte e Nordeste. In: Araújo EL, Moura NA, Sampaio EVSB, Gestinari LM & Carneiro JMT (eds.) Biodiversidade, conservação e uso sustentável da flora do Brasil. Sociedade Botânica do Brasil. Universidade Federal Rural de Pernambuco, Recife. Pp. 209-216., 2010Cavalcanti LH (2010) Myxomycota. In: Forzza RC, Baumgratz JFA, Bicudo CDM, Carvalho Jr AA, Costa A, Costa DP & Martinelli G (eds.) Catálogo de plantas e fungos do Brasil. Andrea Jakobsson Estúdio: Instituto de Pesquisas Jardim Botânico do Rio de Janeiro, Rio de Janeiro. Pp. 94-104.; Putzke 1996Putzke J (1996) Myxomycetes no Brasil. Cadernos de Pesquisa, Série Botânica 8: 1-133.; Bezerra et al. 2009Bezerra ACC, Cavalcanti LH & Dianese JC (2009) Species of Hemitrichia (Trichiaceae, Myxomycetes) in Brazil. Mycotaxon 107: 34-48.; Cavalcanti et al. 2020Cavalcanti LH, Bezerra ACC & Agra LAN (2020) Myxomycetes. In: Lista de Espécies da Flora do Brasil. Instituto de Pesquisas Jardim Botânico do Rio de Janeiro. Available at <http://floradobrasil.jbrj.gov.br/>. Access on 9 October 2020.
http://floradobrasil.jbrj.gov.br/...
).
Hemiarcyria leiocarpa was described by Cooke (1877)Cooke MC (1877) Myxomycetes of the United States. Annals of the Lyceum of Natural History of New York 11: 378-409. based on a specimen collected by Rev. E. C. Bolles in Maine, USA (Zoll & Stephenson 2015Zoll V & Stephenson SL (2015) Records of myxomycetes from Maine. Mycosphere 6: 568-584.). Arthur Lister and his daughter Gulielma Lister included it among the species of Hemitrichia and commented on its similarity to H. clavata (Lister 1894Lister A (1894) A monograph of the Mycetozoa being a descriptive catalogue of the species in the Herbarium of the British Museum. British Museum (Natural History), London. 418p.) and, later, to Arcyria cinerea (Bull.) Pers. (Lister 1925Lister A (1925) A monograph of the Mycetozoa. British Museum of Natural History, London. 30p.), distinguishing it on both occasions mainly by the capillitium with sinistrorse spirals and spines. Martin & Alexopoulos (1969)Martin GW & Alexopoulos CJ (1969) The Myxomycetes. University of Iowa Press, Iowa City. 561p. also commented on its similarity to A. cinerea and considered the characteristics of the sporocarp to be typical of Arcyria, except for the weak spirals on the capillitium, proposing a new combination and suggesting changes in the description of the capillitium in the diagnosis of the genus. Farr (1976)Farr ML (1976) Myxomycetes. Flora Neotropica. Monograph 16. New York Botanical Garden, New York. 304p. agreed on its similarity to A. cinerea, although with a longer stalk, and commented that both species can be easily confused. However, based on the analysis of the exsiccate BPI 833083 from Grenada, Windward Islands, the author considers that the ornamentation of the capillitium is sufficient to distinguish the two species, an opinion shared by Nannenga-Bremekamp (1991)Nannenga-Bremekamp NE (1991) A guide to temperate Myxomycetes: an english translation of De Nederlandse Myxomycetesn. Biopress, Bristol. 409p.. Illana et al. (1999)Illana C, Moreno G, Lizarraga M & Castillo A (1999) Hemitrichia pseudoleiocarpa, spec. nova, a species confused with Arcyria leiocarpa (Myxomycetes). Österreichische Zeitschrift für Pilzkunde 8: 63-70. analyzed the type material and agreed with its inclusion in the genus Arcyria, making comments on its similarity to A. cinerea, except for the capillitial ornamentation. Eliasson (2015)Eliasson UH (2015) Review and remarks on current generic delimitations in the myxomycetes, with special emphasis on Licea, Listerella and Perichaena. Nova Hedwigia 104: 343-350. DOI: 10.1127/nova_hedwigia/2015/0283
https://doi.org/10.1127/nova_hedwigia/20...
commented that certain species of myxomycetes bridge the gap between two genera by combining morphological characters from both and cited H. leiocarpa as an example of that, which could well be placed in either Hemitrichia or Arcyria. Despite being included among Hemitrichia species in Lado (2005–2020), the taxonomic status of the species is still controversial, being treated in recent articles sometimes as Arcyria, as in Walker et al. (2019)Walker LM, Cedeño-Sanchez M, Carbonero F, Herre EA, Turner BL, Wright SJ & Stephenson SL (2019) The response of litter-associated myxomycetes to long-term nutrient addition in a Lowland Tropical Forest. Journal of Eukaryotic Microbiology 66: 757-770., and other times as Hemitrichia, as in Lado et al. (2017)Lado C, Estrada-Torres A, Basanta DW, Schnittler M & Stephenson SL (2017) A rapid biodiversity assessment of myxomycetes from a primary tropical moist forest of the Amazon basin in Ecuador. Nova Hedwigia 104: 293-321. .
Information on the occurrence of species in different parts of the world based on fieldwork is needed in order to build consistent distribution patterns of myxomycetes using computational biology techniques (Rojas et al. 2011Rojas C, Stephenson SL & Pavlich M (2011) New additions to the myxobiota of Peru. Mycosphere 2: 583-592.). The present work reports the rediscovery of H. leiocarpa in Brazil, collected in Pedra Talhada Biological Reserve and the microhabitats in which it has been found, as well as updates and expands the knowledge about its worldwide geographical distribution and proposes its inclusion in the Red List of Brazil.
Located in the transition region between the Atlantic Forest and the Caatinga, the Pedra Talhada Biological Reserve (PTBR) represents one of the main forest fragments of the Pernambuco Endemism Center and plays an important role in the conservation of endangered species (Fig. 1).
Created in 1989, the PTBR is located on the boundaries between the states of Pernambuco and Alagoas, northeastern Brazil (09°14’45.5’’S, 36°25’14.06’’W), with an area of 4.469 hectare. The reserve protects fragments of Submontane Ombrophilous Forest, which host plant species considered vulnerable or near threatened, such as the palm heart (Euterpe edulis Martius), “sucupira” (Bowdichia virgilioides Kunth) and “sapucarana” (Eschweilera alvimii Mori), among others mentioned by Nusbaumer et al. (2015)Nusbaumer L, Barbosa, MRV, Thomas WW, Alves MV, Loizeau PA & Spichiger R (2015) Flora e vegetação da Reserva Biológica de Pedra Talhada. In: Studer A, Nusbaumer L & Spichiger R (eds.) Biodiversidade da Reserva Biológica de Pedra Talhada (Alagoas, Pernambuco - Brasil). Boissiera 68: 59-121.. Endangered and endemic fauna also inhabit these forest fragments, including Leptodon forbesi Swann, 1922, the white-collared kite, Xipholena atropurpurea Wied, 1820, the white-winged cotinga, Hemitriccus mirandae Snethlage, 1925, the “Maria-do-nordeste”, and Procnias averano Hermann, 1783, the “araponga-do-nordeste” (Studer 2015Studer A (2015) Aves. In: Studer A, Nusbaumer L & Spichiger R (eds.) Biodiversidade da Reserva Biológica de Pedra Talhada. Alagoas / Pernambuco. Brasil. Boissiera 68: 59-121. ).
The elevation varies between 459 and 883 m, the average annual temperature is 25 °C, and average rainfall rates ranges from 1,250 to 1,500 mm (Guimarães et al. 2014Guimarães JRA, Studer A &Trivellato C (2014) Educação ambiental no entorno da Reserva Biológica de Pedra Talhada. Fórum ambiental da Alta Paulista 10: 32-45.). In the rainy season, when excursions were made, rainfall rates reach peaks between May and July, with more than 250 mm/month; the months from October to February are drier, with less than 50 mm/month (Tscharner et al. 2015Tscharner T, Duda GP, Oliveira VP, Silva CMS, Nusbaumer L & Silva Filho AF (2015) Parâmetros abióticos da Reserva Biológica de Pedra Talhada. In: Studer A, Nusbaumer L & Spichiger R (eds.) Biodiversidade da Reserva Biológica de Pedra Talhada (Alagoas, Pernambuco - Brasil). Boissiera 68: 39-57.). The region has tropical rainy climate (As’), according to the Köppen classification (Barros et al. 2012Barros AHC, Araújo Filho JC, Silva AB & Santiago GACF (2012) Climatologia do estado de Alagoas. Boletim de Pesquisa e Desenvolvimento (INFOTECA-E). Embrapa Solos, Recife. 39p.), determined by the elevation, with higher rainfall rates than those of surrounding areas, typical of the semi-arid region of Northeastern Brazil; as a result, the forest maintains its green appearance throughout the year, with non-deciduous trees (Studer 1985Studer A (1985) Estudo ecológico do conjunto florestal da Serra das Guaribas e da Serra do Cavaleiro. Pedido para a Salvaguarda desta Floresta. Monografia. Quebrangulo, Alagoas. 61p.; Nusbaumer et al. 2015Nusbaumer L, Barbosa, MRV, Thomas WW, Alves MV, Loizeau PA & Spichiger R (2015) Flora e vegetação da Reserva Biológica de Pedra Talhada. In: Studer A, Nusbaumer L & Spichiger R (eds.) Biodiversidade da Reserva Biológica de Pedra Talhada (Alagoas, Pernambuco - Brasil). Boissiera 68: 59-121.).
Two excursions were made to the PTBR, each lasting six days, exploring the ground litter of areas defined in Management Plan of the reserve as primitive and recovering. Leaf litter samples were collected, packed in plastic bags and transported to the laboratory for mounting in moist chambers, according to Rojas et al. (2011)Rojas C, Stephenson SL & Pavlich M (2011) New additions to the myxobiota of Peru. Mycosphere 2: 583-592.. The cultures were kept at room temperature (22–25 °C) under diffuse light and observed for a period of four consecutive months. The sporocarps were removed from the moist chamber still attached to the fragment of substrate where they were fixed and placed in a semi-open Petri dish, for gradual drying.
The macroscopic morphological characteristics of the specimens were observed under a stereomicroscope, and microscopic characteristics were observed and measured in an optical microscope equipped with an ocular micrometer under magnifications of 400x and 1,000x. These measurements were used to identify the species, using the keys, illustrations and descriptions provided by Lister (1925)Lister A (1925) A monograph of the Mycetozoa. British Museum of Natural History, London. 30p., Martin & Alexopoulos (1969)Martin GW & Alexopoulos CJ (1969) The Myxomycetes. University of Iowa Press, Iowa City. 561p. and Poulain et al. (2011)Poulain M, Meyer M & Bozonnet J (2011) Les Myxomycètes. Fédération Mycologique et Botanique Dauphiné-Savoie, Sévrier. 1119p.. Data from the Eumycetozoan Project (<http://slimemold.uark.edu/>) and online database <https://eumycetozoa.com/> (Lado 2005Lado C (2005-2020) An online nomenclatural information system of Eumycetozoa. Available at <https://eumycetozoa.com/>. Access on 5 October 2020.
https://eumycetozoa.com/...
–2020) were also consulted. Exsiccates from the material examined were deposited at the UFP herbarium.
Occurrence records (country, location, elevation, type of environment, substrate, year of collection, collector, determiner) were obtained from the DiscoverLife (<https://www.discoverlife.org/>), SpeciesLink (<https://specieslink.net/>), Flora do Brasil (<http://floradobrasil.jbrj.gov.br/r>), and Global Biodiversity Information Facility - GBIF (<https://www.gbif.org/>) online databases and complemented with information about the species existing in the BPI, MA-fungi, M, and UARK herbaria (acronyms according to Thiers, continuously updated). Exsiccates of Trichiales from the following Brazilian herbaria were examined in person, with special attention to those identified as Arcyria cinerea: HFSL, ICN, INPA, IPA, JPB, SP-Fun, TEPB, UFP, UFRR, URM (Thiers, continuously updated). Data on location, type of environment, microhabitat, and substrate of sporulation were complemented with information provided by Adamonyte et al. (2013)Adamonyte G, Iršėnaitė R, Motiejūnaitė J, Taraškevičius R & Matulevičiūtė D (2013) Myxomycetes in a forest affected by great cormorant colony: a case study in Western Lithuania. Fungal Diversity 59: 131-146., Cavalcanti (1976)Cavalcanti LH (1976) Mixomicetos novos para Pernambuco II. Memórias do Instituto de Biociências. Universidade Federal de Pernambuco, Série Botânica 4: 1-19., Farr (1976)Farr ML (1976) Myxomycetes. Flora Neotropica. Monograph 16. New York Botanical Garden, New York. 304p., Ing (1999)Ing B (1999) The Myxomycetes of Britain and Ireland: an identification handbook. Berkshire, The Richmond Publishing, Slough. 20p., Lado et al. (2013)Lado C, Basanta DW, Estrada-Torres A & Stephenson SL (2013) The biodiversity of myxomycetes in central Chile. Fungal Diversity 59: 3-32., Lister (1925)Lister A (1925) A monograph of the Mycetozoa. British Museum of Natural History, London. 30p., Liu et al. (2013)Liu QS, Yan SZ, Dai JY & Chen SL (2013) Species diversity of corticolous myxomycetes in Tianmu Mountain National Nature Reserve, China. Canadian Journal of Microbiology 59: 803-813., Martin & Alexopoulos (1969)Martin GW & Alexopoulos CJ (1969) The Myxomycetes. University of Iowa Press, Iowa City. 561p., Nannenga-Bremekamp (1991)Nannenga-Bremekamp NE (1991) A guide to temperate Myxomycetes: an english translation of De Nederlandse Myxomycetesn. Biopress, Bristol. 409p., Neubert et al. (1993)Neubert H, Nowotney W & Baumann K (1993) Die Myxomyceten Deutschlands und des angrenzenden Alpenraumes unter besondererBerücksichtigung Österreichs. V. I Ceratiomyxiales, Echinosteliales, Liceales, Trichiales. Verlag Karlheinz Baumann, Gomaringen. 343p., Novozhilov et al. (2006)Novozhilov YK, Zemlianskaia IV, Schnittler M & Stephenson SL (2006) Myxomycete diversity and ecology in the arid regions of the Lower Volga River Basin (Russia). Fungal Diversity 23: 193-241., Oran & Ergül (2004)Oran RB & Ergül CC (2004) New records for the myxobiota of Turkey. Turkish Journal of Botany 28: 511-515., Poulain et al. (2011)Poulain M, Meyer M & Bozonnet J (2011) Les Myxomycètes. Fédération Mycologique et Botanique Dauphiné-Savoie, Sévrier. 1119p., Rojas et al. (2011, 2018), and Yamamoto (1998)Yamamoto Y (1998) The myxomycete biota of Japan. Tojo Shorin, Tokyo. 700p..
The criteria and guidelines of the International Union for Conservation of Nature IUCN (2012)IUCN (2012) Guidelines for application of IUCN red list criteria at regional and national levels: version 4.0. IUCN, Gland and Cambridge. 41p., version 4.0, were followed for the classification of the conservation status of the species.
Hemitrichia leiocarpa (Cooke) Lister, Monogr. Mycetozoa, ed. 1, 177 (1894).
Hemiarcyria leiocarpa Cooke, Annals Lyceum Nat. Hist. N.Y. 11(12): 405 (1877).
Arcyria leiocarpa (Cooke) Massee, Monogr. Myxogastr. (London): 167 (1892).
Arcyria leiocarpa (Cooke) G.W. Martin & Alexop., Myxomycetes 131 (1969).
Hyporrama leiocarpa (Cooke) Lado, Cuadernos de Trabajo de Flora Micológica Ibérica (Madrid) 16: 47 (2001).
Sporocarp stalked, 0.95–1.2 mm total height, sparse. Hypothallus inconspicuous, membranous. Sporotheca obovoid, gray, 0.2–0.5mm diam. Peridium simple, membranous, partially evanescent, remaining as a shallow calyculus at the base of the sporotheca. Stalk 0.8–1.0 mm high, erect, cylindrical, striate, grayish yellow under transmitted light, filled with subglobose cysts of 15.72–26.2 µm in diameter. Capillitium 4.86–5.24 µm diam., pale yellow under transmitted light, tubular, ornamented with sinistrorse spirals. Spores nearly smooth, subglobose, colorless under transmitted light, 7.14–10.48 µm diam.
Examined material: Quebrangulo, Pedra Talhada Biological Reserve, 09°14’45.5’’S, 36°25’14.06’’W, in ground litter. Cultivation 1, 23.V.2019, sporulation 23.VIII.2019, L.H. Cavalcanti and J.M.S. Parentes (UFP 87524); cultivation 2, 23.V.2019, sporulation 29.VIII.2019, L.H. Cavalcanti and J.M.S. Parentes (UFP 86379).
Additional material: BRAZIL. PERNAMBUCO: Recife, Espinheiro, 08º03’43.2”S, 34º55’22.8”W, on dead trunk of Cocos nucifera L., 28.III.1968, L.H. Cavalcanti 55 (UFP 2762; duplicate BPI 833075).
In descriptions of different authors, the spore diameter of H. leiocarpa differs significantly, being 12.5–14 µm in Cooke (1877)Cooke MC (1877) Myxomycetes of the United States. Annals of the Lyceum of Natural History of New York 11: 378-409., 12–14 µm in Massee (1892)Massee GE (1892) A monograph of the Myxogastres. Mathuen & Co., London. 367p., 6–8 µm in Lister (1894)Lister A (1894) A monograph of the Mycetozoa being a descriptive catalogue of the species in the Herbarium of the British Museum. British Museum (Natural History), London. 418p., and (6)7–9 µm in Macbride & Martin (1934)Macbride TH & Martin GW (1934) The Myxomycetes: a descriptive list of the known species with special reference to those occurring in North America. The MacMillan, New York. 358p., Martin & Alexopoulos (1969)Martin GW & Alexopoulos CJ (1969) The Myxomycetes. University of Iowa Press, Iowa City. 561p., and Farr (1976)Farr ML (1976) Myxomycetes. Flora Neotropica. Monograph 16. New York Botanical Garden, New York. 304p.. Spore size is one of the main characters used in the identification key of Poulain et al. (2011), who place H. leiocarpa in the group with spores measuring 7–9 µm in diameter and the closest species, H. pseudoleiocarpa, in the group with spores measuring 8–10 µm in diameter, with overlapping values around 8 µm. Illana et al. (1999)Illana C, Moreno G, Lizarraga M & Castillo A (1999) Hemitrichia pseudoleiocarpa, spec. nova, a species confused with Arcyria leiocarpa (Myxomycetes). Österreichische Zeitschrift für Pilzkunde 8: 63-70. examined a slide of the type material of H. leiocarpa deposited at the BPI Herbarium and informed that the spore diameter was effectively in the range of 8–9 µm, that is, fitting both the first and the second alternatives in the identification key by Poulain et al. (2011)Poulain M, Meyer M & Bozonnet J (2011) Les Myxomycètes. Fédération Mycologique et Botanique Dauphiné-Savoie, Sévrier. 1119p.. Characters of the capillitium must therefore be of greater taxonomic value for the morphological distinction of the two species.
Lado et al. (2017)Lado C, Estrada-Torres A, Basanta DW, Schnittler M & Stephenson SL (2017) A rapid biodiversity assessment of myxomycetes from a primary tropical moist forest of the Amazon basin in Ecuador. Nova Hedwigia 104: 293-321. identified two specimens collected in Ecuador as Hemitrichia cf. pseudoleiocarpa. These had a capillitium with dextrorse spirals, spores measuring 6.5–8 µm in diameter and ornamentation differing from the type material but similar to the exsiccate Nannenga-Bremekamp 11887 analyzed by Illana et al. (1999)Illana C, Moreno G, Lizarraga M & Castillo A (1999) Hemitrichia pseudoleiocarpa, spec. nova, a species confused with Arcyria leiocarpa (Myxomycetes). Österreichische Zeitschrift für Pilzkunde 8: 63-70.. These authors commented that specimens collected in other Neotropical countries and identified as H. leiocarpa may in fact be H. pseudoleiocarpa. In Brazilian specimens, the spores are nearly smooth and the capillitium is marked with well-defined spiral bands with sinistral arrangement, very similar to the illustrations of the type material of H. leiocarpa presented by Illana et al. (1999)Illana C, Moreno G, Lizarraga M & Castillo A (1999) Hemitrichia pseudoleiocarpa, spec. nova, a species confused with Arcyria leiocarpa (Myxomycetes). Österreichische Zeitschrift für Pilzkunde 8: 63-70., confirming its identification and occurrence in Brazil.
The type locality of H. leiocarpa is Portland, on the coastal region of Maine, a state located at the northeastern corner of the United States of America (USA), with much of the territory covered by forests (Zoll & Stephenson 2015Zoll V & Stephenson SL (2015) Records of myxomycetes from Maine. Mycosphere 6: 568-584.). Lister (1925)Lister A (1925) A monograph of the Mycetozoa. British Museum of Natural History, London. 30p. cited its occurrence in that state and extends its distribution to Pennsylvania, still in northeastern USA. Martin & Alexopoulos (1969)Martin GW & Alexopoulos CJ (1969) The Myxomycetes. University of Iowa Press, Iowa City. 561p. made reference to a similar distribution and add the South (Florida), Mid-South (Louisiana, Texas), and West (Oregon) regions of the United States; they also included Central-Eastern Canada, probably based on material collected between 1931–1932 by R. F. Cain at different locations in Ontario, identified by G. W. Martin and deposited in the BPI herbarium under numbers 833074, 833086, 833088 (Farr & Rossman 2020Farr DF & Rossman AY (2020) Fungal Databases, U.S. National Fungus Collections, ARS, USDA. Available at <https://nt.ars-grin.gov/fungaldatabases/>. Access on 12 October 2020.
https://nt.ars-grin.gov/fungaldatabases/...
). Specimens collected between 1971–2008 and deposited in different herbaria (GBIF 2020bGBIF (2020b) Ochoa Morales C, Comisión nacional para el conocimiento y uso de la biodiversidad C. Computarización de la colección de myxomycetes y líquenes de Baja California. Version 1.8. Comisión nacional para el conocimiento y uso de la biodiversidad. <https://doi.org/10.15468/1lutoa> Available at <https://www.gbif.org/occurrence/1421468812>. Access on 19 October 2020.
https://www.gbif.org/occurrence/14214688...
) indicate that the distribution area of H. leiocarpa covers Mexico, with the first records in the southwest region of the country, in Guerrero, Taxco, in elevation above 5,000 m; in the northern region, in El Ranchito and La Tinaja (Chihuahua), at elevations above 1,000 m, on bark of Picea chihuahuana Martinez; in the northernmost region of the country, bathed by the Pacific Ocean and the Gulf of California, it was found on logs of Acacia greggii A. Gray, in the Sierra de Paredones, Baja California, and also on fragments of Fabaceae in another location, near Loreto (Lado 2018Lado C (2018) Neotropicmyxo. A database of Myxomycetes from the Neotropics. Version 1.4. CSIC-Real Jardín Botánico. <https://doi.org/10.1007/s13225-012-0209-2> accessed via GBIF.org. Available at <https://www.gbif.org/occurrence/1209612727>. Access on 20 November 2020.
https://www.gbif.org/occurrence/12096127...
).
In Central American islands, there are records of the occurrence of the species in Grenada, Windward Islands, with one specimen collected by C. J. Alexopoulos in 1965, deposited in the BPI herbarium under number 833083 (Farr & Rossman 2020Farr DF & Rossman AY (2020) Fungal Databases, U.S. National Fungus Collections, ARS, USDA. Available at <https://nt.ars-grin.gov/fungaldatabases/>. Access on 12 October 2020.
https://nt.ars-grin.gov/fungaldatabases/...
), and in central Cuba, where it is included in the Red List of 21 species threatened with extinction (Camino 1991Camino M (1991) Myxomycetes de Cuba I. Revista del Jardín Botánico Nacional 12: 127-131. ; Camino-Vilaro & Kryvomaz 2018Camino-Vilaro M & Kryvomaz TI (2018) IUCN SSC Chytrid, Zygomycete, Downy Mildew, Slime Mould Specialist Group. Report. Available at <https://www.iucn.org/sites/>. Access on 10 September 2020.
https://www.iucn.org/sites/...
; Lado & Basanta 2008Lado C & Basanta DW (2008) Review of Neotropical Myxomycetes (1828-2008). Anales del Jardín Botánico de Madrid 65: 211-254. ). On the mainland, it was found by C. Haynes in 1994 in the vicinity of the Mayan Xunantunich ruins, in western Belize, on living trunks of the palm tree Orbgnya cohune (Martius) Dahlgren ex Standley (Ing & Haynes 1999Ing B & Haynes C (1999) Corticolous Myxomycetes from Belize. Kew Bulletin 54: 723.-730.; Lado 2018Lado C (2018) Neotropicmyxo. A database of Myxomycetes from the Neotropics. Version 1.4. CSIC-Real Jardín Botánico. <https://doi.org/10.1007/s13225-012-0209-2> accessed via GBIF.org. Available at <https://www.gbif.org/occurrence/1209612727>. Access on 20 November 2020.
https://www.gbif.org/occurrence/12096127...
). It also occurs in Costa Rica, growing on microhabitats offered by dead logs and leaf litter of montane rainforests in San José and Puntarenas (Rojas et al. 2018Rojas C, Rojas PA & Lado C (2018) Myxomycete diversity in Costa Rica. Mycosphere 9: 227-255.). Its distribution area reaches the southernmost part of Central America, as evidenced by a specimen obtained from moist chamber cultures by G. W. Martin, collected in Juan Diaz, Panama, in 1935, deposited at the BPI Herbarium under number 833080 (Farr & Rossman 2020Farr DF & Rossman AY (2020) Fungal Databases, U.S. National Fungus Collections, ARS, USDA. Available at <https://nt.ars-grin.gov/fungaldatabases/>. Access on 12 October 2020.
https://nt.ars-grin.gov/fungaldatabases/...
); new records for the country were obtained after six decades in a study carried out by Walker et al. (2019)Walker LM, Cedeño-Sanchez M, Carbonero F, Herre EA, Turner BL, Wright SJ & Stephenson SL (2019) The response of litter-associated myxomycetes to long-term nutrient addition in a Lowland Tropical Forest. Journal of Eukaryotic Microbiology 66: 757-770. in the Gigante peninsula, in the Barro Colorado Nature Monument, which included the species among the 10 most abundant in the leaf litter and bark remains of trees and twigs collected in a lowland evergreen forest.
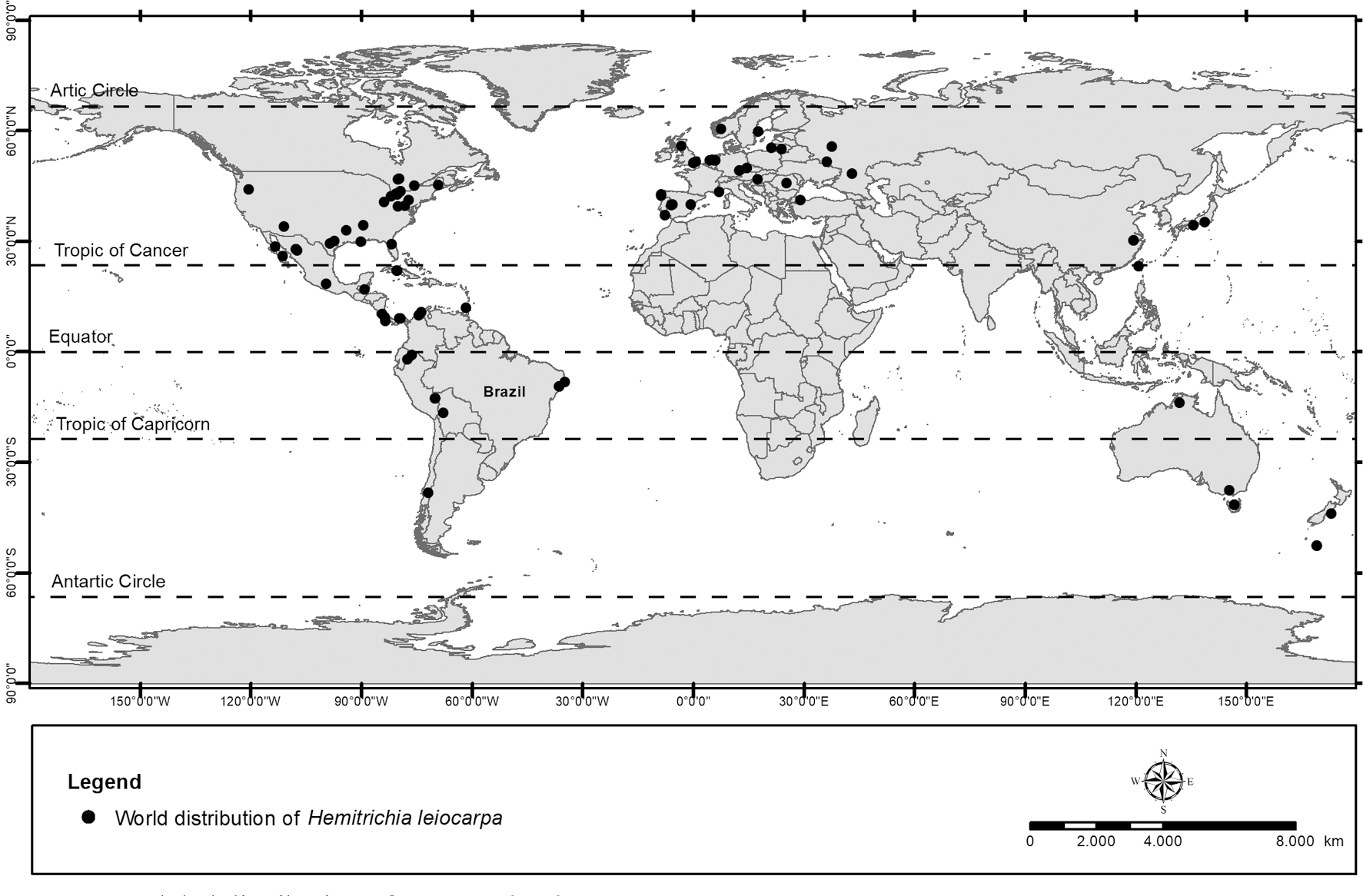
In South America, Martin (1938)Martin GW (1938) Myxomycetes from Colombia. Transactions of the American Microscopical Society 57: 123-126. included H. leiocarpa in the list of myxomycetes from Colombia based on sporangia developed in moist chambers, and commented on its macroscopic resemblance to A. cinerea, but considered the capillitial filaments, with left-handed spirals and small spines, a remarkable difference. In a compilation of the knowledge about the Neotropical myxomycetes biota, Farr (1976)Farr ML (1976) Myxomycetes. Flora Neotropica. Monograph 16. New York Botanical Garden, New York. 304p. and Lado & Basanta (2008)Lado C & Basanta DW (2008) Review of Neotropical Myxomycetes (1828-2008). Anales del Jardín Botánico de Madrid 65: 211-254. did not include Bolivia in the area of distribution of H. leiocarpa, but in 1956 a specimen was collected by the mycologist Rolf Singer in Mururata, in Nor-Yungas province, Bolivian department of La Paz, and deposited it in the Bernard Lowy Mycological Herbarium of Louisiana State University, USA (LSU00156053). Its presence in other areas of the South American continent was only discovered 30 years later, in locations quite distant from each other (Fig. 2; Tab. 1). In Brazil, it was recorded in 1968 in the Northeast, in an urban environment, on dead trunk of the palm Cocos nucifera L. (Cavalcanti 1976Cavalcanti LH (1976) Mixomicetos novos para Pernambuco II. Memórias do Instituto de Biociências. Universidade Federal de Pernambuco, Série Botânica 4: 1-19.). The first record of its occurrence in Chile, and the third in South America, was published 38 years later by Lado et al. (2013)Lado C, Basanta DW, Estrada-Torres A & Stephenson SL (2013) The biodiversity of myxomycetes in central Chile. Fungal Diversity 59: 3-32., based on specimens found in 2006 in Malleco, Araucania region, on Nothofagus dombeyi (Mirbel) Blume wood, in a mixed forest with N. dombeyi, N. pumilio (Poepp. & Endl.) Krasser, and Chusquea culeou É.Desv. The occurrence of the species in Peru was only documented in a research carried out in 2007, when five specimens developed in moist chambers assembled with ground and aerial leaf litter collected from Amazonian lowland and seasonally flooded forests (Rojas et al. 2011Rojas C, Stephenson SL & Pavlich M (2011) New additions to the myxobiota of Peru. Mycosphere 2: 583-592.). The presence of H. leiocarpa in Ecuador was not mentioned by Lado & Basanta (2008)Lado C & Basanta DW (2008) Review of Neotropical Myxomycetes (1828-2008). Anales del Jardín Botánico de Madrid 65: 211-254. but in 2000, M. Schnittler had the opportunity to collect some specimens in a fragment of humid primary forest in the Yasuni National Park, on the right bank of the Tiputini river, and deposited it in the SNSB-M herbarium.
Records of Hemitrichia leiocarpa (Cooke) Lister in different continents and countries documented in herbaria and in the literature (1873-2020).
On the European continent, the oldest records, from the end of the 19th century, indicate its occurrence in Bohemia, in the western part of the Czech Republic, and in a greenhouse in the Edinburgh Botanical Garden, in Scotland (Lister 1925Lister A (1925) A monograph of the Mycetozoa. British Museum of Natural History, London. 30p.). Ing (1999)Ing B (1999) The Myxomycetes of Britain and Ireland: an identification handbook. Berkshire, The Richmond Publishing, Slough. 20p. comments that, since 1925 until that date, H. leiocarpa had no new records in the United Kingdom, but that the species was not uncommon in the warmest parts of the center and south of the continent. In western Europe, there are one or more records of H. leiocarpa, old and recent, in Portugal, Spain, France, Germany, the Netherlands, reaching Sweden and Norway (Fig. 2; Tab. 1). The Netherlands stands out in the number of records, but most from the same location, in residential areas. Under the binomium Arcyria leiocarpa, the species is mentioned but not described by Neubert et al. (1993)Neubert H, Nowotney W & Baumann K (1993) Die Myxomyceten Deutschlands und des angrenzenden Alpenraumes unter besondererBerücksichtigung Österreichs. V. I Ceratiomyxiales, Echinosteliales, Liceales, Trichiales. Verlag Karlheinz Baumann, Gomaringen. 343p. in the monograph on myxomycetes of Germany and the neighboring alpine region, with special attention to Austria, and the authors indicated that its known worldwide distribution is restricted to Great Britain, Czech Republic, the Netherlands, and the United Sates. In eastern Europe, the known range of distribution of H. leiocarpa beyond the Czech Republic includes Hungary, Russia and Lithuania, with sporadic records between 1900–2002 (Fig. 2; Tab. 1). Although the first record was in 1915, in the Kursk region (Matveev et al. 2016Matveev AV, Bortnikov FM, Gmoshinskiy VI & Novozhilov YK (2016-2020) Myxomycetes of Russia. Lomonosov Moscow State University, Komarov Botanical Institute of the Russian Academy of Sciences, Moscow, St. Petersburg. Available at <http://myxomycetes.org/russia>. Access on 25 October 2020.
http://myxomycetes.org/russia...
–2020), the occurrence of H. leiocarpa in Russia was not mentioned in the classic works of Lister (1925)Lister A (1925) A monograph of the Mycetozoa. British Museum of Natural History, London. 30p., Macbride & Martin (1934)Macbride TH & Martin GW (1934) The Myxomycetes: a descriptive list of the known species with special reference to those occurring in North America. The MacMillan, New York. 358p., Martin & Alexopoulos (1969)Martin GW & Alexopoulos CJ (1969) The Myxomycetes. University of Iowa Press, Iowa City. 561p., Nannenga-Bremekamp (1991)Nannenga-Bremekamp NE (1991) A guide to temperate Myxomycetes: an english translation of De Nederlandse Myxomycetesn. Biopress, Bristol. 409p., and Ing (1999)Ing B (1999) The Myxomycetes of Britain and Ireland: an identification handbook. Berkshire, The Richmond Publishing, Slough. 20p.. Considering that the second record was only in 1997, in Moscow, and the third in 1999, in steppes of the Volgograd region (Matveev et al. 2016Matveev AV, Bortnikov FM, Gmoshinskiy VI & Novozhilov YK (2016-2020) Myxomycetes of Russia. Lomonosov Moscow State University, Komarov Botanical Institute of the Russian Academy of Sciences, Moscow, St. Petersburg. Available at <http://myxomycetes.org/russia>. Access on 25 October 2020.
http://myxomycetes.org/russia...
–2020; GBIF 2020aGBIF (2020a) - The Myxomycetes Collection. V. L. Komarov Botanical Institute, Russian Academy of Sciences, St. Petersburg. Occurrence dataset <https://doi.org/10.15468/i83r9k> Available at <https://www.gbif.org/occurrence/1135606485>. Access on 19 October 2020.
https://www.gbif.org/occurrence/11356064...
), the species was classified as rare by Novozhilov et al. (2006)Novozhilov YK, Zemlianskaia IV, Schnittler M & Stephenson SL (2006) Myxomycete diversity and ecology in the arid regions of the Lower Volga River Basin (Russia). Fungal Diversity 23: 193-241.. In Lithuania, the first record of H. leiocarpa was made in the Kuršių Nerija National Park, in the west of the country, in a pine forest where a large colony of cormorants (Phalacrocorax carbo sinensis L. 1758) was established; the species was among the ten most abundant, with 26 specimens obtained both in the field and in moist chamber cultures of ground litter and bark of Quercus robur L., Juniperus communis L., Pinus sylvestris L., Sambucus nigra L. and S. racemosa L. (Adamonyte et al. 2013Adamonyte G, Iršėnaitė R, Motiejūnaitė J, Taraškevičius R & Matulevičiūtė D (2013) Myxomycetes in a forest affected by great cormorant colony: a case study in Western Lithuania. Fungal Diversity 59: 131-146.; Telenius 2016Telenius A (2016) Gothenburg Herbarium - General (GBIF:IH:GB:Herbarium). GBIF-Sweden. Occurrence dataset <https://doi.org/10.15468/afkfpi> accessed via GBIF.org. Available at <https://www.gbif.org/occurrence/1043002216>. Access on 19 October 2020.
https://www.gbif.org/occurrence/10430022...
). The area of occurrence of H. leiocarpa reaches the Bosphorus Strait, on the borders between the European and Asian continents, where it inhabits forested areas of Turkey, with the first records made in 2002 in the province of Istanbul, Bahçeköy and Topkoru Stream districts (90–120 m alt.), on dead tree trunks of species typical of the region such as Carpinus betulus L. and Fagus orientalis Lipsky (Oran & Ergül 2004Oran RB & Ergül CC (2004) New records for the myxobiota of Turkey. Turkish Journal of Botany 28: 511-515.; Dülger 2007Dülger B (2007) Checklist of the myxomycetes in Turkey. Mycologia Balcanica 4: 151-155.; Sesli & Denchev 2014Sesli E & Denchev CM (2014) Checklists of the myxomycetes, larger ascomycetes, and larger basidiomycetes in Turkey. Mycotaxon Checklists Online. 136p. Available at <http://www.mycotaxon.com/resources/checklists/sesli-v106-checklist.pdf>. Access on 9 october 2020.
http://www.mycotaxon.com/resources/check...
).
In Oceania, records made between 2000–2012 show that its area of distribution reaches Australia and New Zealand (Fig. 3; Tab. 1). The species was first recorded in Australia on the Atherton plateau, Queensland, in the northeastern region of the country; it was collected on dead wood and sporulated in moist chambers prepared with Dysoxylum cerebriforme F. M. Bailey bark (McHugh et al. 2003McHugh R, Stephenson SL, Mitchell DW & Brims MH (2003) New records of Australian Myxomycota. New Zealand Journal of Botany 41: 487-500. <https://doi.org/10.1080/0028825X.2003.9512865>). In the Australian islands, it occurs in Eucalyptus forests of Tasmania, and in the southeast of the country it was found for the first time in Victoria, in the Kinglake National Park Jehosaphat Gulley, on bark of Olearia sp. (Rosing et al. 2007Rosing WC, Mitchell DW & Stephenson SL (2007) Corticolous myxomycetes from Victoria. Australasian Mycologist 26: 9-15.). It sporulated in moist chamber culture prepared with bark of Lysiphyllum gilvum (F.M.Bailey) Pedley collected in 2006 in Pine Creek, being the first record of the species for the Northern Territory, in the mid-north region of the country (McHugh et al. 2009McHugh R, Mitchell DW, Brims MH & Stephenson SL (2009) New additions to the Myxomycota of Australia. Australasian Mycologist 28: 56-64.). The New Zealand Fungal and Plant Disease Collection (PDD) has records on Ericaceae (Dracophyllum sp.) and Asteraceae (Pleurophyllum sp.) from 2000 by S. L Stephenson, based on material from Campbell Island, and on Nothofagus fusca (Hook.f.) Oerst, collected in 2003 by J. A. Cooper at the Hinewai Private Reserve in Akaroa (Wilton 2020Wilton A (2020) New Zealand fungal and plant disease collection (PDD). Landcare Research. <https://doi.org/10.15468/nrq12b> accessed via GBIF.org. Available at <https://www.gbif.org/occurrence/1135719082>. Access on 9 October 2020.
https://www.gbif.org/occurrence/11357190...
).
Variation in the number of records of Hemitrichia leiocarpa (Cooke) Lister in different continents over a period of 120 years.
Asia was not included in the known area of distribution of the species in classic monographs, whether those of a global scope such as Massee (1892)Massee GE (1892) A monograph of the Myxogastres. Mathuen & Co., London. 367p., Lister (1925)Lister A (1925) A monograph of the Mycetozoa. British Museum of Natural History, London. 30p. and Martin & Alexopoulos (1969)Martin GW & Alexopoulos CJ (1969) The Myxomycetes. University of Iowa Press, Iowa City. 561p., or those of a little more restricted scope such as Nannenga-Bremekamp (1991)Nannenga-Bremekamp NE (1991) A guide to temperate Myxomycetes: an english translation of De Nederlandse Myxomycetesn. Biopress, Bristol. 409p. and Neubert et al. (1993)Neubert H, Nowotney W & Baumann K (1993) Die Myxomyceten Deutschlands und des angrenzenden Alpenraumes unter besondererBerücksichtigung Österreichs. V. I Ceratiomyxiales, Echinosteliales, Liceales, Trichiales. Verlag Karlheinz Baumann, Gomaringen. 343p.. Only Ing (1999)Ing B (1999) The Myxomycetes of Britain and Ireland: an identification handbook. Berkshire, The Richmond Publishing, Slough. 20p. and Poulain et al. (2011)Poulain M, Meyer M & Bozonnet J (2011) Les Myxomycètes. Fédération Mycologique et Botanique Dauphiné-Savoie, Sévrier. 1119p. indicated the occurrence of H. leiocarpa on this continent, in Japan. However, old exsiccates of the collection of the National Museum of Nature and Science (Tsukuba, Japan) document the occurrence of H. leiocarpa in China from a collection carried out in 1924 in the Alishan region. Liu et al. (2013)Liu QS, Yan SZ, Dai JY & Chen SL (2013) Species diversity of corticolous myxomycetes in Tianmu Mountain National Nature Reserve, China. Canadian Journal of Microbiology 59: 803-813. reported an occasional occurrence of the species in the Tianmu Mountain National Nature Reserve, eastern China, in an evergreen broadleaved forest at 430 m and in a semi-deciduous mixed broadleaved forest at 800 m of elevation. In Korea, Shiro Koban obtained one specimen in 1930 from Mount Kongo, which was identified by Shiro Koase. In Japan, it was collected in 1932 on Mount Fuji by Y. Emoto, in Shizuoka, and mentioned by Yamamoto (1998)Yamamoto Y (1998) The myxomycete biota of Japan. Tojo Shorin, Tokyo. 700p..
In the survey of the literature and herbaria collections, no records were found of the occurrence of H. leiocarpa in Antarctica and on the African continent. Figure 2 shows the world distribution known for the species until present date.
According to the number and date of records in the 33 countries listed in Table 1, in which H. leiocarpa is currently known, it appears that, in 51% of them, the species was collected only once or twice and there have not been further records for over 30 (Portugal, Sweden), 50 (Bolivia, Brazil, Colombia, Korea, Grenada, Japan, Norway) and even 100 (Scotland, England, Czech Republic) years.
Herbarium records and the consulted literature show that H. leiocarpa occurs in different ecosystems, natural or altered by humans, occupying several microhabitats and substrates. A tendency towards synanthropic conditions was observed, with the ability to colonize urban environments, as evidenced by several records in the Netherlands, on manure of domestic animals, and in Brazil, in residential gardens, as reported by Cavalcanti (1976)Cavalcanti LH (1976) Mixomicetos novos para Pernambuco II. Memórias do Instituto de Biociências. Universidade Federal de Pernambuco, Série Botânica 4: 1-19. (Tab. 2). The species was not included in the list of fimicolous myxomycetes recently published by Calaça et al. (2020)Calaça FS, Araújo JC, Cacialli G, Silva NC, Rojas C & Xavier-Santos S (2020) Fimicolous myxomycetes: overview of their global distribution and scientific production. Biologia 75: 2159-2174. <https://doi.org/10.2478/s11756-020-00578-9> but data from herbarium exsiccates from different countries confirm the information of Farr (1976)Farr ML (1976) Myxomycetes. Flora Neotropica. Monograph 16. New York Botanical Garden, New York. 304p., Nannenga-Bremekamp (1991)Nannenga-Bremekamp NE (1991) A guide to temperate Myxomycetes: an english translation of De Nederlandse Myxomycetesn. Biopress, Bristol. 409p., Poulain et al. (2011)Poulain M, Meyer M & Bozonnet J (2011) Les Myxomycètes. Fédération Mycologique et Botanique Dauphiné-Savoie, Sévrier. 1119p., Adamonyte et al. (2013)Adamonyte G, Iršėnaitė R, Motiejūnaitė J, Taraškevičius R & Matulevičiūtė D (2013) Myxomycetes in a forest affected by great cormorant colony: a case study in Western Lithuania. Fungal Diversity 59: 131-146., and Eliasson (2013)Eliasson UH (2013) Coprophilous myxomycetes: recent advances and future research directions. Fungal Diversity 59: 85-90. about its occurrence in faeces of herbivorous animals (Tab. 2).
Microhabitats and substrates colonized by Hemitrichia leiocarpa (Cooke) Lister with records for different countries. 1 = GBIF; 2 = MA-fungi; 3 = Farr & Rossman 2020Farr DF & Rossman AY (2020) Fungal Databases, U.S. National Fungus Collections, ARS, USDA. Available at <https://nt.ars-grin.gov/fungaldatabases/>. Access on 12 October 2020.
https://nt.ars-grin.gov/fungaldatabases/... ; 4 = Adamonyte et al. (2013)Adamonyte G, Iršėnaitė R, Motiejūnaitė J, Taraškevičius R & Matulevičiūtė D (2013) Myxomycetes in a forest affected by great cormorant colony: a case study in Western Lithuania. Fungal Diversity 59: 131-146.; 5 = Cavalcanti (1976)Cavalcanti LH (1976) Mixomicetos novos para Pernambuco II. Memórias do Instituto de Biociências. Universidade Federal de Pernambuco, Série Botânica 4: 1-19.; 6 = Farr (1976)Farr ML (1976) Myxomycetes. Flora Neotropica. Monograph 16. New York Botanical Garden, New York. 304p.; 7 = Ing & Haynes (1999)Ing B & Haynes C (1999) Corticolous Myxomycetes from Belize. Kew Bulletin 54: 723.-730.; 8 = Ing (1999)Ing B (1999) The Myxomycetes of Britain and Ireland: an identification handbook. Berkshire, The Richmond Publishing, Slough. 20p.; 9 = Lado et al. (2013)Lado C, Basanta DW, Estrada-Torres A & Stephenson SL (2013) The biodiversity of myxomycetes in central Chile. Fungal Diversity 59: 3-32.; 10 = Liu et al. (2013)Liu QS, Yan SZ, Dai JY & Chen SL (2013) Species diversity of corticolous myxomycetes in Tianmu Mountain National Nature Reserve, China. Canadian Journal of Microbiology 59: 803-813.; 11 = Novozhilov et al. (2006)Novozhilov YK, Zemlianskaia IV, Schnittler M & Stephenson SL (2006) Myxomycete diversity and ecology in the arid regions of the Lower Volga River Basin (Russia). Fungal Diversity 23: 193-241.; 12 = Oran & Ergül (2004)Oran RB & Ergül CC (2004) New records for the myxobiota of Turkey. Turkish Journal of Botany 28: 511-515.; 13 = Rojas et al. (2011)Rojas C, Stephenson SL & Pavlich M (2011) New additions to the myxobiota of Peru. Mycosphere 2: 583-592.; 14 = Rojas et al. (2018)Rojas C, Rojas PA & Lado C (2018) Myxomycete diversity in Costa Rica. Mycosphere 9: 227-255.; 15 = McHugh et al. (2009)McHugh R, Mitchell DW, Brims MH & Stephenson SL (2009) New additions to the Myxomycota of Australia. Australasian Mycologist 28: 56-64.; 16 = Rosing et al. (2007)Rosing WC, Mitchell DW & Stephenson SL (2007) Corticolous myxomycetes from Victoria. Australasian Mycologist 26: 9-15.; 17 = McHugh et al. (2003)McHugh R, Stephenson SL, Mitchell DW & Brims MH (2003) New records of Australian Myxomycota. New Zealand Journal of Botany 41: 487-500. <https://doi.org/10.1080/0028825X.2003.9512865>; 18 = This paper.
Environmental disturbance caused by human activities, such as habitat destruction and poorly planned exploitation of natural resources, have accelerated the extinction of species from different groups of living beings, which present different levels of vulnerability (Leão et al. 2014Leão TCC, Fonseca CR, Peres CA & Tabarelli M (2014) Predicting extinction risk of Brazilian Atlantic Forest Angiosperms. Conservation Biology 28: 1349-1350.; Stork et al. 2009Stork NE, Coddington JA, Colwell RK, Chazdon RL, Dick CW, Peres CA, Sloan S & Willis K (2009) Vulnerability and resilience of tropical forest species to land-use change. Conservation Biology 23: 1438-1447. <https://doi.org/10.1111/j.1523-1739.2009.01335.x> ). Little is known about attributes that could allow predicting the sensitivity of myxomycetes to such disturbances. Considering the evidence from better studied groups, Kryvomaz et al. (2012)Kryvomaz T, Camino M & Minter D (2012) Myxomycetes from a conservation perspective. Fungal Conservation, International Society for Fungal Conservation issue 2. Esk Terrace, Whitby. 48p. elaborated a list of threats related to climate change, pollution and habitat destruction, questioning which of them affect myxomycetes and whether there could be others, still unknown.
Currently available data on the global distribution of myxomycetes do not support the claim that they are mostly cosmopolitan, and recent estimates indicate that about half of the species have an area of distribution limited to only one continent or natural climatic zone (Estrada-Torres et al. 2013Estrada-Torres A, Basanta DW & Lado C (2013) Biogeographic patterns of the myxomycete biota of the Americas using a parsimony analysis of endemicity. Fungal Diversity 59: 159-177. DOI 10.1007/s13225-012-0209-2; Leontyev et al. 2020Leontyev DV, Yatsiuk II & Kochergina AV (2020) Inclusion of myxomycetes in the Red Data Book of Ukraine: feasibility, selection criteria and recommended species. Ukrainian Botanical Journal 77: 189-203. <https://doi.org/10.15407/ukrbotj77.03.189>). In the case of H. leiocarpa, its known geographic distribution is very wide, with records in at least 33 countries until 2020, distributed in both Hemispheres (Fig. 2; Tab. 1). However, the data indicate that the species is rare in most countries within this range. The analysis of the information existing in the GBIF database, for example, revealed that 50% of the records were made between 1950 and 2000, mainly in Europe and North America, and in 17 countries there is only one record (Fig. 3).
In Brazil, until 2019, the only record available was that of a specimen collected in Pernambuco in 1968. The species was not found in the various researches developed since the 1970s, which is especially notable for the Northeast, where the myxomycete biota of different ecosystems has been inventoried. Specimens of the species were not found in the review of Trichiales either, in which collections from herbaria in the North (INPA, HFSL, UFRR), Northeast (IPA, JPB, TEPB, UFP, URM), Southeast (SP-Fun), and South (ICN) regions of the country were personally examined by one of the authors. In the inventory of the myxomycete biota of PTBR, the species was found 225 km far from the site of the first collection, and sporulated on ground litter in moist chamber cultures. The two sites of occurrence in Brazil are located within the area of the Pernambuco Endemism Center (Fig. 1), in the Atlantic Forest domain and considered a biodiversity hotspot for conservation priority worldwide (Barbosa et al. 2016Barbosa DI, Bezerra ACC, Lima VX & Cavalcanti LH (2016) Corticolous myxobiota of the Pernambuco Center of Endemism, Brazil. Acta Botanica Brasilica 30: 549-559. <https://doi.org/10.1590/0102-33062016abb0209> ).
Despite having a multi-zone distribution area, H. leiocarpa is considered rare by several authors, namely Martin & Alexopoulos (1969)Martin GW & Alexopoulos CJ (1969) The Myxomycetes. University of Iowa Press, Iowa City. 561p., Novozhilov et al. (2006)Novozhilov YK, Zemlianskaia IV, Schnittler M & Stephenson SL (2006) Myxomycete diversity and ecology in the arid regions of the Lower Volga River Basin (Russia). Fungal Diversity 23: 193-241., Adamonyte et al. (2013)Adamonyte G, Iršėnaitė R, Motiejūnaitė J, Taraškevičius R & Matulevičiūtė D (2013) Myxomycetes in a forest affected by great cormorant colony: a case study in Western Lithuania. Fungal Diversity 59: 131-146., and Eliasson (2015)Eliasson UH (2015) Review and remarks on current generic delimitations in the myxomycetes, with special emphasis on Licea, Listerella and Perichaena. Nova Hedwigia 104: 343-350. DOI: 10.1127/nova_hedwigia/2015/0283
https://doi.org/10.1127/nova_hedwigia/20...
, and current data indicate that it needs protection in some countries, like Cuba, where it was included in the Red List. In Brazil, H. leiocarpa can also be considered rare, as only two records were obtained over an interval of five decades and restricted to the Northeast. This fact, associated with the loss of habitats resulting from the deforestation of the Atlantic Forest, points to the classification of H. leiocarpa in the near threatened (NT) category based on IUCN criteria.
The data of the present work allow the conclusion that H. leiocarpa is part of the Brazilian myxomycete biota, of rare occurrence, with records restricted to the Northeast region in the Atlantic Forest domain. Considering the long time elapsed between the records and the restricted distribution in the Brazilian territory, the species was considered to present a near threatened conservation status in the country and its inclusion in the Brazilian Red List of Threatened Species, in the NT category, is recommended.
Acknowledgements
The authors thank the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), for the financial support (process 131525/2019-0, master’s scholarship to the first author; process 150054/2011-4 PROTAX, grant to the second author; process 421241/2017-9, project Conservation of fungal diversity in Atlantic Forest areas of Northeast Brazil); the Chico Mendes Institute for Biodiversity Conservation (ICMBio), for authorizing the collection of biological material for scientific purposes; the NORDESTA - Reforestation and Education association, for the logistical support provided during fieldwork.
References
- Adamonyte G, Iršėnaitė R, Motiejūnaitė J, Taraškevičius R & Matulevičiūtė D (2013) Myxomycetes in a forest affected by great cormorant colony: a case study in Western Lithuania. Fungal Diversity 59: 131-146.
- Barbosa DI, Bezerra ACC, Lima VX & Cavalcanti LH (2016) Corticolous myxobiota of the Pernambuco Center of Endemism, Brazil. Acta Botanica Brasilica 30: 549-559. <https://doi.org/10.1590/0102-33062016abb0209>
- Barros AHC, Araújo Filho JC, Silva AB & Santiago GACF (2012) Climatologia do estado de Alagoas. Boletim de Pesquisa e Desenvolvimento (INFOTECA-E). Embrapa Solos, Recife. 39p.
- Bezerra ACC, Cavalcanti LH & Dianese JC (2009) Species of Hemitrichia (Trichiaceae, Myxomycetes) in Brazil. Mycotaxon 107: 34-48.
- Calaça FS, Araújo JC, Cacialli G, Silva NC, Rojas C & Xavier-Santos S (2020) Fimicolous myxomycetes: overview of their global distribution and scientific production. Biologia 75: 2159-2174. <https://doi.org/10.2478/s11756-020-00578-9>
- Camino M (1991) Myxomycetes de Cuba I. Revista del Jardín Botánico Nacional 12: 127-131.
- Camino-Vilaro M & Kryvomaz TI (2018) IUCN SSC Chytrid, Zygomycete, Downy Mildew, Slime Mould Specialist Group. Report. Available at <https://www.iucn.org/sites/>. Access on 10 September 2020.
» https://www.iucn.org/sites/ - Cavalcanti LH (1976) Mixomicetos novos para Pernambuco II. Memórias do Instituto de Biociências. Universidade Federal de Pernambuco, Série Botânica 4: 1-19.
- Cavalcanti LH (2002) Biodiversidade e distribuição de mixomicetos em ambientes naturais e antropogênicos no Brasil: espécies ocorrentes nas Regiões Norte e Nordeste. In: Araújo EL, Moura NA, Sampaio EVSB, Gestinari LM & Carneiro JMT (eds.) Biodiversidade, conservação e uso sustentável da flora do Brasil. Sociedade Botânica do Brasil. Universidade Federal Rural de Pernambuco, Recife. Pp. 209-216.
- Cavalcanti LH (2010) Myxomycota. In: Forzza RC, Baumgratz JFA, Bicudo CDM, Carvalho Jr AA, Costa A, Costa DP & Martinelli G (eds.) Catálogo de plantas e fungos do Brasil. Andrea Jakobsson Estúdio: Instituto de Pesquisas Jardim Botânico do Rio de Janeiro, Rio de Janeiro. Pp. 94-104.
- Cavalcanti LH, Bezerra ACC & Agra LAN (2020) Myxomycetes. In: Lista de Espécies da Flora do Brasil. Instituto de Pesquisas Jardim Botânico do Rio de Janeiro. Available at <http://floradobrasil.jbrj.gov.br/>. Access on 9 October 2020.
» http://floradobrasil.jbrj.gov.br/ - Cooke MC (1877) Myxomycetes of the United States. Annals of the Lyceum of Natural History of New York 11: 378-409.
- Dülger B (2007) Checklist of the myxomycetes in Turkey. Mycologia Balcanica 4: 151-155.
- Eliasson UH (2013) Coprophilous myxomycetes: recent advances and future research directions. Fungal Diversity 59: 85-90.
- Eliasson UH (2015) Review and remarks on current generic delimitations in the myxomycetes, with special emphasis on Licea, Listerella and Perichaena Nova Hedwigia 104: 343-350. DOI: 10.1127/nova_hedwigia/2015/0283
» https://doi.org/10.1127/nova_hedwigia/2015/0283 - Estrada-Torres A, Basanta DW & Lado C (2013) Biogeographic patterns of the myxomycete biota of the Americas using a parsimony analysis of endemicity. Fungal Diversity 59: 159-177. DOI 10.1007/s13225-012-0209-2
- Farr ML (1976) Myxomycetes. Flora Neotropica. Monograph 16. New York Botanical Garden, New York. 304p.
- Farr DF & Rossman AY (2020) Fungal Databases, U.S. National Fungus Collections, ARS, USDA. Available at <https://nt.ars-grin.gov/fungaldatabases/>. Access on 12 October 2020.
» https://nt.ars-grin.gov/fungaldatabases/ - GBIF (2020a) - The Myxomycetes Collection. V. L. Komarov Botanical Institute, Russian Academy of Sciences, St. Petersburg. Occurrence dataset <https://doi.org/10.15468/i83r9k> Available at <https://www.gbif.org/occurrence/1135606485>. Access on 19 October 2020.
» https://www.gbif.org/occurrence/1135606485 - GBIF (2020b) Ochoa Morales C, Comisión nacional para el conocimiento y uso de la biodiversidad C. Computarización de la colección de myxomycetes y líquenes de Baja California. Version 1.8. Comisión nacional para el conocimiento y uso de la biodiversidad. <https://doi.org/10.15468/1lutoa> Available at <https://www.gbif.org/occurrence/1421468812>. Access on 19 October 2020.
» https://www.gbif.org/occurrence/1421468812 - Guimarães JRA, Studer A &Trivellato C (2014) Educação ambiental no entorno da Reserva Biológica de Pedra Talhada. Fórum ambiental da Alta Paulista 10: 32-45.
- Illana C, Moreno G, Lizarraga M & Castillo A (1999) Hemitrichia pseudoleiocarpa, spec. nova, a species confused with Arcyria leiocarpa (Myxomycetes). Österreichische Zeitschrift für Pilzkunde 8: 63-70.
- Ing B (1999) The Myxomycetes of Britain and Ireland: an identification handbook. Berkshire, The Richmond Publishing, Slough. 20p.
- Ing B & Haynes C (1999) Corticolous Myxomycetes from Belize. Kew Bulletin 54: 723.-730.
- IUCN (2012) Guidelines for application of IUCN red list criteria at regional and national levels: version 4.0. IUCN, Gland and Cambridge. 41p.
- Kryvomaz T, Camino M & Minter D (2012) Myxomycetes from a conservation perspective. Fungal Conservation, International Society for Fungal Conservation issue 2. Esk Terrace, Whitby. 48p.
- Lado C (2005-2020) An online nomenclatural information system of Eumycetozoa. Available at <https://eumycetozoa.com/>. Access on 5 October 2020.
» https://eumycetozoa.com/ - Lado C (2018) Neotropicmyxo. A database of Myxomycetes from the Neotropics. Version 1.4. CSIC-Real Jardín Botánico. <https://doi.org/10.1007/s13225-012-0209-2> accessed via GBIF.org. Available at <https://www.gbif.org/occurrence/1209612727>. Access on 20 November 2020.
» https://www.gbif.org/occurrence/1209612727 - Lado C & Basanta DW (2008) Review of Neotropical Myxomycetes (1828-2008). Anales del Jardín Botánico de Madrid 65: 211-254.
- Lado C, Estrada-Torres A, Basanta DW, Schnittler M & Stephenson SL (2017) A rapid biodiversity assessment of myxomycetes from a primary tropical moist forest of the Amazon basin in Ecuador. Nova Hedwigia 104: 293-321.
- Lado C, Basanta DW, Estrada-Torres A & Stephenson SL (2013) The biodiversity of myxomycetes in central Chile. Fungal Diversity 59: 3-32.
- Leão TCC, Fonseca CR, Peres CA & Tabarelli M (2014) Predicting extinction risk of Brazilian Atlantic Forest Angiosperms. Conservation Biology 28: 1349-1350.
- Leontyev DV, Schnittler M, Stephenson SL, Novozhilov YK & Shchepin ON (2019) Towards a phylogenetic classification of the Myxomycetes. Phytotaxa 399: 209-238.
- Leontyev DV, Yatsiuk II & Kochergina AV (2020) Inclusion of myxomycetes in the Red Data Book of Ukraine: feasibility, selection criteria and recommended species. Ukrainian Botanical Journal 77: 189-203. <https://doi.org/10.15407/ukrbotj77.03.189>
- Lister A (1894) A monograph of the Mycetozoa being a descriptive catalogue of the species in the Herbarium of the British Museum. British Museum (Natural History), London. 418p.
- Lister A (1925) A monograph of the Mycetozoa. British Museum of Natural History, London. 30p.
- Liu QS, Yan SZ, Dai JY & Chen SL (2013) Species diversity of corticolous myxomycetes in Tianmu Mountain National Nature Reserve, China. Canadian Journal of Microbiology 59: 803-813.
- Macbride TH & Martin GW (1934) The Myxomycetes: a descriptive list of the known species with special reference to those occurring in North America. The MacMillan, New York. 358p.
- Martin GW (1938) Myxomycetes from Colombia. Transactions of the American Microscopical Society 57: 123-126.
- Martin GW & Alexopoulos CJ (1969) The Myxomycetes. University of Iowa Press, Iowa City. 561p.
- Massee GE (1892) A monograph of the Myxogastres. Mathuen & Co., London. 367p.
- Matveev AV, Bortnikov FM, Gmoshinskiy VI & Novozhilov YK (2016-2020) Myxomycetes of Russia. Lomonosov Moscow State University, Komarov Botanical Institute of the Russian Academy of Sciences, Moscow, St. Petersburg. Available at <http://myxomycetes.org/russia>. Access on 25 October 2020.
» http://myxomycetes.org/russia - McHugh R, Mitchell DW, Brims MH & Stephenson SL (2009) New additions to the Myxomycota of Australia. Australasian Mycologist 28: 56-64.
- McHugh R, Stephenson SL, Mitchell DW & Brims MH (2003) New records of Australian Myxomycota. New Zealand Journal of Botany 41: 487-500. <https://doi.org/10.1080/0028825X.2003.9512865>
- Nannenga-Bremekamp NE (1991) A guide to temperate Myxomycetes: an english translation of De Nederlandse Myxomycetesn. Biopress, Bristol. 409p.
- Neubert H, Nowotney W & Baumann K (1993) Die Myxomyceten Deutschlands und des angrenzenden Alpenraumes unter besondererBerücksichtigung Österreichs. V. I Ceratiomyxiales, Echinosteliales, Liceales, Trichiales. Verlag Karlheinz Baumann, Gomaringen. 343p.
- Novozhilov YK, Zemlianskaia IV, Schnittler M & Stephenson SL (2006) Myxomycete diversity and ecology in the arid regions of the Lower Volga River Basin (Russia). Fungal Diversity 23: 193-241.
- Nusbaumer L, Barbosa, MRV, Thomas WW, Alves MV, Loizeau PA & Spichiger R (2015) Flora e vegetação da Reserva Biológica de Pedra Talhada. In: Studer A, Nusbaumer L & Spichiger R (eds.) Biodiversidade da Reserva Biológica de Pedra Talhada (Alagoas, Pernambuco - Brasil). Boissiera 68: 59-121.
- Oran RB & Ergül CC (2004) New records for the myxobiota of Turkey. Turkish Journal of Botany 28: 511-515.
- Poulain M, Meyer M & Bozonnet J (2011) Les Myxomycètes. Fédération Mycologique et Botanique Dauphiné-Savoie, Sévrier. 1119p.
- Putzke J (1996) Myxomycetes no Brasil. Cadernos de Pesquisa, Série Botânica 8: 1-133.
- Rojas C, Stephenson SL & Pavlich M (2011) New additions to the myxobiota of Peru. Mycosphere 2: 583-592.
- Rojas C, Rojas PA & Lado C (2018) Myxomycete diversity in Costa Rica. Mycosphere 9: 227-255.
- Rosing WC, Mitchell DW & Stephenson SL (2007) Corticolous myxomycetes from Victoria. Australasian Mycologist 26: 9-15.
- Sesli E & Denchev CM (2014) Checklists of the myxomycetes, larger ascomycetes, and larger basidiomycetes in Turkey. Mycotaxon Checklists Online. 136p. Available at <http://www.mycotaxon.com/resources/checklists/sesli-v106-checklist.pdf>. Access on 9 october 2020.
» http://www.mycotaxon.com/resources/checklists/sesli-v106-checklist.pdf - Stork NE, Coddington JA, Colwell RK, Chazdon RL, Dick CW, Peres CA, Sloan S & Willis K (2009) Vulnerability and resilience of tropical forest species to land-use change. Conservation Biology 23: 1438-1447. <https://doi.org/10.1111/j.1523-1739.2009.01335.x>
- Studer A (1985) Estudo ecológico do conjunto florestal da Serra das Guaribas e da Serra do Cavaleiro. Pedido para a Salvaguarda desta Floresta. Monografia. Quebrangulo, Alagoas. 61p.
- Studer A (2015) Aves. In: Studer A, Nusbaumer L & Spichiger R (eds.) Biodiversidade da Reserva Biológica de Pedra Talhada. Alagoas / Pernambuco. Brasil. Boissiera 68: 59-121.
- Telenius A (2016) Gothenburg Herbarium - General (GBIF:IH:GB:Herbarium). GBIF-Sweden. Occurrence dataset <https://doi.org/10.15468/afkfpi> accessed via GBIF.org. Available at <https://www.gbif.org/occurrence/1043002216>. Access on 19 October 2020.
» https://www.gbif.org/occurrence/1043002216 - Thiers B [continuously updated] Index Herbariorum: a global directory of public herbaria and associated staff. New York Botanical Garden’s Virtual Herbarium. Available at <http://sweetgum.nybg.org/science/ih/>. Access on 17 October 2020.
» http://sweetgum.nybg.org/science/ih/ - Tscharner T, Duda GP, Oliveira VP, Silva CMS, Nusbaumer L & Silva Filho AF (2015) Parâmetros abióticos da Reserva Biológica de Pedra Talhada. In: Studer A, Nusbaumer L & Spichiger R (eds.) Biodiversidade da Reserva Biológica de Pedra Talhada (Alagoas, Pernambuco - Brasil). Boissiera 68: 39-57.
- Walker LM, Cedeño-Sanchez M, Carbonero F, Herre EA, Turner BL, Wright SJ & Stephenson SL (2019) The response of litter-associated myxomycetes to long-term nutrient addition in a Lowland Tropical Forest. Journal of Eukaryotic Microbiology 66: 757-770.
- Wilton A (2020) New Zealand fungal and plant disease collection (PDD). Landcare Research. <https://doi.org/10.15468/nrq12b> accessed via GBIF.org. Available at <https://www.gbif.org/occurrence/1135719082>. Access on 9 October 2020.
» https://www.gbif.org/occurrence/1135719082 - Yamamoto Y (1998) The myxomycete biota of Japan. Tojo Shorin, Tokyo. 700p.
- Zoll V & Stephenson SL (2015) Records of myxomycetes from Maine. Mycosphere 6: 568-584.
Edited by
Publication Dates
-
Publication in this collection
11 Apr 2022 -
Date of issue
2022
History
-
Received
01 Dec 2020 -
Accepted
03 May 2021