Abstract:
Aim
This study verified if the macrophyte banks found in two reservoirs of small hydroelectric plants (SHPs) have a functional ecological role for the initial development of ichthyofauna. Additionally, we compared the differences in the structure of the fish assemblages along each reservoir compartment and between reservoirs.
Methods
Sampling was performed in March 2018, in lotic, intermediate, and lentic compartments of Palmeiras and Retiro reservoirs, Sapucaí-Mirim River (SP). Three distinct macrophyte banks in each stretch (triplicates), considering the most representative in terms of composition (recurrency/dominance), were sampled, resulting in 18 samples (nine per reservoir). For fish collection we used a sieve of 1 m2 of area, mesh size of 1 mm, which was manually hauled, from the boat, in the marginal aquatic vegetation (three hauls per sample). Simultaneously, we measured basic limnological parameters with a Horiba U-52 water probe, adjacent to the banks.
Results
The presence of juveniles of all fish orders found in the river, Characiformes, Siluriformes, Synbranchiformes, Gymnotiformes and Cichliformes, demonstrates that, at least in certain moment of their development cycle, the macrophyte banks are used by species with different ecological requirements. It was observed significant statistical differences in the structure of the ichthyofauna along the longitudinal axes of the reservoirs, but not between reservoirs. The same was seen for the limnological data (PCA).
Conclusions
The macrophyte banks found in the SHPs reservoirs have a potential role as nurseries for the local ichthyofauna, mainly, but not exclusively, for sedentary species and with parental care. This association is ecologically relevant, given the absence of typical lateral habitats for the initial development of the fish fauna. Additionally, despite the reservoirs small size, spatial organization was also important for the assemblage’s structure, with differences in terms of composition, size and development stages along the distinct sampling stretches.
Keywords:
reservoir cascade; ichthyofauna; juveniles; larvae; spatial organization
Resumo:
Objetivo
O presente estudo verificou se os bancos de macrófitas presentes nos reservatórios de pequenas centrais hidrelétricas desempenham um papel ecológico funcional no desenvolvimento inicial da ictiofauna. Adicionalmente, comparou-se a estrutura das assembleias de peixes ao longo dos compartimentos de cada reservatório e entre os reservatórios.
Métodos
A amostragem foi realizada em março de 2018 nos compartimentos lótico, intermediário e lêntico dos reservatórios de Palmeiras e Retiro, rio Sapucaí-Mirim (SP). Três bancos diferentes de macrófitas por trecho (tréplicas), considerando os mais representativos em termos de composição (recorrência/dominância), foram amostrados, no total de 18 amostras (nove por reservatório). Para a coleta dos peixes foi utilizada peneira de 1 m2 de área, abertura de malha de 1 mm, introduzida manualmente, da proa do barco, sob a vegetação aquática marginal e erguida rapidamente (três lances por amostra). Simultaneamente, foram medidos parâmetros limnológicos básicos, utilizando sonda Horiba U-52, adjacente aos bancos.
Resultados
A presença de estágios juvenis para todas as ordens de peixes encontradas no rio, Characiformes, Siluriformes, Synbranchiformes, Gymnotiformes e Cichliformes, demonstra que os bancos de macrófitas são utilizados, em algum momento do seu ciclo de desenvolvimento, por espécies com diferentes requerimentos ecológicos. Foram observadas diferenças estatísticas significativas na estrutura da ictiofauna no eixo longitudinal dos reservatórios, mas não entre os reservatórios. O mesmo foi verificado para os dados limnológicos (ACP).
Conclusões
Os bancos de macrófitas encontrados nos reservatórios de pequeno porte das PCHs do rio Sapucaí-Mirim são berçários potenciais para a ictiofauna, principalmente, mas não exclusivamente, para espécies sedentárias e com cuidado parental. Esta associação é ecologicamente relevante, dado a ausência de habitats laterais típicos para o desenvolvimento inicial da ictiofauna. Adicionalmente, a despeito do pequeno porte dos reservatórios, a organização espacial também se mostrou importante, com diferenças em termos de composição, tamanho e estágios de desenvolvimento ao longo dos distintos trechos de amostragem.
Palavras-chave:
cascata de reservatórios; ictiofauna; juvenis; larvas; organização espacial
1. Introduction
In Brazil, the energy matrix is still highly dependent on hydropower generation, which represents around 65% of the production (EPE, 2020EMPRESA DE PESQUISA ENERGÉTICA – EPE. Relatório síntese do balanço energético nacional – ano base 2020. Rio de Janeiro, 2020.). In this scenario, the contribution of small hydroelectric plants (SHPs) has increased, with hundreds of generation units in operation, summing 5.5 x106 kW, distributed all over the country (ANEEL, 2019AGÊNCIA NACIONAL DE ENERGIA ELÉTRICA – ANEEL. Capacidade de geração do Brasil [online]. 2019 [viewed 20 June 2019]. Available from: http://www.aneel.gov.br/siga
http://www.aneel.gov.br/siga...
). The SHPs have lower construction cost, attracting more investors; losses in the energy transportation are lower, as they usually supply nearby consumers; there is also higher feasibility to be built in smaller and widely distributed rivers; with shorter implementation times and less restrictive legislation (Sharma et al., 2013SHARMA, K.N., TIWARI, P.K. and SOOD, Y.R. A comprehensive analysis of strategies, policies and development of hydropower in India: Special emphasis on small hydro power. Renewable & Sustainable Energy Reviews, 2013, 18, 460-470. http://dx.doi.org/10.1016/j.rser.2012.10.017.
http://dx.doi.org/10.1016/j.rser.2012.10...
). Despite the benefits hydropower brings to society in terms of energy supply, river damming may cause major changes in aquatic environments, such as fragmentation, loss of original habitats, changes in the physical and chemical water conditions, in the structure and functioning of aquatic communities and in the interactions with terrestrial ecosystems (e.g. Tundisi 1999TUNDISI, J.G. Reservatórios como sistemas complexos: teoria, aplicações e perspectivas para usos múltiplos. In: R. HENRY, ed. Ecologia de reservatórios: estrutura, função e aspectos sociais. Botucatu: FUNDIBIO, 1999, pp. 19-38., 2018TUNDISI, J.G. Reservoirs: new challenges for ecosystem studies and environmental management. Water Security, 2018, 4-5, 1-7. http://dx.doi.org/10.1016/j.wasec.2018.09.001.
http://dx.doi.org/10.1016/j.wasec.2018.0...
; Agostinho et al., 2005AGOSTINHO, A.A., THOMAZ, S.M. and GOMES, L.C. Conservation of the Biodiversity of Brazil’s Inland Waters. Biological Conservation, 2005, 19(3), 646-652. http://dx.doi.org/10.1111/j.1523-1739.2005.00701.x.
http://dx.doi.org/10.1111/j.1523-1739.20...
, 2016AGOSTINHO, A.A., GOMES, L.C., SANTOS, N.C.L., ORTEGA, J.C.G. and PELICICE, F.M. Fish assemblages in Neotropical reservoirs: colonization patterns, impacts and management. Fisheries Research, 2016, 173, 26-36. http://dx.doi.org/10.1016/j.fishres.2015.04.006.
http://dx.doi.org/10.1016/j.fishres.2015...
; Soares et al., 2008SOARES, M.C.S., MARINHO, M.M., HUSZAR, V.L.M., BRANCO, C.W.C. and AZEVEDO, S.M.F.O. The effects of water retention time and watershed features on the limnology of two tropical reservoirs in Brazil. Lakes and Reservoirs: Research and Management, 2008, 13(4), 257-269. http://dx.doi.org/10.1111/j.1440-1770.2008.00379.x.
http://dx.doi.org/10.1111/j.1440-1770.20...
; Nogueira et al., 2012NOGUEIRA, M.G., PERBICHE-NEVES, G. and NALIATO, D.A.O. Limnology of two contrasting hydroelectric reservoirs (storage and run-of-river) in southeast Brazil. In: H.S. BOROUGENI, ed. Hydropower: practice and application. Rijeka: Intech, 2012, pp. 167-784.). Most studies are focused on large hydropower reservoirs and the environmental impacts generated by SHPs in Brazil are still scarce (Ruocco et al., 2019RUOCCO, A.M.C., PORTINHO, J.L. and NOGUEIRA, M.G. Potential impact of small hydroelectric power plants on river biota: a case study on macroinvertebrates associated to basaltic knickzones. Brazilian Journal of Biology = Revista Brasileira de Biologia, 2019, 79(4), 722-734. http://dx.doi.org/10.1590/1519-6984.191148. PMid:30088529.
http://dx.doi.org/10.1590/1519-6984.1911...
).
Distinct hydrological and limnological characteristics, from the original fluvial conditions, including physical compartmentalization, are the main determinants for the restructuring of the aquatic biota in reservoirs (Agostinho et al., 2008AGOSTINHO, A.A., PELICICE, F.M. and GOMES, L.C. Dams and the fish fauna of the Neotropical region: impacts and management related to diversity and fisheries. Brazilian Journal of Biology = Revista Brasileira de Biologia, 2008, 68(4), 1119-1132, Supplement. http://dx.doi.org/10.1590/S1519-69842008000500019. PMid:19197482.
http://dx.doi.org/10.1590/S1519-69842008...
; Nogueira et al., 2008NOGUEIRA, M.G., OLIVEIRA, P.C.R. and DE BRITTO, Y.T. Zooplankton assemblages (Copepoda and Cladocera) in a cascade of reservoirs of a large tropical river (SE Brazil). Limnetica, 2008, 27, 151-170.; 2010NOGUEIRA, M.G., FERRAREZE, M., MOREIRA, M.L. and GOUVÊA, R.M. Phytoplankton assemblages in a reservoir cascade of a large tropical - subtropical river (SE, Brazil). Brazilian Journal of Biology = Revista Brasileira de Biologia, 2010, 70(3), 781-793, Supplement. http://dx.doi.org/10.1590/S1519-69842010000400009. PMid:21085783.
http://dx.doi.org/10.1590/S1519-69842010...
). Despite the lack of information, such longitudinal organizational pattern may also occur in SHP reservoirs. Another important aspect to be considered are the effects of reservoirs arranged in sequence – cascade systems, when several dams are present in the same river (Nogueira & Pomari, 2019NOGUEIRA, M.G. and POMARI, J. Limnological patterns in a large subtropical reservoir cascade. In: D. GOKCE, ed. Limnology: some new aspects of inland water ecology. Rijeka: IntechOpen, 2019, pp. 96-129. http://dx.doi.org/10.5772/intechopen.80632.
http://dx.doi.org/10.5772/intechopen.806...
). The effects of dam’s sequences on fish assemblages depend on the position of the reservoirs in the river basin (Ferrareze et al., 2014FERRAREZE, M., CASATTI, L. and NOGUEIRA, M.G. Spatial heterogeneity affecting fish fauna in cascade reservoirs of the Upper Paraná Basin, Brazil. Hydrobiologia, 2014, 738(1), 97-109. http://dx.doi.org/10.1007/s10750-014-1922-5.
http://dx.doi.org/10.1007/s10750-014-192...
; Loures & Pompeu, 2018LOURES, R.C. and POMPEU, P.S. Long-term study of reservoir cascade in south-eastern Brazil reveals spatio-temporal gradient in fish assemblages. Marine and Freshwater Research, 2018, 69(12), 1983-1994. http://dx.doi.org/10.1071/MF18109.
http://dx.doi.org/10.1071/MF18109...
; Pelicice et al., 2018PELICICE, F.M., AZEVEDO-SANTOS, V.M., ESGUÍCERO, A.L.H., AGOSTINHO, A.A. and ARCIFA, M.S. Fish diversity in the cascade of reservoirs along the Paranapanema River, southeast Brazil. Neotropical Ichthyology, 2018, 16(2), 1-18. http://dx.doi.org/10.1590/1982-0224-20170150.
http://dx.doi.org/10.1590/1982-0224-2017...
). Negative impacts of river damming on fishes are notorious, even in SHP reservoirs (Arcifa & Esguícero, 2012ARCIFA, M.S. and ESGUÍCERO, A.L.H. The fish fauna in the fish passage at the Ourinhos Dam, Paranapanema River. Neotropical Ichthyology, 2012, 10(4), 715-722. http://dx.doi.org/10.1590/S1679-62252012000400004.
http://dx.doi.org/10.1590/S1679-62252012...
; Bakken et al., 2012BAKKEN, T.H., SUNDT, H., RUUD, A. and HARBY, A. Development of small versus large hydropower in Norway comparison of environmental impacts. Energy Procedia, 2012, 20, 185-199. http://dx.doi.org/10.1016/j.egypro.2012.03.019.
http://dx.doi.org/10.1016/j.egypro.2012....
). The ichthyofauna affected by SHPs may exhibit reduction in abundance, average length, total weight, and condition factor (Benejam et al., 2016BENEJAM, L., SAURA-MAS, S., BARDINA, M., SOLÀ, C., MUNNÉ, A. and GARCÍA-BERTHOU, E. Ecological impacts of small hydropower plants on headwater stream fish: From individual to community effects. Ecology Freshwater Fish, 2016, 25(2), 295-306. http://dx.doi.org/10.1111/eff.12210.
http://dx.doi.org/10.1111/eff.12210...
); interruption of the individual’s free movement along the river (Bakken et al., 2012BAKKEN, T.H., SUNDT, H., RUUD, A. and HARBY, A. Development of small versus large hydropower in Norway comparison of environmental impacts. Energy Procedia, 2012, 20, 185-199. http://dx.doi.org/10.1016/j.egypro.2012.03.019.
http://dx.doi.org/10.1016/j.egypro.2012....
; Kucukali, 2014KUCUKALI, S. Environmental risk assessment of small hydropower (SHP) plants: a case study for Tefen SHP plant on Filyos River. Energy for Sustainable Development, 2014, 19, 102-110.) and changes in ichthyoplankton dispersion (Suzuki et al., 2011SUZUKI, F.M., PIRES, L.V. and POMPEU, P.S. Passage of fish larvae and eggs through the Funil, Itutinga and Camargos reservoirs on the upper Rio Grande (Minas Gerais, Brazil). Neotropical Ichthyology, 2011, 9(3), 617-622. http://dx.doi.org/10.1590/S1679-62252011000300014.
http://dx.doi.org/10.1590/S1679-62252011...
; Pelicice et al., 2014PELICICE, F.M., POMPEU, P.S. and AGOSTINHO, A.A. Large reservoirs as ecological barriers to downstream movements of Neotropical migratory fish. Fish and Fisheries, 2014, 16, 1-19., Brambilla et al., 2020BRAMBILLA, E.M., SILVA, L.G.M., BAUMGARTNER, L.J., BIALETZKI, A. and NOGUEIRA, M.G. Dispersal of fish eggs and larvae in a cascade of small hydropower plants with fish ladders. Hydrobiologia, 2020. In press. http://dx.doi.org/10.1007/s10750-020-04425-5.
http://dx.doi.org/10.1007/s10750-020-044...
).
For the neotropics it has been proven the importance of lateral habitats associated with rivers and reservoirs, such as marginal lagoons and tributaries, for fish reproduction and development (e.g. Vianna & Nogueira, 2008VIANNA, N.C. and NOGUEIRA, M.G. Ichthyoplankton and limnological factors in the Cinzas River – an alternative spawning site for fishes in the middle Paranapanema River basin, Brazil. Acta Limnologica Brasiliensia, 2008, 20, 139-151.; Ferrareze & Nogueira, 2011FERRAREZE, M. and NOGUEIRA, M.G. Importance of lateral lagoons for the ichthyofauna in a large tropical reservoir. Brazilian Journal of Biology = Revista Brasileira de Biologia, 2011, 71(4), 807-820. http://dx.doi.org/10.1590/S1519-69842011000500002.
http://dx.doi.org/10.1590/S1519-69842011...
; Agostinho et al., 2016AGOSTINHO, A.A., GOMES, L.C., SANTOS, N.C.L., ORTEGA, J.C.G. and PELICICE, F.M. Fish assemblages in Neotropical reservoirs: colonization patterns, impacts and management. Fisheries Research, 2016, 173, 26-36. http://dx.doi.org/10.1016/j.fishres.2015.04.006.
http://dx.doi.org/10.1016/j.fishres.2015...
). Especially in marginal lagoons, fish larvae and juveniles find refuge from predators and availability of food resource, essential conditions for early stages survival (Orsi et al., 2016ORSI, M.L., ALMEIDA, F.S., SWARÇA, A.C., CLARO-GARCÍA, A., VIANNA, N.C., GARCIA, D.A.Z. and BIALETZKI, A. Ovos, larvas e juvenis dos peixes da Bacia do Rio Paranapanema uma avaliação para a conservação. Assis: Triunfal Gráfica e Editora, Duke Energy, 2016.). The presence of macrophyte banks, when they are not excessive in coverage and biomass (Pompêo, 2017POMPÊO, M. Monitoramento e manejo de macrófitas aquáticas em reservatórios tropicais brasileiros. São Paulo: Instituto de Biociências da USP, 2017. http://dx.doi.org/10.11606/9788585658670.
http://dx.doi.org/10.11606/9788585658670...
), increases the complexity of habitats, which contributes for the maintenance of fish diversity (Agostinho et al., 2003AGOSTINHO, A.A., GOMES, L.C. and JÚLIO, J.H. Relações entre macrófitas aquáticas e fauna de peixes. In: S.M. THOMAZ & L.M. BINI, eds. Ecologia e manejo de macrófitas aquáticas. Maringá: EDUEM, 2003, pp. 261-279.; 2007AGOSTINHO, A.A., THOMAZ, S.M., GOMES, L.C. and BALTAR, S.L.S.M.A. Influence of Eichhornia azurea on fish assemblages of the Upper Paraná River Floodplain (Brazil). Aquatic Ecology, 2007, 41(4), 611-619. http://dx.doi.org/10.1007/s10452-007-9122-2.
http://dx.doi.org/10.1007/s10452-007-912...
; Pelicice et al., 2008PELICICE, F.M., THOMAZ, S.M. and AGOSTINHO, A.A. Simple relationships to predict attributes of fish assemblages in patches of submerged macrophytes. Neotropical Ichthyology, 2008, 6(4), 543-550. http://dx.doi.org/10.1590/S1679-62252008000400001.
http://dx.doi.org/10.1590/S1679-62252008...
; Dibble & Pelicice, 2010DIBBLE, E.D. and PELICICE, F.M. Influence of aquatic plant-specific habitat on an assemblage of small neotropical floodplain fishes. Ecology Freshwater Fish, 2010, 19(3), 381-389. http://dx.doi.org/10.1111/j.1600-0633.2010.00420.x.
http://dx.doi.org/10.1111/j.1600-0633.20...
; Hermes-Silva & Zaniboni-Filho, 2012HERMES-SILVA, S. and ZANIBONI-FILHO, E. Structure of the littoral fish assemblage in an impounded tributary: the effects of macrophytes presence (subtropical region, Brazil). Brazilian Journal of Biology = Revista Brasileira de Biologia, 2012, 72(3), 489-495. http://dx.doi.org/10.1590/S1519-69842012000300011. PMid:22990819.
http://dx.doi.org/10.1590/S1519-69842012...
). Nevertheless, the role of aquatic plants for the fish fauna in SHPs reservoirs is still poorly investigated. In some SHPs reservoirs, due to their small size and simplified morphometry, ecological functional connectivity with lateral environments may not properly exist. In this case, the occurrence of certain habitats, such as the aquatic macrophyte banks, can ensure the initial survival and consequently the regional maintenance of several fish species (Kirjasniemi & Valtonen, 1997KIRJASNIEMI, M. and VALTONEN, T. Size-dependent over-winter mortality of young- of-the-year roach, Rutilus rutilus. Environmental Biology of Fishes, 1997, 50(4), 451-456. http://dx.doi.org/10.1023/A:1007302931943.
http://dx.doi.org/10.1023/A:100730293194...
; Wilzbach et al., 2002WILZBACH, M.A., CUMMINS, K.W., BARNES, T.K. and TREXLER, J.C. Channel- floodplain coupling in the Kisimmee River, Florida (USA): invertebrate movement and fish feeding. Verhandlungen - Internationale Vereinigung für Theoretische und Angewandte Limnologie, 2002, 28(1), 164-172. http://dx.doi.org/10.1080/03680770.2001.11902567.
http://dx.doi.org/10.1080/03680770.2001....
; Bulla et al., 2011BULLA, C.K., GOMES, L.C., MIRANDA, L.E. and AGOSTINHO, A.A. The ichthyofauna of drifting macrophyte mats in the Ivinhema River, upper Paraná River basin. Neotropical Ichthyology, 2011, 9(2), 403-409. http://dx.doi.org/10.1590/S1679-62252011005000021.
http://dx.doi.org/10.1590/S1679-62252011...
; Hermes-Silva & Zaniboni-Filho, 2012HERMES-SILVA, S. and ZANIBONI-FILHO, E. Structure of the littoral fish assemblage in an impounded tributary: the effects of macrophytes presence (subtropical region, Brazil). Brazilian Journal of Biology = Revista Brasileira de Biologia, 2012, 72(3), 489-495. http://dx.doi.org/10.1590/S1519-69842012000300011. PMid:22990819.
http://dx.doi.org/10.1590/S1519-69842012...
).
Two SHP’s reservoirs were selected in our study, located in Sapucaí-Mirim River, north of São Paulo State, Brazil. This river basin has a diverse fish fauna, with at least 105 species (Oliveira et al., 2016OLIVEIRA, A.K., GARAVELLO, J.C., CESARIO, V.V. and CARDOSO, R.T. Fish fauna from Sapucaí-Mirim River, tributary of Grande River, upper Paraná River basin, Southeastern Brazil. Biota Neotropica, 2016, 16(1), 1-9. http://dx.doi.org/10.1590/1676-0611-BN-2014-0192.
http://dx.doi.org/10.1590/1676-0611-BN-2...
; Brambilla et al., 2019BRAMBILLA, E.M., UIEDA, V.S. and NOGUEIRA, M.G. Does seasonality and pool connectivity influence fish trophic ecology in knickzone habitats? Studies on Neotropical Fauna and Environment, 2019, 54(1), 22-30. http://dx.doi.org/10.1080/01650521.2018.1518118.
http://dx.doi.org/10.1080/01650521.2018....
; Diniz et al., 2019DINIZ, P.B., SIQUEIRA, H.D.O., FALEIROS, T.D.O., PEREIRA, N.L., SENHORINI, J.A., ESGUÍCERO, A.L.H. and BERTELLI, C. Fishes from lakes and tributaries of the Rio Santa Bárbara, Sapucaí Mirim/Grande hydrographic basin, São Paulo, Brazil. Check List, 2019, 15(4), 629-640. http://dx.doi.org/10.15560/15.4.629.
http://dx.doi.org/10.15560/15.4.629...
), and it is an important direct tributary of the Grande River, upper Paraná Basin. The fish fauna is mainly represented by small and medium-size species, with only three large-sized migrators (Oliveira et al., 2016OLIVEIRA, A.K., GARAVELLO, J.C., CESARIO, V.V. and CARDOSO, R.T. Fish fauna from Sapucaí-Mirim River, tributary of Grande River, upper Paraná River basin, Southeastern Brazil. Biota Neotropica, 2016, 16(1), 1-9. http://dx.doi.org/10.1590/1676-0611-BN-2014-0192.
http://dx.doi.org/10.1590/1676-0611-BN-2...
). A recent study, based on ichthyoplankton analyzes, provided consistent information about the presence of quantitatively important spawning sites, even for migratory species, in the Sapucaí-Mirim SHP reservoirs (Brambilla et al., 2020BRAMBILLA, E.M., SILVA, L.G.M., BAUMGARTNER, L.J., BIALETZKI, A. and NOGUEIRA, M.G. Dispersal of fish eggs and larvae in a cascade of small hydropower plants with fish ladders. Hydrobiologia, 2020. In press. http://dx.doi.org/10.1007/s10750-020-04425-5.
http://dx.doi.org/10.1007/s10750-020-044...
). Authors concluded that the reproductive products drift downstream, passing through the dam’s physical structures, including the fish-ladders. However, it is unknown if recruitment processes are taking place along the dammed stretches, given the absence of typical nursery areas, such as lagoons and tributaries. A similar question was posed by Ávila-Simas et al. (2014)ÁVILA-SIMAS, S., REYNALTE-TATAJE, D.A. and ZANIBONI-FILHO, E. Pools and rapids as spawning and nursery areas for fish in a river stretch without floodplains. Neotropical Ichthyology, 2014, 12(3), 611-622. http://dx.doi.org/10.1590/1982-0224-20130116.
http://dx.doi.org/10.1590/1982-0224-2013...
, which searched for alternative spawning and nursery areas – pools and rapids, in a tributary without floodplains of the upper Uruguay River.
Based on the premise that littoral habitats are important for early fish development, the aim of this study was to verify if the macrophyte banks found in two reservoirs of small hydroelectric plants (SHPs) have a functional ecological role for the initial development of fishes. The investigation comprised the analysis of representative macrophyte banks, independent on their species composition or life-forms, distributed along the main axis of both reservoirs. We hypothesize that the aquatic plants have an important functional role as fish nursery areas. Additionally, we also searched for longitudinal differences in the structure of the plant-associated fish assemblages, as well as in the limnological variables, within each reservoir and between both reservoirs.
2. Material and Methods
2.1. Study area
The Sapucaí-Mirim River springs are located in the Atlantic Plateau (Serra da Mantiqueira), between the states of São Paulo and Minas Gerais. The river mouth is located in the left margin of Porto Colômbia Reservoir, middle stretch of Grande River (Paiva, 1982PAIVA, M.M. Grandes represas do Brasil. Brasília: Editerra, 1982.). It is a plateau river, with several rapids and small waterfalls in the upper section, becoming less turbulent with decreased water velocity in the lower section. Another distinctive characteristic of this river is the presence of several knickzones mesohabitats, bed rock (basaltic) platforms popularly known as “pedrais” (Brambilla et al., 2019BRAMBILLA, E.M., UIEDA, V.S. and NOGUEIRA, M.G. Does seasonality and pool connectivity influence fish trophic ecology in knickzone habitats? Studies on Neotropical Fauna and Environment, 2019, 54(1), 22-30. http://dx.doi.org/10.1080/01650521.2018.1518118.
http://dx.doi.org/10.1080/01650521.2018....
; Ruocco et al., 2019RUOCCO, A.M.C., PORTINHO, J.L. and NOGUEIRA, M.G. Potential impact of small hydroelectric power plants on river biota: a case study on macroinvertebrates associated to basaltic knickzones. Brazilian Journal of Biology = Revista Brasileira de Biologia, 2019, 79(4), 722-734. http://dx.doi.org/10.1590/1519-6984.191148. PMid:30088529.
http://dx.doi.org/10.1590/1519-6984.1911...
). The river total length is 290 km, with its course entirely in the State of São Paulo (Paiva, 1982PAIVA, M.M. Grandes represas do Brasil. Brasília: Editerra, 1982.).
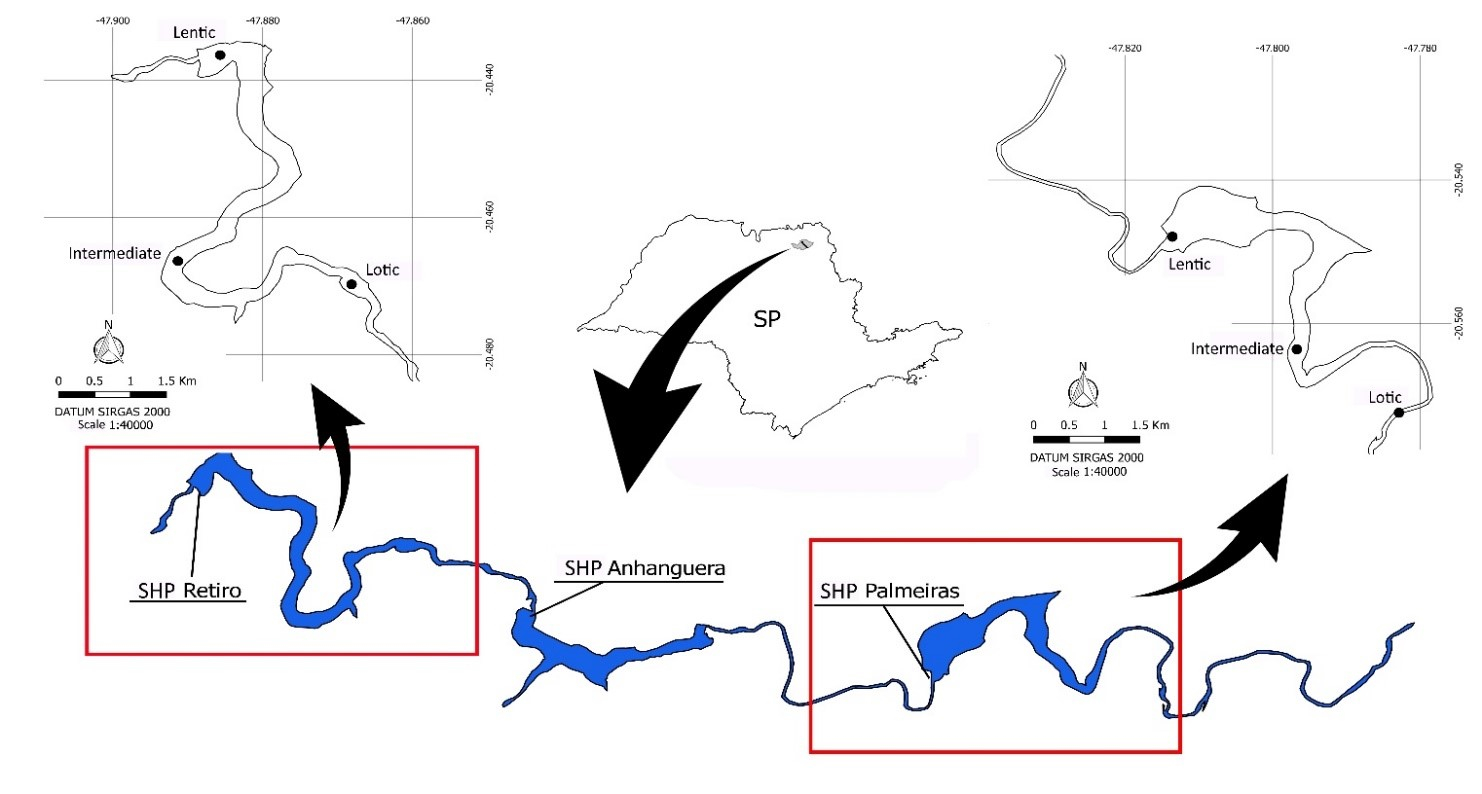
In the main channel of the Sapucaí-Mirim River there are three SHPs arranged in a reservoir cascade system, Palmeiras, Anhanguera and Retiro, which started to operate in 2011, 2009 and 2013, respectively. For this research, two reservoirs were selected, Palmeiras and Retiro, the most upstream and the most downstream in the cascade (Figure 1). The reservoirs are similar in area, Palmeiras 2.6 km2 and Retiro 2.8 km2, and despite the second has a longitudinal axis (from the lotic zone to dam) which is 1.5 times larger than the first one (11 and 7.5 km, respectively), both are considered short. The average retention time is 3.3 days for Palmeiras and 3.4 days for Retiro (Brambilla et al., 2020BRAMBILLA, E.M., SILVA, L.G.M., BAUMGARTNER, L.J., BIALETZKI, A. and NOGUEIRA, M.G. Dispersal of fish eggs and larvae in a cascade of small hydropower plants with fish ladders. Hydrobiologia, 2020. In press. http://dx.doi.org/10.1007/s10750-020-04425-5.
http://dx.doi.org/10.1007/s10750-020-044...
). These reservoirs were chosen because they belong to the same stakeholder (CTG Brazil), which allowed us the access into the area, and have similar monitoring requirements determined by the Environmental Company of São Paulo State (CETESB).
Map of the Sapucaí-Mirim River (SP, Brazil) stretch, where are located the studied Small Hydropower Plants (SHPs) reservoirs – Palmeiras and Retiro, with indication of the sampling sites.
2.2. Sampling
Sampling was performed in March 2018, at the end of the annual reproductive period (“piracema”) for most native fish species (Vazzoler, 1982VAZZOLER, A.E.A.M. Manual de métodos para estudos biológicos de populações de peixes: reprodução e crescimento. Brasília: Programa Nacional de Zoologia (CNPq), 1982.). In each lotic, intermediate, and lentic compartment of Palmeiras and Retiro reservoirs (Figure 1), three distinct macrophyte banks (nine per reservoir) were sampled in triplicates (Table 1), resulting in a total of 18 samples. The macrophyte banks were randomly chosen within each compartment, considering the recurrent/dominant species determined through visual inspection. It is important to mention that submerged macrophytes do not occur in these reservoirs, probably due to the excessive water turbidity mainly observed in the summer-rainy season. For fish collection we used a sieve of 1 m2 of area, with mesh size of 1 mm, which was manually introduced bellow the marginal aquatic vegetation and raised quickly (three times per sample). It was assumed that most fish larvae with exogenous feeding, therefore with active swimming (not in passive derive), should be captured with a 1 mm mesh size. Sampling was carried out from the forward part of the boat (bow), which was moving (engine propulsion) towards the plants. The collected material was stored in plastic bags and eugenol solution was immediately added to anesthetize the organisms. Subsequently, the samples were fixed in 4% formalin solution. Sampling was performed under the license SISBIO 13.794-1.
The main aquatic macrophytes present and the sampling points geographic coordinates in Palmeiras and Retiro reservoirs, Sapucaí-Mirim River (SP) (*exotic species).
Simultaneously to the fish samplings, the following environmental variables were measured in the water sub-surface: temperature, pH, electrical conductivity (K), turbidity, dissolved oxygen (DO) and oxide-reduction potential (ORP), with a previously calibrated Horiba U-52 multiparameter probe.
2.3. Sorting and identification
The sampled fish was sorted and transferred to polyethylene bottles with a 4% formalin solution and calcium carbonate for preservation of the bone structures. Larger fishes were immediately separated for posterior identification. The detailed sorting for larvae and small juveniles was made in laboratory using a Bogorov acrylic plate under stereo-microscope ZEISS (STEMI SV 6). The individuals captured were classified into three stages (larval, juvenile, and adult) and identified to the lowest taxonomic level possible. Individuals in larval phase were identified following Ahlstrom & Moser (1976)AHLSTROM, E.H. and MOSER, G. Eggs and larvae of fishes and their role in systematic investigations and in fisheries. Revue des Travaux de l’Institut des Pêches Maritimes, 1976, 40, 379-398. and Nakatani et al. (2001)NAKATANI, K., AGOSTINHO, A.A., BAUMGARTNER, G., BIALETZKI, A., SANCHES, P.V., MAKRAKIS, M.C. and PAVANELLI, C.S. Ovos e larvas de peixes de água doce: desenvolvimento e manual de identificação. Maringá: Eduem. 2001.. The fish in juvenile and adult stages were identified following specialized references (Castro et al., 2004CASTRO, R.M.C., CASATTI, L., SANTOS, H.F., MELO, A.L.A., MARTINS, L.S.F., FERREIRA, K.M., GIBRAN, F.Z., BENINE, R.C., CARVALHO, M., RIBEIRO, A.C., ABREU, T.X., BOCKMANN, F.A., PELIÇÃO, G.Z., STOPIGLIA, R. and LANGEANI, F. Estrutura e composição da ictiofauna de riachos da bacia do rio Grande no Estado de São Paulo, sudeste do Brasil. Biota Neotropica, 2004, 4(1), 1-39. http://dx.doi.org/10.1590/S1676-06032004000100006.
http://dx.doi.org/10.1590/S1676-06032004...
; Graça & Pavanelli, 2007GRAÇA W.J. and PAVANELLI C.S. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: EDUEM, 2007.; Langeani & Rego, 2014LANGEANI F. and REGO A.C.L. Guia ilustrado dos peixes da bacia do Rio Araguari. Uberlândia: Grupo de Mídia Brasil Central, 2014.; Ota et al., 2018OTA, R.R., DEPRÁ, G.C., GRAÇA, W.J. and PAVANELLI, C.S. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes: revised, annotated and updated. Neotropical Ichthyology, 2018, 16(2), e170094. http://dx.doi.org/10.1590/1982-0224-20170094.
http://dx.doi.org/10.1590/1982-0224-2017...
). The taxonomic framework followed Fricke et al. (2019)FRICKE, R., ESCHMEYER, W.N. and VAN DER LAAN, R. Eschmeyer’s catalog of fishes: genera, species, references [online]. 2019 [viewed 20 June 2019]. Available from: http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcat main.asp
http://researcharchive.calacademy.org/re...
.
The term “larvae” refers to the period of development between the egg hatching and the total formation of the fins (with rays) and scales (depending on the taxonomical group). The “juvenile” term refers to the period between the initial development of fin and scales and sexual maturity (Nakatani et al., 2001NAKATANI, K., AGOSTINHO, A.A., BAUMGARTNER, G., BIALETZKI, A., SANCHES, P.V., MAKRAKIS, M.C. and PAVANELLI, C.S. Ovos e larvas de peixes de água doce: desenvolvimento e manual de identificação. Maringá: Eduem. 2001.). For differentiation between juveniles and adults, it was used the length at first maturity (LS50) (Langeani & Rego, 2014LANGEANI F. and REGO A.C.L. Guia ilustrado dos peixes da bacia do Rio Araguari. Uberlândia: Grupo de Mídia Brasil Central, 2014.). Using a caliper, we obtained the total length of the animals, snout tip to the end of the caudal fin. Individuals with total length smaller than LS50 described in the literature were classified as juveniles, and individuals with total length equal to or larger than LS50 were classified as adults.
2.4. Statistical analysis
Initially, a descriptive approach was performed through the elaboration of graphics (SigmaPlot) of richness, absolute abundance, relative abundance by order and by development phase and size. Representations include the entire data set as well as their central (mean) and dispersion (standard deviation) descriptors. The same pattern of representation was used for limnological variables. These environmental data were tested through analysis of variance (ANOVA) for significant differences (p < 0.05) among compartments (lotic, intermediate, and lentic) and between reservoirs (Palmeiras and Retiro). A principal component analysis (PCA) was performed to ordinate the spatial trends in limnological conditions. Data were previously transformed using log (x + 1) (except for pH) and normalized. Finally, a Multivariate Permutation Variance Analysis (PERMANOVA), based on the Bray-Curtis similarity matrix, derived from log (x + 1) fish abundance and richness data, was used to test for differences in assemblage structure among the compartments and between the reservoirs.
3. Results
3.1. Limnology
The values of the limnological variables (temperature, pH, ORP, electrical conductivity, turbidity, and dissolved oxygen) (mean and standard deviation and statistical differences) measured in the different reservoir’s compartments are presented in Table 2.
Mean values and standard deviations (into parentheses) of the limnological variables measured in the different compartments of Retiro and Palmeiras SHPs reservoirs, Sapucaí-Mirim River (SP).
For the lentic compartment, all variables showed significant statistical differences between reservoirs. For the intermediate compartment, half of variables showed differences. For the lotic compartment, only dissolved oxygen was statistically different. Comparing lotic, intermediate, and lentic compartments, we observed difference for three variables within Palmeiras, and for four variables in Retiro.
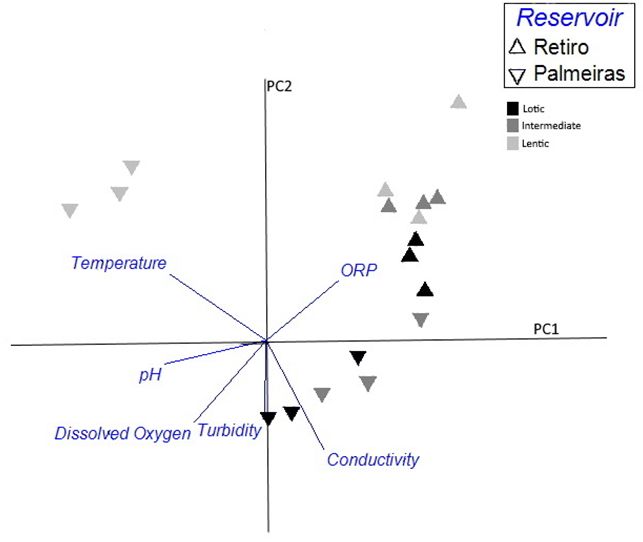
The Principal Component Analysis (Figure 2) explained 71.1% of data variability (43.7% in the first component and 27.4% in the second component). All variables, except turbidity, showed a high correlation (> 0.4) with at least one of the two first components (Table 3). Component one separated the lentic zone of Palmeiras, which was associated with higher pH and temperature values, while other compartments were related to higher conductivity. For component two there was the separation of Palmeiras and Retiro reservoirs, the first associated with higher pH and dissolved oxygen values, and Retiro with higher ORP values.
Graphic results of principal component analysis (components 1 and 2), based on the limnological characteristics adjacent to macrophyte banks in lotic, intermediate, and lentic compartments of Palmeiras and Retiro SHPs reservoirs, Sapucaí-Mirim River (SP, Brazil).
Correlation values (scores) among limnological variables adjacent to macrophyte banks and components one and two of principal component analysis. Data from lotic, intermediate, and lentic compartments of Palmeiras and Retiro SHPs reservoirs, Sapucaí-Mirim River (SP, Brazil).
3.2. Ichthyofauna
We collected 100 fish individuals in the macrophyte banks of Palmeiras and Retiro reservoirs, represented by 20 taxa, including 15 species, 3 genera without species discrimination and 2 individuals identified at the family level (Table 4). Limitation in identification was due to the early development stage of some sampled individuals.
Taxonomic list of fish fauna associated to macrophyte banks in the distinct compartments of Palmeiras and Retiro SHPs reservoirs, Sapucaí-Mirim River (SP, Brazil).
In Palmeiras the fish fauna was represented by 11 taxa. The order Characiformes had five species and two families (not identified), followed by Gymnotiformes and Siluriformes with two species and two families (not identified) each, and Cichliformes and Synbranchiformes with one species and one family (not identified) each. Only four species were captured in more than one compartment. Intermediate exhibited higher richness (six taxa) and the lotic and lentic zones had five and four taxa, respectively (Figure 3; Table 4). In Retiro it was found 14 taxa, comprising nine families. Characiformes also exhibited higher richness, with four species and three families (not identified), followed by Gymnotiformes and Siluriformes, both with two species and two families (not identified), Cichliformes with two species and one family (not identified), and Synbranchiformes with one species and one family (not identified). Two species were captured in more than one compartment, with the intermediate being the richest place (seven taxa) and the lotic and lentic compartments with four and five taxa, respectively (Table 4).
Richness values for the fish fauna associated to macrophyte banks in the distinct compartments of Palmeiras and Retiro SHPs reservoirs, Sapucaí-Mirim River (SP, Brazil).
In Palmeiras, we collected 38 individuals and the compartment with higher abundance was the intermediate, followed by the lotic and lentic (Figure 4). In Retiro, 62 individuals were collected, with higher abundance in the lentic zone (Figure 4). Considering the abundance of each developmental stage, both reservoirs exhibited a higher number of juveniles in the intermediate and lentic compartments. In the lotic compartment, the proportion of juveniles and adults were similar in Retiro, while adults prevailed in Palmeiras (Figure 4).
Abundance per development stage for the fish fauna associated to macrophyte banks in the distinct compartments of Palmeiras (A) and Retiro (B) SHPs reservoirs, Sapucaí-Mirim River (SP, Brazil).
Considering the relative abundance per order, in Palmeiras 42.1% were Synbranchiformes, followed by 31.5% of Characiformes, 13.1% of Gymnotiformes, 10.5% of Siluriformes and 2.6% of Cichliformes. In Retiro, 54.8% of individuals were Synbranchiformes, followed by 16.1% of Characiformes, 12.9% of Gymnotiformes, 9.7% of Siluriformes and 6.4% of Cichliformes. Figure 5 shows the relative abundance for each reservoir compartment, showing that Synbranchiformes, despite being dominant in the intermediate and lentic environments of both reservoirs, were not observed in the lotic ones.
Relative abundance values of each order of the fish fauna associated to macrophyte banks in the distinct compartments of Palmeiras (A) and Retiro (B) SHPs reservoirs, Sapucaí-Mirim River (SP, Brazil).
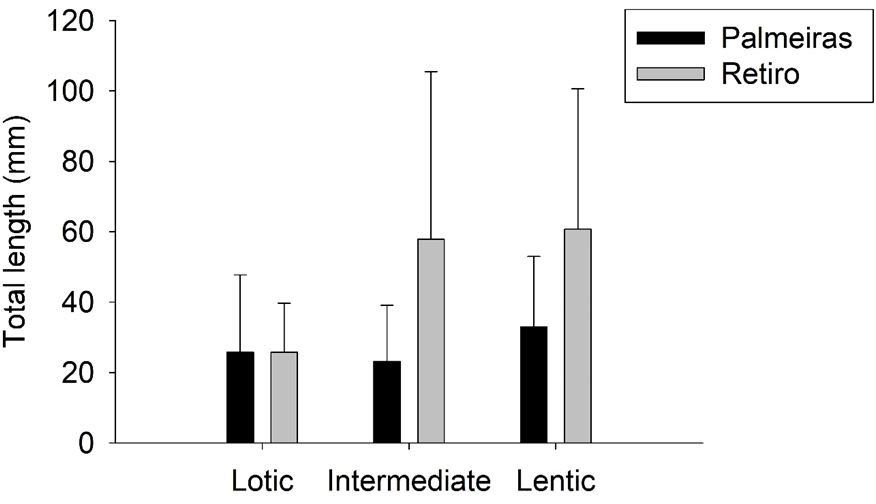
Variation in fish size is presented in Figure 6. For Palmeiras, higher values were found in the lentic compartment, followed by lotic and intermediate. In Retiro, much higher values were found in the lentic and intermediate compared to the lotic compartment. For both reservoirs, higher sizes were influenced by the dominance of Synbranchiformes.
Values (mean and standard deviation) of the total length of the fish fauna associated to macrophyte banks in the distinct compartments of Palmeiras and Retiro SHPs reservoirs, Sapucaí-Mirim River (SP).
The PERMANOVA, based on richness and abundance, indicated a significant difference in the ichthyofauna structure when comparing the distinct compartments (F = 4.700; p = 0.014).
4. Discussion
The main hypothesis of this study was supported by the obtained data. The macrophyte banks of the SHPs reservoirs of the Sapucaí-Mirim River are used by the ichthyofauna as habitats for initial development. Most sampled fishes were juveniles, which evidences the potential ecological function of macrophytes as nursery areas. It was also observed, confirming our additional hypothesis, that assemblage structure is affected by the spatial position along the longitudinal gradient of the reservoirs, despite their small size. The macrophyte banks distributed in the SHPs reservoirs of the Sapucaí-Mirim River are particularly important, but not exclusively, for sedentary species and those with parental care. For some species that do not require long drift displacements, macrophytes may represent a functional alternative for early fish development. For a proper interpretation of the results, it must be considered that the Sapucaí-Mirim fish fauna is mainly represented by small and medium-size non-migratory species (Oliveira et al., 2016OLIVEIRA, A.K., GARAVELLO, J.C., CESARIO, V.V. and CARDOSO, R.T. Fish fauna from Sapucaí-Mirim River, tributary of Grande River, upper Paraná River basin, Southeastern Brazil. Biota Neotropica, 2016, 16(1), 1-9. http://dx.doi.org/10.1590/1676-0611-BN-2014-0192.
http://dx.doi.org/10.1590/1676-0611-BN-2...
).
The number of taxa we found, at least 20 species, is comparable to other studies that also searched for fish inside macrophyte banks in Brazilian rivers and reservoirs, which varied between 22 to 26 species, but with a much higher sampling efforts – 5 to 50 times more captures (Pelicice et al., 2008PELICICE, F.M., THOMAZ, S.M. and AGOSTINHO, A.A. Simple relationships to predict attributes of fish assemblages in patches of submerged macrophytes. Neotropical Ichthyology, 2008, 6(4), 543-550. http://dx.doi.org/10.1590/S1679-62252008000400001.
http://dx.doi.org/10.1590/S1679-62252008...
; Dibble & Pelicice, 2010DIBBLE, E.D. and PELICICE, F.M. Influence of aquatic plant-specific habitat on an assemblage of small neotropical floodplain fishes. Ecology Freshwater Fish, 2010, 19(3), 381-389. http://dx.doi.org/10.1111/j.1600-0633.2010.00420.x.
http://dx.doi.org/10.1111/j.1600-0633.20...
; Hermes-Silva & Zaniboni-Filho, 2012HERMES-SILVA, S. and ZANIBONI-FILHO, E. Structure of the littoral fish assemblage in an impounded tributary: the effects of macrophytes presence (subtropical region, Brazil). Brazilian Journal of Biology = Revista Brasileira de Biologia, 2012, 72(3), 489-495. http://dx.doi.org/10.1590/S1519-69842012000300011. PMid:22990819.
http://dx.doi.org/10.1590/S1519-69842012...
). In terms of composition, it is also important to mention that we sampled fish species of all orders occurring in this river (Oliveira et al., 2016OLIVEIRA, A.K., GARAVELLO, J.C., CESARIO, V.V. and CARDOSO, R.T. Fish fauna from Sapucaí-Mirim River, tributary of Grande River, upper Paraná River basin, Southeastern Brazil. Biota Neotropica, 2016, 16(1), 1-9. http://dx.doi.org/10.1590/1676-0611-BN-2014-0192.
http://dx.doi.org/10.1590/1676-0611-BN-2...
; Brambilla et al., 2019BRAMBILLA, E.M., UIEDA, V.S. and NOGUEIRA, M.G. Does seasonality and pool connectivity influence fish trophic ecology in knickzone habitats? Studies on Neotropical Fauna and Environment, 2019, 54(1), 22-30. http://dx.doi.org/10.1080/01650521.2018.1518118.
http://dx.doi.org/10.1080/01650521.2018....
; Diniz et al., 2019DINIZ, P.B., SIQUEIRA, H.D.O., FALEIROS, T.D.O., PEREIRA, N.L., SENHORINI, J.A., ESGUÍCERO, A.L.H. and BERTELLI, C. Fishes from lakes and tributaries of the Rio Santa Bárbara, Sapucaí Mirim/Grande hydrographic basin, São Paulo, Brazil. Check List, 2019, 15(4), 629-640. http://dx.doi.org/10.15560/15.4.629.
http://dx.doi.org/10.15560/15.4.629...
), namely Characiformes, Siluriformes, Gymnotiformes, Synbranchiformes and Cichliformes. This mean that fish species with distinct evolutionary histories, reproductive biology, and ecological traits (Reis et al., 2003REIS, R.E., KULLANDER, S.O. and FERRARIS, C.J. Check list of the freshwater fishes of South and Central America. Porto Alegre: EDIPUCRS. 2003.), are using, in some way or in some period of their life cycle, the macrophyte habitats. The fact that the reservoirs have a lifetime of 7 and 5 years, Palmeiras and Retiro, respectively, was probably enough for different species to start using these areas. The order with higher number of taxa, in both reservoirs, was Characiformes, followed by Gymnotiformes. In terms of abundance, Synbranchiformes was predominant, except in the lotic stretches. Most sampled individuals belong to sedentary species, with parental care, which use these macrophyte banks as refuge against predation. In addition to fish in the early stages of development, adults of small sedentary species were also found. Similar to larvae and juveniles, they also use these banks as refuge against visual predators and feeding sites (Grenouillet & Pont, 2001GRENOUILLET, G. and PONT, D. Juvenile fishes in macrophyte beds: influence of food resources, habitat structure and body size. Journal of Fish Biology, 2001, 59(4), 939-959. http://dx.doi.org/10.1111/j.1095-8649.2001.tb00163.x.
http://dx.doi.org/10.1111/j.1095-8649.20...
; Sánchez-Botero & Araújo-Lima, 2001SÁNCHEZ-BOTERO, J.I. and ARAÚJO-LIMA, C.A.R.M. As macrófitas aquáticas como berçário para a ictiofauna da várzea do rio Amazonas. Acta Amazonica, 2001, 31(3), 437-447. http://dx.doi.org/10.1590/1809-43922001313447.
http://dx.doi.org/10.1590/1809-439220013...
; Ferrareze & Nogueira, 2011FERRAREZE, M. and NOGUEIRA, M.G. Importance of lateral lagoons for the ichthyofauna in a large tropical reservoir. Brazilian Journal of Biology = Revista Brasileira de Biologia, 2011, 71(4), 807-820. http://dx.doi.org/10.1590/S1519-69842011000500002.
http://dx.doi.org/10.1590/S1519-69842011...
).
The presence of small sedentary species, especially in the lotic compartment of Palmeiras reservoir, may be associated with the proximity with a knickzone, located immediately upstream, where 82% of the species were of small size, representing 98% of total abundance observed in this macrohabitat (Brambilla et al., 2019BRAMBILLA, E.M., UIEDA, V.S. and NOGUEIRA, M.G. Does seasonality and pool connectivity influence fish trophic ecology in knickzone habitats? Studies on Neotropical Fauna and Environment, 2019, 54(1), 22-30. http://dx.doi.org/10.1080/01650521.2018.1518118.
http://dx.doi.org/10.1080/01650521.2018....
). The higher abundance of individuals of the Synbranchidae family in the macrophyte banks, as well as the presence of the order Gymnotiformes, can be explained by the adaptations they have such as the elongated body shape, easily camouflaged between plants, insectivorous feeding behavior, and tolerance to low oxygen concentrations (Henderson & Hamilton, 1995HENDERSON, P.A. and HAMILTON, H.F. Standing crop and distribution of fish in drifting and attached floating meadow within an Upper Amazonian varzea lake. Journal of Fish Biology, 1995, 47(2), 266-276. http://dx.doi.org/10.1111/j.1095-8649.1995.tb01894.x.
http://dx.doi.org/10.1111/j.1095-8649.19...
; Crampton & Hopkins, 2005CRAMPTON, W.G.R. and HOPKINS, C.D. Nesting and paternal care in the weakly electric fish Gymnotus (Gymnotiformes: Gymnotidae) with descriptions of larval and adult electric organs discharges of two species. Copeia, 2005, 2005(1), 48-60. http://dx.doi.org/10.1643/CI-04-056R1.
http://dx.doi.org/10.1643/CI-04-056R1...
; Bulla et al., 2011BULLA, C.K., GOMES, L.C., MIRANDA, L.E. and AGOSTINHO, A.A. The ichthyofauna of drifting macrophyte mats in the Ivinhema River, upper Paraná River basin. Neotropical Ichthyology, 2011, 9(2), 403-409. http://dx.doi.org/10.1590/S1679-62252011005000021.
http://dx.doi.org/10.1590/S1679-62252011...
). In case of Gymnotiformes, an intensive study of Hermes-Silva & Zaniboni-Filho (2012)HERMES-SILVA, S. and ZANIBONI-FILHO, E. Structure of the littoral fish assemblage in an impounded tributary: the effects of macrophytes presence (subtropical region, Brazil). Brazilian Journal of Biology = Revista Brasileira de Biologia, 2012, 72(3), 489-495. http://dx.doi.org/10.1590/S1519-69842012000300011. PMid:22990819.
http://dx.doi.org/10.1590/S1519-69842012...
, comparing the ichthyofauna of macrophyte and non-macrophyte areas, found the species Gymnotus carapo only inside macrophyte banks.
The fact that we found a low abundance of fish larvae, including those of migratory fish, probably is related to the sampling period (late March), which correspond to the end of the seasonal reproductive period (“Piracema”). Another possibility to be checked in future studies is that the reproduction of migratory fish is happening further upstream of this sequence of reservoirs.
The sampled macrophyte banks were randomly chosen to represent the distinct reservoirs and their respective compartments. They had a mixed composition of plant species. Therefore, it was not possible to discriminate if any specific type of plant had a direct influence on the composition of the associated fish fauna. However, independent on the species composition or their life-form, it has already been proven that macrophyte banks can support high abundance of individuals and species of fish due to their role as spawning substrate, refuge against predators and high food availability (Dibble et al., 1996DIBBLE, E.D., KILLGORE, K.J. and HARREL, S.L. Assessment of fish plant interactions. In: E. MIRANDA and D. R. DEVRIES, eds. Multidimensional approaches to reservoir fisheries management. Bethesda: American Fisheries Society, 1996, pp. 357-372.; Agostinho et al., 2003AGOSTINHO, A.A., GOMES, L.C. and JÚLIO, J.H. Relações entre macrófitas aquáticas e fauna de peixes. In: S.M. THOMAZ & L.M. BINI, eds. Ecologia e manejo de macrófitas aquáticas. Maringá: EDUEM, 2003, pp. 261-279., 2007AGOSTINHO, A.A., THOMAZ, S.M., GOMES, L.C. and BALTAR, S.L.S.M.A. Influence of Eichhornia azurea on fish assemblages of the Upper Paraná River Floodplain (Brazil). Aquatic Ecology, 2007, 41(4), 611-619. http://dx.doi.org/10.1007/s10452-007-9122-2.
http://dx.doi.org/10.1007/s10452-007-912...
; Pelicice et al., 2005PELICICE, F.M., AGOSTINHO, A.A. and THOMAZ, S.M. Fish assemblages associated with Egeria in a tropical reservoir: investigating the effects of plant biomass and diel period. Acta Oecologica, 2005, 27(1), 9-16. http://dx.doi.org/10.1016/j.actao.2004.08.004.
http://dx.doi.org/10.1016/j.actao.2004.0...
, 2008PELICICE, F.M., THOMAZ, S.M. and AGOSTINHO, A.A. Simple relationships to predict attributes of fish assemblages in patches of submerged macrophytes. Neotropical Ichthyology, 2008, 6(4), 543-550. http://dx.doi.org/10.1590/S1679-62252008000400001.
http://dx.doi.org/10.1590/S1679-62252008...
; Dibble & Pelicice, 2010DIBBLE, E.D. and PELICICE, F.M. Influence of aquatic plant-specific habitat on an assemblage of small neotropical floodplain fishes. Ecology Freshwater Fish, 2010, 19(3), 381-389. http://dx.doi.org/10.1111/j.1600-0633.2010.00420.x.
http://dx.doi.org/10.1111/j.1600-0633.20...
). Commonly, there is a high abundance of insects and other aquatic invertebrates associated with the roots and leaves of aquatic plants, which potentially serve as food for most fish species (Neiff & Carignan, 1997NEIFF, A.P. and CARIGNAN, R. Macroinvertebrates on Eichhornia crassipes roots in two lakes of the Paraná River floodplain. Hydrobiologia, 1997, 345(2/3), 185-196. http://dx.doi.org/10.1023/A:1002949528887.
http://dx.doi.org/10.1023/A:100294952888...
; Padial et al., 2009PADIAL, A.A., THOMAZ, S.M. and AGOSTINHO, A.A. Effects of structural heterogeneity provided by the floating macrophyte Eichhornia azurea on the predation efficiency and habitat use of the small Neotropical fish Moenkhausia sanctaefilomenae. Hydrobiologia, 2009, 624(1), 161-170. http://dx.doi.org/10.1007/s10750-008-9690-8.
http://dx.doi.org/10.1007/s10750-008-969...
; Ferrareze et al., 2015FERRAREZE, M., NOGUEIRA, M.G. and CASATTI, L. Differences in ichthyofauna feeding habits among lateral lagoons and the river channel in a large reservoir. Brazilian Journal of Biology = Revista Brasileira de Biologia, 2015, 75(2), 380-390. http://dx.doi.org/10.1590/1519-6984.14713. PMid:26132022.
http://dx.doi.org/10.1590/1519-6984.1471...
). According to Rossi & Parma de Croux (1992)ROSSI, L.M. and PARMA DE CROUX, M.J. Influencia de la vegetación acuática en la distribución de peces del río Paraná, Argentina. Ambiente Subtropical, 1992, 2, 65-75., these plants can also be used directly as food, as well the associated periphyton and organic detritus. However, it is important to note that excessive macrophyte growth can disrupt the ecological functioning of aquatic ecosystems and cause major problems in terms of hydropower reservoir management (Thomaz & Bini, 2003THOMAZ, S.M. and BINI, L.M. Ecologia e manejo de macrófitas aquáticas. Maringá: EDUEM, 2003.; Pompêo, 2008POMPÊO, M. Monitoramento e manejo de macrófitas aquáticas. Oecologia Brasiliensis, 2008, 12(3), 406-424.; 2017POMPÊO, M. Monitoramento e manejo de macrófitas aquáticas em reservatórios tropicais brasileiros. São Paulo: Instituto de Biociências da USP, 2017. http://dx.doi.org/10.11606/9788585658670.
http://dx.doi.org/10.11606/9788585658670...
).
The limnological characterization indicates a tendency of differentiation between the reservoirs due to their positioning (opposite extremes in the dam cascade), as well as the longitudinal intra-reservoir variability. Significant differences for limnological variables such as temperature, turbidity, pH, and electrical conductivity were determined between reservoirs and among intra-reservoir compartments.
Studies show the relationship between the composition and structure of the ichthyofauna along dammed rivers (Ferrareze et al., 2014FERRAREZE, M., CASATTI, L. and NOGUEIRA, M.G. Spatial heterogeneity affecting fish fauna in cascade reservoirs of the Upper Paraná Basin, Brazil. Hydrobiologia, 2014, 738(1), 97-109. http://dx.doi.org/10.1007/s10750-014-1922-5.
http://dx.doi.org/10.1007/s10750-014-192...
; Nobile et al., 2019NOBILE, A.B., FREITAS-SOUZA, D., LIMA, F.P., QUEIROZ, J., BAYONA-PEREZ, I.L., CARVALHO, E.D. and RAMOS, I.P. Damming and seasonality as modulators of fish community structure in a small tributary. Ecology Freshwater Fish, 2019, 28(4), 563-572. http://dx.doi.org/10.1111/eff.12475.
http://dx.doi.org/10.1111/eff.12475...
). The lotic, intermediate, and lentic environments have distinct environmental conditions, with particular limnological features as function of differential water flow and its effects on suspended particles (Nogueira et al., 1999NOGUEIRA, M.G., HENRY, R. and MARICATTO, F.E. Spatial and temporal heterogeneity in the Jurumirim Reservoir, São Paulo, Brazil. Lakes and Reservoirs: Research and Management, 1999, 4(3-4), 107-120. http://dx.doi.org/10.1046/j.1440-1770.1999.00086.x.
http://dx.doi.org/10.1046/j.1440-1770.19...
, 2012NOGUEIRA, M.G., PERBICHE-NEVES, G. and NALIATO, D.A.O. Limnology of two contrasting hydroelectric reservoirs (storage and run-of-river) in southeast Brazil. In: H.S. BOROUGENI, ed. Hydropower: practice and application. Rijeka: Intech, 2012, pp. 167-784.; Nogueira & Pomari, 2019NOGUEIRA, M.G. and POMARI, J. Limnological patterns in a large subtropical reservoir cascade. In: D. GOKCE, ed. Limnology: some new aspects of inland water ecology. Rijeka: IntechOpen, 2019, pp. 96-129. http://dx.doi.org/10.5772/intechopen.80632.
http://dx.doi.org/10.5772/intechopen.806...
). Despite of the lack of limnological studies for SHP´s reservoirs, the theoretical model of spatial compartmentalization should be investigated in these systems. For Palmeiras, the PCA showed a clear separation of the lentic compartment compared to the lotic and intermediate ones. In case of Retiro, the same analysis indicated a gradient of conditions between lotic, intermediate, and lentic compartments. Probably the different kind of separation among compartments observed in each reservoir must be associated with the relatively higher extension of Retiro longitudinal axis when compared to Palmeiras. Therefore, the influence of the spatial organization on the fish fauna cannot be neglected, directly, through the limnological variables and passive drift of eggs and larvae, or indirectly, through the macrophytes composition and abundance.
In this study, the larger number of individuals, mainly juveniles, was found in the intermediate zone of Palmeiras Reservoir and in the lentic zone of Retiro Reservoir. This shows that these compartments may have better biological, physical and chemical characteristics for the initial development of fish than lotic zones, especially for sedentary species, such as Synbranchus marmoratus, which was the most abundant species. These conditions include food availability, relatively low water velocity compared to the lotic stretches, higher temperatures, shallow depth (littoral), high microhabitat heterogeneity, and shelter availability (Silva et al., 2012SILVA, P.A., REYNALTE-TATAJE, D.A. and ZANIBONI-FILHO, E. Identification of fish nursery areas in a free tributary of an impoundment region, upper Uruguay River, Brazil. Neotropical Ichthyology, 2012, 10(2), 425-438. http://dx.doi.org/10.1590/S1679-62252012005000012.
http://dx.doi.org/10.1590/S1679-62252012...
; Price et al., 2013PRICE, A.E., HUMPHRIES, P., GAWNE, B. and THOMS, M.C. Effects of discharge regulation on slackwater characteristics at multiple scales in a lowland river. Canadian Journal of Fisheries and Aquatic Sciences, 2013, 70(2), 253-262. http://dx.doi.org/10.1139/cjfas-2012-0164.
http://dx.doi.org/10.1139/cjfas-2012-016...
). In turn, lotic environments are more likely to function as spawning areas (Brambilla et al., 2020BRAMBILLA, E.M., SILVA, L.G.M., BAUMGARTNER, L.J., BIALETZKI, A. and NOGUEIRA, M.G. Dispersal of fish eggs and larvae in a cascade of small hydropower plants with fish ladders. Hydrobiologia, 2020. In press. http://dx.doi.org/10.1007/s10750-020-04425-5.
http://dx.doi.org/10.1007/s10750-020-044...
), due to low water transparency and high velocity, which minimize sedimentation rates and protect the offspring (mainly egg stages) from visual predators (Pompeu et al., 2012POMPEU, P.S., AGOSTINHO, A.A. and PELICICE, F.M. Existing and future challenges: the concept of successful fish passage in South America. River Research and Applications, 2012, 28(4), 504-512. http://dx.doi.org/10.1002/rra.1557.
http://dx.doi.org/10.1002/rra.1557...
).
Our findings are relevant for the local and regional context, considering that typical habitats for the initial development of fishes do not exist in the study area, such as tributaries and marginal lagoons. Furthermore, there was a statistically significant difference in the limnological conditions among the lotic, intermediate, and lentic compartments, which should contribute to the differential distribution of the ichthyofauna, including the species associated with aquatic vegetation. Our results point to the need of future studies to evaluate in detail the effects of the SHPs reservoirs on the distribution of early life stages, as well as the longitudinal compartmentalization and position in relation to the cascade (upper, intermediate, and lower), phases of the reproductive cycle and particularities in terms of macrophyte composition.
Acknowledgements
We thank to Conselho Nacional de Pesquisa e Desenvolvimento Científico (CNPq/PIBIC) for the scholarship conceived to the first author and to Limnética Consultoria em Recursos Hídricos for logistic support. We also thank the Anonymous Reviewers and the Associate Editor for their valuable suggestions.
References
- AGÊNCIA NACIONAL DE ENERGIA ELÉTRICA – ANEEL. Capacidade de geração do Brasil [online]. 2019 [viewed 20 June 2019]. Available from: http://www.aneel.gov.br/siga
» http://www.aneel.gov.br/siga - AGOSTINHO, A.A., GOMES, L.C. and JÚLIO, J.H. Relações entre macrófitas aquáticas e fauna de peixes. In: S.M. THOMAZ & L.M. BINI, eds. Ecologia e manejo de macrófitas aquáticas. Maringá: EDUEM, 2003, pp. 261-279.
- AGOSTINHO, A.A., GOMES, L.C., SANTOS, N.C.L., ORTEGA, J.C.G. and PELICICE, F.M. Fish assemblages in Neotropical reservoirs: colonization patterns, impacts and management. Fisheries Research, 2016, 173, 26-36. http://dx.doi.org/10.1016/j.fishres.2015.04.006
» http://dx.doi.org/10.1016/j.fishres.2015.04.006 - AGOSTINHO, A.A., PELICICE, F.M. and GOMES, L.C. Dams and the fish fauna of the Neotropical region: impacts and management related to diversity and fisheries. Brazilian Journal of Biology = Revista Brasileira de Biologia, 2008, 68(4), 1119-1132, Supplement. http://dx.doi.org/10.1590/S1519-69842008000500019 PMid:19197482.
» http://dx.doi.org/10.1590/S1519-69842008000500019 - AGOSTINHO, A.A., THOMAZ, S.M. and GOMES, L.C. Conservation of the Biodiversity of Brazil’s Inland Waters. Biological Conservation, 2005, 19(3), 646-652. http://dx.doi.org/10.1111/j.1523-1739.2005.00701.x
» http://dx.doi.org/10.1111/j.1523-1739.2005.00701.x - AGOSTINHO, A.A., THOMAZ, S.M., GOMES, L.C. and BALTAR, S.L.S.M.A. Influence of Eichhornia azurea on fish assemblages of the Upper Paraná River Floodplain (Brazil). Aquatic Ecology, 2007, 41(4), 611-619. http://dx.doi.org/10.1007/s10452-007-9122-2
» http://dx.doi.org/10.1007/s10452-007-9122-2 - AHLSTROM, E.H. and MOSER, G. Eggs and larvae of fishes and their role in systematic investigations and in fisheries. Revue des Travaux de l’Institut des Pêches Maritimes, 1976, 40, 379-398.
- ARCIFA, M.S. and ESGUÍCERO, A.L.H. The fish fauna in the fish passage at the Ourinhos Dam, Paranapanema River. Neotropical Ichthyology, 2012, 10(4), 715-722. http://dx.doi.org/10.1590/S1679-62252012000400004
» http://dx.doi.org/10.1590/S1679-62252012000400004 - ÁVILA-SIMAS, S., REYNALTE-TATAJE, D.A. and ZANIBONI-FILHO, E. Pools and rapids as spawning and nursery areas for fish in a river stretch without floodplains. Neotropical Ichthyology, 2014, 12(3), 611-622. http://dx.doi.org/10.1590/1982-0224-20130116
» http://dx.doi.org/10.1590/1982-0224-20130116 - BAKKEN, T.H., SUNDT, H., RUUD, A. and HARBY, A. Development of small versus large hydropower in Norway comparison of environmental impacts. Energy Procedia, 2012, 20, 185-199. http://dx.doi.org/10.1016/j.egypro.2012.03.019
» http://dx.doi.org/10.1016/j.egypro.2012.03.019 - BENEJAM, L., SAURA-MAS, S., BARDINA, M., SOLÀ, C., MUNNÉ, A. and GARCÍA-BERTHOU, E. Ecological impacts of small hydropower plants on headwater stream fish: From individual to community effects. Ecology Freshwater Fish, 2016, 25(2), 295-306. http://dx.doi.org/10.1111/eff.12210
» http://dx.doi.org/10.1111/eff.12210 - BRAMBILLA, E.M., SILVA, L.G.M., BAUMGARTNER, L.J., BIALETZKI, A. and NOGUEIRA, M.G. Dispersal of fish eggs and larvae in a cascade of small hydropower plants with fish ladders. Hydrobiologia, 2020. In press. http://dx.doi.org/10.1007/s10750-020-04425-5
» http://dx.doi.org/10.1007/s10750-020-04425-5 - BRAMBILLA, E.M., UIEDA, V.S. and NOGUEIRA, M.G. Does seasonality and pool connectivity influence fish trophic ecology in knickzone habitats? Studies on Neotropical Fauna and Environment, 2019, 54(1), 22-30. http://dx.doi.org/10.1080/01650521.2018.1518118
» http://dx.doi.org/10.1080/01650521.2018.1518118 - BULLA, C.K., GOMES, L.C., MIRANDA, L.E. and AGOSTINHO, A.A. The ichthyofauna of drifting macrophyte mats in the Ivinhema River, upper Paraná River basin. Neotropical Ichthyology, 2011, 9(2), 403-409. http://dx.doi.org/10.1590/S1679-62252011005000021
» http://dx.doi.org/10.1590/S1679-62252011005000021 - CASTRO, R.M.C., CASATTI, L., SANTOS, H.F., MELO, A.L.A., MARTINS, L.S.F., FERREIRA, K.M., GIBRAN, F.Z., BENINE, R.C., CARVALHO, M., RIBEIRO, A.C., ABREU, T.X., BOCKMANN, F.A., PELIÇÃO, G.Z., STOPIGLIA, R. and LANGEANI, F. Estrutura e composição da ictiofauna de riachos da bacia do rio Grande no Estado de São Paulo, sudeste do Brasil. Biota Neotropica, 2004, 4(1), 1-39. http://dx.doi.org/10.1590/S1676-06032004000100006
» http://dx.doi.org/10.1590/S1676-06032004000100006 - CRAMPTON, W.G.R. and HOPKINS, C.D. Nesting and paternal care in the weakly electric fish Gymnotus (Gymnotiformes: Gymnotidae) with descriptions of larval and adult electric organs discharges of two species. Copeia, 2005, 2005(1), 48-60. http://dx.doi.org/10.1643/CI-04-056R1
» http://dx.doi.org/10.1643/CI-04-056R1 - DIBBLE, E.D. and PELICICE, F.M. Influence of aquatic plant-specific habitat on an assemblage of small neotropical floodplain fishes. Ecology Freshwater Fish, 2010, 19(3), 381-389. http://dx.doi.org/10.1111/j.1600-0633.2010.00420.x
» http://dx.doi.org/10.1111/j.1600-0633.2010.00420.x - DIBBLE, E.D., KILLGORE, K.J. and HARREL, S.L. Assessment of fish plant interactions. In: E. MIRANDA and D. R. DEVRIES, eds. Multidimensional approaches to reservoir fisheries management Bethesda: American Fisheries Society, 1996, pp. 357-372.
- DINIZ, P.B., SIQUEIRA, H.D.O., FALEIROS, T.D.O., PEREIRA, N.L., SENHORINI, J.A., ESGUÍCERO, A.L.H. and BERTELLI, C. Fishes from lakes and tributaries of the Rio Santa Bárbara, Sapucaí Mirim/Grande hydrographic basin, São Paulo, Brazil. Check List, 2019, 15(4), 629-640. http://dx.doi.org/10.15560/15.4.629
» http://dx.doi.org/10.15560/15.4.629 - EMPRESA DE PESQUISA ENERGÉTICA – EPE. Relatório síntese do balanço energético nacional – ano base 2020 Rio de Janeiro, 2020.
- FERRAREZE, M. and NOGUEIRA, M.G. Importance of lateral lagoons for the ichthyofauna in a large tropical reservoir. Brazilian Journal of Biology = Revista Brasileira de Biologia, 2011, 71(4), 807-820. http://dx.doi.org/10.1590/S1519-69842011000500002
» http://dx.doi.org/10.1590/S1519-69842011000500002 - FERRAREZE, M., CASATTI, L. and NOGUEIRA, M.G. Spatial heterogeneity affecting fish fauna in cascade reservoirs of the Upper Paraná Basin, Brazil. Hydrobiologia, 2014, 738(1), 97-109. http://dx.doi.org/10.1007/s10750-014-1922-5
» http://dx.doi.org/10.1007/s10750-014-1922-5 - FERRAREZE, M., NOGUEIRA, M.G. and CASATTI, L. Differences in ichthyofauna feeding habits among lateral lagoons and the river channel in a large reservoir. Brazilian Journal of Biology = Revista Brasileira de Biologia, 2015, 75(2), 380-390. http://dx.doi.org/10.1590/1519-6984.14713 PMid:26132022.
» http://dx.doi.org/10.1590/1519-6984.14713 - FRICKE, R., ESCHMEYER, W.N. and VAN DER LAAN, R. Eschmeyer’s catalog of fishes: genera, species, references [online]. 2019 [viewed 20 June 2019]. Available from: http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcat main.asp
» http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcat - GRAÇA W.J. and PAVANELLI C.S. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: EDUEM, 2007.
- GRENOUILLET, G. and PONT, D. Juvenile fishes in macrophyte beds: influence of food resources, habitat structure and body size. Journal of Fish Biology, 2001, 59(4), 939-959. http://dx.doi.org/10.1111/j.1095-8649.2001.tb00163.x
» http://dx.doi.org/10.1111/j.1095-8649.2001.tb00163.x - HENDERSON, P.A. and HAMILTON, H.F. Standing crop and distribution of fish in drifting and attached floating meadow within an Upper Amazonian varzea lake. Journal of Fish Biology, 1995, 47(2), 266-276. http://dx.doi.org/10.1111/j.1095-8649.1995.tb01894.x
» http://dx.doi.org/10.1111/j.1095-8649.1995.tb01894.x - HERMES-SILVA, S. and ZANIBONI-FILHO, E. Structure of the littoral fish assemblage in an impounded tributary: the effects of macrophytes presence (subtropical region, Brazil). Brazilian Journal of Biology = Revista Brasileira de Biologia, 2012, 72(3), 489-495. http://dx.doi.org/10.1590/S1519-69842012000300011 PMid:22990819.
» http://dx.doi.org/10.1590/S1519-69842012000300011 - KIRJASNIEMI, M. and VALTONEN, T. Size-dependent over-winter mortality of young- of-the-year roach, Rutilus rutilus. Environmental Biology of Fishes, 1997, 50(4), 451-456. http://dx.doi.org/10.1023/A:1007302931943
» http://dx.doi.org/10.1023/A:1007302931943 - KUCUKALI, S. Environmental risk assessment of small hydropower (SHP) plants: a case study for Tefen SHP plant on Filyos River. Energy for Sustainable Development, 2014, 19, 102-110.
- LANGEANI F. and REGO A.C.L. Guia ilustrado dos peixes da bacia do Rio Araguari. Uberlândia: Grupo de Mídia Brasil Central, 2014.
- LOURES, R.C. and POMPEU, P.S. Long-term study of reservoir cascade in south-eastern Brazil reveals spatio-temporal gradient in fish assemblages. Marine and Freshwater Research, 2018, 69(12), 1983-1994. http://dx.doi.org/10.1071/MF18109
» http://dx.doi.org/10.1071/MF18109 - NAKATANI, K., AGOSTINHO, A.A., BAUMGARTNER, G., BIALETZKI, A., SANCHES, P.V., MAKRAKIS, M.C. and PAVANELLI, C.S. Ovos e larvas de peixes de água doce: desenvolvimento e manual de identificação Maringá: Eduem. 2001.
- NEIFF, A.P. and CARIGNAN, R. Macroinvertebrates on Eichhornia crassipes roots in two lakes of the Paraná River floodplain. Hydrobiologia, 1997, 345(2/3), 185-196. http://dx.doi.org/10.1023/A:1002949528887
» http://dx.doi.org/10.1023/A:1002949528887 - NOBILE, A.B., FREITAS-SOUZA, D., LIMA, F.P., QUEIROZ, J., BAYONA-PEREZ, I.L., CARVALHO, E.D. and RAMOS, I.P. Damming and seasonality as modulators of fish community structure in a small tributary. Ecology Freshwater Fish, 2019, 28(4), 563-572. http://dx.doi.org/10.1111/eff.12475
» http://dx.doi.org/10.1111/eff.12475 - NOGUEIRA, M.G. and POMARI, J. Limnological patterns in a large subtropical reservoir cascade. In: D. GOKCE, ed. Limnology: some new aspects of inland water ecology Rijeka: IntechOpen, 2019, pp. 96-129. http://dx.doi.org/10.5772/intechopen.80632
» http://dx.doi.org/10.5772/intechopen.80632 - NOGUEIRA, M.G., FERRAREZE, M., MOREIRA, M.L. and GOUVÊA, R.M. Phytoplankton assemblages in a reservoir cascade of a large tropical - subtropical river (SE, Brazil). Brazilian Journal of Biology = Revista Brasileira de Biologia, 2010, 70(3), 781-793, Supplement. http://dx.doi.org/10.1590/S1519-69842010000400009 PMid:21085783.
» http://dx.doi.org/10.1590/S1519-69842010000400009 - NOGUEIRA, M.G., HENRY, R. and MARICATTO, F.E. Spatial and temporal heterogeneity in the Jurumirim Reservoir, São Paulo, Brazil. Lakes and Reservoirs: Research and Management, 1999, 4(3-4), 107-120. http://dx.doi.org/10.1046/j.1440-1770.1999.00086.x
» http://dx.doi.org/10.1046/j.1440-1770.1999.00086.x - NOGUEIRA, M.G., OLIVEIRA, P.C.R. and DE BRITTO, Y.T. Zooplankton assemblages (Copepoda and Cladocera) in a cascade of reservoirs of a large tropical river (SE Brazil). Limnetica, 2008, 27, 151-170.
- NOGUEIRA, M.G., PERBICHE-NEVES, G. and NALIATO, D.A.O. Limnology of two contrasting hydroelectric reservoirs (storage and run-of-river) in southeast Brazil. In: H.S. BOROUGENI, ed. Hydropower: practice and application. Rijeka: Intech, 2012, pp. 167-784.
- OLIVEIRA, A.K., GARAVELLO, J.C., CESARIO, V.V. and CARDOSO, R.T. Fish fauna from Sapucaí-Mirim River, tributary of Grande River, upper Paraná River basin, Southeastern Brazil. Biota Neotropica, 2016, 16(1), 1-9. http://dx.doi.org/10.1590/1676-0611-BN-2014-0192
» http://dx.doi.org/10.1590/1676-0611-BN-2014-0192 - ORSI, M.L., ALMEIDA, F.S., SWARÇA, A.C., CLARO-GARCÍA, A., VIANNA, N.C., GARCIA, D.A.Z. and BIALETZKI, A. Ovos, larvas e juvenis dos peixes da Bacia do Rio Paranapanema uma avaliação para a conservação Assis: Triunfal Gráfica e Editora, Duke Energy, 2016.
- OTA, R.R., DEPRÁ, G.C., GRAÇA, W.J. and PAVANELLI, C.S. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes: revised, annotated and updated. Neotropical Ichthyology, 2018, 16(2), e170094. http://dx.doi.org/10.1590/1982-0224-20170094
» http://dx.doi.org/10.1590/1982-0224-20170094 - PADIAL, A.A., THOMAZ, S.M. and AGOSTINHO, A.A. Effects of structural heterogeneity provided by the floating macrophyte Eichhornia azurea on the predation efficiency and habitat use of the small Neotropical fish Moenkhausia sanctaefilomenae. Hydrobiologia, 2009, 624(1), 161-170. http://dx.doi.org/10.1007/s10750-008-9690-8
» http://dx.doi.org/10.1007/s10750-008-9690-8 - PAIVA, M.M. Grandes represas do Brasil Brasília: Editerra, 1982.
- PELICICE, F.M., AGOSTINHO, A.A. and THOMAZ, S.M. Fish assemblages associated with Egeria in a tropical reservoir: investigating the effects of plant biomass and diel period. Acta Oecologica, 2005, 27(1), 9-16. http://dx.doi.org/10.1016/j.actao.2004.08.004
» http://dx.doi.org/10.1016/j.actao.2004.08.004 - PELICICE, F.M., AZEVEDO-SANTOS, V.M., ESGUÍCERO, A.L.H., AGOSTINHO, A.A. and ARCIFA, M.S. Fish diversity in the cascade of reservoirs along the Paranapanema River, southeast Brazil. Neotropical Ichthyology, 2018, 16(2), 1-18. http://dx.doi.org/10.1590/1982-0224-20170150
» http://dx.doi.org/10.1590/1982-0224-20170150 - PELICICE, F.M., POMPEU, P.S. and AGOSTINHO, A.A. Large reservoirs as ecological barriers to downstream movements of Neotropical migratory fish. Fish and Fisheries, 2014, 16, 1-19.
- PELICICE, F.M., THOMAZ, S.M. and AGOSTINHO, A.A. Simple relationships to predict attributes of fish assemblages in patches of submerged macrophytes. Neotropical Ichthyology, 2008, 6(4), 543-550. http://dx.doi.org/10.1590/S1679-62252008000400001
» http://dx.doi.org/10.1590/S1679-62252008000400001 - POMPÊO, M. Monitoramento e manejo de macrófitas aquáticas em reservatórios tropicais brasileiros São Paulo: Instituto de Biociências da USP, 2017. http://dx.doi.org/10.11606/9788585658670
» http://dx.doi.org/10.11606/9788585658670 - POMPÊO, M. Monitoramento e manejo de macrófitas aquáticas. Oecologia Brasiliensis, 2008, 12(3), 406-424.
- POMPEU, P.S., AGOSTINHO, A.A. and PELICICE, F.M. Existing and future challenges: the concept of successful fish passage in South America. River Research and Applications, 2012, 28(4), 504-512. http://dx.doi.org/10.1002/rra.1557
» http://dx.doi.org/10.1002/rra.1557 - PRICE, A.E., HUMPHRIES, P., GAWNE, B. and THOMS, M.C. Effects of discharge regulation on slackwater characteristics at multiple scales in a lowland river. Canadian Journal of Fisheries and Aquatic Sciences, 2013, 70(2), 253-262. http://dx.doi.org/10.1139/cjfas-2012-0164
» http://dx.doi.org/10.1139/cjfas-2012-0164 - REIS, R.E., KULLANDER, S.O. and FERRARIS, C.J. Check list of the freshwater fishes of South and Central America Porto Alegre: EDIPUCRS. 2003.
- ROSSI, L.M. and PARMA DE CROUX, M.J. Influencia de la vegetación acuática en la distribución de peces del río Paraná, Argentina. Ambiente Subtropical, 1992, 2, 65-75.
- RUOCCO, A.M.C., PORTINHO, J.L. and NOGUEIRA, M.G. Potential impact of small hydroelectric power plants on river biota: a case study on macroinvertebrates associated to basaltic knickzones. Brazilian Journal of Biology = Revista Brasileira de Biologia, 2019, 79(4), 722-734. http://dx.doi.org/10.1590/1519-6984.191148 PMid:30088529.
» http://dx.doi.org/10.1590/1519-6984.191148 - SÁNCHEZ-BOTERO, J.I. and ARAÚJO-LIMA, C.A.R.M. As macrófitas aquáticas como berçário para a ictiofauna da várzea do rio Amazonas. Acta Amazonica, 2001, 31(3), 437-447. http://dx.doi.org/10.1590/1809-43922001313447
» http://dx.doi.org/10.1590/1809-43922001313447 - SHARMA, K.N., TIWARI, P.K. and SOOD, Y.R. A comprehensive analysis of strategies, policies and development of hydropower in India: Special emphasis on small hydro power. Renewable & Sustainable Energy Reviews, 2013, 18, 460-470. http://dx.doi.org/10.1016/j.rser.2012.10.017
» http://dx.doi.org/10.1016/j.rser.2012.10.017 - SILVA, P.A., REYNALTE-TATAJE, D.A. and ZANIBONI-FILHO, E. Identification of fish nursery areas in a free tributary of an impoundment region, upper Uruguay River, Brazil. Neotropical Ichthyology, 2012, 10(2), 425-438. http://dx.doi.org/10.1590/S1679-62252012005000012
» http://dx.doi.org/10.1590/S1679-62252012005000012 - SOARES, M.C.S., MARINHO, M.M., HUSZAR, V.L.M., BRANCO, C.W.C. and AZEVEDO, S.M.F.O. The effects of water retention time and watershed features on the limnology of two tropical reservoirs in Brazil. Lakes and Reservoirs: Research and Management, 2008, 13(4), 257-269. http://dx.doi.org/10.1111/j.1440-1770.2008.00379.x
» http://dx.doi.org/10.1111/j.1440-1770.2008.00379.x - SUZUKI, F.M., PIRES, L.V. and POMPEU, P.S. Passage of fish larvae and eggs through the Funil, Itutinga and Camargos reservoirs on the upper Rio Grande (Minas Gerais, Brazil). Neotropical Ichthyology, 2011, 9(3), 617-622. http://dx.doi.org/10.1590/S1679-62252011000300014
» http://dx.doi.org/10.1590/S1679-62252011000300014 - THOMAZ, S.M. and BINI, L.M. Ecologia e manejo de macrófitas aquáticas Maringá: EDUEM, 2003.
- TUNDISI, J.G. Reservatórios como sistemas complexos: teoria, aplicações e perspectivas para usos múltiplos. In: R. HENRY, ed. Ecologia de reservatórios: estrutura, função e aspectos sociais Botucatu: FUNDIBIO, 1999, pp. 19-38.
- TUNDISI, J.G. Reservoirs: new challenges for ecosystem studies and environmental management. Water Security, 2018, 4-5, 1-7. http://dx.doi.org/10.1016/j.wasec.2018.09.001
» http://dx.doi.org/10.1016/j.wasec.2018.09.001 - VAZZOLER, A.E.A.M. Manual de métodos para estudos biológicos de populações de peixes: reprodução e crescimento Brasília: Programa Nacional de Zoologia (CNPq), 1982.
- VIANNA, N.C. and NOGUEIRA, M.G. Ichthyoplankton and limnological factors in the Cinzas River – an alternative spawning site for fishes in the middle Paranapanema River basin, Brazil. Acta Limnologica Brasiliensia, 2008, 20, 139-151.
- WILZBACH, M.A., CUMMINS, K.W., BARNES, T.K. and TREXLER, J.C. Channel- floodplain coupling in the Kisimmee River, Florida (USA): invertebrate movement and fish feeding. Verhandlungen - Internationale Vereinigung für Theoretische und Angewandte Limnologie, 2002, 28(1), 164-172. http://dx.doi.org/10.1080/03680770.2001.11902567
» http://dx.doi.org/10.1080/03680770.2001.11902567
Edited by
Publication Dates
-
Publication in this collection
26 Nov 2021 -
Date of issue
2021
History
-
Received
09 Mar 2021 -
Accepted
26 Oct 2021