Abstracts
AIM: The Sinos River, a tributary of Guaíba Lake, is 200 km long from the headwaters to the mouth and is influenced by several sources of pollution. A study to learn more about conditions in this river was performed in the middle and lower reaches. The study evaluated the effects of sediment contamination by xenobiotics on the survival and reproduction of Daphnia magna. METHODS: Eight sediment samples were collected per site from Dec/2007 to Aug/2009, as a substrate for 480 young cladocera (2-26 h old at the beginning of the trials) in chronic toxicity tests (21 days). For this purpose, D. magna individuals were exposed to sediment and M4 culture medium, at a proportion of 1:4 (v:v) using 50 mL beakers, kept at 20 ºC ± 2 ºC in 16h light:8h dark cycles. The test organisms came from lots with LC50-24h = 1.04 mgK2Cr2O7 ± 0.11 mg. Percentage survival and reproduction were considered to define acute and/or chronic responses. Duncan (p < 0.05) and T (p < 0.05) Tests, ANOVA and Spearman correlation of the biological data were used as statistical complementation. The Spearman correlation was also applied looking for dependencies between the rainfall measured at the sites and the biological parameters but there was no evidence of correlation. The presence of acute toxicity was diagnosed for the sample in which survival was less than 80%, and for chronic toxicity when the mean number of neonates was less than 20 daphniids. RESULTS: Reproductive delays were found coinciding with physical and chemical parameters, as well as the presence of metals at values that were not in accordance with the law. Statistical analysis suggested the predominance of point sources of contamination. An effect on survival was observed in 17% of the samples, and on reproduction in 87%. CONCLUSIONS: This study found that pollutants discharged into the Sinos River remain and can interfere in the equilibrium of the trophic network, since they increase mortality and diminish the production of offspring among the cladocera, representatives of the primary consumers in the food chain.
Daphnia magna; ecotoxicology; pollution; sediment
OBJETIVO: O rio dos Sinos, tributário do Lago Guaíba, percorre cerca de 200km desde a nascente até a foz , recebendo influência de diversas fontes poluidoras. Visando melhor conhecer as condições deste rio foi desenvolvido estudo nas zonas média e inferior avaliando os efeitos na sobrevivência e na reprodução de Daphnia magna, decorrentes da contaminação do sedimento por xenobióticos. MÉTODOS: Entre dez/2007 e ago/2009 foram coletadas oito amostras de sedimento por site, para serem usadas como substrato para 480 cladóceros jovens (2-26h de vida no início dos ensaios) em testes de toxicidade crônica (21 dias). Para tanto indivíduos de D. magna foram expostos a sedimento e meio de cultivo M4, na proprção1:4 (v:v), utilizando béqueres de 50 mL, mantidos a 20 ºC ± 2 ºC em ciclos de 16h luz: 8h escuro. Os organismos-teste originaram-se de lotes com LC50-24h = 1,04 mg K2Cr2O7 ± 0.11 mg. A definição de respostas agudas e/ou crônicas considerou a porcentagem de sobrevivência e a média reprodutiva. Como complementação estatística foram aplicados os testes T (p < 0,05) e Duncan (p < 0,05), ANOVA e Correlação de Spearman dos dados biológicos. Buscando constatar dependências entre a pluviometria dos sites e os parâmetros biológicos também foi aplicado o mesmo cálculo, porém não houve evidência de correlação. Presença de toxicidade aguda foi diagnosticada para a amostra em que a sobrevivência foi inferior a 80% e crônica quando a média de neonatos por ninhada foi inferior a 20 dafinídeos. RESULTADOS: Foram encontrados atrasos reprodutivos coincidentes com parâmetros físicos e químicos e presença de metais em valores em desconformidade com a legislação. A análise estatística sugeriu a predominância de fontes pontuais de contaminação. Observou-se efeito na sobrevivência em 17% das amostras e na reprodução em 87%. CONCLUSÕES: Este estudo mostrou que a descarga de poluentes persiste rio dos Sinos o que pode interferir no equilíbrio da rede trófica, já que houve elevação da mortalidade e queda da fecundidade entre os organismos expostos.
Daphnia magna; ecotoxicologia; poluição; sedimento
Daphnia magna Straus, 1820 response to sediment samples from a contaminated river (Rio Grande do Sul, Brazil)
Resposta de Daphnia magna Straus, 1820 a amostras de sedimento de um rio contaminado (Rio Grande do Sul, Brasil)
Nara Regina Terra; Silvana Pereira Gonçalves
Divisão de Biologia, Programa de Pesquisas, Departamento de Laboratórios, Fundação Estadual de Proteção Ambiental Henrique Luis Roessler FEPAM, Av. Dr. Salvador França, 1707, CEP 90690-000, Porto Alegre, RS, Brazil e-mail: nara.terra@ufrgs.br; silgoncalves1405@gmail.com
ABSTRACT
AIM: The Sinos River, a tributary of Guaíba Lake, is 200 km long from the headwaters to the mouth and is influenced by several sources of pollution. A study to learn more about conditions in this river was performed in the middle and lower reaches. The study evaluated the effects of sediment contamination by xenobiotics on the survival and reproduction of Daphnia magna.
METHODS: Eight sediment samples were collected per site from Dec/2007 to Aug/2009, as a substrate for 480 young cladocera (2-26 h old at the beginning of the trials) in chronic toxicity tests (21 days). For this purpose, D. magna individuals were exposed to sediment and M4 culture medium, at a proportion of 1:4 (v:v) using 50 mL beakers, kept at 20 ºC ± 2 ºC in 16h light:8h dark cycles. The test organisms came from lots with LC50-24h = 1.04 mgK2Cr2O7 ± 0.11 mg. Percentage survival and reproduction were considered to define acute and/or chronic responses. Duncan (p < 0.05) and T (p < 0.05) Tests, ANOVA and Spearman correlation of the biological data were used as statistical complementation. The Spearman correlation was also applied looking for dependencies between the rainfall measured at the sites and the biological parameters but there was no evidence of correlation. The presence of acute toxicity was diagnosed for the sample in which survival was less than 80%, and for chronic toxicity when the mean number of neonates was less than 20 daphniids.
RESULTS: Reproductive delays were found coinciding with physical and chemical parameters, as well as the presence of metals at values that were not in accordance with the law. Statistical analysis suggested the predominance of point sources of contamination. An effect on survival was observed in 17% of the samples, and on reproduction in 87%.
CONCLUSIONS: This study found that pollutants discharged into the Sinos River remain and can interfere in the equilibrium of the trophic network, since they increase mortality and diminish the production of offspring among the cladocera, representatives of the primary consumers in the food chain.
Keywords:Daphnia magna, ecotoxicology, pollution, sediment.
RESUMO
OBJETIVO: O rio dos Sinos, tributário do Lago Guaíba, percorre cerca de 200km desde a nascente até a foz , recebendo influência de diversas fontes poluidoras. Visando melhor conhecer as condições deste rio foi desenvolvido estudo nas zonas média e inferior avaliando os efeitos na sobrevivência e na reprodução de Daphnia magna, decorrentes da contaminação do sedimento por xenobióticos.
MÉTODOS: Entre dez/2007 e ago/2009 foram coletadas oito amostras de sedimento por site, para serem usadas como substrato para 480 cladóceros jovens (2-26h de vida no início dos ensaios) em testes de toxicidade crônica (21 dias). Para tanto indivíduos de D. magna foram expostos a sedimento e meio de cultivo M4, na proprção1:4 (v:v), utilizando béqueres de 50 mL, mantidos a 20 ºC ± 2 ºC em ciclos de 16h luz: 8h escuro. Os organismos-teste originaram-se de lotes com LC50-24h = 1,04 mg K2Cr2O7 ± 0.11 mg. A definição de respostas agudas e/ou crônicas considerou a porcentagem de sobrevivência e a média reprodutiva. Como complementação estatística foram aplicados os testes T (p < 0,05) e Duncan (p < 0,05), ANOVA e Correlação de Spearman dos dados biológicos. Buscando constatar dependências entre a pluviometria dos sites e os parâmetros biológicos também foi aplicado o mesmo cálculo, porém não houve evidência de correlação. Presença de toxicidade aguda foi diagnosticada para a amostra em que a sobrevivência foi inferior a 80% e crônica quando a média de neonatos por ninhada foi inferior a 20 dafinídeos.
RESULTADOS: Foram encontrados atrasos reprodutivos coincidentes com parâmetros físicos e químicos e presença de metais em valores em desconformidade com a legislação. A análise estatística sugeriu a predominância de fontes pontuais de contaminação. Observou-se efeito na sobrevivência em 17% das amostras e na reprodução em 87%.
CONCLUSÕES: Este estudo mostrou que a descarga de poluentes persiste rio dos Sinos o que pode interferir no equilíbrio da rede trófica, já que houve elevação da mortalidade e queda da fecundidade entre os organismos expostos.
Palavras-chaves:Daphnia magna, ecotoxicologia, poluição, sedimento.
1. Introduction
The Guaíba Lake Hydrographic Basin which supplies water to Porto Alegre, capital of Rio Grande do Sul State, Brazil, is formed by five rivers, including the Sinos River which is influenced by sources of pollution along its 200 km course. Although this river receives raw or partly treated domestic and industrial wastes, it is the main source of water supply for the Sinos Valley River. Previous studies (Vargas et al., 2001; Terra et al., 2008; Feiden and Terra, 2009) indicate the presence of environmental stressors that interfere in the quality of the river bed and of the waters that form it.
This study evaluated the effects of contaminants on the river bed on Daphnia magna. Many substances are deposited in the sediments, including metals, agricultural pesticides and PAHs, that may be included in the fauna food through biological activities, chemical reactions and streamflow. When this species is â¥48h old it grazes the surface sediment layer releasing xenobiotics that are part of the river bottom system (Suedel et al., 1996). The level of response of the daphniids is conditioned to the level of contamination and to the time of exposure to the toxic substance (Ren et al., 2009a, b). Gillis et al. (2006) observed up to 50% reduction in the survival of this cladocera when exposed to the sample of contaminated sediment.
This study assesses changes in survival and especially in the reproductive activity of test-organisms, to define the quality of Sinos River, which presents successive ichthyofauna death events.
The main pollutants in this river are domestic, hospital and industrial sewage, garbage, tanneries, agriculture, pesticides, oil refinery, sand dredging and navigation.
Particles that flow directly into rivers in effluents or are transported through the atmosphere, or also by runoff can be deposited on the bed or recycled by living beings. Reis et al. (2010) highlight the importance of these particles containing associated metals, because they are available to the liquid mass resulting from the variation of the currents or biological activity. According to Weltens et al. (2000) sediments present constant toxicity since they are permanent sources of xenobiotics, besides integrating food particles, highlighting the importance of the metals available for daphniids. Yu and Wang (2002) observed that cladocera exuviae are major sources of metals in the environment.
Successful trials with Daphnia magna have transformed the species into a test-organism widely used in environmental sample bioassays. Since the daphniids are non-selective filterers (Weltens et al., 2000), they ingest sediment particles containing toxic compounds that, even in small doses, are sufficient to cause damage. Thus local toxicity can be characterized without identifying the agent responsible for the problem.
The different types of industries located in this river valley are responsible for frequent fish death events in the region. Moreover, many of the contaminants discharged and/or their byproducts, such as phenol ((Kühn et al., 1989) and agricultural pesticides are toxic (Ren et al., 2009b). Along the course of this river, agricultural pesticides with different active principles are applied and may interfere in the survival and reproduction of aquatic biota. The main active ingredients of the pesticides used in the Sinos River Basin are abamectin, deltametrin, paraquat dichloride, diphenoconazole, sulphur, gliphosate, mancozeb, sulfonamide, trifluraline and cuprous oxide (CRQ, 2008). Other elements, while not toxic, indicate contamination and alter the ecosystem equilibrium, as in the case of P and N (Ballantine et al., 2009).
The main purpose of this study was to identify the level of environmental alteration of the sites sampled by looking at changes in the survival and reproduction of D. magna.
2. Material and Methods
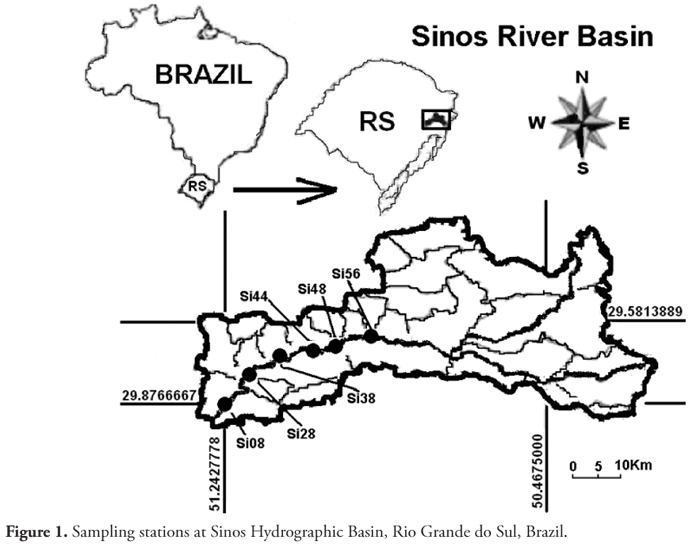
This study evaluated eight sediment samples from six sites on the Sinos River. These sites were to provide domestic water supply after conventional treatment, primary contact recreation and protection of aquatic communities, according to Brazilian Law (CONAMA, 2005) in Class 2 (Si56). Others were in Class 3 (Si08, Si28, Si38, Si44, Si48) for public supply, after conventional or advanced treatment, for irrigation, fishing, watering animals and secondary contact. The samples were collected between Dec/2007 and Aug/2009, and all the sites were located in the lower reach of Sinos River.
Lots (LC50 - 24h = 1.04 mg K2Cr2O7 ± 0.11 mg) containing 25 individuals per 1000 mL with M4 culture medium (Elendt and Bias, 1990), kept under controlled lighting conditions (16h light daily) and 20 ºC ± 2 ºC (ABNT, 2009) were the source of the test-organisms. The Trimmed Spearman-Karber method was used to calculate sensitivity (Hamilton et al., 1977).
The sediment was used as a substrate in long duration tests (21 days) with Daphnia magna (Clone A). Time of exposure and feeding habits are essential in the cladoceran response process. Work with sediment samples emphasizes the importance of time of exposure as related to the concentration of a toxic substance for the organism response (Robinson et al., 2010).
The sampling sites are named using the river name initials (Si) followed by the number of kilometers from the mouth (Figure 1). The geographical coordinates and sources of pollution at each site are shown in Table 1.
Ten young cladocera, 2-26 hours old at initial exposure, were exposed to each sediment sample (ABNT, 2009), a total of 480 individuals (80 per site, six assays,). The microcrustaceans were monitored for percentage of survival, total reproductive mean and per brood. During the assays at least 80% survivors per sample were expected and an average of â¥20 neonates per brood at each point. The samples that presented these values were characterized as free of acute or chronic toxicity, respectively. Spearman Correlation, ANOVA (two factors), Duncan Test (variation in time and between the points), ANOVA (single factors) and T Test complemented the evaluation of the results. The Spearman correlation also evaluated the relationship among the data on survival, reproduction and rainfall.
The sediment was collected using a Petersen grab sampler. The samples were placed in a glass flask (1000 g), kept in a thermal box with ice during transport to the laboratory, and transferred to the refrigerator at 4 ºC until they are used (Suedel et al., 1996). The experiments were performed until a month after the samples were collected, using bulk sediment, removing the larger organisms that were sometimes part of the sample.
The microcrustaceans were exposed in 50 mL beaker, covered with laboratory film containing sediment and M4 (1:4; v:v) (Suedel et al., 1996; Terra et al., 2008). Sediment samples were aliquoted into the test-containers which were kept covered under refrigeration until the day before the exposure began, when M4 culture medium (pH: 7.8; hardness: 230 CaCO3/L) was placed over the sediment, taking care to avoid disrupting the upper layer of sediment. At this point, the test-containers were taken overnight to a germinator to acclimate the medium. The next morning one microcrustacean was included per replica (10) in each sample. A control group submitted to the same conditions as the samples accompanied these assays with exposure only to the M4 medium. The tests were performed under the same temperature and light conditions as the original lots, but in different germinators to avoid contamination.
The observations occurred on Mondays, Wednesdays and Fridays, recording the dead daphniids and the neonates. At the end of the exposure period, the survivors that did not produce offspring were examined for sex. After this procedure the old culture medium was removed, new medium was introduced into the beaker, and the organisms were fed Desmodesmus subspicatus (Chodat, 1926) Hegewald and Schmidt, 2000, (0.7 mL; 107 cells.cm3) algae and fermented fish chow complemented by biological yeast (0.1 mL) according to the national standard (ABNT, 2009). These amounts of food ensure that the nutritional needs of the test-organism are met until the next observation.
Complementary data on physical-chemical analyses and on metals in water sampled at the same time as the sediment were considered helpful to interpret the responses. These parameters were defined according to the risk of local contamination due to the sources of emission.
Nitric acid (5 mL) was added in dark glass flasks (1000 mL) with samples of water for the dosage of heavy metals. Physical-chemical parameters followed the methodology advocated in APHA (2005) both for collection and for analysis. Acid Digestion of Samples and Determination by FlameAtomic Absorption Spectrophotometry were used to verify the presence of total heavy metals. Dissolved oxygen (DO) and pH assessments were obtained from direct readings performed with appropriate equipment, Oximeter and Potentiometer, respectively.
Heavy metals, even below the allowable limit or during a month when no sediment sampling was performed, were considered because they belong to the group of persistent xenobiotics. Physical-chemical parameters were related to thedata only when present at values above the legally allowed value at the time of sediment sampling.
Rainfall indices of the period of study provided by Defesa Civil (Civil Defense) do Rio Grande do Sul were used to complement the study.
3. Results
Acute toxicity was seen in 17% of the samples, as shown in Figure 2. Si28 and Si48 presented 6% of the samples that could potentially cause the death of the organisms and Si38, 2% of samples with this characteristic. On the other hand, 87% of samples were seen to induce chronic effects (Figure 3), and in Dec/07, Feb/09 and Apr/09 all sites presented chronic action on the microcrustaceans. The total number of births per month of collection showed that Si48 reached the expected number of neonates on two occasions, Si08 on one, while the other points were below expectations in all samplings (Figure 4).
When the Duncan Test was applied to compare the production of neonates per month of sampling, a difference was seen among the sites (Table 2). Three (June/08, Oct/08, Dec/08, Aug/09) and four (Aug/08, Feb/09, Apr/09) differentiated groups of neonates predominated, although in Dec/07 there were only two. The reproductive response behavior pattern was absent when the months of collection or the sites were considered using this statistical method. Sometimes lower neonate production was observed at sites close to the mouth of Sinos River compared to those that are closer to the headwaters, and on other occasions the contrary occurred.
The samples from each point compared to each other applying the Duncan Test showed homogeneity (one cluster) at Si38 among the months, considering the production of neonates, while Si44 showed two clusters (Figure 5). Si28 separated the data into three groups, while Si08 separated them into four and Si56 into five clusters.
If the overall production of young is considered, the mean number of neonates shows Si28 as the least fertile site (325 neonates), while Si38 had the greatest number of young (637 neonates). Figure 6 shows the total number of neonates per site, the control group and the minimum number of neonates expected in environments without toxicity at any level.
The Spearman correlation showed an inverse relationship for reproduction among sites Si28 with Si38 and Si44 with Si48, i.e., when the number of neonates rose at one of the sites production diminished at the other. The same statistical calculation comparing reproduction and survival only showed a correlation among the data at site Si48. At this site (Si48) 750 females survived, while at the others survival varied from 640 to 760 test-organisms. The Spearman correlation showed a correspondence of survival between sites Si08 and Si44.
The single factor ANOVA showed a difference between the variances of the sites, considering the total number of neonates. The T test initially compared the groups two by two and later each group was compared to the control, showing a significant difference (p < 0.05) between the control group and the others. The results were similar only for Si48 in relation to Si56 and Si08. The means varied from 79 neonates for Si38 to 41 for Si23, while the mean of the Control group reached 103 neonates.
Table 3 shows the comparison of reproductive delays observed and the parameters that do not conform to CONAMA.
P element presented mean values and SD above the legal level at all sites by law at all sites (Figure 7).
To evaluate the biological results, the presence of metals was considered according to Brazilian law, for each class of water. Heavy metals were sometimes detected above the current Brazilian legal levels. These occurrences are at Si08 (June/08- Hg: 0,7 mg.L 1), Si28 (Dec/07- Cu: 0,032 mg.L 1; Apr/08- Cu: 0.015 mg.L 1; June/08- Hg: 0.007 mg.L 1), Si38 (Feb/08- Hg: 0.0056 mg.L 1; Apr/08- Cu: 0.016 mg.L 1), Si44 ( Dec/07- Cu: 0.068 mg.L 1; June/08- Fe: 5.15 mg.L 1; June/09- Cu: 0.015 mg.L 1), Si48 (Apr/08- Cu: 0.081 mg.L 1; Aug/08- Cd: 0.54 mg.L 1, Cu: 0.205 mg.L 1; Dec/08- Cu: 0.122 mg.L 1; Feb/09- Cu: 0.26 mg.L 1, Ni: 0.198 mg.L 1; Apr/09- Cd: 0.3 mg.L 1, Cu: 0.048 mg.L 1, Ni: 0.137 mg.L 1; June/09- Cu: 0.032 mg.L 1, Ni: 0.14 mg.L 1; Aug/09- Cu: 0.126 mg.L 1, Ni: 0.147 mg.L 1), Si56 (Dec/07- Fe: 2.36 mg.L 1, Mn: 0.106 mg.L 1; Apr/08- Cu: 0.018 mg.L 1, Fe: 4.72 mg.L 1, Mn: 0.169 mg.L 1, Pb:0.024 mg.L 1, Zn: 0.23 mg.L 1; June/08- Fe: 5.17 mg.L 1; Aug/08- Fe: 3.24 mg.L 1; Dec/08- Zn:0.134 mg.L 1; Feb/09- Fe: 2.22 mg.L 1, Mn: 0.138 mg.L 1; Apr/09- Fe: 1.44 mg.L 1; June/08- Fe: 1.22 mg.L 1.
Sometimes the heavy metals were found at values accepted by CONAMA. All the same they were evaluated according to the interpretation of reproductive changes and/or survival of the test-organisms because they were conservative. To evaluate the contribution of the metals during this period, the mean and standard deviation of each of them was calculated and sometimes mean and/or standard deviation values were observed above the levels allowed by CONAMA (Figure 8).
4. Discussion
Natural events such as precipitation and currents, besides anthropic contributions, affect environmental quality, triggering responses in the flora and fauna. Cities and different types of industry installed along the Sinos River create localized organic and /or chemical pollution, provoking cyclic processes of death of native organisms, especially those situated at the top of the trophic chain, such as fishes, formerly plentiful in the region and often used to feed the riparian populations, but which have now become scarce.
Comparing the mortality results of D. magna generated in previous studies (Terra et al. 2008; Feiden and Terra, 2009) to the current study, it is found that the greatest pressure on survival of the species occurs in October, followed by February. In October there are usually heavy rains in Rio Grande do Sul, while February is characterized by drought. These two situations are critical for sites with high levels of contamination. In the first case (rainfall) the current is stronger and disturbs the river bed, besides bringing more contaminants into the river from contaminated soils. Lemos and Erdtmann (2000) found a positive correlation between precipitation and genotoxic effect using water samples from a river that is also one of those forming the Guaíba Basin.
In this study it was found that acute levels of toxicity persist at Si48. Positive results for cytotoxicity and genotoxicity had already been reported previously (Vargas et al., 2001; Terra et al., 2008). According to Terra et al. (2008) between the years of 2001 and 2005 sediment samples from this site already presented acute toxicity in D. magna.
Si38 showed similar responses for the production of neonates over time, and this may mean a continuous contribution of pollutants without the significant presence of peaks. Si44 exhibited a slight variation in results, while Si56 presented a greater diversity of responses. The diversity of results may be due to point and irregular discharges of effluents, since in this reach there are several industries of different types, mainly tanneries.
Comparing the production of neonates among different sites, the Duncan Test showed different responses, even among the sites located close to each other, such as Si44 and Si48 (4km distant) confirming the presence of point sources of contamination along the river. A pattern of responses was found to be absent, since the generation of neonates fluctuated during the period, sometimes presenting a lower production of neonates at the points located close to the mouth and at other times a higher number of young in these regions. It was not, however, possible to establish a direct relationship between the production of neonates or the mortality of the test-organisms and the rainfall index. Both point sources of effluents such as artificial canals, and streams receiving effluents flow into the main body of the river, causing degradation and the fluctuation of results.
Observing the total number of births per month of collection, it is found that Si48 reached the expected number of neonate production on two occasions, Si08 on one, and the other points remained below the expected levels, independent of rainfall index.
Although the Duncan Test showed a variation of results with the prevailing formation of three clusters, only two occurred in Dec/07. During that month a cluster was formed with points Si38 and Si44, and another cluster with all the rest. It can be recorded that these sites (Si38 and Si44) presented a lower rainfall index as compared with the local historical mean. No reproductive response patterns occurred, either when data were evaluated month by month or when each site was analyzed individually over time.
Low reproduction prevailed at Si28 (mean number of 325 neonates), a point which is contaminated mainly by inputs from rice crops, tanneries, sand dredging, and an agricultural pesticide plant. A similar response was seen in a previous study (Feiden and Terra, 2009), when a low number of offspring occurred in 79% of the samples. This site sometimes presented Cu above the CONAMA standard, and at other times it was detected within Brazilian legal limits. Moreover, pesticides with different active principles are known to be discharged directly or indirectly into this river (CRQ, 2008), and could have contributed to the drop in survival and to the reproduction of aquatic biota. At this site, delaying events were observed at the beginning of reproductive activity in 50% of the samples, coinciding with physical and chemical patterns and metals above the levels recommended in Brazilian law. In some episodes of reproductive delay, there was a high level of mortality, and a low level of reproduction occurred among the survivors.
At site Si08, located near the mouth, a small number of offspring was expected, since this site is impacted by pollutant discharges all along the river as seen in a previous study (Feiden and Terra, 2009). According to Terra et al. (2008) in a study performed in this area between 2001 and 2005, a cytotoxic effect was detected in water samples compatible with the heavy anthropic influence found at this site. According to Cowgill et al. (1986) stressed organisms often produce smaller broods than non-stressed ones. The action of xenobiotics alters the organic functions triggering undernourishment processes and stress in the cladocera.
The best mean reproduction was found at Si38, although at this site the females delayed the beginning of reproduction and one did not have any offspring. At this site, results above the CONAMA standard were also found. At Si38 in Feb/09, a test-organism stopped developing after 48 h, and did not lay any young. The individual was examined to identify the sex, and was confirmed to be female. A similar situation was observed in a cladoceran at site Si48 (Apr/09), when delayed growth occurred. It exhibited a 48h size on the 8th day of exposure and began reproduction only on the 15th day of exposure. Si08 and Si44 did not present acute toxicity, while at Si28 and Si48 this effect was observed in 62.5% of the samples, and at Si38 and Si56, 87.5%. On the other hand, chronic toxicity was found in all samples from Si28 and Si44, 87.5% in those collected at Si08 and Si56 and 75% at the other points (Si38 and Si48).
In the months with toxicity, metals that were not in conformity with the law were found at Si 48 (Cd) and Si56 (Fe). Si38 in Dec/08 presented a high number of young, although 30% deaths occurred. Since only surviving individuals, which probably present higher resistance to environmental changes, are taken into account to calculate the mean reproduction per brood this situation has been observed in several rivers.
The mean number of neonates per test-organism, calculated from the total production of young, shows Si28 as the least fertile site (41 neonates), and this factor may be related to the local sources of contamination (Table 1), since the mean observed in the control group reached 103 neonates.
Correlation (p<0.05) between reproduction and survival was found only at Si48. At Si48 several events of reproductive delay were seen, besides females that did not lay after the foreseen exposure time (21 days).
When ANOVA was applied, it clearly indicated the environmental change that occurred in the area, since, comparing each point to the control group, a significant difference (p < 0.05) was found between births. The points of the river, however, presented some similarity, specifically related to the type of industry at the point sources of contamination than to a direct influence among them, since they are distant sites, because only Si48 presented some similarity to Si56 and Si08. This was underlined when the Spearman Correlation (p < 0.05) identified inverse responses between sites Si28 with Si38 and between sites Si44 with Si48, i.e., when the number of neonates increased at one of the sites, at the other the number of neonates diminished. No cause was found for this, and the rainfall was actually similar at Si28 compared to Si38 and at Si44 compared to Si48. It should be considered that both at Si28 and at Si38 precipitation was above the historical mean. No specific causes for this behavior were found among the parameters analyzed. On the other hand, the Spearman correlation (p < 0.5) was found for Si08 and Si44 when the survival data were compared. In common, the presence of Hg at values above those allowed by CONAMA was found for both points in June/08.
The difference between the sites was identified by single-factor ANOVA, considering the total number of neonates. The analysis of these groups was complemented using the T test, comparing them two by two and then, afterwards, each group to the control. This statistical tests pointed to a significant difference (p<0.05) between the control group and the others. On the other hand, the results for Si48 were similar to those of Si56 and Si08.
Along the Sinos River different pesticides are applied according to CRQ (2008), which provokes changes in the development of D. magna. Neurotoxic signals are observed in Daphnia magna when it is exposed to deltametrin (pyrethroid) (Ren et al., 2009b) used in the Sinos River, reinforcing the choice of the species to evaluate the area. Studies show that glyphosate, an herbicide used in the area of study (CRQ, 2008) is toxic for fish, organisms that occupy high levels in the trophic chain and whose food base are microcrustaceans.
Sampling was performed to evaluate cyanide on one occasion at site Si44 (0.050 mg.L1- June/09) and on two at Si48 (0.144 mg.L1 Dec/08 and 0.336 mg.L1- Apr/09), and presented high values in all samples. According to CONAMA (2005), the value accepted for Class 3 waters should be below 0.022 mg.L1. Although this byproduct of industrial processes is not cumulative, it is toxic for aquatic fauna. According to David et al. (2010) cyanide has an asphyxiating effect interfering strongly in the respiratory rate and in enzyme activity.
Parameters that are not in conformity with CONAMA may cause effects observed in the reproduction and survival of the species exposed. The main environmental changes, which coincided with reproductive delays, were the presence of E. coli and P above the standards and DO below the legally advised standards, all of them related to anthropic contamination. P is an essential element for species development, but excessive amount in the environment suggests the presence of sources of contamination, such as sewers and/or agriculture.
The effects of non metallic elements may also trigger processes that interfere in the development of the microcrustaceans, as in the case of Phosphorus, which runs into the rivers through entrainment of soils that have been treated for crops. Ballantine et al. (2009) suggest that due to the uses of soil entrained into the rivers, the P content is more important than its physical properties. When He and Wang (2008) compared diets rich and poor in P, they found that this element speeds up the passage of the nutrients in the gut, leading to greater growth of the species. At this time it is necessary to consider that when the P element was present in high values, there was no stimulus for growth, probably due to the toxic elements that that come with it in the effluents and soils entrained, since other parameters were also present at least one at of the sites, with values above those recommended by CONAMA (2005). It also became clear that the delay at the beginning of reproductive activity does not depend on the level of survival of the replicas, and chronic activity is clearly marked even in the absence of acute action, through the response of the organism to constant pollutants, even if at low levels. Phenols and their byproducts are toxic for D. magna (Keen and Baillod, 1985; Kühn et al., 1989) and in this study reproductive delay is reported concomitant to the high phenol content.
Evaluating the results of metal analyses at each site, at least three types of metals were found even if sometimes their value was the legally recommended level. Since metals are conservative pollutants, they may accumulate constantly in the biota even at admissible values. Heavy metals present in the river varied along the course and may have interfered in the quality of life of the cladocera, contributing to the lower reproduction of the test-organisms and/or sometimes death. Pb was a frequent finding, but in medium amounts and SD within legally recommended level for Si08, Si28 and Si44. At Si56 the metal was detected only in one of the samples, when its value was very high compared to the CONAMA standard, which placed the SD above the legally recommended level. While for Si56 (Class 2) the CONAMA standard required corresponds to 0.01 mg.L1, at the other points the presence of this metal is accepted at 0.033 mg.L1 since, belonging to Class 3, its purpose is not the protection of aquatic communities.
Toxicity tests performed exposing Daphnia magna to Pb showed that this metal is more toxic to the species, compared to Cyclop spp, and that the toxic effect may be provoked by food or by contact (Offem and Ayotunde, 2008). The same authors suggest that 0.19 mg L1 is potentially dangerous for D. magna and lethal beginning at 1.65 mg L1. The Sinos River presented various events of contamination by Pb, sometimes exceeding the value the value legally recommended in Brazil, and at others with legally accepted values, as seen in Si44 (Dec/07-0.033 mg.L1, Apr/09-0.005 mg.L1), Si08 (Apr/08-0.006 mg.L1), Si28 (Dec/07-0.01 mg.L1, Apr/08-0.021 mg.L1). The metal was not detected at Si48 and Si38.
Other metals found in this river were Cu and Zn, responsible for the low survival of D. magna. Whereas zinc presented low values with a mean and SD within the legally allowed range, Cu presented a mean value and a very high SD at Si48. Si44 and Si28 presented an SD above the the Brazilian legal level. On one occasion Si56 presented double the accepted limit (Apr/08-0.018 mg.L1) with a view to conserving the biota. According to Gillis et al. (2006) sediment contaminated by Cu and Zn is responsible for mortality higher than 50% in exposure tests with D. magna ascribing the action to the water overlying the sediment. Delay in the first reproduction (12th day of exposure) was described by De Schamphelaere et al. (2007) when Daphnia magna was exposed to Cu. In the same study the author cites the 50% reduction of reproduction due to this metal. Changes in the life cycle of the copepoda Notodiaptomus conifer (SARS), especially in fertility and the first egg sac of copepoda were recorded by Gutierrez et al. (2010) when they were exposed to Cu. Progressive reduction in size, beginning with the second brood of Moina monogolica Daday was observed by Wang et al. (2007) when there was exposure to the same metal. Samples of the Sinos River induced a delay in reproduction at Si28 and Si48 when Cu was present at concentrations above the legally recommended level.
De Schamphelaere and Janssen (2004) observed that responses related to the reproductive process are more intense than in the survival of this species due to the presence of Cu. In this study acute action of the sediment samples occurred in the presence of Cu at high doses at sites Si28 (Dec/07) and Si48 (Dec/08 and Feb/09). De Schamphelaere et al. (2007) further mentions that the ingestion rate of Daphnia magna exposed to Cu is inhibited and the metabolic cost rose in order to inhibit the stress caused by metal, leading to the reduction of the reproductive and growth processes.
The same must be said about the zinc that conformed with legal standards only in the Class 3 areas (Si08, Si38, Si44 and Si48), which does not aim at the full conservation of the biota and accepts a higher value for this metal (5 mg L1). Sometimes Zn exceeded legal standards (0.18 mg L1) at Si56. This metal, when accumulated in the sediment, is easily released by grazing daphniids (Twiss and Campbell, 1995). In healthy quantities it is important for the reproductive process (De Schamphelaere et al., 2004), but at high doses it can cause reproductive problems in fauna, as seen by Terra and Schäfer (2000).
Iron, another essential nutrient at high doses, can physiological changes besides provoking significant changes in the structure and function of lotic ecosystems. This metal was detected in all samples of Si44 and Si56, with a value above the legal level. Van Anholt et al. (2002) describe the presence of sublethal effects, such as the strong reduction of the viability of offspring, caused by iron in D. magna.
Vuori (1995) reported that the precipitates of Fe on animals may be the cause of qualitative and quantitative changes in the feeding behavior of the fauna. When Fe was at higher values than the CONAMA standard, it was involved in reproductive delays and low number of neonates, but it did not influence the survival of the test organisms. Van Anholt et al. (2002) reported a strong reduction in offspring viability, reduced growth and high mortality in Daphnia magna when there was an excess of Fe. According to the same author, it is possible that it is possible that when there is an excessive amount of this metal it interferes in food ingestion by filter feeder organisms, triggering chronic and sublethal actions.
The mean Hg was below the CONAMA standard, whereas the SD exceeded the acceptance line at sites Si08, Si28 and Si38. These points presented sporadic values above the legally allowed levels. A study exposing Carcinus maenas to this metal (Costa et al., 2011) showed the cumulative character of mercury. Safahieh et al. (2011) detected a similar problem in fish. On the other hand, when Martin and Wen-Xiong (2005) exposed D. magna to constant concentrations of Mercury, they found that the species has the ability to alter physiological mechanisms and adapt to the metal Biesinger et al. (1982) in assays exposing D. magna to mercury provoked a significant reduction in the production of young already at 0.04 µg Hg/L, a concentration which is the lowest among all those used. The presence of Hg is a matter of concern, since this metal may remain in the sediment for over 100 years (Azevedo, 2003).
Cd exceeded the legal level only at Si48, and the mean values and SD were high. This response is consistent with the presence of metallurgical plants close to the site. The same occurred with Ni, which was found in excess (mean and SD) only at this site, although it was detected at the others. Since this metal is cumulative, it is important to highlight its presence at any concentration. Croteau et al. (2005) warn about the increased concentration of Cd at higher trophic levels due to transfer and bioaccumulation. Teodorovic et al. (2009) report the high toxicity exerted by Cd on D. magna. Geffard et al. (2008) in their experiments show the toxic and additive effect of Cd on the reproduction of D. magna, with a reduction of up to 73% of neonates in 50 µg/L of Cadmium.
Mn was detected at Si44 and Si56, surpassing the legal limits only at the second site. Mejía-Saavedra et al. (2005), in a study on the toxicity of this element, found that Mn had increased toxicity for Daphnia magna in the presence of DDT. Although this agricultural pesticide is no longer one of the most used in the area of study, it is useful to perform a similar study for other combinations, since the environment, particularly when exposed to effluent discharge, may present a great variety of pesticides. Assays with Ceriodaphnia dubia and Hyalella azteca (Lasier et al., 2000) detected the chronic effect of these organisms in the presence of Mn. Although this metal did not present a direct relationship with reproductive delay, mean value (Si44), and standard deviation (Si44 and Si56) were observed, above the legal level. Lasier et al. (2000) indicated municipal and industrial discharges as sources of MN releases.
Nickel presented high results only in Si48, when both the mean and the standard deviation were above the CONAMA standard. Evens et al. (2009) reported this metal as responsible for the reduction of the first brood, size of organism and increased mortality. Similar results were presented by Vandenbrouck et al. (2011) when they found a decrease in the number of offspring born from organisms exposed to Nickel. In this study Nickel was present in episodes of delay and absence of reproduction among the test organisms.
5. Conclusion
Comparing this study to previous ones, it can be seen that the responses of the Sinos River oscillate constantly. So far the quality has not recovered, although a growing number of companies in the area have adopted effluent treatment.
The chronic assays performed using Daphnia magna, in sediment samples from the Sinos River indicate that the contamination present in the Sinos River samples induces sub-lethal toxic effects in the test-organisms.
Acknowledgments
This research was funded by FEPAM. The authors wish to thank FEPAM for sampling, Conselho Nacional de Desenvolvimento Científico e Tecnológico (Processo 108460/2009-6) for the grant to Silvana Pereira Gonçalves); Fundação Estadual de Pesquisa Agropecuária (FEPAGRO) and Defesa Civil do Rio Grande do Sul (www2.defesacivil.rs.gov.br/estatistica/pluviometro_consulta.asp) for the rainfall data. The values informed for heavy metals, physical, chemical and microbiological parameters were obtained from the FEPAM Database.
Received: 13 September 2012
Accepted: 03 May 2013
- American Public Health Association APHA. 2005. Standard methods for the examination of water and wastewater 21th ed. Washington.
- Associação Brasileira de Normas Técnicas – ABNT. 2009. NBR 12713: Ecotoxicologia aquática – Toxicidade aguda – Método de ensaio com Daphnia spp (Cladocera, crustacea). 3. ed. ABNT.
- AZEVEDO, FA. 2003. Toxicologia do Mercúrio São Carlos: RIMA; São Paulo: InterTox.
- BALLANTINE, D., WALLING, DE. and LEEKS, GJL. 2009. Mobilization and transport of sediment-associated phosphorus by surface runoff. Water, Air, & Soil Pollution, vol. 196, p. 311-320. http://dx.doi.org/10.1007/s11270-008-9778-9
- BIESINGER, KE., ANDERSON, LE. and EATON, JG. 1982. Chronic effects of inorganic and toxicity. Accumulation and loss. Archives of Environmental Contamination and Toxicology, vol. 11, p. 769-774.
- Conselho Nacional do Meio Ambiente - CONAMA. Resolução nÂş 357, de 17 de março de 2005. Dispõe sobre a classificação dos corpos de água e diretrizes ambientais para o seu enquadramento, bem como estabelece as condições e padrões de lançamento de efluentes, e dá outras providências. Diário Oficial da República Federativa do Brasil, Poder Executivo, Brasília, DF, mar. 2005. Available from: <http://www.mma.gov.br>. Access in: 17 Apr 2005.
- COSTA, S., VIEGAS, I., PEREIRA, E., DUARTE, AC., PALMEIRA, CM. and PARDAL, MA. 2011. Differential sex, morphotype and tissue accumulation of mercury in the crab Carcinus maenas. Water, Air, & Soil Pollution, vol. 222, p. 65-75. http://dx.doi.org/10.1007/s11270-011-0809-6
- COWGILL, UM., EMMEL, HW., HOPKINS, DL., TAKAHASHI, IT. and PARKER, WM. 1986. Variation in chemical composition reproductive success and body weight of Daphnia magna in relation to diet. Internationale Revue der gesamten Hydrobiologie und Hydrographie, vol. 71, p. 79-99. http://dx.doi.org/10.1002/iroh.19860710111
- CROTEAU, MN., LUOMA, SN. and STEWART, AR. 2005. Trophic transfer of metals along freshwater food webs: Evidence of Cadmium biomagnification in nature. Limnology and Oceanography, vol. 50, p. 1511-1519. http://dx.doi.org/10.4319/lo.2005.50.5.1511
- Conselho Regional de Química da 5ÂŞ Região CRQ. 2008. I Mapeamento dos agrotóxicos utilizados no Rio Grande do Sul Câmara de Agrotóxicos. Porto Alegre: CRQ-V.
- DAVID, M., RAMESH, H., PATIL, VK., MARIGOUDAR, SR. and CHEBBI, SG. 2010. Sodium yanideinduced modulation in the activities of some oxidative enzymes and metabolites in the fingerlings of Cyprinus carpio (Linnaeus). Toxicological & Environmental chemistry, vol. 92, n. 10, p. 1841-1849.
- DE SCHAMPHELAERE, KAC. and JANSSEN, CR. 2004. Effects of chronic dietary copper exposure on growth and reproduction of Daphnia magna. Environmental Toxicology & Chemistry, vol. 23, p. 2038-2047. http://dx.doi.org/10.1897/03-411
- DE SCHAMPHELAERE, KAC., CANLI, M., LIERDE, VV., FORREZ, I., VANHAECKE, F. and JANSSEN, CR. 2004. Reproductive toxicity of dietary zinc to Daphnia magna. Aquatic Toxicology, vol. 70, p. 233-244. http://dx.doi.org/10.1016/j.aquatox.2004.09.008
- DE SCHAMPHELAERE, KAC., FORREZ, I., DIERCKENS, K., SORGELOOS, P. and JANSSEN, CR. 2007. Chronic toxicity of dietary cooper to Daphnia magna. Aquatic Toxicology, vol. 81, p. 409-418. http://dx.doi.org/10.1016/j.aquatox.2007.01.002
- ELENDT, BP. and BIAS, WR. 1990. Trace nutrient deficiency in Daphnia magna cultured in standard medium for toxicity testing. Effects of the optimization of culture conditions on life history parameters of D. magna. Water Research, vol. 24, p. 1157-1167. http://dx.doi.org/10.1016/0043-1354(90)90180-E
- EVENS, MS., FAZAKAS, K. and KEATING, J. 2009. Creosote contamination in sediments of the Grey Owl Marina in Prince Albert National Park, Saskatchewan, Canada. Water, Air, & Soil Pollution, vol. 94, p. 138-144. http://dx.doi.org/10.1016/j.aquatox.2009.06.011
- FEIDEN, IR. and TERRA, NR. 2009. Ecotoxicological evaluation of sediment from a river contaminated by industrial effluents, Sinos River (Rio Grande do Sul, Brazil) using Daphnia magna (Straus, 1820). Acta Limnologica Brasiliensia, vol. 21, n. 4, p. 441-450.
- GEFFARD, O., GEFFARD, A., CHAUMOT, A., VOLLAT, B., ALVAREZ, C., TUSSEAU-VUILLEMIN, M-H. and GARRIC, J. 2008. Effects of chronic dietary and waterborne cadmium exposures on the contamination level and reproduction of Daphnia magna. Environmental Toxicology & Chemistry, vol. 27, n. 5, p. 1128-1134.
- GILLIS, PL., WOOD, CM., RANVILLE, JF. and CHOW-FRASER, P. 2006. Bioavailability of sediment-associated Cu and Zn to Daphnia magna. Aquatic Toxicology, vol. 77, p. 402-411. http://dx.doi.org/10.1016/j.aquatox.2006.01.010
- GUTIERREZ, MF., GAGNETEN, AM. and PAGGI, JC. 2010. Copper and Chromium alter life cycle variables and the equiproportional development of the freshwater copepod Notodiaptomus conifer (SARS). Water, Air, & Soil Pollution, vol. 213, p. 275-286. http://dx.doi.org/10.1007/s11270-010-0383-3
- HAMILTON, MA., RUSSO, RC. and THURSTON, RV. 1977. Trimmed Spearman-Karber method for estimating median lethal concentrations in toxicity bioassays. Environmental Science & Technology, vol. 11, p. 714-719. http://dx.doi.org/10.1021/es60130a004
- HE, X. and WANG, W-X. 2008. Stoichiometric regulation of carbon and phosphorus in P. deficient Daphnia magna. Limnology and Oceanography, vol. 53, p. 244-254. http://dx.doi.org/10.4319/lo.2008.53.1.0244
- KEEN, R. and BAILLOD, CR. 1985. Toxicity to Daphnia of the end products of wet oxidation of phenol and substituted phenols. Water Research, vol. 19, p. 767-772. http://dx.doi.org/10.1016/0043-1354(85)90125-3
- KÜHN, R., PATTARD, M., PERNAK, K-D. and WINTER, A. 1989. Results of the harmful effects of selected water pollutants (Anilines, phenols, aliphatic compounds) to Daphnia magna. Water Research, vol. 23, p. 495-499. http://dx.doi.org/10.1016/0043-1354(89)90141-3
- LASIER, PJ., WINGER, PV. and BOGENRIEDER, KJ. 2000. Toxicity of manganese to Ceriodaphnia dubia and Hyalella azteca. Archives of Environmental Contamination and Toxicology, vol. 38, n. 3, p. 298-304.
- LEMOS, CT. and ERDTMANN, B. 2000 Cytogenetic evaluation of aquatic genotoxicity in human cultured lymphocytes. Mutation Research, vol. 467, p. 1-9.
- MARTIN, TKT. and WEN-XIONG, W. 2005. Multigenerational Acclimation of Daphnia magna to Mercury: Relationships Between Biokinetics and Toxicity. Environmental Toxicology and Chemistry, vol. 24, n. 11, p. 2927-2933.
- MEJÍA-SAAVEDRA, J., SÁNCHEZ-ARMASS, S., SANTOS-MEDRANO, GE., GONZÁLES-AMARO, R., RAZO-SOTO, I., RICO-MARTÍNEZ, R. and DÍAZ-BARRIGA, F. 2005. Effect of coexposure to ddt and manganese on freshwater invertebrates: pore water from contaminated rivers and laboratory studies. Environmental Toxicology and Chemistry, vol. 24, p. 2037-2044. http://dx.doi.org/10.1897/04-438R
- OFFEM, BO. and AYOTUNDE, EO. 2008. Toxicity of Lead to Freshwater Invertebrates (Water fleas; Daphnia magna and Cyclop sp.) in Fish Ponds in a Tropical Floodplain. Water, Air, & Soil Pollution, vol. 192, p. 39-46. http://dx.doi.org/10.1007/s11270-008-9632-0
- REIS, A., PARKER, A. and ALENCOÃO, A. 2010. Avaliação da qualidade de sedimento s em rios de montanha: um caso de estudo no norte de Portugal. Associação Portuguesa dos Recursos Hídricos, vol. 31, n. 1, p. 87-97.
- REN, Z., LI, Z., MA, M., WANG, Z. and FU, R. 2009a. Behavioral responses of Daphnia magna to stresses of chemicals with different toxic characteristics. Bulletin of Environmental Contamination and Toxicology, vol. 82, p. 310-316. http://dx.doi.org/10.1007/s00128-008-9588-1
- REN, Z-M., LI, Z-L., ZHA, J-M., RAO, K-F., MA, M., WANG, Z. and FU, R-S. 2009b. The avoidance responses of Daphnia magna to the exposure of organophosphorus pesticides in an on-line biomonitoring system. Environmental Modeling & Assessment,, vol. 14, p. 405-410.
- ROBINSON, SE., CAPPER, NA. and KLAINE, SJ. 2010. The effects of continuous and pulsed exposures of suspended clay on the survival, growth, and reproduction of Daphnia magna. Environmental Toxicology and Chemistry, vol. 29, p. 168-175. http://dx.doi.org/10.1002/etc.4
- SAFAHIEH, A., HEDAYATI, A., SAVARI, A., and MOVAHEDINIA, A. 2011. Effect of sublethal dose of mercury toxicity on liver cells and tissue of yellowfin seabream. Toxicology and Industrial Health, vol. 23, p. 583-592. http://dx.doi.org/10.1177/0748233711416951
- SUEDEL, BC., DEAVER, E. and RODGER JUNIOR, JH. 1996. Experimental factors that may affect toxicity of aqueous and sediment-bound copper to freshwater organisms. Archives of Environmental Contamination and Toxicology, vol. 30, p. 40-46. http://dx.doi.org/10.1007/BF00211327
- TEODOROVIC, I., PLANOJEVIC, I., KNEZEVIC, P., RADAK, S. and NEMET, I. 2009. Sensitivity of bacterial vs. acute Daphnia magna toxicity test to metals. Central European Journal of Biology, vol. 4, p. 482-492. http://dx.doi.org/10.2478/511535-009-0048-7
- TERRA, NR. and SCHĂFER, A. 2000. Chronic in vivo effects of zinc on Pomacea canaliculata (Lamark, 1822) (Gastropoda, Ampullariidae). Brazilian Journal of Ecology, vol. 4, p. 118-122.
- TERRA, NR., FEIDEN, IR., FACHEL, JMG., LEMOS, CT. and NUNES, EA. 2008. Ecotoxicological evaluation of sediment and water samples from Sinos River, Rio Grande do Sul, Brazil, using Daphnia magna and V79 cells. Acta Limnologica Brasiliensia, vol. 20, n. 1, p. 63-72.
- TWISS, MR. and CAMPBELL, PGC. 1995. Regeneration of trace metals from picoplankton by nanoflagellate grazing. Limnology and Oceanography, vol. 40, n. 8, p. 1418-1429.
- VAN ANHOLT, RD., SPANINGS, FAT., KNOL, AH., VAN DER VELDEN, JA. and WENDELAAR, BONGA, SE. 2002. Effects of iron sulfate dosage on the water flea (Daphnia magna Straus) and early development of carp (Cyprinus carpio L.). Archives of Environmental Contamination and Toxicology, vol. 42, p. 182-192.
- VANDENBROUCK, T., DOM, N., NOVAIS, S., SOETAERT, A., FERREIRA, ALG., LOUREIRO, S., SOARES, AMVM. and COEN, W. 2011. Nickel response in function of temperature differences: Effects at different levels of biological organization in Daphnia magna. Comparative biochemistry and physiology. Part D, Genomics & proteomics, vol. 6, p. 271-281. http://dx.doi.org/10.1016/j.cbd.2011.06.001
- VARGAS, VMF., MIGLIAVACA, SB., MELO, AC., HORN, RC., GUIDOBONO, RR., FERREIRA, ICF. and PESTANA, MHD. 2001. Genotoxicity assessment in aquatic environments under the influence of heavy metals and organic contaminants. Mutation Research, vol. 490, p. 141-158.
- VUORI, K-M. 1995. Direct and indirect effects of iron on river ecosystems. Annales Zoologici Fennici, vol. 32, p. 317-329.
- WANG, Z-S., KONG, H-N. and WU, D-Y. 2007. Reproductive toxicity of dietary copper to a saltwater cladoceran, Moina monogolica daday. Environmental Toxicology & Chemistry, vol. 26, n. 1, p. 126-131.
- WELTENS, R., GOOSSENS, R. and PUYMBROECK, SV. 2000. Ecotoxicity of contaminated suspended solids for filter feeders (Daphnia magna). Archives of Environmental Contamination and Toxicology, vol. 39, p. 315-323.
- YU, RQ. and WANG, W X. 2002. Trace metal assimilation and release budget in Daphnia magna. Limnology and Oceanography, vol. 47, n. 2, p. 495-504.
Publication Dates
-
Publication in this collection
10 Sept 2013 -
Date of issue
Mar 2013
History
-
Received
13 Sept 2012 -
Accepted
03 May 2013