Abstracts
AIM: in this study we present data from the diet of Astyanax vermilion which were used as a tool to compare two sites of streams with different vegetation cover in Ribeirão Limoeiro, Cachoeira River Basin, BA. METHODS: Four samples were taken (March, June, September and December) using electrofishing as the collection method in two contiguous sites of the headwaters: stretch without riparian vegetation and with riparian vegetation. RESULTS: The qualitative composition of the diet was analyzed by the method of frequency of occurrence. The allochthonous resources of vegetal origin made up the bulk of the diet in the stretch without riparian vegetation with a frequency of occurrence of 64%. In the section with riparian vegetation allochthonous resources of animal origin made up the bulk of the diet with a frequency of 62%, which emphasizes the importance of food items from the surrounding environment. The mean Shannon diversity index, calculated from the frequency of occurrence of food items was significantly different (p = 0.04) when comparing reach deforested (H'= 1.44) with reach forested (H'= 1.80). The average weight of stomachs in the deforested reach (WS D = 0.18g) was significantly higher than that of the forested reach (WS F = 0.14g). CONCLUSIONS: in the stretch with riparian vegetation, the food diversity was greater and the fish that are in the riparian stretch do not need as much food to satisfy their physiological needs. These results underscore the importance of the type of riparian vegetation as a food source for fish
headwater environments; lotic systems; food resources; special dependence
OBJETIVO: nesse trabalho utilizamos informações sobre a alimentação de Astyanax vermilion como ferramenta para comparar dois trechos de riachos com diferentes coberturas vegetais, no Ribeirão Limoeiro, bacia do Rio Cachoeira, BA. MÉTODOS: foram realizadas quatro amostragens (março, junho, setembro e dezembro) utilizando-se a pesca elétrica como método de coleta em dois trechos de cabeceira contíguos: trecho sem vegetação ripária e com vegetação ripária. A composição qualitativa da dieta foi analisada por meio do método de freqüência de ocorrência. RESULTADOS: os recursos alóctones de origem vegetal compuseram a maior parte da dieta no trecho sem mata ripária, com 64% em freqüência de ocorrência; no trecho com mata ciliar, os recursos alóctones de origem animal compuseram a maior parte da dieta, com 62% de ocorrência, o que ressalta a importância do alimento proveniente do ambiente no entorno. O valor médio do índice de diversidade de Shannon, calculado a partir da freqüência de ocorrência dos itens alimentares, foi significativamente diferente (p = 0,007) quando se comparou o trecho sem mata (H' = 1,44) e com mata (H' = 1,80). O peso médio dos estômagos no trecho sem mata (PeD = 0,18g) foi significativamente maior que aqueles provenientes do trecho com mata (PeF = 0,14g). CONCLUSÕES: no trecho com mata ciliar a diversidade alimentar é maior e os peixes que estão no trecho sem mata ciliar necessitam de maior quantidade de alimento para satisfazer suas necessidades fisiológicas. Estes resultados acentuam a importância do tipo de vegetação ripária como fonte de alimento para os peixes.
ambiente de cabeceira; sistemas lóticos; recursos alimentares; dependência espacial
Variation in the diet of a small characin according to the riparian zone coverage in an Atlantic Forest stream, northeastern Brazil
Variação na dieta de um pequeno Characidae de acordo com a cobertura da mata ripária em um riacho da Mata Atlântica, nordeste do Brasil
Márcia Emília de Jesus TrindadeI; Alexandre PeressinII; Maurício CetraIII; Ricardo Jucá-ChagasIV
IInstituto de Desenvolvimento Sustentável Mamirauá IDSM, Estrada do Bexiga, 2584, Fonte Boa, CEP 69470-000, Tefé, AM, Brazil e-mail: mejtrindade@hotmail.com
IIPrograma de Pós-graduação em Diversidade Biológica e Conservação, Universidade Federal de São Carlos UFSCar, Rod. João Leme dos Santos, Km 110, SP 264, CEP 18052-780, Sorocaba, SP, Brazil e-mail: alex_peressin@yahoo.com.br
IIIDepartamento de Ciências Ambientais, Universidade Federal de São Carlos UFSCar, Rod. João Leme dos Santos, Km 110, SP 264, CEP 18052-780, Sorocaba, SP, Brazil e-mail: mcetra@ufscar.br
IVUniversidade Estadual do Sudoeste da Bahia UESB, Rua José Moreira Sobrinho, s/n, Jequiezinho, CEP 45200-000, Jequié, BA, Brazil e-mail: rjchagas@hotmail.com
ABSTRACT
AIM: in this study we present data from the diet of Astyanax vermilion which were used as a tool to compare two sites of streams with different vegetation cover in Ribeirão Limoeiro, Cachoeira River Basin, BA.
METHODS: Four samples were taken (March, June, September and December) using electrofishing as the collection method in two contiguous sites of the headwaters: stretch without riparian vegetation and with riparian vegetation.
RESULTS: The qualitative composition of the diet was analyzed by the method of frequency of occurrence. The allochthonous resources of vegetal origin made up the bulk of the diet in the stretch without riparian vegetation with a frequency of occurrence of 64%. In the section with riparian vegetation allochthonous resources of animal origin made up the bulk of the diet with a frequency of 62%, which emphasizes the importance of food items from the surrounding environment. The mean Shannon diversity index, calculated from the frequency of occurrence of food items was significantly different (p = 0.04) when comparing reach deforested (H'= 1.44) with reach forested (H'= 1.80). The average weight of stomachs in the deforested reach (WSD = 0.18g) was significantly higher than that of the forested reach (WSF = 0.14g).
CONCLUSIONS: in the stretch with riparian vegetation, the food diversity was greater and the fish that are in the riparian stretch do not need as much food to satisfy their physiological needs. These results underscore the importance of the type of riparian vegetation as a food source for fish.
Keywords: headwater environments, lotic systems, food resources, special dependence.
RESUMO
OBJETIVO: nesse trabalho utilizamos informações sobre a alimentação de Astyanax vermilion como ferramenta para comparar dois trechos de riachos com diferentes coberturas vegetais, no Ribeirão Limoeiro, bacia do Rio Cachoeira, BA.
MÉTODOS: foram realizadas quatro amostragens (março, junho, setembro e dezembro) utilizando-se a pesca elétrica como método de coleta em dois trechos de cabeceira contíguos: trecho sem vegetação ripária e com vegetação ripária. A composição qualitativa da dieta foi analisada por meio do método de freqüência de ocorrência.
RESULTADOS: os recursos alóctones de origem vegetal compuseram a maior parte da dieta no trecho sem mata ripária, com 64% em freqüência de ocorrência; no trecho com mata ciliar, os recursos alóctones de origem animal compuseram a maior parte da dieta, com 62% de ocorrência, o que ressalta a importância do alimento proveniente do ambiente no entorno. O valor médio do índice de diversidade de Shannon, calculado a partir da freqüência de ocorrência dos itens alimentares, foi significativamente diferente (p = 0,007) quando se comparou o trecho sem mata (H' = 1,44) e com mata (H' = 1,80). O peso médio dos estômagos no trecho sem mata (PeD = 0,18g) foi significativamente maior que aqueles provenientes do trecho com mata (PeF = 0,14g).
CONCLUSÕES: no trecho com mata ciliar a diversidade alimentar é maior e os peixes que estão no trecho sem mata ciliar necessitam de maior quantidade de alimento para satisfazer suas necessidades fisiológicas. Estes resultados acentuam a importância do tipo de vegetação ripária como fonte de alimento para os peixes.
Palavras-chave: ambiente de cabeceira, sistemas lóticos, recursos alimentares, dependência espacial.
1. Introduction
Rivers and streams are being greatly altered by degradation of riparian vegetation, erosion, siltation, chemical and domestic sewage pollution, removal of pebbles and sand, and deforestation (Lambin et al., 2003). This includes streams located in the Atlantic Forest (Menezes et al., 2007). These human actions generate great environmental impacts for stream structure (Lorion and Kennedy, 2009) and ichthyofauna (Casatti et al., 2012), making it difficult to maintain the integrity of these ecosystems (Ferreira and Casatti, 2006; Casatti et al., 2006). These alterations are particularly critical because, in headwater streams, the availability of resources, especially food, depends mostly on the surrounding environment, which is constantly providing organic and inorganic matter (Benstead et al., 2003; Pusey and Arthington, 2003; Allan, 1995; Lorion and Kennedy, 2009). Furthermore, leaves and branches, originating from riparian forest, serve as substrate for the development of microorganisms taken in as food by invertebrates and fishes (Pusey and Arthington, 2003). Thus, riparian cover has an important effect on the feeding ecology of stream fishes (Wootton, 1990). As the contribution of autochthonous items increases with the order of the streams, in low order stretches, the contribution of allochthonous material is very important for the ichthyofauna, providing the starting point for the food chain (Vannote et al., 1980), through allochthonous resources (Rezende and Mazzoni, 2005). In these water systems, primary production and food chains could not be sustained without the input of allochthonous resources (Braga and Gomiero, 2009).
Besides providing food resources, the riparian zone has other important functions for the fish species in river and stream ecosystems: it plays a mitigating role, filtering sediments from human activities; it serves as shelter for ichthyofauna (Angermeier and Karr, 1983); it influences the geomorphological process, especially the maintenance of the stream structure (Brooks et al., 2004; Sweeney et al., 2004); it provides shade and cover; it maintains the quality of the water (Growns and Gehrke, 2003). When the riparian vegetation or the morphology of the river channel is modified, the aquatic biota become affected by high sediment transport, rise in temperature and disruption of the food chain (Dufech et al., 2003), causing the disappearance of some species from certain sites (Melo et al., 2004).
Studies on the feeding of different species of Characidae show that species of Astyanax usually feed on items of both plant and animal origin and therefore have an omnivorous diet (Ferreira, 2007; Mazzoni et al., 2010). It is known that species of Characidae have shown important changes in their feeding habits as a response to environmental fluctuations and shortages of their preferential food items (Casatti et al., 2001). According to several authors (Bojsen and Barriga, 2002; Casatti et al., 2009) alterations of feeding habits and tactics can be related to altered environmental conditions.
As well as these negative impacts, streams are also suffering great antropic impacts, like riparian forest deforestation. Given the known dependency relationship between stream ecosystems and adjacent riparian areas (Naiman and Décamps, 1997), it is necessary to develop strategies for maintaining the ecological integrity of tropical stream ecosystems (Lorion and Kennedy, 2009). In this context, this study intends to compare the diet of Astyanax vermilion Zanata and Camelier, 2009 (Zanata and Camelier, 2009) in two reaches with different riparian coverage.
2. Material and Methods
2.1. Study area
The Cachoeira River Basin is located in the southeast of the state of Bahia between the coordinates 14º 42' and 15º 20' S and 39º 01' and 40º 09' W. It is limited by the Contas and Almada River basins to the north, the Pardo and Una River basins to the south, the Pardo River basin to the west and its eastern limit is the Atlantic Ocean. Cachoeira River is about 50 km long and its main tributaries are the Colônia, Salgado and Piabanhas Rivers. The drainage area of the basin is 4,600 km2 with a population of approximately 600,000 inhabitants, mostly cattle and Theobroma cacao cocoa farmers, distributed among 12 municipalities: Firmino Alves, Floresta Azul, Jussari, Itajú do Colônia, Ibicaraí, Ilhéus, Itabuna, Itapé, Itapetinga, Itororó, Lomanto Junior and Santa Cruz da Vitória. The Cachoeira River begins in the Serra de Ouricana at an altitude of 800 m in the city of Itororó where it is called Colônia River. It meets the Salgado River and reaches its lowest level in the coastal city of Ilhéus (Torres et al., 2001).
The Cachoeira River basin is inserted into the type Af climatic band, typical of tropical forests, with an annual precipitation that exceeds 1,000 mm and is well distributed throughout the year, and an average temperature of 24 ºC. It also flows through a transition zone of the type Am climate, characterized by the occurrence of a dry season in August-September, compensated by high total rainfall and high monthly average temperatures and, in the west, it runs through a typical area of semi-humid tropical climate near the plateau region of Vitória da Conquista, with annual rainfall of 800 mm (Schiavetti et al., 2005).
The Ribeirão Limoeiro is a small permanent tributary, about 11 km long, located near to the Floresta Azul municipality. The sampling sites were defined according to their position in the hydrographic basin and their accessibility. The deforested reach (D) (i.e. without riparian vegetation) (14º 57' 57.6" S and 39º 41' 42.9" W) has rapid and shallow waters due to the steep terrain and only few ponds. The channel is only slightly sinuous. The area has no riparian forest and is surrounded by pastures. The substrate is predominantly sandy composed of gravel, pebbles and rocks that are exposed during the course of the river. Its depth varies from 0.10 to 0.33 m, its width between 0.72 to 3.11 m and its average water surface velocity is 0.50 ms1. The forested reach (F) (i.e. with riparian vegetation) (14º 57' 56.5" S and 39º 41' 49.7" W) is a headwater environment, sinuous, with rapid and shallow waters due to the steep slope, a few ponds, especially on the meanders or after any obstacles. The riparian forest is preserved. The substrate is predominantly rocky composed of gravel, pebbles and rocks that are exposed during the course of the river, with submerged logs and branches and leaf litter from the marginal vegetation. Its depth varies from 0.12 to 0.67 m, its width from 0.71 to 2.85 m and its mean surface water velocity is 0.47 ms1 (Figure 1).
2.2. Sampling processing and data analyses
Four samples of fish were taken in 2007 (March, June, September and December) using electrofishing. The sampling activities were started in the morning usually from 10:00 AM to 16:00 PM. The specimens collected were fixed, still in the field, in aqueous 10% formaldehyde and later transferred in the laboratory to a container with aqueous ethyl alcohol 70%.
Stomachs were removed from 80 individuals (i.e. 10 stomachs of each sample) of Astyanax vermilion of similar size. The items were identified with the aid of a stereomicroscope to the lowest taxonomic level possible according to specialized literature (Stehr, 1987, 1991). The stomach contents were analyzed according to the method of frequency of occurrence (Hyslop, 1980). We recorded the number of stomachs in which each item occurred, obtaining the percentage of total recorded items, calculated using the following formula (Equação 1):
where: ni = number of stomachs in the sample containing the food item i, nt = total number of stomachs with contents in the sample.
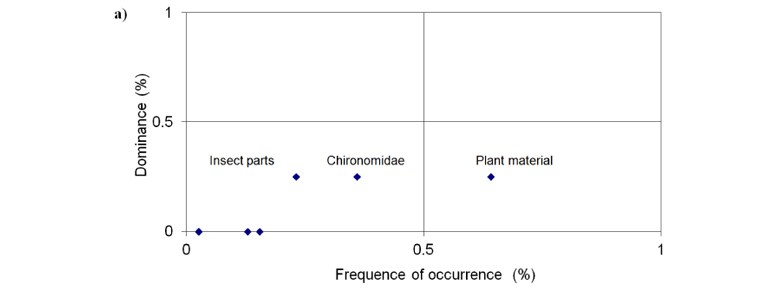
In order to identify the most important food item we used a graphical visualization of the relationship between the frequency of occurrence and the dominance of food items. Dominance is given as the number of times the item occupies most of stomach contents divided by the total number of samples (Bennemann et al., 2006).
To determine whether the diversity of the diet of Astyanax vermilion differed significantly between reaches deforested and forested, we applied a randomization paired t-test. In a randomization the factor levels are repeatedly shuffled so as to yield a probability distribution for the paired t-test to the sample data and do not have any distributional assumptions. Randomization tests examine whether the sample patterns could have occurred by chance and do not pertain to populations (Logan, 2010). The dependent variable was the Shannon diversity index, Pielou equitability index and Margalef richness index, calculated from the frequency of occurrence of food items.
To verify the existence of a significant difference in the average weight of the stomach in different environmental conditions, we applied an ANCOVA in which the total weight of the individuals was used as a covariate. We do not use the classic condition factor due to the fact that total weight and average weight of the stomach are not independent variables (Cetra, 2005).
3. Results
Of the 80 analyzed stomachs of Astyanax vermilion only two were empty. There was a significant difference between the Shannon diversity index values of deforested and forested reaches (p = 0.04). The equitability was the component of the diversity that promoted this difference (p = 0.028), once the richness index was statistically equal (p = 0.07) (Table 1).
Plant material (64%), Chironomidae (36%) and insect debris (23%) were the most frequent items at deforested reach, while at forested reach the items Hymenoptera (Formicidae) (62%), Coleoptera (adult) (31%) and insect debris (23%) had the higher frequencies of occurrence. The diet of Astyanax vermilion at forested reach was quite varied when compared to the diet at deforested reach with items such as Hymenoptera (wasp), Ephemeroptera (larvae), Lepidoptera (larvae), Trichoptera (larvae), Araneae, Nematoda, Odonata and Coleoptera, that only occurred at forested reach (Table 2).
The graphical analysis demonstrated that the dominant food items at deforested reach were insect parts, Chironomidae and plant material, with the plant material occurring at a higher frequency than the others (Figure 2a). At forested reach insect parts, Coleoptera (adult) and Hymenoptera (Formicidae) were the dominant items, with Hymenoptera (Formicidae) occurring at a higher frequency than the others, throughout the study period (Figure 2b).
There was also a significant difference (p = 0.016) between the average weight of the stomachs of fish caught in deforested reach (WSD = 0.18 g; sd = 0.09 g) compared to those from forested reach (WSF = 0.14 g; sd = 0.05 g).
4. Discussion
The greater diversity of food items in forested reach, as evidenced by the higher value of the Shannon index, reflects the input of sources from the riparian vegetation, mainly terrestrial insects, whose participation in deforested reach is reduced substantially. This dependence of streams on allochtonous items from the riparian vegetation has been widely reported in the literature (Deus and Petrere Junior, 2003; Goulding, 1980; Lowe-McConnell, 1975; Roque et al., 2003; Sabino and Castro, 1990). The removal of the plant cover causes an increase in the incidence of light and nutrient load, triggering eutrophication of water and increase in primary productivity (Bunn et al., 1997; Luiz et al. 1998, Tundisi and Tundisi, 2008). Thus, the food items available for fish are altered, with reduced quantities of terrestrial insects and an increase in the amount of algae, macrophytes and debris.
The presence of material of plant origin and a large variety of invertebrates, especially insects, are evidence for the omnivorous diet of Astyanax vermilion, that has a tendency towards insectivory, a condition that has been widely reported for species of the genus (Casatti et al., 2012; Castro and Casatti, 1997; Ferreira, 2007; Luiz et al., 1998).
The composition of the diet of this species changes when areas with and without surrounding vegetation are compared, indicating that the species displays an opportunistic behavior. This adaptation to adverse environmental conditions is common in small characids with nektonic habits (Dufech et al., 2003, Rezende and Mazzoni, 2005; Ceneviva-Bastos and Casatti, 2007; Mazzoni et al., 2010), possibly as a result of a series of morphological and behavioral adaptations (Ferreira et al., 2012).
The changes in the diet of a species according to the degradation of the surrounding vegetation may occur directly, by reducing the amount of food items that are loaded into the system, or indirectly, as a consequence of changes in the channel or in the penetration of light (Esteves and Aranha, 1999; Hicks, 1997).
Nektonic characids have laterally compressed bodies, eyes in a lateral position, are visually oriented, and active swimmers and foragers of the water column and surface (Casatti and Castro, 2006). Thus, changes caused by sediment loading, such as changes in depth and increased turbidity, can affect the search and finding of prey, influencing the diet of these fish (Ferreira et al., 2012). Ferreira et al. (2012) reported that fish with these foraging habits are constantly exposed to predation. Therefore, the camouflage provided by the riparian vegetation shade is of great importance for the species and, without it, these fish seek refuge on the banks of the streams, obtaining their food from the margins or in the substrate, which would explain the great presence of Chironomidae larvae in the diet of the fish collected in deforested reach (D).
A greater mean weight in the stomachs was registered in fish from open areas, indicating that individuals of A. vermilion collected in deforested reach consumed larger quantities of food than those collected in forested reach. This evidence suggests the need for these individuals to feed themselves in larger quantities to meet nutritional deficiencies, since the preferred food, when in the preserved area, was terrestrial insects, a scarce item in areas without riparian vegetation.
These results validate the importance of riparian vegetation in maintaining the integrity of low-order streams and show that knowledge of the food habits of stream fishes can be used as an important tool for providing information in studies of punctual impacts.
Acknowledgements
We are grateful to PPGDBC/UFSCar-Sorocaba for the financial support to translate this manuscript.
Received: 20 March 2012
Accepted: 20 May 2013
- ALLAN, JD. 1995. Stream ecology structure and function of running waters London: Chapman and Hall. 436 p.
- ANGERMEIER, PL. and KARR, JR. 1983. Fish communities along environmental gradients in a system of tropical streams. Environmental Biology of Fishes, vol. 9, p. 117-135. http://dx.doi.org/10.1007/BF00690857
- BENNEMANN, ST., CASATTI, L. and OLIVEIRA, DC. 2006. alimentação de peixes: proposta para análise de itens registrados em conteúdos gástricos. Biota Neotropica, vol. 6, no. 2, p. 1-8.
- BENSTEAD, JP., DOUGLAS, MM. and PRINGLE, CM. 2003. Relationship of stream invertebrate communities to deforestation in eastern Madagascar. Ecological Applications, vol. 13, p. 1473-1490. http://dx.doi.org/10.1890/02-5125
- BOJSEN, BH. and BARRIGA, R. 2002. Effects of deforestation on fish community structure in Ecuadorian Amazon streams. Freshwater Biology, vol. 47, p. 2246-2260. http://dx.doi.org/10.1046/j.1365-2427.2002.00956.x
- BRAGA, FMS. and GOMIERO, LM. 2009. Alimentação de peixes na microbacia do ribeirão Grande, serra da Mantiqueira Oriental, SP. Biota Neotropica, vol. 9, no. 3, p. 208-212.
- BROOKS, AP., GEHRKE, P., JANSEN, JD. and ABBE, TB. 2004. Experimental reintroduction of woody debris on the Williams River, NSW: Geomorphic and ecological responses. River Research & Applications, vol. 20, p. 513-536. http://dx.doi.org/10.1002/rra.764
- BUNN, SE., DAVIES, PM. and KELLAWAY, DM. 1997. Contributions of sugar cane and invasive pasture grass to the aquatic food web of a tropical lowland stream. Marine and Freshwater Research, vol. 48, p. 173-179. http://dx.doi.org/10.1071/MF96055
- CASATTI, L. and CASTRO, RMC. 2006. Testing the ecomorphological hypothesis in a headwater riffles fish assemblage of the rio São Francisco, southeastern Brazil. Neotropical Ichthyology, vol. 4, no. 2, p. 203-214. http://dx.doi.org/10.1590/S1679-62252006000200006
- CASATTI, L., FERREIRA, CP. and CARVALHO, FR. 2009. Grass-dominated stream sites exhibit low fish species diversity and dominance by guppies: an assessment of two tropical pasture river basins. Hydrobiologia, vol. 632, p. 273-283. http://dx.doi.org/10.1007/s10750-009-9849-y
- CASATTI, L., LANGEANI, F. and CASTRO, RMC. 2001. Peixes de riacho do parque Estadual Morro do Diabo, bacia do alto Rio Paraná, SP. Biota Neotropica, vol. 1, no. 1, p. 1-15.
- CASATTI, L., LANGEANI, F. and FERREIRA, CP. 2006. Effects of physical habitat degradation on the stream fish assemblage structure in a pasture region. Environmental Management, vol. 38, p. 974-982. PMid:16990983. http://dx.doi.org/10.1007/s00267-005-0212-4
- CASATTI, L., TERESA, FB., GONÇALVES-SOUZA, T., BESSA, E., MANZOTTI, AR., GONÇALVES, CS. and ZENI, JO. 2012. From forests to cattail: how does the riparian zone influence stream fish? Neotropical Ichthyology, vol. 10, no. 1, p. 205-214. http://dx.doi.org/10.1590/S1679-62252012000100020
- CASTRO, RMC. and CASATTI, L. 1997. The fish fauna from a small forest stream of the upper Paraná river basin, southeastern Brazil. Ichthyological Exploration of Freshwaters, vol. 7, no. 4, p. 337-352.
- CENEVIVA-BASTOS, M. and CASATTI, L. 2007. Oportunismo alimentar de Knodus moenkhausii (Teleostei, Characidae): uma espécie abundante em riachos do noroeste do Estado de São Paulo, Brasil. Iheringia, vol. 97, p. 7-15.
- CETRA, M. 2005. Avaliação do bem estar em peixes: ANCOVA uma alternativa para estudos da relação peso-comprimento. Boletim Sociedade Brasileira de Ictiologia, Brasil, vol. 81, p. 3-5.
- DEUS, CP. and PETRERE-JUNIOR, M. 2003. Seazonal diet shifts of seven fish species in an atlantic rainforest stream in southeastern Brazil. Brazilian Journal of Biology, vol. 63, no. 4, p. 579-588. PMid:15029369.
- DUFECH, APS., AZEVEDO, MA. and FIALHO, C.B. 2003. Comparative dietary analysis of two populations of Mimagoniates rhocharis (Characidae: Glandulocaudinae) from two streams of southern Brazil. Neotropical Ichthyology, vol. 1, no. 1, p. 67-74. http://dx.doi.org/10.1590/S1679-62252003000100008
- ESTEVES, KE. and ARANHA, JMR. 1999. Ecologia trófica de peixes de riachos. In CARAMASCHI, EP., MAZZONNI, R. and PERES-NETO, PR. (Eds.). Ecologia de peixes de riachos Rio de Janeiro: PPGE-UFRJ. Série Oecologia Brasiliensis, vol. 7, p. 157-182.
- FERREIRA, KM. 2007. Biology and ecomorphology of stream fishes from the rio Mogi-Guaçu basin, Southeastern Brazil. Neotropical Ichthyology, vol. 5, no. 3, p. 311-326.
- FERREIRA, CP. and CASATTI, L. 2006. Integridade biótica de um córrego na bacia do alto Rio Paraná avaliada por meio da comunidade de peixes. Biota Neotropica, vol. 6, no. 3, p. 1-25.
- FERREIRA, A., DE PAULA, FR., FERRAZ, SFB., GERHARD, P., KASHIWAQUI, EAL., CYRINO, JEP. and MARTINELLI, LA. 2012. Riparian coverage affects diets of characids in neotropical streams. Ecology of Freshwater Fish, vol. 21, p. 12-22. http://dx.doi.org/10.1111/j.1600-0633.2011.00518.x
- GOULDING, M. 1980. The fishes and the forest: explorations in Amazon natural history. Berkeley: University of California Press. 280 p.
- GROWNS, I. and GEHRKE, C. 2003. A comparison of fish assemblages associated with different riparian vegetation types in the Hawkesbury- Nepean river system. Fisheries Management and Ecology, vol. 10, p. 209-220. http://dx.doi.org/10.1046/j.1365-2400.2003.00337.x
- HICKS, BJ. 1997. Food webs in forest and pasture streams in the Waikato region, New Zealand: a study based on analyses of stable isotopes of carbon and nitrogen, and fish gut contents. New Zealand Journal of Marine and Freshwater Research, vol. 31, p. 651-664. http://dx.doi.org/10.1080/00288330.1997.9516796
- HYSLOP, EJ. 1980. Stomach contents analysis, a review of methods and their application. Journal of Fish Biology, vol. 17, p. 411-429. http://dx.doi.org/10.1111/j.1095-8649.1980.tb02775.x
- LAMBIN, EF., GEIST, HJ. and LEPERS, E. 2003. Dynamics of land-use and land-cover change in tropical regions. Annual Review of Environment and Resources, vol. 28, p. 205-241. http://dx.doi.org/10.1146/annurev.energy.28.050302.105459
- LOGAN, M. 2010. Biostatistical design and analysis using R: a practical guide. Oxford: Wiley-Blackwell. 546 p. http://dx.doi.org/10.1002/9781444319620
- LORION, CM. and KENNEDY, BP. 2009. Riparian forest buffers mitigate the effects of deforestation on fish assemblages in tropical headwater streams. Ecological Applications, vol. 19, p. 468-479. PMid:19323203. http://dx.doi.org/10.1890/08-0050.1
- LOWE-McCONNELL, RH. 1975. Fish communities in tropical freshwater: their distribution, ecology and evolution. London: Longman. 337 p.
- LUIZ, EA., AGOSTINHO, AA., GOMES, LC. and HAHN, NS. 1998. Ecologia trófica de peixes em dois riachos da bacia do rio Paraná. Brazilian Journal of Biology, vol. 58, no. 2, p. 273-285.
- MAZZONI, R., NERY, LL. and IGLESIAS-RIOS, R. 2010. Ecologia e ontogenia da alimentação de Astyanax janeiroensis (Osteichthyes, Characidae) de um riacho costeiro do Sudeste do Brasil. Biota Neotropica, vol. 10, no. 3, p. 53-60. http://dx.doi.org/10.1590/S1676-06032010000300005
- MELO, CE., MACHADO, FA. and PINTO-SILVA, V. 2004. Feeding habits of fish from a stream in the savanna of Central Brazil, Araguaia basin. Neotropical Ichthyology, vol. 2, no. 1, p. 37-44. http://dx.doi.org/10.1590/S1679-62252004000100006
- MENEZES, NA., WEITZMAN, SH., OYAKAWA, OT., LIMA, FCT., CASTRO, RMC. and WEITZMAN, MJ. 2007. Peixes de água doce da Mata Atlântica: lista preliminar das espécies e comentários sobre conservação de peixes de água doce neotropicais. São Paulo: Museu de Zoologia, Universidade de São Paulo. 408 p.
- NAIMAN, R J. and DÉCAMPS, H. 1997. The ecology of interfaces: riparian zones. Annual Review of Ecology and Systematics, vol. 28, p. 621-658. http://dx.doi.org/10.1146/annurev.ecolsys.28.1.621
- PUSEY, B J. and ARTHINGTON, AH. 2003. Importance of the riparian zone to the conservation and management of freshwater fish: a review. Marine and Freshwater Research, vol. 54, p. 1-16. http://dx.doi.org/10.1071/MF02041
- REZENDE, CF. and MAZZONI, R. 2005. Seasonal variation in the input of allochthonous matter in an Atlantic rain forest stream, Ilha Grande-RJ. Acta Limnologica Brasiliensia, vol. 17, no. 2, p. 167-175.
- ROQUE, FO., PEPINELLI, M., FRAGOSO, EM., FERREIRA, WA., BARILLARI, PR., YOSHINAGA, MY., STRIXINO, ST., VERANI, NF. and LIMA, MIS. 2003. Ecologia de macroinvertebrados, peixes e vegetação ripária de um córrego de primeira ordem em região de cerrado do estado de São Paulo (São Carlos, SP). In HENRY, R. (Ed.). Ecótonos nas Interfaces dos Ecossistemas Aquáticos São Carlos: Rima Editora. p. 313-338.
- SABINO, J. and CASTRO, RMC. 1990. Alimentação, período de atividade e distribuição espacial dos peixes de um riacho de Floresta Atlântica (Sudeste do Brasil). Brazilian Journal of Biology, vol. 50, no. 1, p. 23-36.
- SCHIAVETTI, A., SCHILLING, AC. and OLIVEIRA, HT. 2005. Caracterização socioambiental da bacia hidrográfica do rio Cachoeira Sul da Bahia, Brasil In SCHIAVETTI, A. and CAMARGO, AFM. (Eds.). Conceitos de bacias hidrográficas teorias e aplicações. Ilhéus: Editus. 293 p.
- STEHR, FW. 1987. Immature insects Dubuque: Kendall/Hunt Publishing Company. vol. 1, 754 p.
- STEHR, FW. 1991. Immature insects Dubuque: Kendall/Hunt Publishing Company. vol. 2., 974 p.
- SWEENEY, BW., BOTT, TL., JACKSON, JK., KAPLAN, LA., NEWBOLD, JD., STANDLEY, LJ., HESSION, WC. and HORWITZ, RJ. 2004. Riparian deforestation, stream narrowing, and loss of stream ecosystem services. Proceedings of the National Academy of Sciences (USA), vol. 101, p. 14132-14137. PMid:15381768 PMCid:521096.
- TORRES, MLM., REGO, NC., GEUZ, F., LEVY, MC. and MOREAU, M. 2001. Programa de recuperação das bacias dos rios Cachoeira e Almada Diagnóstico Regional Núcleo de bacias hidrográficas da UESC, Superintendência de Recursos Hídricos do Estado da Bahia. 21 p.
- TUNDISI, JG. and TUNDISI, TM. 2008. Limnologia São Paulo: Oficina de Textos. 631 p.
- VANNOTE, RL., MINSHALL, GW., CUMMINS, KW., SEDELL, JR. and CUSHING, CE. 1980. The river continuum concept. Canadian Journal of Fisheries and Aquatic Sciences, vol. 37, p.130-137. http://dx.doi.org/10.1139/f80-017
- ZANATA, AM. and CAMELIER, P. 2009. Astyanax vermillion and Astyanax burgerai: new characid fishes (Ostariophysis: Characiformes) from Northeastern Bahia, Brazil. Neotropical Ichthyology , vol. 7, no. 2, p. 175-184. http://dx.doi.org/10.1590/S1679-62252009000200007
- WOOTTON, RL. 1990. Ecology of teleost fishes London: Chapman and Hall.
Publication Dates
-
Publication in this collection
10 Sept 2013 -
Date of issue
Mar 2013
History
-
Received
20 Mar 2012 -
Accepted
20 May 2013