Abstract:
Aim
We evaluated changes in periphyton biomass and the composition of benthic diatom communities along a gradient of urbanization in 10 coastal streams located on the coastal plain of southernmost Brazil.
Methods
At each coastal stream, we obtained limnological variables and periphytic material from the stolon of the aquatic macrophyte Hydrocotyle ranunculoides for further analyses of chlorophyll a and diatoms.
Results
Total phosphorus was the only limnological variable selected by the statistical models, showing a positive relationship with periphyton biomass and a negative relationship with diatom species richness in these streams. Species composition (for both presence-absence and abundance data) was also explained by total phosphorus. Further, we observed a nested distribution of diatom species along the streams, in which poorer communities of streams with higher concentrations of phosphorous are subsets of richer communities from streams with lower concentrations of the nutrient.
Conclusions
Our study shows that water quality modifications caused by eutrophication are leading to the loss of species and changes in the structure of biological communities in ecotones such as coastal streams.
Keywords:
gradient of urbanization; washouts; lotic ecosystem; ecotones; Brazil
Resumo:
Objetivo
Avaliamos as mudanças na biomassa do perifíton e na composição das comunidades de diatomáceas bentônicas ao longo de um gradiente de urbanização em 10 riachos costeiros localizados na planície costeira do extremo sul do Brasil.
Métodos
Em cada riacho coletamos variáveis limnológicas e o material perifítico do estolão da macrófita aquática Hydrocotyle ranunculoides para posterior análises de clorofila a e de diatomáceas.
Resultados
Fósforo total foi a única variável limnológica selecionada pelos modelos, mostrando uma relação positiva com a biomassa e negativa com a riqueza de espécies de diatomáceas nesses riachos. A composição de espécies (para dados de presença e ausência e de abundância) também foi explicada pelas concentrações de fósforo total. Além disso, observamos uma distribuição aninhada de espécies ao longo dos riachos, de forma que as comunidades mais pobres de riachos com maiores concentrações de fósforo são subconjuntos de comunidades mais ricas com menores concentrações do nutriente.
Conclusões
Evidenciamos em nosso estudo que as modificações na qualidade da água causadas pela eutrofização estão levando à perda de espécies e mudanças na estrutura de comunidades em ecótonos como os riachos costeiros.
Palavras-chave:
gradiente de urbanização; sangradouros, ecossistema lótico, ecótonos, Brasil
1. Introduction
Freshwater ecosystems are biodiversity hotspots, supporting more species per area than terrestrial ecosystems and accounting for approximately 10% of all known species (Strayer & Dudgeon, 2010Strayer, D.L., & Dudgeon, D., 2010. Freshwater biodiversity conservation: recent progress and future challenges. J. N. Am. Benthol. Soc. 29(1), 344-358. http://dx.doi.org/10.1899/08-171.1.
http://dx.doi.org/10.1899/08-171.1...
). However, these ecosystems are also among the most threatened environments on the planet, presenting declines in biodiversity superior to terrestrial ecosystems (Sala et al., 2000Sala, O.E., Stuart Chapin 3rd, F., Armesto, J.J., Berlow, E., Bloomfield, J., Dirzo, R., Huber-Sanwald, E., Huenneke, L.F., Jackson, R.B., Kinzig, A., Leemans, R., Lodge, D.M., Mooney, H.A., Oesterheld, M., Poff, N.L.R., Sykes, M.T., Walker, B.H., Walker, M., & Wall, D.H., 2000. Global biodiversity scenarios for the year 2010. Science 287(5459), 1770-1774. PMid:10710299. http://dx.doi.org/10.1126/science.287.5459.1770.
http://dx.doi.org/10.1126/science.287.54...
; Strayer & Dudgeon, 2010Strayer, D.L., & Dudgeon, D., 2010. Freshwater biodiversity conservation: recent progress and future challenges. J. N. Am. Benthol. Soc. 29(1), 344-358. http://dx.doi.org/10.1899/08-171.1.
http://dx.doi.org/10.1899/08-171.1...
), as a consequence of human-induced disturbances such as landscape modification for land use, the introduction of invasive species, damming of rivers, and water pollution by nutrient enrichment and other sources (Dudgeon et al., 2006Dudgeon, D., Arthington, A.H., Gessner, M.O., Kawabata, Z.-I., Knowler, D.J., Lévêque, C., Naiman, R.J., Prieur-Richard, A.H., Soto, D., Stiassny, M.L.J., & Sullivan, C.A., 2006. Freshwater biodiversity: importance, threats, status and conservation challenges. Biol. Rev. Camb. Philos. Soc. 81(2), 163-182. PMid:16336747. http://dx.doi.org/10.1017/S1464793105006950.
http://dx.doi.org/10.1017/S1464793105006...
; Vörösmarty et al., 2010Vörösmarty, C.J., McIntyre, P.B., Gessner, M.O., Dudgeon, D., Prusevich, A., Green, P., Glidden, S., Bunn, S.E., Sullivan, C.A., Liermann, C.R., & Davies, P.M., 2010. Global threats to human water security and river biodiversity. Nature 467(7315), 555-561. PMid:20882010. http://dx.doi.org/10.1038/nature09440.
http://dx.doi.org/10.1038/nature09440...
; Petsch, 2016Petsch, D.K., 2016. Causes and consequences of biotic homogenization in freshwater ecosystems. Int. Rev. Hydrobiol. 101(3-4), 113-122. http://dx.doi.org/10.1002/iroh.201601850.
http://dx.doi.org/10.1002/iroh.201601850...
; Dudgeon, 2019Dudgeon, D., 2019. Multiple threats imperil freshwater biodiversity in the Anthropocene. Curr. Biol. 29(19), 960-967. PMid:31593677. http://dx.doi.org/10.1016/j.cub.2019.08.002.
http://dx.doi.org/10.1016/j.cub.2019.08....
). Given the threats faced by freshwater ecosystems, it is pivotal to better understand the effects of anthropogenic impacts on freshwater biota to enhance conservation strategies and ecosystem management policies (Vörösmarty et al., 2010Vörösmarty, C.J., McIntyre, P.B., Gessner, M.O., Dudgeon, D., Prusevich, A., Green, P., Glidden, S., Bunn, S.E., Sullivan, C.A., Liermann, C.R., & Davies, P.M., 2010. Global threats to human water security and river biodiversity. Nature 467(7315), 555-561. PMid:20882010. http://dx.doi.org/10.1038/nature09440.
http://dx.doi.org/10.1038/nature09440...
; Jacobson et al., 2017Jacobson, P.C., Hansen, G.J.A., Bethke, B.J., & Cross, T.K., 2017. Disentangling the effects of a century of eutrophication and climatic warming on freshwater lake fish assemblages. PLoS One 12(8), e0182667. PMid:28777816. http://dx.doi.org/10.1371/journal.pone.0182667.
http://dx.doi.org/10.1371/journal.pone.0...
).
Different phenomena are responsible for the degradation of streams in urban areas, which is commonly referred to as the “urban stream syndrome”. Among the main symptoms of streams draining urban areas are (i) changes in stream hydrology (Walsh et al., 2005Walsh, C.J., Roy, A.H., Feminella, J.W., Cottingham, P.D., Groffman, P.M., & Morgan 2nd, R.P., 2005. The urban stream syndrome: current knowledge and the search for a cure. J. N. Am. Benthol. Soc. 24(3), 706-723. http://dx.doi.org/10.1899/04-028.1.
http://dx.doi.org/10.1899/04-028.1...
; Grimm et al., 2008Grimm, N.B., Faeth, S.H., Golubiewski, N.E., Redman, C.L., Wu, J., Bai, W., & Briggs, J.M., 2008. Global change and the ecology of cities. Science 319(5864), 756-760. PMid:18258902. http://dx.doi.org/10.1126/science.1150195.
http://dx.doi.org/10.1126/science.115019...
), as increased frequency of erosive and overland flow (Walsh et al., 2005Walsh, C.J., Roy, A.H., Feminella, J.W., Cottingham, P.D., Groffman, P.M., & Morgan 2nd, R.P., 2005. The urban stream syndrome: current knowledge and the search for a cure. J. N. Am. Benthol. Soc. 24(3), 706-723. http://dx.doi.org/10.1899/04-028.1.
http://dx.doi.org/10.1899/04-028.1...
); (ii) reduction of the diversity of aquatic species, which may result in biotic homogenization (e.g., Barnum et al., 2017Barnum, T.R., Weller, D.E., & Williams, M., 2017. Urbanization reduces and homogenizes traits diversity in stream macroinvertebrate communities. Ecol. Appl. 27(8), 2428-2442. PMid:28872731. http://dx.doi.org/10.1002/eap.1619.
http://dx.doi.org/10.1002/eap.1619...
); and (iii) anthropogenic eutrophication, which in urban areas occurs mainly due to disposal of domestic sewage (with high concentrations of nutrients) without proper treatment (Walsh et al., 2005Walsh, C.J., Roy, A.H., Feminella, J.W., Cottingham, P.D., Groffman, P.M., & Morgan 2nd, R.P., 2005. The urban stream syndrome: current knowledge and the search for a cure. J. N. Am. Benthol. Soc. 24(3), 706-723. http://dx.doi.org/10.1899/04-028.1.
http://dx.doi.org/10.1899/04-028.1...
; Tromboni & Dodds, 2017Tromboni, F., & Dodds, W.K., 2017. Relationships between land use and stream nutrient concentrations in a highly urbanized tropical region of Brazil: thresholds and riparian zones. Environ. Manage. 60(1), 30-40. PMid:28405753. http://dx.doi.org/10.1007/s00267-017-0858-8.
http://dx.doi.org/10.1007/s00267-017-085...
).
Anthropogenic eutrophication, resulting from excessive nutrient loading – mostly nitrogen and phosphorus – is among the most severe impacts threatening freshwater ecosystems around the world (Smith, 2003Smith, V.H., 2003. Eutrophication of freshwater and coastal marine ecosystems: a global problem. Environ. Sci. Pollut. Res. Int. 10(2), 126-139. PMid:12729046. http://dx.doi.org/10.1065/espr2002.12.142.
http://dx.doi.org/10.1065/espr2002.12.14...
; Smith & Schindler, 2009Smith, V.H., & Schindler, W.D., 2009. Eutrophication science: where do we go from here? Trends Ecol. Evol. 24(4), 201-207. PMid:19246117. http://dx.doi.org/10.1016/j.tree.2008.11.009.
http://dx.doi.org/10.1016/j.tree.2008.11...
; Dodds & Smith, 2016Dodds, W.K., & Smith, V.H., 2016. Nitrogen, phosphorus and eutrophication in streams. Inland Waters 6(2), 155-164. http://dx.doi.org/10.5268/IW-6.2.909.
http://dx.doi.org/10.5268/IW-6.2.909...
). Among its consequences are the increase in primary producer biomass and the occurrence of toxin-producing cyanobacterial blooms, resulting in the degradation of water conditions, reduction of species diversity, and changes in species composition (Smith & Schindler, 2009Smith, V.H., & Schindler, W.D., 2009. Eutrophication science: where do we go from here? Trends Ecol. Evol. 24(4), 201-207. PMid:19246117. http://dx.doi.org/10.1016/j.tree.2008.11.009.
http://dx.doi.org/10.1016/j.tree.2008.11...
; Wojciechowski et al., 2017Wojciechowski, J., Heino, J., Bini, L.M., & Padial, A.A., 2017. Temporal variation in phytoplankton beta diversity patterns and metacommunity structures across subtropical reservoirs. Freshw. Biol. 62(4), 751-766. http://dx.doi.org/10.1111/fwb.12899.
http://dx.doi.org/10.1111/fwb.12899...
), with a predominance of generalist and tolerant species and local extinction of sensitive ones (Chen et al., 2016Chen, X., Zhou, W., Pickett, S.T.A., Li, W., Han, L., & Ren, Y., 2016. Diatoms are better indicators of urban stream conditions: a case study in Beijing, China. Ecol. Indic. 60, 265-274. http://dx.doi.org/10.1016/j.ecolind.2015.06.039.
http://dx.doi.org/10.1016/j.ecolind.2015...
; Jacobson et al., 2017Jacobson, P.C., Hansen, G.J.A., Bethke, B.J., & Cross, T.K., 2017. Disentangling the effects of a century of eutrophication and climatic warming on freshwater lake fish assemblages. PLoS One 12(8), e0182667. PMid:28777816. http://dx.doi.org/10.1371/journal.pone.0182667.
http://dx.doi.org/10.1371/journal.pone.0...
). If sensitive species are selectively removed along a eutrophication gradient and only tolerant species are able to establish, a nested distribution pattern could be expected, in which communities with fewer species form a subset of richer communities (Patterson & Atmar, 1986Patterson, B.D., & Atmar, W., 1986. Nested subsets and the structure of insular mammalian and archipelagos. Biol. J. Linn. Soc. Lond. 28(1-2), 65-82. http://dx.doi.org/10.1111/j.1095-8312.1986.tb01749.x.
http://dx.doi.org/10.1111/j.1095-8312.19...
; Baselga, 2010Baselga, A., 2010. Partitioning the turnover and nestedness components of beta diversity. Glob. Ecol. Biogeogr. 19(1), 134-143. http://dx.doi.org/10.1111/j.1466-8238.2009.00490.x.
http://dx.doi.org/10.1111/j.1466-8238.20...
). This pattern can be explained by habitat quality and its abiotic characteristics, in addition to area and isolation (Wright et al., 1997Wright, D.H., Patterson, B.D., Mikkelson, G.M., Cutler, A., & Atmar, W., 1997. A comparative analysis of nested subset patterns of species composition. Oecologia. 113(1), 1-20. PMid:28307284. http://dx.doi.org/10.1007/s004420050348.
http://dx.doi.org/10.1007/s004420050348...
). In fact, nestedness was already observed along gradients of anthropogenic impacts, such as presence of heavy metals in water (Gutiérrez-Cánovas et al., 2013Gutiérrez-Cánovas, C., Millán, A., Velasco, J., Vaughan, I.P., & Ormerod, S.J., 2013. Contrasting effects of natural and anthropogenic stressors on beta diversity in river organisms. Glob. Ecol. Biogeogr. 22(7), 796-805. http://dx.doi.org/10.1111/geb.12060.
http://dx.doi.org/10.1111/geb.12060...
), and experimental habitat homogenization (Schneck et al., 2011Schneck, F., Schwarzbold, A., & Melo, A.S., 2011. Substrate roughness affects stream benthic algal diversity, assemblage composition, and nestedness. J. N. Am. Benthol. Soc. 30(4), 1049-1056. http://dx.doi.org/10.1899/11-044.1.
http://dx.doi.org/10.1899/11-044.1...
). Nutrient enrichment has also been described as a factor leading to nestedness of phytoplankton and zooplankton communities in experimental microcosms (Di Carvalho & Wickham, 2019Di Carvalho, J.A., & Wickham, S.A., 2019. Simulating eutriphication in a metacommunity landscape: an aquatic model ecosystem. Oecologia 189(2), 461-474. PMid:30523402. http://dx.doi.org/10.1007/s00442-018-4319-8.
http://dx.doi.org/10.1007/s00442-018-431...
), zooplankton in streams (Gutierrez et al., 2020Gutierrez, M.F., Simões, N.R., Frau, D., Saigo, M., & Licursi, M., 2020. Responses of stream zooplankton diversity metrics to eutrophication and temporal environmental variability in agricultural catchments. Environ. Monit. Assess. 192(12), 1-17. PMid:33242179. http://dx.doi.org/10.1007/s10661-020-08766-5.
http://dx.doi.org/10.1007/s10661-020-087...
), and diatoms in streams (Jamoneau et al., 2017Jamoneau, A., Passy, S.I., Soininen, J., Leboucher, T., & Tison-Rosebery, J., 2017. Beta diversity of diatoms species and ecological guilds: response to environmental and spatial mechanisms along the stream watercourse. Freshw. Biol. 63(1), 62-73. http://dx.doi.org/10.1111/fwb.12980.
http://dx.doi.org/10.1111/fwb.12980...
) and reservoirs (Zorzal-Almeida et al., 2021Zorzal-Almeida, S., Bartozek, E.C.R., & Bicudo, D.C., 2021. Homogenization of diatom assemblages is driven by eutrophication in tropical reservoirs. Environ. Pollut. 288, 117778. PMid:34280747. http://dx.doi.org/10.1016/j.envpol.2021.117778.
http://dx.doi.org/10.1016/j.envpol.2021....
).
The coastal plain of southernmost Brazil harbors several coastal streams (or washouts; sangradouros in Figueiredo & Calliari, 2006Figueiredo, S.A., & Calliari, L.J., 2006. Washouts in the central and northern litoral of Rio Grande do Sul state, Brazil: distribution and implications. J. Coast. Res. 39, 366-370.), that are considered marine-freshwater ecotones, essential for the establishment of freshwater, estuarine and marine biological communities (Figueiredo & Calliari, 2006Figueiredo, S.A., & Calliari, L.J., 2006. Washouts in the central and northern litoral of Rio Grande do Sul state, Brazil: distribution and implications. J. Coast. Res. 39, 366-370.; Bastos et al., 2013Bastos, R.F., Condini, M.V., & Garcia, A.M., 2013. Fish species list of coastal streams in Southern Brazil, with notes on austral distribution limits of marine and freshwater endangered species. Pan-Am. J. Aquat. Sci. 8, 347-351.). These channels drain coastal lakes and wetlands through the foredunes arriving at the sea surf zone (Figueiredo & Calliari, 2006Figueiredo, S.A., & Calliari, L.J., 2006. Washouts in the central and northern litoral of Rio Grande do Sul state, Brazil: distribution and implications. J. Coast. Res. 39, 366-370.), being responsible for the transport of a considerable amount of organic matter, sediment, and nutrients from the continent to the marine environment (Baumgarten et al., 2007Baumgarten, M.G.Z., Millão, D., Costa, P.G., Attisano, K.K., Costa, N.B.D., Gutierres, F.B., Giordano, S.B., & Araújo, E.A.C., 2007. Praia do Cassino (Rio Grande -RS): qualidade da água dos sangradouros da área central – antes (2003) e depois (2005) da instalação de estação de tratamento de esgotos (ETE). Cad Ecol Aquat 2, 1-12.). Studies about sangradouros geology and genesis classify these ecosystems as (i) permanent, that are permanently connected to the sea; (ii) intermittent, that can occasionally lose connection with the sea and; (iii) ephemeral, which originated momentarily due to heavy rains and soon disappear (Pereira da Silva, 1998Pereira da Silva, R., 1998. Ocorrência, distribuição e características morfodinâmicas dos sangradouros na zona costeira do Rio Grande do Sul: trecho Rio Grande – Chuí, RS [Dissertação de mestrado em Geociências]. Porto Alegre: Universidade Federal do Rio Grande do Sul.). Moreover, these ecosystems are strongly influenced by precipitation and evaporation (Figueiredo & Calliari, 2006Figueiredo, S.A., & Calliari, L.J., 2006. Washouts in the central and northern litoral of Rio Grande do Sul state, Brazil: distribution and implications. J. Coast. Res. 39, 366-370.). Studies about these ecosystems show that in urbanized areas they are mostly impacted by eutrophication (Albertoni & Palma-Silva, 2006Albertoni, E.F., & Palma-Silva, C., 2006. Macroinvertebrados associados a macrófitas aquáticas flutuantes em canais urbanos de escoamento pluvial (Balneário Cassino, Rio Grande, RS). Neotrop. Biol. Conserv. 1(2), 90-100.; Baumgarten et al., 2007Baumgarten, M.G.Z., Millão, D., Costa, P.G., Attisano, K.K., Costa, N.B.D., Gutierres, F.B., Giordano, S.B., & Araújo, E.A.C., 2007. Praia do Cassino (Rio Grande -RS): qualidade da água dos sangradouros da área central – antes (2003) e depois (2005) da instalação de estação de tratamento de esgotos (ETE). Cad Ecol Aquat 2, 1-12.). However, there is a scarcity of studies on the effects of eutrophication gradients on these streams and their biodiversity.
In streams, benthic algae, especially diatoms, are among the most important primary producers and account for an important portion of species diversity (Biggs, 1996Biggs, B.J.F., 1996. Patterns in benthic algae of streams. In: Stevenson, J., Bothwell, M.L. & Lowe, R.L., eds. Algal ecology: freshwater benthic ecosystems. San Diego: Elsevier. http://dx.doi.org/10.1016/B978-012668450-6/50031-X.
http://dx.doi.org/10.1016/B978-012668450...
). Diatoms respond quickly to environmental changes and are commonly used in studies evaluating human-induced impacts on aquatic environments, such as those related to urbanization (Jüttner et al., 2012Jüttner, I., Chimonides, P.J., & Ormerod, S.J., 2012. Developing a diatom monitoring network in an urban river-basin: initial assessment and site selection. Hydrobiologia 695(1), 137-151. http://dx.doi.org/10.1007/s10750-012-1123-z.
http://dx.doi.org/10.1007/s10750-012-112...
; Chen et al., 2016Chen, X., Zhou, W., Pickett, S.T.A., Li, W., Han, L., & Ren, Y., 2016. Diatoms are better indicators of urban stream conditions: a case study in Beijing, China. Ecol. Indic. 60, 265-274. http://dx.doi.org/10.1016/j.ecolind.2015.06.039.
http://dx.doi.org/10.1016/j.ecolind.2015...
; Mbao et al., 2020Mbao, E.O., Gao, J., Wang, Y., Sitoki, L., Pan, Y., & Wang, B., 2020. Sensitivity and reliability of diatom metrics and guilds in detecting the impact of urbanization on streams. Ecol. Indic. 116, 106506. http://dx.doi.org/10.1016/j.ecolind.2020.106506.
http://dx.doi.org/10.1016/j.ecolind.2020...
) and eutrophication (Hering et al., 2006Hering, D., Johnson, R.K., Kramm, S., Schmutz, S., Szoszkiewicz, K., & Verdonschot, P.F.M., 2006. Assessment of European streams with diatoms, macrophytes, macroinvertebrates and fishes: a comparative metric-based analysis of organism response to stress. Freshw. Biol. 51(9), 1757-1785. http://dx.doi.org/10.1111/j.1365-2427.2006.01610.x.
http://dx.doi.org/10.1111/j.1365-2427.20...
; Vilmi et al., 2015Vilmi, A., Karjalainen, S.M., Landeiro, V.L., & Heino, J., 2015. Freshwater diatoms as environmental indicators: evaluating the effects of eutrophication using species morphology and biological indices. Environ. Monit. Assess. 187(5), 243. PMid:25864081. http://dx.doi.org/10.1007/s10661-015-4485-7.
http://dx.doi.org/10.1007/s10661-015-448...
; Licursi et al., 2016Licursi, M., Gómez, N., & Sabater, S., 2016. Effects of nutrient enrichment on epipelic diatom assemblages in a nutrient-rich lowland stream, Pampa region, Argentina. Hydrobiologia 766(1), 135-150. http://dx.doi.org/10.1007/s10750-015-2450-7.
http://dx.doi.org/10.1007/s10750-015-245...
; Zorzal-Almeida et al., 2021Zorzal-Almeida, S., Bartozek, E.C.R., & Bicudo, D.C., 2021. Homogenization of diatom assemblages is driven by eutrophication in tropical reservoirs. Environ. Pollut. 288, 117778. PMid:34280747. http://dx.doi.org/10.1016/j.envpol.2021.117778.
http://dx.doi.org/10.1016/j.envpol.2021....
). The composition of diatom communities usually presents substantial changes in response to eutrophication, resulting mostly from the dominance of some tolerant taxa (Chen et al., 2016Chen, X., Zhou, W., Pickett, S.T.A., Li, W., Han, L., & Ren, Y., 2016. Diatoms are better indicators of urban stream conditions: a case study in Beijing, China. Ecol. Indic. 60, 265-274. http://dx.doi.org/10.1016/j.ecolind.2015.06.039.
http://dx.doi.org/10.1016/j.ecolind.2015...
; Xiao et al., 2018Xiao, W., Liu, X., Irwin, A.J., Laws, E.A., Wang, L., Chen, B., Zeng, Y., & Huang, H., 2018. Warming and eutrophication combine to restructure diatoms and dinoflagellates. Water Res. 128, 206-216. PMid:29107905. http://dx.doi.org/10.1016/j.watres.2017.10.051.
http://dx.doi.org/10.1016/j.watres.2017....
).
In this context, we aimed to evaluate the effects of eutrophication, as a consequence of a gradient of urbanization, on benthic algal biomass and diatom communities from coastal streams, important marine-freshwater ecotones. Specifically, we expected to find a reduction in diatom species richness and an alteration in diatom community composition along the eutrophication gradient, causing a nested pattern of species distribution.
2. Material and Methods
2.1. Study area
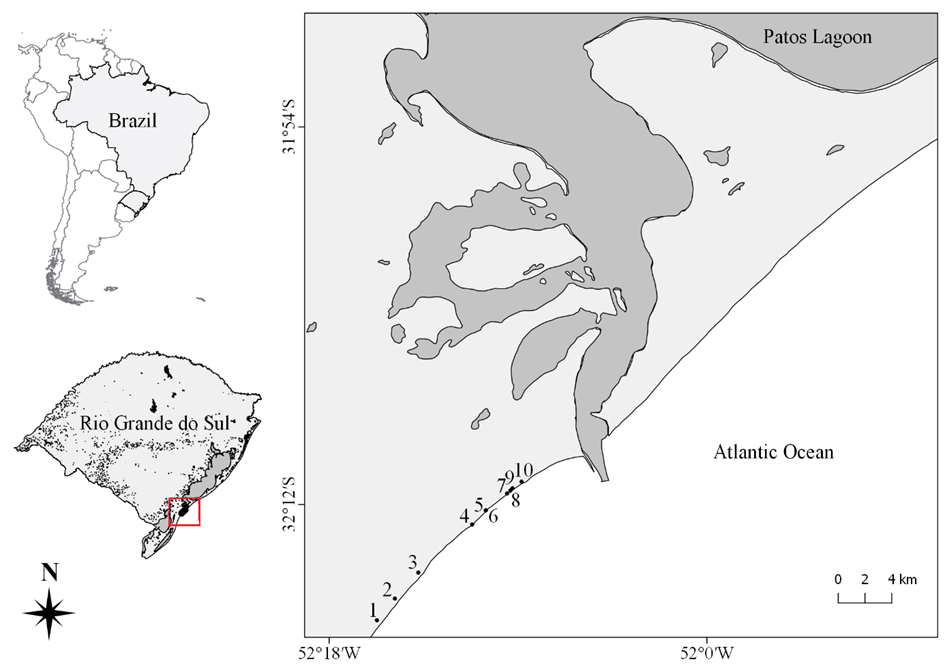
We sampled 10 coastal streams (intermittent and permanent ones) in the Coastal Plain of Rio Grande do Sul State, southern Brazil (Figure 1). The Coastal Plain of Rio Grande do Sul was formed in the Holocene from the deposition of sediments in events of marine transgressions and regressions, which formed a complex system of coastal lakes and wetlands (Schwarzbold & Schäfer, 1984Schwarzbold, A., & Schäfer, A., 1984. Gênese e morfologia das lagoas costeiras do Rio Grande do Sul, Brasil. Amazoniana 9, 87-104.). The average annual temperature and precipitation of the region are 18 ºC and 1200 mm, respectively (Cordazzo & Seeliger, 1995Cordazzo, V.C., & Seeliger, U., 1995. Guia Ilustrado da vegetação Costeira no Extremo Sul do Brasil. Rio Grande: Editora da FURG.). The climate of the region is subtropical super-humid mesothermic (Vieira, 1983Vieira, E.F. 1983. Rio Grande: geografia física, humana e econômica. Porto Alegre: Sagra.). The 10 coastal streams in which the samplings were carried are located in Cassino beach, Rio Grande (in the extreme south of Brazil), and are distributed along a remarkable urbanization gradient on 16.8 km of shore extension. Streams 1, 2, and 3 corresponded to a less urbanized area and consequently to less anthropogenic influence concerning sewage disposal. Streams 4 to 10 are located in an area of strong urban influence, although in regions with distinct degrees of urbanization. We sampled periphyton and obtained limnological variables on the same day in all streams (17 March 2016). In each stream, we selected a point with no shading near the foredunes and away from the sea surf zone.
Location of the 10 studied coastal streams in the Coastal Plain of Rio Grande do Sul State, Brazil.
2.2. Limnological variables
We obtained data on dissolved oxygen (DO), electrical conductivity (COND), pH, salinity (SAL), and total dissolved solids (TDS) using an in-situ Horiba U-50 multi-parameter probe. In addition, we sampled 500 ml of surface water at each stream for analyses of total nitrogen (TN; Allen et al., 1974Allen, S., Grimsha, W.N., Parkinson, J.A., & Quarmby, C., 1974. Chemical analysis of ecological materials. London: Blackwell Scientific Publishers.) and total phosphorus (TP; Valderrama, 1981Valderrama, J.C., 1981. The simultaneous analysis of total nitrogen and total phosphorus in natural waters. Mar. Chem. 10(2), 109-122. http://dx.doi.org/10.1016/0304-4203(81)90027-X.
http://dx.doi.org/10.1016/0304-4203(81)9...
; Baumgarten et al., 1996Baumgarten, M.G.Z., Rocha, J.M.B., & Niencheski, L.F.H., 1996. Manual de análises em oceanografia química. Rio Grande: Editora da FURG.).
2.3. Sampling of benthic algae and diatom counts
We sampled the benthic material from stolons of the aquatic plant Hydrocotyle ranunculoides L f., an herb belonging to the Araliaceae family associated with the foredune streams (Souza & Lorenzi, 2008Souza, V.C., & Lorenzi, H., 2008. Botânica Sistemática. São Paulo: Instituto Plantarum.). This substrate was chosen because it was found in all streams. We scraped the stolons with a soft brush and washed them with distilled water. Three stolons of similar sizes were scraped to obtain material for chlorophyll-a analysis and other three for community analysis. Immediately after sampling, we preserved the material for diatom community analysis with 4% formalin. Chlorophyll-a concentrations were determined according to Mackinney (1941)Mackinney, G., 1941. Absorption of light by chlorophyll solutions. J. Biol. Chem. 140(2), 315-322. http://dx.doi.org/10.1016/S0021-9258(18)51320-X.
http://dx.doi.org/10.1016/S0021-9258(18)...
, adapted by Paranhos (1996)Paranhos, R., 1996. Alguns métodos para análise de água. Rio de Janeiro: UFRJ. Cadernos Didáticos. and Chorus & Bartram (1999)Chorus, I., & Bartram, J., 1999. Water Resources. In Chorus, I. & Bartram, J., eds. Toxic cyanobacteria in water: a guide to their public health consequences, monitoring and management. New York: E & FN Spon. http://dx.doi.org/10.4324/9780203478073.
http://dx.doi.org/10.4324/9780203478073...
. We measured the scraped area of the stolons (using a pachymeter) to obtain the concentration of chlorophyll-a per unit area as a measure of benthic algal biomass (in µg cm-2). The aliquots reserved for the analyses of diatom communities were oxidized according to Simonsen (1974)Simonsen, R., 1974. The diatom plankton of the Indian Ocean Expedition of RV Meteor. Forsch Ergeb. 19, 1-107. adapted by Moreira-Filho & Valente-Moreira (1981)Moreira-Filho, H., & Valente-Moreira, I.M., 1981. Avaliação taxonômica e ecológica das diatomáceas (Bacillariophyceae) epífitas em algas pluricelulares obtidas nos litorais nos estados do Paraná, Santa Catarina e São Paulo. Bol Mus Mun 47, 1-17.. After this, we mounted permanent slides using Naphrax® (Brunel Microscopes Ltd., Chippenham, UK). To evaluate the species composition of the diatom communities we counted 300 valves from each stream using an optical microscope with a magnification of 1000×. All specimens were identified to the lowest possible taxonomic level, usually species, using specialized bibliography such Metzeltin et al. (2005)Metzeltin, D., Lange-Bertalot, H., & García-Rodríguez, F., 2005. Diatoms of Uruguay – Taxonomy Biogeography, Diversity. Königstein: A.R.G. Gantner Verlag., Metzeltin & García-Rodríguez (2012)Metzeltin, D., & García-Rodríguez, F., 2012. Las Diatomeas Uruguayas. Montevideo: DIRAC. and Round et al. (1990)Round, F.E., Crawford, R.M., & Mann, D.G., 1990. The diatoms: biology and morphology of the genera. Cambridge: Cambridge University Press..
2.4. Data analyses
We calculated the Trophic State Index for each stream using total phosphorus, according to Lamparelli (2004)Lamparelli, M.C., 2004. Graus de trofia em corpos d’água do Estado de São Paulo: avaliação dos métodos de monitoramento [Tese de doutorado em Ciências]. São Paulo: Departamento de Ecologia, Universidade de São Paulo.. To assess sampling sufficiency, we constructed a species accumulation curve using the rarefaction method (accumulation of individuals). We used multiple linear regressions to evaluate the influence of limnological variables on chlorophyll-a and on diatom species richness. To produce a minimum adequate model for each response variable, we performed a manual selection by sequentially removing non-significant explanatory variables until only significant variables remained in the final model. Five explanatory variables were included in the regressions (pH, DO, SAL, TN, and TP), all of which have variance inflation factor (VIF) smaller than 4 (Zuur et al., 2009Zuur, A., Ieno, E.N., Walker, N.J., Saveliev, A.A., & Smith, G.M., 2009. Mixed effects models and extensions in Ecology with R. New York: Springer. http://dx.doi.org/10.1007/978-0-387-87458-6.
http://dx.doi.org/10.1007/978-0-387-8745...
). Chlorophyll a was log-transformed to improve normality. Regression residuals meet the assumptions of homogeneity of variance, normality, and independence.
To explore differences in the composition of diatom communities among the coastal streams, we performed Nonmetric Multidimensional Scaling (NMDS) analyses on presence-absence and logarithmically transformed abundance data using Sørensen and Bray-Curtis dissimilarity coefficients (Legendre & Legendre, 1998Legendre, P., & Legendre, L., 1998. Numerical ecology. Amsterdam: Elsevier.), respectively, followed by the adjustment of environmental variables (envfit function available in the R vegan package; Oksanen et al., 2017Oksanen, J., Blanchet, F.G., Kindt, R., Legendre, P., Minchin, P.R., O’Hara, R.B., Simpsom, G.L., Solymos, P., Stevens, M.H.H., & Wagner, H., 2017. vegan: Community Ecology Package. R Package version 2.4-3 [online]. Retrieved in 2022, January 4, from http://CRAN.R-project.org/package=vegan
http://CRAN.R-project.org/package=vegan...
). We used Permutational Multivariate Analysis of Variance (PERMANOVA; Anderson, 2001Anderson, M.J., 2001. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 26, 32-46. https://doi.org/10.1111/j.1442-9993.2001.01070.pp.x.
https://doi.org/10.1111/j.1442-9993.2001...
) using the same dissimilarity coefficients described above and 999 permutations to verify which environmental variables influence the composition of communities. Finally, we used the Nestedness metric based on Overlap and Decreasing Fill (NODF; Almeida-Neto et al., 2008Almeida-Neto, M., Guimarães, P., Guimarães Junior, P.R., Loyola, R.D., & Ulrich, W., 2008. A consistente metric for nestedness analysis in ecological systems: reconciling concept and measurement. Oikos 117(8), 1227-1239. http://dx.doi.org/10.1111/j.0030-1299.2008.16644.x.
http://dx.doi.org/10.1111/j.0030-1299.20...
) to calculate nestedness. NODF values range from 0 to 100, with NODF = 100 representing perfectly nested communities. We calculated NODF only for rows (sites) since we aimed to test the occurrence of a nested pattern in species composition among streams. We generated two null models (with 999 permutations) to test the significance of the observed nestedness metric: 1) rows equiprobable and columns equiprobable (total number of species is preserved, but species richness and frequency of occurrence are not); 2) rows equiprobable and columns fixed (species frequencies are preserved, but site frequencies are not) (Wright et al., 1997Wright, D.H., Patterson, B.D., Mikkelson, G.M., Cutler, A., & Atmar, W., 1997. A comparative analysis of nested subset patterns of species composition. Oecologia. 113(1), 1-20. PMid:28307284. http://dx.doi.org/10.1007/s004420050348.
http://dx.doi.org/10.1007/s004420050348...
; Gotelli, 2000Gotelli, N.J., 2000. Null model analysis of species co-occurrence patterns. Ecology 81(9), 2606-2621. http://dx.doi.org/10.1890/0012-9658(2000)081[2606:NMAOSC]2.0.CO;2.
http://dx.doi.org/10.1890/0012-9658(2000...
; Jonsson, 2001Jonsson, B.G., 2001. A null model for randomization tests of nestedness in species assemblages. Oecologia 127(3), 309-313. PMid:28547100. http://dx.doi.org/10.1007/s004420000601.
http://dx.doi.org/10.1007/s004420000601...
). Spearman’s coefficient was applied to evaluate the correlation between the TP concentrations and the nestedness order of the coastal streams. We ran all analyses in the R environment (R Core Team, 2017R Core Team, 2017. R: A language and environment for statistical computing [online]. Vienna: R Foundation for Statistical Computing. Retrieved in 2022, January 4, from www.R-project.org
www.R-project.org...
), using the packages car (Fox & Weisberg, 2011Fox, J., & Weisberg, S., 2011. An {R} Companion to Applied Regression. 2nd ed. Thousand Oaks: Sage.) and vegan (Oksanen et al., 2017Oksanen, J., Blanchet, F.G., Kindt, R., Legendre, P., Minchin, P.R., O’Hara, R.B., Simpsom, G.L., Solymos, P., Stevens, M.H.H., & Wagner, H., 2017. vegan: Community Ecology Package. R Package version 2.4-3 [online]. Retrieved in 2022, January 4, from http://CRAN.R-project.org/package=vegan
http://CRAN.R-project.org/package=vegan...
).
3. Results
The coastal streams varied strongly in their limnological characteristics, mainly in relation to nutrients and dissolved oxygen (Table 1). The most striking gradient was of total phosphorus, so that the streams more distant from the urban area had six times lower concentrations of total phosphorus than the streams at the urbanized area (Table 1). Thus, the streams varied from mesotrophic to hypereutrophic environments.
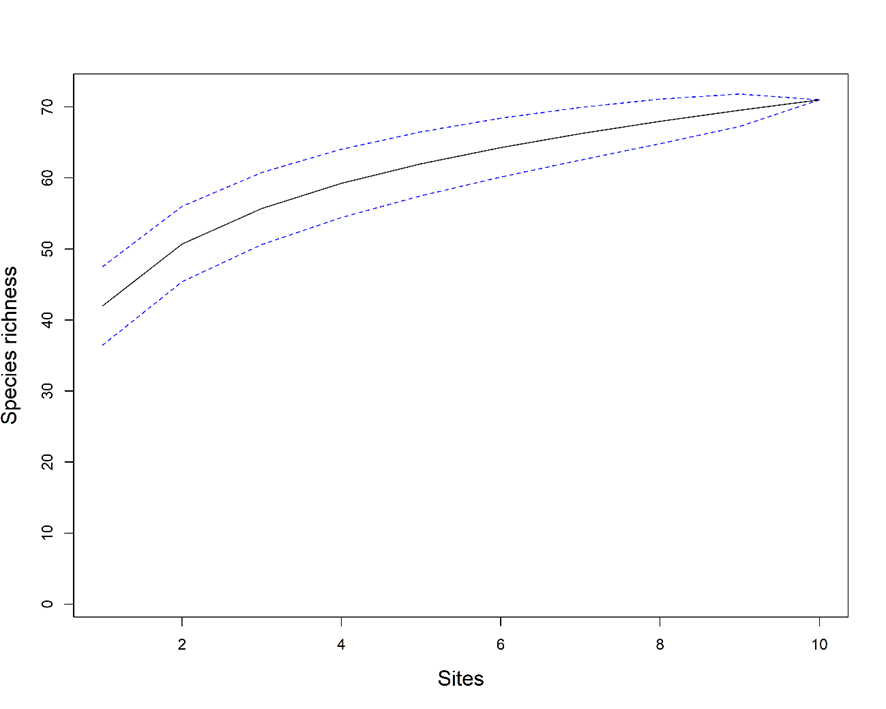
We found 71 taxa of diatoms, ranging from 15 to 34 taxa per stream (Table 1). However, the species accumulation curve did not reach an asymptote (Figure 2), indicating that species richness should be even greater. The more abundant species was Nitzschia palea, followed by Gomphonema parvulum and Nitzschia amphibia (Table 2). These species dominated in eutrophic streams, whereas species such as Capartogramma crucicola, Cocconeis placentula, and Nupela sp1 were commonly found in streams away from urban areas (streams 1, 2, and 3; Table 2).
Diatom’s species accumulation curve constructed by using the rarefaction method (black line with 95% confidence interval).
List of diatom taxa found in 10 coastal streams on southernmost Brazil, indicating the relative abundance of each species and the streams where each species occurred.
Benthic algal biomass measured as chlorophyll-a and diatom species richness were explained only by total phosphorus concentrations. Whereas chlorophyll-a presented a positive relationship with total phosphorus (P = 0.006; adjusted R2 = 0.58; Figure 3a), diatom species richness presented a negative relationship with it (P= 0.013; adjusted R2 = 0.50; Figure 3b).
(a) Periphytic biomass represented by chlorophyll-a and (b) species richness of diatoms in relation to the concentrations of total phosphorus in the sampled coastal streams.
We also found a significant nested pattern among diatom communities (NODFrows = 59.16; P = 0.001 for null model 1; P = 0.035 for null model 2; Figure 4). The nestedness of diatom communities strongly correlated with total phosphorus (rho = -0.83; P = 0.005), such that communities in streams with higher phosphorus concentrations were nested subsets of communities in streams with lower concentrations.
Nestedness order of the composition of diatom communities among the 10 coastal streams in southernmost Brazil. Black lines indicate species occurrence, line numbers indicate the streams, column numbers indicate species (respective species names are in Table 2).
Finally, the streams presented distinct communities along the eutrophication gradient for both presence-absence (Figure 5A) and abundance (Figure 5B) data. Corroborating this result, PERMANOVA analyses showed that differences in species composition among streams are explained by salinity (presence-absence: R2 = 0.17, P = 0.013; abundance: R2 = 0.16, P = 0.025), pH (presence-absence: R2 = 0.20, P = 0.001; abundance: R2 = 0.20, P = 0.002) and total phosphorus (presence-absence: R2= 0.16, P = 0.014; abundance: R2 = 0.15, P = 0.021).
NMDS of benthic diatom communities using (a) presence-absence data and (b) abundance data. The numbers represent the 10 sampled streams; DO = dissolved oxygen; TN = total nitrogen; TP = total phosphorus; pH = potential of hydrogen; Sal = salinity.
4. Discussion
In adequate concentrations, nitrogen and phosphorus are essential for the health of aquatic ecosystems (Søndergaard et al., 2017Søndergaard, M., Lauridsen, T.L., Johansson, L.S., & Jeppesen, E., 2017. Nitrogen or phosphorus limitation in lakes and its impact on phytoplankton biomass and submerged macrophyte cover. Hydrobiologia 795(1), 35-48. http://dx.doi.org/10.1007/s10750-017-3110-x.
http://dx.doi.org/10.1007/s10750-017-311...
). However, at high levels, these nutrients can have negative effects on water quality, resulting in a eutrophication process (Søndergaard et al., 2017Søndergaard, M., Lauridsen, T.L., Johansson, L.S., & Jeppesen, E., 2017. Nitrogen or phosphorus limitation in lakes and its impact on phytoplankton biomass and submerged macrophyte cover. Hydrobiologia 795(1), 35-48. http://dx.doi.org/10.1007/s10750-017-3110-x.
http://dx.doi.org/10.1007/s10750-017-311...
). Eutrophication is among the main causes of species losses and changes in the composition of aquatic communities worldwide (Allan & Flecker, 1993Allan, J.D., & Flecker, A.S., 1993. Biodiversity conservation in running waters: identifying the major factors that threaten destruction of riverine species and ecosystems. Bioscience 43(1), 32-43. http://dx.doi.org/10.2307/1312104.
http://dx.doi.org/10.2307/1312104...
; Strayer & Dudgeon, 2010Strayer, D.L., & Dudgeon, D., 2010. Freshwater biodiversity conservation: recent progress and future challenges. J. N. Am. Benthol. Soc. 29(1), 344-358. http://dx.doi.org/10.1899/08-171.1.
http://dx.doi.org/10.1899/08-171.1...
). Those changes affect ecosystem functioning by changing, for example, the quality and quantity of primary consumer food sources and the decomposition of detritus (Dodds & Smith, 2016Dodds, W.K., & Smith, V.H., 2016. Nitrogen, phosphorus and eutrophication in streams. Inland Waters 6(2), 155-164. http://dx.doi.org/10.5268/IW-6.2.909.
http://dx.doi.org/10.5268/IW-6.2.909...
). Our results showed that eutrophication increased benthic algal biomass in the studied marine-freshwater ecotones, but reduced diatom species richness and changed community composition by selectively removing less tolerant species, as indicated by the observed nested distribution.
Studies in urban freshwater ecosystems have increased in the last decades (Grimm et al., 2000Grimm, N.B., Morgan Grove, J., Pickett, S.T.A., & Redman, C.L., 2000. Integrated approaches to long-term studies of urban ecological systems. Bioscience 50(7), 571-584. http://dx.doi.org/10.1641/0006-3568(2000)050[0571:IATLTO]2.0.CO;2.
http://dx.doi.org/10.1641/0006-3568(2000...
), mainly after the elaboration of the urban stream syndrome concept (Walsh et al., 2005Walsh, C.J., Roy, A.H., Feminella, J.W., Cottingham, P.D., Groffman, P.M., & Morgan 2nd, R.P., 2005. The urban stream syndrome: current knowledge and the search for a cure. J. N. Am. Benthol. Soc. 24(3), 706-723. http://dx.doi.org/10.1899/04-028.1.
http://dx.doi.org/10.1899/04-028.1...
). Our results are in accordance with this concept since we found that streams within urban areas are more prone to eutrophication, which leads to lower species richness than in streams less influenced by urbanization. The excessive increase of nutrients due to urbanization has already been shown to occur in other Brazilian regions (Tromboni & Dodds, 2017Tromboni, F., & Dodds, W.K., 2017. Relationships between land use and stream nutrient concentrations in a highly urbanized tropical region of Brazil: thresholds and riparian zones. Environ. Manage. 60(1), 30-40. PMid:28405753. http://dx.doi.org/10.1007/s00267-017-0858-8.
http://dx.doi.org/10.1007/s00267-017-085...
), as well as the negative impacts generated by sewage from urban areas for the biodiversity in several regions of the world (Barnum et al., 2017Barnum, T.R., Weller, D.E., & Williams, M., 2017. Urbanization reduces and homogenizes traits diversity in stream macroinvertebrate communities. Ecol. Appl. 27(8), 2428-2442. PMid:28872731. http://dx.doi.org/10.1002/eap.1619.
http://dx.doi.org/10.1002/eap.1619...
; Cunningham & Gharipour, 2018Cunningham, C., & Gharipour, M., 2018. Pipe dreams: urban wastewater treatment for biodiversity conservation. Urban Sci. 2(1), 2-18. http://dx.doi.org/10.3390/urbansci2010010.
http://dx.doi.org/10.3390/urbansci201001...
).
In flowing waters, both phosphorus and nitrogen are recognized as playing important roles in the eutrophication process (Dodds & Smith, 2016Dodds, W.K., & Smith, V.H., 2016. Nitrogen, phosphorus and eutrophication in streams. Inland Waters 6(2), 155-164. http://dx.doi.org/10.5268/IW-6.2.909.
http://dx.doi.org/10.5268/IW-6.2.909...
), despite phosphorus being usually considered the most important limiting nutrient in freshwater environments and thus, the main responsible for eutrophication (Hecky & Kilham, 1988Hecky, R.E., & Kilham, P., 1988. Nutrient limitation of phytoplankton in freshwater and marine environments: a review of recent evidence on the effects of enrichment. Limnol. Oceanogr. 33(4 Pt 2), 796-822. http://dx.doi.org/10.4319/lo.1988.33.4part2.0796.
http://dx.doi.org/10.4319/lo.1988.33.4pa...
; Schindler, 2012Schindler, D.W., 2012. The dilemma of controlling cultural eutrophication of lakes. Proc Royal Soc B. 279(1746), 4322-4333. PMid:22915669. http://dx.doi.org/10.1098/rspb.2012.1032.
http://dx.doi.org/10.1098/rspb.2012.1032...
). Empirical evidence shows that stream benthic algal communities strongly respond to both nitrogen and phosphorus (Dodds & Smith, 2016Dodds, W.K., & Smith, V.H., 2016. Nitrogen, phosphorus and eutrophication in streams. Inland Waters 6(2), 155-164. http://dx.doi.org/10.5268/IW-6.2.909.
http://dx.doi.org/10.5268/IW-6.2.909...
). However, in our study, only total phosphorus (in addition to pH and salinity) explained the observed changes.
The observed decrease in species richness contradicts part of the literature on nutrient enrichment. For instance, in a meta-analysis, Hillebrand et al. (2007)Hillebrand, H., Gruner, D.S., Borer, E.T., Bracken, M.E., Cleland, E.E., Elser, J.J., Harpole, W.S., Ngai, J.T., Seabloom, E.W., Shurin, J.B., & Smith, J.E., 2007. Consumer versus resource control of producer diversity depends on ecosystem type and producer community structure. Proc. Natl. Acad. Sci. USA 104(26), 10904-10909. PMid:17581875. http://dx.doi.org/10.1073/pnas.0701918104.
http://dx.doi.org/10.1073/pnas.070191810...
found that species richness of primary producers increases with nutrient enrichment in freshwater ecosystems, results corroborated by Schneider et al. (2013)Schneider, S.C., Kahlert, M., & Kelly, M.G., 2013. Interactions between pH and nutrients on benthic algae in streams and consequences for ecological status assessment and species richness patterns. Sci. Total Environ. 444(1), 73-84. PMid:23266552. http://dx.doi.org/10.1016/j.scitotenv.2012.11.034.
http://dx.doi.org/10.1016/j.scitotenv.20...
and Soininen et al. (2016)Soininen, J., Jamoneau, A., Rosebery, J., & Passy, S.I., 2016. Global patterns and drivers of species and trait composition in diatoms. Glob. Ecol. Biogeogr. 25(8), 940-950. http://dx.doi.org/10.1111/geb.12452.
http://dx.doi.org/10.1111/geb.12452...
for stream benthic diatoms. However, our study sites presented high levels of nutrient concentrations, such that the minimum total phosphorus values we found equal the maximum values from Schneider et al. (2013)Schneider, S.C., Kahlert, M., & Kelly, M.G., 2013. Interactions between pH and nutrients on benthic algae in streams and consequences for ecological status assessment and species richness patterns. Sci. Total Environ. 444(1), 73-84. PMid:23266552. http://dx.doi.org/10.1016/j.scitotenv.2012.11.034.
http://dx.doi.org/10.1016/j.scitotenv.20...
, which, in turn, are 6-fold smaller than the maximum phosphorus concentration found in our study. Thus, the reduction in diatom species richness and the nested distribution of communities related to the gradient of phosphorus concentrations among the studied streams suggest that the high levels of eutrophication resulted in harsh environmental conditions in which only a subset of generalist and eutrophic-tolerant species from the regional species pool were able to persist (Goldenberg Vilar et al., 2014Goldenberg Vilar, A.G., Van Dam, H., Van Loon, E.E., Vonk, J.A., Van Der Geest, H.G., & Admiraal, W., 2014. Eutrophication decrease distance decay of similarity in diatom communities. Freshw. Biol. 59(7), 1522-1531. http://dx.doi.org/10.1111/fwb.12363.
http://dx.doi.org/10.1111/fwb.12363...
). On the other hand, the less eutrophic streams harbored both sensitive and generalist/tolerant species, resulting in higher species richness than in eutrophic streams. For example, Gomphonema parvulum and different species of the genus Nitzschia are widely known to be tolerant to and indicators of anthropogenic eutrophication in lotic environments (Bere & Tundisi, 2010Bere, T., & Tundisi, J.G., 2010. Epipsammic diatoms in streams influenced by urban pollution, São Carlos, SP, Brazil. Braz. J. Biol. 70(4), 921-930. PMid:21180895. http://dx.doi.org/10.1590/S1519-69842010000500002.
http://dx.doi.org/10.1590/S1519-69842010...
; Bere & Tundisi, 2011Bere, T., & Tundisi, J.G., 2011. Diatom-based water quality assessment in streams influence by urban pollution: effects of natural and two selected artificial substrates, São Carlos-SP, Brazil. Braz. J. Aquat. Sci. Tech. 15(1), 54-63. http://dx.doi.org/10.14210/bjast.v15n1.p54-63.
http://dx.doi.org/10.14210/bjast.v15n1.p...
; Moresco et al., 2015Moresco, C., Gubiani, E.A., & Rodrigues, L., 2015. Periphytic diatoms as indicators in a tropical stream: from urban to rural environments. Acta Sci. Biol. Sci. 37(4), 427-437. http://dx.doi.org/10.4025/actascibiolsci.v37i4.27426.
http://dx.doi.org/10.4025/actascibiolsci...
). These species occurred in all streams, but were more abundant in those streams with the highest concentrations of phosphorus. In contrast, species such as Capartogramma crucicola and Cocconeis placentula, considered to have a low tolerance to eutrophication (e.g., Silva-Benavides, 1996Silva-Benavides, A.M., 1996. The epilithic diatom flora of a pristine and a polluted river in Costa Rica, Central America. Diatom Res. 11(1), 105-142. http://dx.doi.org/10.1080/0269249X.1996.9705368.
http://dx.doi.org/10.1080/0269249X.1996....
; Lobo et al., 2004Lobo, E.A., Callegaro, V.L.M., Hermany, G., Bes, D., Wetzel, C.E., & Oliveira, C.A., 2004. Use of epilithics diatoms as bioindicators from lotic systems in Southern Brazil, with special emphasis on eutrophication. Acta Limnol. Bras. 16(1), 25-40.; Salomoni et al., 2006Salomoni, S.E., Rocha, O., Callegaro, V.L., & Lobo, E.A., 2006. Epilithic diatoms as indicators of water quality in the Gravataí river, Rio Grande do Sul, Brazil. Hydrobiologia 559(1), 233-246. http://dx.doi.org/10.1007/s10750-005-9012-3.
http://dx.doi.org/10.1007/s10750-005-901...
), occurred more frequently in the streams with low nutrient concentrations in our study. Moreover, we identified one species of the genus Nupela in streams 1 and 2 (with the lowest phosphorus concentrations) and several authors emphasize the preference of this genus of oligotrophic environments (Wojtal, 2009Wojtal, A.Z., 2009. Nupela marvanii sp. nov., and N. lapidosa (Krasske) Lange-Bertalot in Poland with notes on the distribuition and ecology of the genus of Nupela (Bacillariophyta). Fottea. 9(2), 233-242. http://dx.doi.org/10.5507/fot.2009.024.
http://dx.doi.org/10.5507/fot.2009.024...
) or with moderated nutrient concentrations (Potapova et al., 2003Potapova, M.G., Ponader, K.C., Lowe, R.L., Clason, T.A., & Bahls, L.L., 2003. Small-celled Nupela species from North America. Diatom Res. 18(2), 293-306. http://dx.doi.org/10.1080/0269249X.2003.9705593.
http://dx.doi.org/10.1080/0269249X.2003....
).
Further, the observed changes in the species composition of benthic diatom communities along the eutrophication gradient is an expected result, since this group is known for rapidly responding to changes in physical and chemical characteristics of water (Hering et al., 2006Hering, D., Johnson, R.K., Kramm, S., Schmutz, S., Szoszkiewicz, K., & Verdonschot, P.F.M., 2006. Assessment of European streams with diatoms, macrophytes, macroinvertebrates and fishes: a comparative metric-based analysis of organism response to stress. Freshw. Biol. 51(9), 1757-1785. http://dx.doi.org/10.1111/j.1365-2427.2006.01610.x.
http://dx.doi.org/10.1111/j.1365-2427.20...
; Jyrkänkallio-Mikkola et al., 2017Jyrkänkallio-Mikkola, J., Meier, S., Heino, J., Laamanen, T., Pajunen, V., Tolonen, K.T., Tolkkinen, M., & Soininen, J., 2017. Disentangling multi-scale environmental effects on stream microbial communities. J. Biogeogr. 44(7), 1512-1523. http://dx.doi.org/10.1111/jbi.13002.
http://dx.doi.org/10.1111/jbi.13002...
). In addition to phosphorus, pH and salinity also affected the organization of communities in the studied streams. These two variables were probably important because of the characteristics of the studied ecosystems. Many of the coastal streams in the study region are formed in lakes and wetlands located behind the foredunes (Figueiredo & Calliari, 2005Figueiredo, S.A., & Calliari, L.J., 2005. Sangradouros: distribuição espacial, variação sazonal, padrões morfológicos e implicações no gerenciamento costeiro. Gravel 3, 45-57.), thus being responsible for the drainage of these systems and of pluvial waters towards the sea, carrying a considerable amount of organic matter and sediment (Figueiredo & Calliari, 2005Figueiredo, S.A., & Calliari, L.J., 2005. Sangradouros: distribuição espacial, variação sazonal, padrões morfológicos e implicações no gerenciamento costeiro. Gravel 3, 45-57., 2006Figueiredo, S.A., & Calliari, L.J., 2006. Washouts in the central and northern litoral of Rio Grande do Sul state, Brazil: distribution and implications. J. Coast. Res. 39, 366-370.). Moreover, these streams are constantly affected by seawater, increasing their salinity.
One main caveat of our study is that it was based on only one sampling at each coastal stream. This snapshot sampling did not capture seasonal changes in the nutrient loads of the streams, related either to variation in rainfall or to variation on the discharge of sewage. The latter is mostly related to the increased population during austral summer since this neighborhood attracts thousands of tourists every year (Silva, 2012Silva, L.C. 2012. O desenvolvimento do turismo no balneário Cassino: um problema de gerenciamento costeiro integrado [Dissertação de mestrado em Gerenciamento Costeiro]. Rio Grande. Universidade Federal do Rio Grande - FURG.). Further, during summer, the high evaporation rates reduce the volume and the number of these streams (Figueiredo & Calliari, 2006Figueiredo, S.A., & Calliari, L.J., 2006. Washouts in the central and northern litoral of Rio Grande do Sul state, Brazil: distribution and implications. J. Coast. Res. 39, 366-370.). Thus, despite the snapshot sampling and the reduced dataset, our sampling at the beginning of March was able to capture the synergistic effects of reduced water volume and increased nutrient inputs in the streams.
It is known that the science of eutrophication in lotic environments is not as advanced as in lentic environments (Dodds & Smith, 2016Dodds, W.K., & Smith, V.H., 2016. Nitrogen, phosphorus and eutrophication in streams. Inland Waters 6(2), 155-164. http://dx.doi.org/10.5268/IW-6.2.909.
http://dx.doi.org/10.5268/IW-6.2.909...
), especially when considering these coastal freshwater-marine ecotones. Therefore, we showed in this study the importance of such environments to biological diversity, as shown by the elevated number of diatom species found. We also found that modifications in water quality caused by eutrophication due to the urbanization process are leading to the local loss of species and changes in the composition of biological communities. It is fundamental that more studies on these coastal ecotones evaluate their biodiversity and how anthropogenic impacts are affecting their functioning.
Acknowledgements
We thank Camila Bosenbecker and Fernanda Marques for helping with the map of the studied area, and Clara Lima, Claudio Trindade and Leonardo Furlanetto for helping with nutrient analyses. APTC, ECC and CFMS received student fellowships from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brazil (CAPES) – Finance Code 001.
-
Cite as: Costa, A.P.T. et al. Eutrophication changes community composition and drives nestedness of benthic diatoms from coastal streams. Acta Limnologica Brasiliensia, 2022, vol. 34, e14.
References
- Albertoni, E.F., & Palma-Silva, C., 2006. Macroinvertebrados associados a macrófitas aquáticas flutuantes em canais urbanos de escoamento pluvial (Balneário Cassino, Rio Grande, RS). Neotrop. Biol. Conserv. 1(2), 90-100.
- Allan, J.D., & Flecker, A.S., 1993. Biodiversity conservation in running waters: identifying the major factors that threaten destruction of riverine species and ecosystems. Bioscience 43(1), 32-43. http://dx.doi.org/10.2307/1312104
» http://dx.doi.org/10.2307/1312104 - Allen, S., Grimsha, W.N., Parkinson, J.A., & Quarmby, C., 1974. Chemical analysis of ecological materials. London: Blackwell Scientific Publishers.
- Almeida-Neto, M., Guimarães, P., Guimarães Junior, P.R., Loyola, R.D., & Ulrich, W., 2008. A consistente metric for nestedness analysis in ecological systems: reconciling concept and measurement. Oikos 117(8), 1227-1239. http://dx.doi.org/10.1111/j.0030-1299.2008.16644.x
» http://dx.doi.org/10.1111/j.0030-1299.2008.16644.x - Anderson, M.J., 2001. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 26, 32-46. https://doi.org/10.1111/j.1442-9993.2001.01070.pp.x
» https://doi.org/10.1111/j.1442-9993.2001.01070.pp.x - Barnum, T.R., Weller, D.E., & Williams, M., 2017. Urbanization reduces and homogenizes traits diversity in stream macroinvertebrate communities. Ecol. Appl. 27(8), 2428-2442. PMid:28872731. http://dx.doi.org/10.1002/eap.1619
» http://dx.doi.org/10.1002/eap.1619 - Baselga, A., 2010. Partitioning the turnover and nestedness components of beta diversity. Glob. Ecol. Biogeogr. 19(1), 134-143. http://dx.doi.org/10.1111/j.1466-8238.2009.00490.x
» http://dx.doi.org/10.1111/j.1466-8238.2009.00490.x - Bastos, R.F., Condini, M.V., & Garcia, A.M., 2013. Fish species list of coastal streams in Southern Brazil, with notes on austral distribution limits of marine and freshwater endangered species. Pan-Am. J. Aquat. Sci. 8, 347-351.
- Baumgarten, M.G.Z., Millão, D., Costa, P.G., Attisano, K.K., Costa, N.B.D., Gutierres, F.B., Giordano, S.B., & Araújo, E.A.C., 2007. Praia do Cassino (Rio Grande -RS): qualidade da água dos sangradouros da área central – antes (2003) e depois (2005) da instalação de estação de tratamento de esgotos (ETE). Cad Ecol Aquat 2, 1-12.
- Baumgarten, M.G.Z., Rocha, J.M.B., & Niencheski, L.F.H., 1996. Manual de análises em oceanografia química. Rio Grande: Editora da FURG.
- Bere, T., & Tundisi, J.G., 2010. Epipsammic diatoms in streams influenced by urban pollution, São Carlos, SP, Brazil. Braz. J. Biol. 70(4), 921-930. PMid:21180895. http://dx.doi.org/10.1590/S1519-69842010000500002
» http://dx.doi.org/10.1590/S1519-69842010000500002 - Bere, T., & Tundisi, J.G., 2011. Diatom-based water quality assessment in streams influence by urban pollution: effects of natural and two selected artificial substrates, São Carlos-SP, Brazil. Braz. J. Aquat. Sci. Tech. 15(1), 54-63. http://dx.doi.org/10.14210/bjast.v15n1.p54-63
» http://dx.doi.org/10.14210/bjast.v15n1.p54-63 - Biggs, B.J.F., 1996. Patterns in benthic algae of streams. In: Stevenson, J., Bothwell, M.L. & Lowe, R.L., eds. Algal ecology: freshwater benthic ecosystems. San Diego: Elsevier. http://dx.doi.org/10.1016/B978-012668450-6/50031-X
» http://dx.doi.org/10.1016/B978-012668450-6/50031-X - Chen, X., Zhou, W., Pickett, S.T.A., Li, W., Han, L., & Ren, Y., 2016. Diatoms are better indicators of urban stream conditions: a case study in Beijing, China. Ecol. Indic. 60, 265-274. http://dx.doi.org/10.1016/j.ecolind.2015.06.039
» http://dx.doi.org/10.1016/j.ecolind.2015.06.039 - Chorus, I., & Bartram, J., 1999. Water Resources. In Chorus, I. & Bartram, J., eds. Toxic cyanobacteria in water: a guide to their public health consequences, monitoring and management. New York: E & FN Spon. http://dx.doi.org/10.4324/9780203478073
» http://dx.doi.org/10.4324/9780203478073 - Cordazzo, V.C., & Seeliger, U., 1995. Guia Ilustrado da vegetação Costeira no Extremo Sul do Brasil. Rio Grande: Editora da FURG.
- Cunningham, C., & Gharipour, M., 2018. Pipe dreams: urban wastewater treatment for biodiversity conservation. Urban Sci. 2(1), 2-18. http://dx.doi.org/10.3390/urbansci2010010
» http://dx.doi.org/10.3390/urbansci2010010 - Di Carvalho, J.A., & Wickham, S.A., 2019. Simulating eutriphication in a metacommunity landscape: an aquatic model ecosystem. Oecologia 189(2), 461-474. PMid:30523402. http://dx.doi.org/10.1007/s00442-018-4319-8
» http://dx.doi.org/10.1007/s00442-018-4319-8 - Dodds, W.K., & Smith, V.H., 2016. Nitrogen, phosphorus and eutrophication in streams. Inland Waters 6(2), 155-164. http://dx.doi.org/10.5268/IW-6.2.909
» http://dx.doi.org/10.5268/IW-6.2.909 - Dudgeon, D., 2019. Multiple threats imperil freshwater biodiversity in the Anthropocene. Curr. Biol. 29(19), 960-967. PMid:31593677. http://dx.doi.org/10.1016/j.cub.2019.08.002
» http://dx.doi.org/10.1016/j.cub.2019.08.002 - Dudgeon, D., Arthington, A.H., Gessner, M.O., Kawabata, Z.-I., Knowler, D.J., Lévêque, C., Naiman, R.J., Prieur-Richard, A.H., Soto, D., Stiassny, M.L.J., & Sullivan, C.A., 2006. Freshwater biodiversity: importance, threats, status and conservation challenges. Biol. Rev. Camb. Philos. Soc. 81(2), 163-182. PMid:16336747. http://dx.doi.org/10.1017/S1464793105006950
» http://dx.doi.org/10.1017/S1464793105006950 - Figueiredo, S.A., & Calliari, L.J., 2005. Sangradouros: distribuição espacial, variação sazonal, padrões morfológicos e implicações no gerenciamento costeiro. Gravel 3, 45-57.
- Figueiredo, S.A., & Calliari, L.J., 2006. Washouts in the central and northern litoral of Rio Grande do Sul state, Brazil: distribution and implications. J. Coast. Res. 39, 366-370.
- Fox, J., & Weisberg, S., 2011. An {R} Companion to Applied Regression. 2nd ed. Thousand Oaks: Sage.
- Goldenberg Vilar, A.G., Van Dam, H., Van Loon, E.E., Vonk, J.A., Van Der Geest, H.G., & Admiraal, W., 2014. Eutrophication decrease distance decay of similarity in diatom communities. Freshw. Biol. 59(7), 1522-1531. http://dx.doi.org/10.1111/fwb.12363
» http://dx.doi.org/10.1111/fwb.12363 - Gotelli, N.J., 2000. Null model analysis of species co-occurrence patterns. Ecology 81(9), 2606-2621. http://dx.doi.org/10.1890/0012-9658(2000)081[2606:NMAOSC]2.0.CO;2
» http://dx.doi.org/10.1890/0012-9658(2000)081[2606:NMAOSC]2.0.CO;2 - Grimm, N.B., Faeth, S.H., Golubiewski, N.E., Redman, C.L., Wu, J., Bai, W., & Briggs, J.M., 2008. Global change and the ecology of cities. Science 319(5864), 756-760. PMid:18258902. http://dx.doi.org/10.1126/science.1150195
» http://dx.doi.org/10.1126/science.1150195 - Grimm, N.B., Morgan Grove, J., Pickett, S.T.A., & Redman, C.L., 2000. Integrated approaches to long-term studies of urban ecological systems. Bioscience 50(7), 571-584. http://dx.doi.org/10.1641/0006-3568(2000)050[0571:IATLTO]2.0.CO;2
» http://dx.doi.org/10.1641/0006-3568(2000)050[0571:IATLTO]2.0.CO;2 - Gutierrez, M.F., Simões, N.R., Frau, D., Saigo, M., & Licursi, M., 2020. Responses of stream zooplankton diversity metrics to eutrophication and temporal environmental variability in agricultural catchments. Environ. Monit. Assess. 192(12), 1-17. PMid:33242179. http://dx.doi.org/10.1007/s10661-020-08766-5
» http://dx.doi.org/10.1007/s10661-020-08766-5 - Gutiérrez-Cánovas, C., Millán, A., Velasco, J., Vaughan, I.P., & Ormerod, S.J., 2013. Contrasting effects of natural and anthropogenic stressors on beta diversity in river organisms. Glob. Ecol. Biogeogr. 22(7), 796-805. http://dx.doi.org/10.1111/geb.12060
» http://dx.doi.org/10.1111/geb.12060 - Hecky, R.E., & Kilham, P., 1988. Nutrient limitation of phytoplankton in freshwater and marine environments: a review of recent evidence on the effects of enrichment. Limnol. Oceanogr. 33(4 Pt 2), 796-822. http://dx.doi.org/10.4319/lo.1988.33.4part2.0796
» http://dx.doi.org/10.4319/lo.1988.33.4part2.0796 - Hering, D., Johnson, R.K., Kramm, S., Schmutz, S., Szoszkiewicz, K., & Verdonschot, P.F.M., 2006. Assessment of European streams with diatoms, macrophytes, macroinvertebrates and fishes: a comparative metric-based analysis of organism response to stress. Freshw. Biol. 51(9), 1757-1785. http://dx.doi.org/10.1111/j.1365-2427.2006.01610.x
» http://dx.doi.org/10.1111/j.1365-2427.2006.01610.x - Hillebrand, H., Gruner, D.S., Borer, E.T., Bracken, M.E., Cleland, E.E., Elser, J.J., Harpole, W.S., Ngai, J.T., Seabloom, E.W., Shurin, J.B., & Smith, J.E., 2007. Consumer versus resource control of producer diversity depends on ecosystem type and producer community structure. Proc. Natl. Acad. Sci. USA 104(26), 10904-10909. PMid:17581875. http://dx.doi.org/10.1073/pnas.0701918104
» http://dx.doi.org/10.1073/pnas.0701918104 - Jacobson, P.C., Hansen, G.J.A., Bethke, B.J., & Cross, T.K., 2017. Disentangling the effects of a century of eutrophication and climatic warming on freshwater lake fish assemblages. PLoS One 12(8), e0182667. PMid:28777816. http://dx.doi.org/10.1371/journal.pone.0182667
» http://dx.doi.org/10.1371/journal.pone.0182667 - Jamoneau, A., Passy, S.I., Soininen, J., Leboucher, T., & Tison-Rosebery, J., 2017. Beta diversity of diatoms species and ecological guilds: response to environmental and spatial mechanisms along the stream watercourse. Freshw. Biol. 63(1), 62-73. http://dx.doi.org/10.1111/fwb.12980
» http://dx.doi.org/10.1111/fwb.12980 - Jonsson, B.G., 2001. A null model for randomization tests of nestedness in species assemblages. Oecologia 127(3), 309-313. PMid:28547100. http://dx.doi.org/10.1007/s004420000601
» http://dx.doi.org/10.1007/s004420000601 - Jüttner, I., Chimonides, P.J., & Ormerod, S.J., 2012. Developing a diatom monitoring network in an urban river-basin: initial assessment and site selection. Hydrobiologia 695(1), 137-151. http://dx.doi.org/10.1007/s10750-012-1123-z
» http://dx.doi.org/10.1007/s10750-012-1123-z - Jyrkänkallio-Mikkola, J., Meier, S., Heino, J., Laamanen, T., Pajunen, V., Tolonen, K.T., Tolkkinen, M., & Soininen, J., 2017. Disentangling multi-scale environmental effects on stream microbial communities. J. Biogeogr. 44(7), 1512-1523. http://dx.doi.org/10.1111/jbi.13002
» http://dx.doi.org/10.1111/jbi.13002 - Lamparelli, M.C., 2004. Graus de trofia em corpos d’água do Estado de São Paulo: avaliação dos métodos de monitoramento [Tese de doutorado em Ciências]. São Paulo: Departamento de Ecologia, Universidade de São Paulo.
- Legendre, P., & Legendre, L., 1998. Numerical ecology. Amsterdam: Elsevier.
- Licursi, M., Gómez, N., & Sabater, S., 2016. Effects of nutrient enrichment on epipelic diatom assemblages in a nutrient-rich lowland stream, Pampa region, Argentina. Hydrobiologia 766(1), 135-150. http://dx.doi.org/10.1007/s10750-015-2450-7
» http://dx.doi.org/10.1007/s10750-015-2450-7 - Lobo, E.A., Callegaro, V.L.M., Hermany, G., Bes, D., Wetzel, C.E., & Oliveira, C.A., 2004. Use of epilithics diatoms as bioindicators from lotic systems in Southern Brazil, with special emphasis on eutrophication. Acta Limnol. Bras. 16(1), 25-40.
- Mackinney, G., 1941. Absorption of light by chlorophyll solutions. J. Biol. Chem. 140(2), 315-322. http://dx.doi.org/10.1016/S0021-9258(18)51320-X
» http://dx.doi.org/10.1016/S0021-9258(18)51320-X - Mbao, E.O., Gao, J., Wang, Y., Sitoki, L., Pan, Y., & Wang, B., 2020. Sensitivity and reliability of diatom metrics and guilds in detecting the impact of urbanization on streams. Ecol. Indic. 116, 106506. http://dx.doi.org/10.1016/j.ecolind.2020.106506
» http://dx.doi.org/10.1016/j.ecolind.2020.106506 - Metzeltin, D., & García-Rodríguez, F., 2012. Las Diatomeas Uruguayas. Montevideo: DIRAC.
- Metzeltin, D., Lange-Bertalot, H., & García-Rodríguez, F., 2005. Diatoms of Uruguay – Taxonomy Biogeography, Diversity. Königstein: A.R.G. Gantner Verlag.
- Moreira-Filho, H., & Valente-Moreira, I.M., 1981. Avaliação taxonômica e ecológica das diatomáceas (Bacillariophyceae) epífitas em algas pluricelulares obtidas nos litorais nos estados do Paraná, Santa Catarina e São Paulo. Bol Mus Mun 47, 1-17.
- Moresco, C., Gubiani, E.A., & Rodrigues, L., 2015. Periphytic diatoms as indicators in a tropical stream: from urban to rural environments. Acta Sci. Biol. Sci. 37(4), 427-437. http://dx.doi.org/10.4025/actascibiolsci.v37i4.27426
» http://dx.doi.org/10.4025/actascibiolsci.v37i4.27426 - Oksanen, J., Blanchet, F.G., Kindt, R., Legendre, P., Minchin, P.R., O’Hara, R.B., Simpsom, G.L., Solymos, P., Stevens, M.H.H., & Wagner, H., 2017. vegan: Community Ecology Package. R Package version 2.4-3 [online]. Retrieved in 2022, January 4, from http://CRAN.R-project.org/package=vegan
» http://CRAN.R-project.org/package=vegan - Paranhos, R., 1996. Alguns métodos para análise de água. Rio de Janeiro: UFRJ. Cadernos Didáticos.
- Patterson, B.D., & Atmar, W., 1986. Nested subsets and the structure of insular mammalian and archipelagos. Biol. J. Linn. Soc. Lond. 28(1-2), 65-82. http://dx.doi.org/10.1111/j.1095-8312.1986.tb01749.x
» http://dx.doi.org/10.1111/j.1095-8312.1986.tb01749.x - Pereira da Silva, R., 1998. Ocorrência, distribuição e características morfodinâmicas dos sangradouros na zona costeira do Rio Grande do Sul: trecho Rio Grande – Chuí, RS [Dissertação de mestrado em Geociências]. Porto Alegre: Universidade Federal do Rio Grande do Sul.
- Petsch, D.K., 2016. Causes and consequences of biotic homogenization in freshwater ecosystems. Int. Rev. Hydrobiol. 101(3-4), 113-122. http://dx.doi.org/10.1002/iroh.201601850
» http://dx.doi.org/10.1002/iroh.201601850 - Potapova, M.G., Ponader, K.C., Lowe, R.L., Clason, T.A., & Bahls, L.L., 2003. Small-celled Nupela species from North America. Diatom Res. 18(2), 293-306. http://dx.doi.org/10.1080/0269249X.2003.9705593
» http://dx.doi.org/10.1080/0269249X.2003.9705593 - R Core Team, 2017. R: A language and environment for statistical computing [online]. Vienna: R Foundation for Statistical Computing. Retrieved in 2022, January 4, from www.R-project.org
» www.R-project.org - Round, F.E., Crawford, R.M., & Mann, D.G., 1990. The diatoms: biology and morphology of the genera. Cambridge: Cambridge University Press.
- Sala, O.E., Stuart Chapin 3rd, F., Armesto, J.J., Berlow, E., Bloomfield, J., Dirzo, R., Huber-Sanwald, E., Huenneke, L.F., Jackson, R.B., Kinzig, A., Leemans, R., Lodge, D.M., Mooney, H.A., Oesterheld, M., Poff, N.L.R., Sykes, M.T., Walker, B.H., Walker, M., & Wall, D.H., 2000. Global biodiversity scenarios for the year 2010. Science 287(5459), 1770-1774. PMid:10710299. http://dx.doi.org/10.1126/science.287.5459.1770
» http://dx.doi.org/10.1126/science.287.5459.1770 - Salomoni, S.E., Rocha, O., Callegaro, V.L., & Lobo, E.A., 2006. Epilithic diatoms as indicators of water quality in the Gravataí river, Rio Grande do Sul, Brazil. Hydrobiologia 559(1), 233-246. http://dx.doi.org/10.1007/s10750-005-9012-3
» http://dx.doi.org/10.1007/s10750-005-9012-3 - Schindler, D.W., 2012. The dilemma of controlling cultural eutrophication of lakes. Proc Royal Soc B. 279(1746), 4322-4333. PMid:22915669. http://dx.doi.org/10.1098/rspb.2012.1032
» http://dx.doi.org/10.1098/rspb.2012.1032 - Schneck, F., Schwarzbold, A., & Melo, A.S., 2011. Substrate roughness affects stream benthic algal diversity, assemblage composition, and nestedness. J. N. Am. Benthol. Soc. 30(4), 1049-1056. http://dx.doi.org/10.1899/11-044.1
» http://dx.doi.org/10.1899/11-044.1 - Schneider, S.C., Kahlert, M., & Kelly, M.G., 2013. Interactions between pH and nutrients on benthic algae in streams and consequences for ecological status assessment and species richness patterns. Sci. Total Environ. 444(1), 73-84. PMid:23266552. http://dx.doi.org/10.1016/j.scitotenv.2012.11.034
» http://dx.doi.org/10.1016/j.scitotenv.2012.11.034 - Schwarzbold, A., & Schäfer, A., 1984. Gênese e morfologia das lagoas costeiras do Rio Grande do Sul, Brasil. Amazoniana 9, 87-104.
- Silva, L.C. 2012. O desenvolvimento do turismo no balneário Cassino: um problema de gerenciamento costeiro integrado [Dissertação de mestrado em Gerenciamento Costeiro]. Rio Grande. Universidade Federal do Rio Grande - FURG.
- Silva-Benavides, A.M., 1996. The epilithic diatom flora of a pristine and a polluted river in Costa Rica, Central America. Diatom Res. 11(1), 105-142. http://dx.doi.org/10.1080/0269249X.1996.9705368
» http://dx.doi.org/10.1080/0269249X.1996.9705368 - Simonsen, R., 1974. The diatom plankton of the Indian Ocean Expedition of RV Meteor. Forsch Ergeb. 19, 1-107.
- Smith, V.H., & Schindler, W.D., 2009. Eutrophication science: where do we go from here? Trends Ecol. Evol. 24(4), 201-207. PMid:19246117. http://dx.doi.org/10.1016/j.tree.2008.11.009
» http://dx.doi.org/10.1016/j.tree.2008.11.009 - Smith, V.H., 2003. Eutrophication of freshwater and coastal marine ecosystems: a global problem. Environ. Sci. Pollut. Res. Int. 10(2), 126-139. PMid:12729046. http://dx.doi.org/10.1065/espr2002.12.142
» http://dx.doi.org/10.1065/espr2002.12.142 - Soininen, J., Jamoneau, A., Rosebery, J., & Passy, S.I., 2016. Global patterns and drivers of species and trait composition in diatoms. Glob. Ecol. Biogeogr. 25(8), 940-950. http://dx.doi.org/10.1111/geb.12452
» http://dx.doi.org/10.1111/geb.12452 - Søndergaard, M., Lauridsen, T.L., Johansson, L.S., & Jeppesen, E., 2017. Nitrogen or phosphorus limitation in lakes and its impact on phytoplankton biomass and submerged macrophyte cover. Hydrobiologia 795(1), 35-48. http://dx.doi.org/10.1007/s10750-017-3110-x
» http://dx.doi.org/10.1007/s10750-017-3110-x - Souza, V.C., & Lorenzi, H., 2008. Botânica Sistemática. São Paulo: Instituto Plantarum.
- Strayer, D.L., & Dudgeon, D., 2010. Freshwater biodiversity conservation: recent progress and future challenges. J. N. Am. Benthol. Soc. 29(1), 344-358. http://dx.doi.org/10.1899/08-171.1
» http://dx.doi.org/10.1899/08-171.1 - Tromboni, F., & Dodds, W.K., 2017. Relationships between land use and stream nutrient concentrations in a highly urbanized tropical region of Brazil: thresholds and riparian zones. Environ. Manage. 60(1), 30-40. PMid:28405753. http://dx.doi.org/10.1007/s00267-017-0858-8
» http://dx.doi.org/10.1007/s00267-017-0858-8 - Valderrama, J.C., 1981. The simultaneous analysis of total nitrogen and total phosphorus in natural waters. Mar. Chem. 10(2), 109-122. http://dx.doi.org/10.1016/0304-4203(81)90027-X
» http://dx.doi.org/10.1016/0304-4203(81)90027-X - Vieira, E.F. 1983. Rio Grande: geografia física, humana e econômica. Porto Alegre: Sagra.
- Vilmi, A., Karjalainen, S.M., Landeiro, V.L., & Heino, J., 2015. Freshwater diatoms as environmental indicators: evaluating the effects of eutrophication using species morphology and biological indices. Environ. Monit. Assess. 187(5), 243. PMid:25864081. http://dx.doi.org/10.1007/s10661-015-4485-7
» http://dx.doi.org/10.1007/s10661-015-4485-7 - Vörösmarty, C.J., McIntyre, P.B., Gessner, M.O., Dudgeon, D., Prusevich, A., Green, P., Glidden, S., Bunn, S.E., Sullivan, C.A., Liermann, C.R., & Davies, P.M., 2010. Global threats to human water security and river biodiversity. Nature 467(7315), 555-561. PMid:20882010. http://dx.doi.org/10.1038/nature09440
» http://dx.doi.org/10.1038/nature09440 - Walsh, C.J., Roy, A.H., Feminella, J.W., Cottingham, P.D., Groffman, P.M., & Morgan 2nd, R.P., 2005. The urban stream syndrome: current knowledge and the search for a cure. J. N. Am. Benthol. Soc. 24(3), 706-723. http://dx.doi.org/10.1899/04-028.1
» http://dx.doi.org/10.1899/04-028.1 - Wojciechowski, J., Heino, J., Bini, L.M., & Padial, A.A., 2017. Temporal variation in phytoplankton beta diversity patterns and metacommunity structures across subtropical reservoirs. Freshw. Biol. 62(4), 751-766. http://dx.doi.org/10.1111/fwb.12899
» http://dx.doi.org/10.1111/fwb.12899 - Wojtal, A.Z., 2009. Nupela marvanii sp. nov., and N. lapidosa (Krasske) Lange-Bertalot in Poland with notes on the distribuition and ecology of the genus of Nupela (Bacillariophyta). Fottea. 9(2), 233-242. http://dx.doi.org/10.5507/fot.2009.024
» http://dx.doi.org/10.5507/fot.2009.024 - Wright, D.H., Patterson, B.D., Mikkelson, G.M., Cutler, A., & Atmar, W., 1997. A comparative analysis of nested subset patterns of species composition. Oecologia. 113(1), 1-20. PMid:28307284. http://dx.doi.org/10.1007/s004420050348
» http://dx.doi.org/10.1007/s004420050348 - Xiao, W., Liu, X., Irwin, A.J., Laws, E.A., Wang, L., Chen, B., Zeng, Y., & Huang, H., 2018. Warming and eutrophication combine to restructure diatoms and dinoflagellates. Water Res. 128, 206-216. PMid:29107905. http://dx.doi.org/10.1016/j.watres.2017.10.051
» http://dx.doi.org/10.1016/j.watres.2017.10.051 - Zorzal-Almeida, S., Bartozek, E.C.R., & Bicudo, D.C., 2021. Homogenization of diatom assemblages is driven by eutrophication in tropical reservoirs. Environ. Pollut. 288, 117778. PMid:34280747. http://dx.doi.org/10.1016/j.envpol.2021.117778
» http://dx.doi.org/10.1016/j.envpol.2021.117778 - Zuur, A., Ieno, E.N., Walker, N.J., Saveliev, A.A., & Smith, G.M., 2009. Mixed effects models and extensions in Ecology with R. New York: Springer. http://dx.doi.org/10.1007/978-0-387-87458-6
» http://dx.doi.org/10.1007/978-0-387-87458-6
Edited by
Publication Dates
-
Publication in this collection
03 June 2022 -
Date of issue
2022
History
-
Received
04 Jan 2022 -
Accepted
11 May 2022