Abstract:
Aim
This study investigated whether seasonal variations have an effect on diet composition of Serrapinnus notomelas in a marginal lagoon located under the area of influence of the Tibagi River, Upper Paraná Basin.
Methods
Samples were carried out monthly between February 2017 and January 2018, and fish specimens were caught with two sieves (2 mm mesh). The volumetric and occurrence method were used to quantify stomach contents.
Results
A total of 358 stomachs had their contents analyzed. The autochthonous resources were dominant in the diet of S. notomelas in all seasons, with a predominance of algae, detritus and Tecamebas. Meanwhile, plant material was the most abundant allochthonous resource in the diet. Diet composition showed significant differences between all seasons, while in the autumn and summer seasons, individuals showed greater trophic niche breadth, respectively.
Conclusions
Our results show the great importance of autochthonous resources for the maintenance of the S. notomelas population, and that seasonality can influence the trophic composition in the species' diet. We observed difference in the diet during the seasons and that during autumn and summer, individuals presented a greater breadth of the trophic niche. Thus, our results corroborate with knowledge to the preservation of small fish species, which are fundamental for the trophic network in ecosystems.
Keywords:
resource availability; Tibagi river; trophic ecology; seasonal variation
Resumo:
Objetivo
Este estudo investigou se variações sazonais induzem mudança na composição da dieta de Serrapinnus notomelas em uma lagoa marginal situada sob a área de influência do rio Tibagi, Bacia do Alto Paraná.
Métodos
As coletas foram realizadas mensalmente de fevereiro de 2017 a janeiro de 2018, e os exemplares foram capturados com duas peneiras (malha de 2 mm). O método volumétrico e de ocorrência foram usados para quantificar o conteúdo estomacal.
Resultados
Um total de 358 estômagos tiveram os seus conteúdos analisados. Os recursos autóctones foram dominantes na dieta de S. notomelas em todas as estações, com predominância de algas, detritos e Tecamebas. Vegetais foram os recursos alóctones mais abundante na dieta. A composição da dieta apresentou diferenças significativas entre todas as estações, e nas estações do outono e verão os indivíduos apresentaram maior amplitude de nicho trófico, respectivamente.
Conclusões
Nossos resultados mostram a grande importância dos recursos autóctones para a manutenção da população de S. notomelas, e que a sazonalidade pode influenciar a composição trófica na dieta da espécie. Observamos diferenças na dieta durante as estações, e que durante o outono e verão, os indivíduos apresentaram uma maior amplitude do nicho trófico. Assim, nossos resultados corroboram com informações para a preservação das espécies de pequeno porte, fundamentais para a rede trófica nos ecossistemas.
Palavras-chave:
disponibilidade de recursos; rio Tibagi; ecologia trófica; variação sazonal
1. Introduction
Aquatic environments present high spatial-temporal heterogeneity and annually suffer influences from temperature variation, rainfall, and hydrological regime (Oliveira et al., 2016Oliveira, J.F., Costa, R.S., Novaes, J.L.C., Rebouças, L.G.F., Morais-Segundo, A.L.N., & Peretti, D., 2016. Efeito da seca e da variação espacial na abundância de indivíduos nas guildas tróficas da ictiofauna em um reservatório no Semiárido Brasileiro. Bol. Inst. Pesca 42(1), 51-64. http://dx.doi.org/10.20950/1678-2305.2016v42n1p51.
http://dx.doi.org/10.20950/1678-2305.201...
; Souza et al., 2017Souza, A.E.F., Oliveira, J.F., Peretti, D., Fernandes, R., Costa, R.S., & Novaes, J.L.C., 2017. Effects of a supraseasonal drought on the ecological attributes of Plagioscion squamosissimus (Heckel, 1840) (Pisces, Sciaenidae) in a Brazilian Reservoir. ScientificWorldJournal 2017, 5930516. PMid:28326431. http://dx.doi.org/10.1155/2017/5930516.
http://dx.doi.org/10.1155/2017/5930516...
). These seasonal factors can influence the structure of communities and promote variations mainly in the abundance and availability of food resources in the ecosystem (Zavala-Camin, 1996Zavala-Camin, L.A., 1996. Introdução aos estudos sobre alimentação natural em peixes. Maringá: EDUEM, 129 p.). Therefore, species tend to develop behavioral strategies to deal with these variations in resources, which would affect their growth and survival rates, in addition to ecological interactions (Carnicer et al., 2008Carnicer, J., Abrams, P.A., & Jordano, P., 2008. Switching behavior, coexistence and diversification: comparing empirical community‐wide evidence with theoretical predictions. Ecol. Lett. 11(8), 802-808. PMid:18445033. http://dx.doi.org/10.1111/j.1461-0248.2008.01195.x.
http://dx.doi.org/10.1111/j.1461-0248.20...
; Barger & Kitaysky, 2012Barger, C.P., & Kitaysky, A.S., 2012. Isotopic segregation between sympatric seabird species increases with nutritional stress. Biol. Lett. 8(3), 442-445. PMid:22171022. http://dx.doi.org/10.1098/rsbl.2011.1020.
http://dx.doi.org/10.1098/rsbl.2011.1020...
; O’Callaghan et al., 2013O’Callaghan, M.J., Hannah, D.M., Boomer, I., Williams, M., & Sadler, J.P., 2013. Responses to river inundation pressures control prey selection of riparian beetles. PLoS One 8(4), e61866. PMid:23613958. http://dx.doi.org/10.1371/journal.pone.0061866.
http://dx.doi.org/10.1371/journal.pone.0...
; Correa & Winemiller, 2014Correa, S., & Winemiller, K.O., 2014. Niche partitioning among frugivorous fishes in response to fluctuating resources in the Amazonian floodplain forest. Ecology 95(1), 210-224. PMid:24649660. http://dx.doi.org/10.1890/13-0393.1.
http://dx.doi.org/10.1890/13-0393.1...
).
An example of adaptive strategy to seasonal variations is trophic plasticity, present in several species of Neotropical fish, which, depending on the availability of food resources, can explore different sources expanding their trophic niche (Abelha et al., 2001Abelha, M.C.F., Agostinho, A.A., & Goulart, E., 2001. Plasticidade trófica em peixes de água doce. Acta Scientiarum 23(2), 425-434. https://doi.org/10.4025/actascibiolsci.v23i0.2696.
https://doi.org/10.4025/actascibiolsci.v...
). This strategy can be verified by the constant variations of food items in their diet (Corrêa et al., 2009Corrêa, C.E., Petry, A.C., & Hahn, N.S., 2009. Influência do ciclo hidrológico na dieta e estrutura trófica da ictiofauna do rio Cuiabá, Pantanal Mato-Grossense. Iheringia Ser. Zool. 99(4), 456-463. http://dx.doi.org/10.1590/S0073-47212009000400018.
http://dx.doi.org/10.1590/S0073-47212009...
). Serrasalmids of the genus Mylossoma and Myleus present morphology of the oral structure adapted to break fruits and seeds, resources that are abundant in the Amazon region during the flood period. However, items such as leaves, flowers, fish, zooplankton, arthropods, and monkey droppings are occasionally found in their diet (Goulding, 1980Goulding, M., 1980. The fishes and the forest: exploration in Amazonian Natural History. Berkeley: University of California, 280 p. http://dx.doi.org/10.1525/9780520316133.
http://dx.doi.org/10.1525/9780520316133...
; Abelha et al., 2001Abelha, M.C.F., Agostinho, A.A., & Goulart, E., 2001. Plasticidade trófica em peixes de água doce. Acta Scientiarum 23(2), 425-434. https://doi.org/10.4025/actascibiolsci.v23i0.2696.
https://doi.org/10.4025/actascibiolsci.v...
).
Lagoons and flood areas that border the main river channel are highly influenced by seasonality during the wet and dry seasons (Henry et al., 2011Henry, R., Panarelli, E.A., Casanova, S.M.C., Granado, D.C., Mortari, R.C., & Abra, J., 2011. Plankton richness and abundance in several different hydrological situations in lakes lateral to a river: a case study in the mouth zone of a tributary into a tropical Reservoir. Oecol. Aust. 15(3), 537-558. http://dx.doi.org/10.4257/oeco.2011.1503.08.
http://dx.doi.org/10.4257/oeco.2011.1503...
; Quirino et al., 2015Quirino, B.A., Carniatto, N., Gaiotto, J.V., & Fugi, R., 2015. Seasonal variation in the use of food resources by small fishes inhabiting the littoral zone in a Neotropical floodplain lake. Aquat. Ecol. 49(4), 431-440. http://dx.doi.org/10.1007/s10452-015-9535-2.
http://dx.doi.org/10.1007/s10452-015-953...
; Brambilla et al., 2018Brambilla, E.M., Uieda, V.S., & Nogueira, M.G., 2018. Influence of habitat connectivity and seasonality on the ichthyofauna structure of a riverine knickzone. Iheringia Ser. Zool. 108, 1-7. http://dx.doi.org/10.1590/1678-4766e2018035.
http://dx.doi.org/10.1590/1678-4766e2018...
). In periods of high-water level, the increase of river volume connects it with marginal lakes. As a result, there is an expansion of water bodies promoting the dispersion, displacement and grouping of the ichthyofauna that inhabit marginal regions of these lakes to new habitats, by consequence to a greater supply of resources (Corrêa et al., 2009Corrêa, C.E., Petry, A.C., & Hahn, N.S., 2009. Influência do ciclo hidrológico na dieta e estrutura trófica da ictiofauna do rio Cuiabá, Pantanal Mato-Grossense. Iheringia Ser. Zool. 99(4), 456-463. http://dx.doi.org/10.1590/S0073-47212009000400018.
http://dx.doi.org/10.1590/S0073-47212009...
; Gomes et al., 2012Gomes, L.C., Bulla, C.K., Agostinho, A.A., Vasconcelos, L.P., & Miranda, L.E., 2012. Fish assemblage dynamics in a Neotropical floodplain relative to aquatic macrophytes and the homogenizing effect of a flood pulse. Hydrobiologia 685(1), 97-107. http://dx.doi.org/10.1007/s10750-011-0870-6.
http://dx.doi.org/10.1007/s10750-011-087...
). On the other hand, in times of low rainfall, river level decrease, making marginal lakes isolated again, restricting the ichthyofauna and its foraging area (Ferrareze & Nogueira, 2011Ferrareze, M., & Nogueira, M.G., 2011. Importance of lateral lagoons for the ichthyofauna in a large tropical reservoir. Braz. J. Biol. 71(4), 807-820. http://dx.doi.org/10.1590/S1519-69842011000500002.
http://dx.doi.org/10.1590/S1519-69842011...
; Cunha et al., 2018Cunha, A.F., Wolff, L.L., & Hahn, N.S., 2018. Seasonal changes at population and individual levels in the diet of juvenile catfish in a Neotropical floodplain. J. Freshwat. Ecol. 33(1), 273-284. http://dx.doi.org/10.1080/02705060.2018.1442371.
http://dx.doi.org/10.1080/02705060.2018....
). This retraction of water bodies causes a decrease in dissolved oxygen concentration and an increase in the density of individuals, influencing important ecological relationships such as inter and intraspecific predation (Oliveira et al., 2016Oliveira, J.F., Costa, R.S., Novaes, J.L.C., Rebouças, L.G.F., Morais-Segundo, A.L.N., & Peretti, D., 2016. Efeito da seca e da variação espacial na abundância de indivíduos nas guildas tróficas da ictiofauna em um reservatório no Semiárido Brasileiro. Bol. Inst. Pesca 42(1), 51-64. http://dx.doi.org/10.20950/1678-2305.2016v42n1p51.
http://dx.doi.org/10.20950/1678-2305.201...
).
The Neotropical region is home to a great diversity of species belonging to the order Characiformes, with different morphologies and ecological niches, fundamental for the structuring of the ecosystem (Lévêque et al., 2008Lévêque, C., Oberdorff, T., Paugy, D., Stiassny, M.L.J., & Tedesco, P.A., 2008. Global diversity of fish (Pisces) in freshwater. Hydrobiologia 595(1), 545-567. http://dx.doi.org/10.1007/s10750-007-9034-0.
http://dx.doi.org/10.1007/s10750-007-903...
). Cheirodontinae is one of the most well-known phylogenetic and taxonomic groups of Characidae (Malabarba, 2003Malabarba, L.R., 2003. Subfamily Cheirodontinae. In: Reis, R.E., Kullander, S.O. & Ferraris-Jr, C.J., eds. Check list of the freshwater fishes of South and Central America. Porto Alegre: Edipucrs, 215-221.; Bührnheim et al., 2008Bührnheim, C.M., Carvalho, T.P., Malabarba, L.R., & Weitzman, S.W., 2008. A new genus and species of characid fish from the Amazon basin: the recognition of a relictual lineage of characid fishes (Ostariophysi: Cheirodontinae: Cheirodontini). Neotrop. Ichthyol. 6(4), 663-678. http://dx.doi.org/10.1590/S1679-62252008000400016.
http://dx.doi.org/10.1590/S1679-62252008...
). Serrapinnus notomelas (Eigenmann, 1915), Cheirodontinae, is recorded across the Upper Paraná River basin inhabiting places of low current velocity and depth such as lakes and swamps, (Malabarba, 2003Malabarba, L.R., 2003. Subfamily Cheirodontinae. In: Reis, R.E., Kullander, S.O. & Ferraris-Jr, C.J., eds. Check list of the freshwater fishes of South and Central America. Porto Alegre: Edipucrs, 215-221.; Piana et al., 2006Piana, P.A., Gomes, L.C., & Cortez, E.M., 2006. Factors influencing Serrapinnus notomelas (Characiformes: Characidae) populations in upper Paraná river floodplain lagoons. Neotrop. Ichthyol. 4(1), 81-86. http://dx.doi.org/10.1590/S1679-62252006000100008.
http://dx.doi.org/10.1590/S1679-62252006...
, Súarez et al., 2007Súarez, Y.R., Valério, S.B., Tondato, K.K., Ximenes, L.Q.L., & Felipe, T.R.A., 2007. Determinantes ambientais da ocorrência de espécies de peixes em riachos de cabeceira da bacia do rio Ivinhema, Alto Rio Paraná. Acta Sci. Biol. Sci. 19(2), 145-150.). It is considered a small-sized species (35 to 40 mm long) with short life cycle and trophic plasticity, consuming plant material, algae, and aquatic invertebrates (Suzuki et al., 2004Suzuki, H.I., Pelicice, F.M., Luiz, E.A., Latini, J.D., & Agostinho, A.A., 2004. Reproductive strategies of the fish community of the upper Paraná River floodplain. In: Agostinho, A.A., Rodrigues, L., Gomes, L.C., Thomaz, S.M., & Miranda, L.E., eds. Structure and functioning of the Paraná River and its floodplain: LTER – Site 6 (PELD – Sítio 6). Maringá: Eduem, 125-130.; Dias & Fialho, 2009Dias, T.S., & Fialho, C.B., 2009. Biologia alimentar de quatro espécies simpátricas de Cheirodontinae (Characiformes, Characidae) do rio Ceará Mirim, Rio Grande do Norte. Iheringia Ser. Zool. 99(3), 242-248. http://dx.doi.org/10.1590/S0073-47212009000300003.
http://dx.doi.org/10.1590/S0073-47212009...
; Brandão-Gonçalves et al., 2010Brandão-Gonçalves, L., Oliveira, S.A.D., & Lima-Junior, S.E., 2010. Hábitos alimentares da ictiofauna do córrego Franco, Mato Grosso do Sul, Brasil. Biota Neotrop. 10(2), 21-30. http://dx.doi.org/10.1590/S1676-06032010000200001.
http://dx.doi.org/10.1590/S1676-06032010...
).
Small fish species such as S. notomelas are important components in the biota of freshwater ecosystems, playing an important role as they present dense populations that are numerically dominant in their assemblages (Súarez et al., 2007Súarez, Y.R., Valério, S.B., Tondato, K.K., Ximenes, L.Q.L., & Felipe, T.R.A., 2007. Determinantes ambientais da ocorrência de espécies de peixes em riachos de cabeceira da bacia do rio Ivinhema, Alto Rio Paraná. Acta Sci. Biol. Sci. 19(2), 145-150.). In addition, this species has great economic importance, especially for aquarists, being exported as ornamental fish (Costa & Rocha, 2017Costa, I.D.D., & Rocha, V.M.D., 2017. Feeding ecology of Serrapinnus notomelas (Characiformes: Cheirodontinae) in small forest streams in the Machado River basin, Rondônia, Brazil. Acta Amazon. 47(1), 19-28. http://dx.doi.org/10.1590/1809-4392201601944.
http://dx.doi.org/10.1590/1809-439220160...
). Considering the ecological importance of S. notomelas, this study aimed to test whether seasonality influences the diet composition of Serrapinus notomelas. In addition, we also sought to assess whether there is expansion of the trophic niche of individuals during the seasons, since there is a tendency for an increase in the amount of food resources during the hottest and rainy season.
2. Material and Methods
2.1. Study area
The studied lagoon is located under the influence area of the Tibagi River, Upper Paraná Basin, in the municipality of Sertanópolis - Paraná State, Brazil (23°00'15”S and 50°58'18”W). The Tibagi River is one of the main tributaries of the Paranapanema River. It is about 550 km long and comprises a watershed with 65 main tributaries and hundreds of sub tributaries (Maack, 1981Maack, R., 1981. Geografia física do Estado do Paraná. Rio de Janeiro: Livraria José Olympio Editora.).
The lagoon has mean area of 24.000,00 m2, and it is located 300 m from the closest margin of the Tibagi River. The water level of the Tibagi River in this region is influenced by the Capivara Dam Reservoir in the Paranapanema River. The connection of the lagoon with the Tibagi River occurs sporadically at high-level peaks during the rainy season, being isolated during most of the year. The margin of the lagoon is mostly dominated by grass of the genus Brachiaria, with a variable width of 1 to 4 m away from the margin. It is surrounded by a matrix of agricultural landscape composed of soybean and corn crops (Figure 1 and 2).
Map of the Tibagi River, Upper Paraná River Basin, Municipality of Sertanópolis – PR, indicating the location of the marginal lagoon sampled.
Satellite image showing the studied marginal lagoon (white arrow) at the left margin of the Tibagi River, Municipality of Sertanópolis – PR, Source: Google Earth.
2.2. Species characterization
Serrapinnus notomelas is considered a small species, with a maximum size of 40.0 mm (Graça & Pavanelli, 2007Graça, W.J., & Pavanelli, C.S., 2007. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: EDUEM, 241 p.) and average weight of 0.5 g (Piana et al., 2006Piana, P.A., Gomes, L.C., & Cortez, E.M., 2006. Factors influencing Serrapinnus notomelas (Characiformes: Characidae) populations in upper Paraná river floodplain lagoons. Neotrop. Ichthyol. 4(1), 81-86. http://dx.doi.org/10.1590/S1679-62252006000100008.
http://dx.doi.org/10.1590/S1679-62252006...
; Figure 3). They live in small shoals searching for food in short migrations among submerged vegetation, roots and debris. They are found in shallow portions of lakes and slow water channels usually close to macrophytes (Carniatto et al., 2012Carniatto, N., Fugi, R., Cantanhêde, G., Gubiani, É.A., & Hahn, N.S., 2012. Effects of flooding regime and diel cycle on diet of a small sized fish associated to macrophytes. Acta Limnol. Bras. 24(4), 363-372. http://dx.doi.org/10.1590/S2179-975X2013005000007.
http://dx.doi.org/10.1590/S2179-975X2013...
). Females usually develop first gonadal maturation when reaching 20 mm, and males around 21 mm (Suzuki et al., 2004Suzuki, H.I., Pelicice, F.M., Luiz, E.A., Latini, J.D., & Agostinho, A.A., 2004. Reproductive strategies of the fish community of the upper Paraná River floodplain. In: Agostinho, A.A., Rodrigues, L., Gomes, L.C., Thomaz, S.M., & Miranda, L.E., eds. Structure and functioning of the Paraná River and its floodplain: LTER – Site 6 (PELD – Sítio 6). Maringá: Eduem, 125-130.). They do not present parental care (Suzuki et al., 2004Suzuki, H.I., Pelicice, F.M., Luiz, E.A., Latini, J.D., & Agostinho, A.A., 2004. Reproductive strategies of the fish community of the upper Paraná River floodplain. In: Agostinho, A.A., Rodrigues, L., Gomes, L.C., Thomaz, S.M., & Miranda, L.E., eds. Structure and functioning of the Paraná River and its floodplain: LTER – Site 6 (PELD – Sítio 6). Maringá: Eduem, 125-130.), and as far as it is known, the species lacks territoriality.
Photography of a live adult male of Serrapinnus notomelas (approximately 35 mm in total length), taken in an aquarium tank.
2.3. Sampling site
Collections were carried out monthly from February 2017 to January 2018. Specimens were captured in the margin of the lagoon with two sieves (1 m diameter; 2 mm mesh) working simultaneously for 40 minutes. After capture, individuals were anesthetized with Eugenol 50-200 mg/L (Neiffer & Stamper, 2009Neiffer, D.L., & Stamper, M.A., 2009. Fish sedation, analgesia, anesthesia, and euthanasia: considerations, methods, and types of drugs. ILAR J. 50(4), 343-360. PMid:19949251. http://dx.doi.org/10.1093/ilar.50.4.343.
http://dx.doi.org/10.1093/ilar.50.4.343...
) and fixed in a 4% formaldehyde solution for 48 hours before stored in alcohol 70%. The samplings were carried out under SISBio 42829-1 license, referring to the project approved by CEUA-UEL under protocol 8781.2015.40. Voucher specimens were deposited at the Zoology Museum of the State University of Londrina (MZUEL 17776, MZUEL 17777, MZUEL 18782, MZUEL 18783, MZUEL 18784, MZUEL 18785, MZUEL 18786, MZUEL 18787, 18788, MZUEL 18789, MZUEL 18790 and MZUEL 18791).
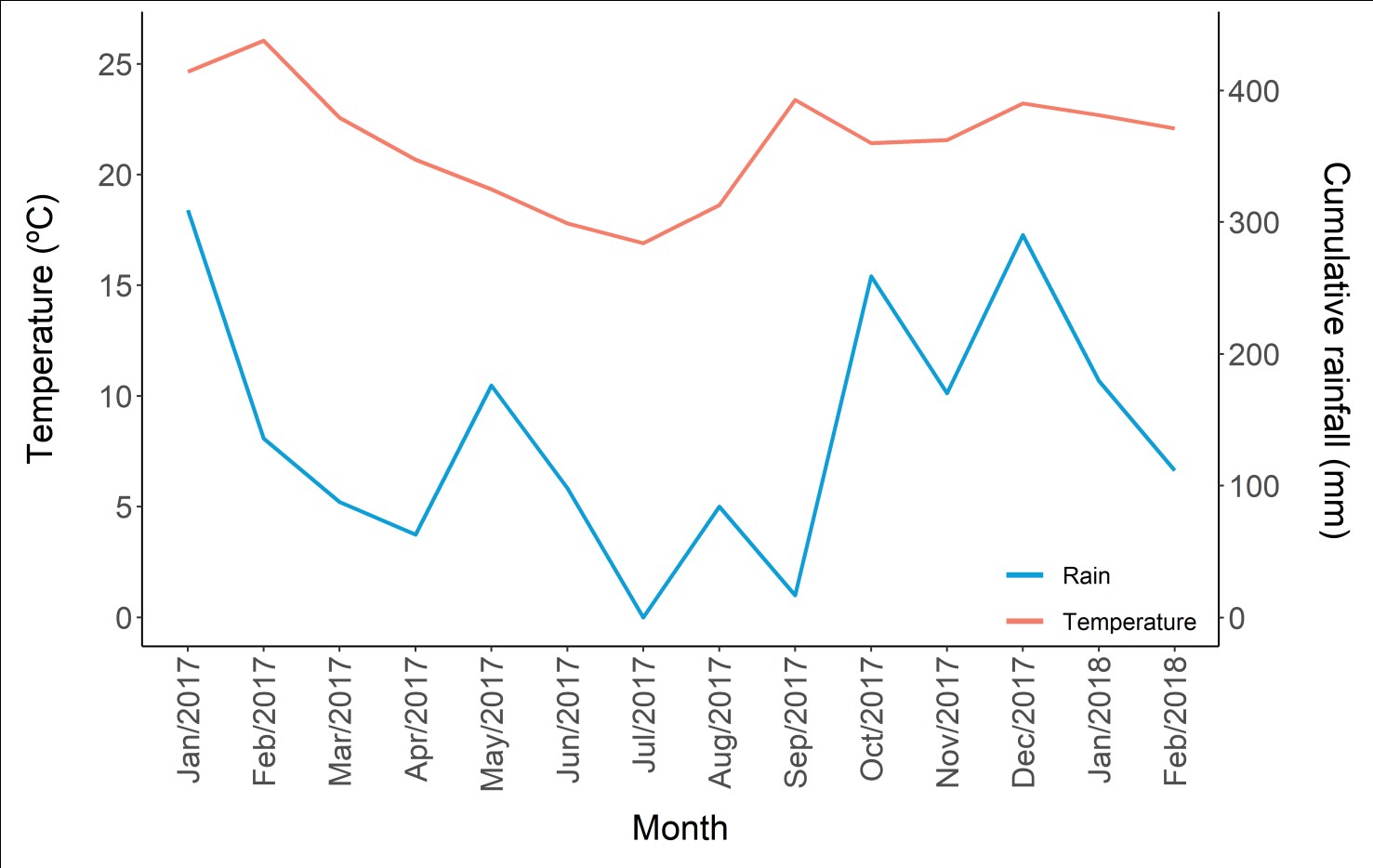
Climatic precipitation (mm) and temperature (°C) data were recorded during the study from January 2017 to February 2018. These data were obtained at the meteorological station of the National Institute of Meteorology (INMET) located in Nova Fátima, PR (-23.42S and -50.58W), being 98.7 km away from the collection point. In addition to this, data from the second closest station located in Maringá (-23.41S and -51.93W) at 130 km away from the collection point, was used to replace the absence (null) of precipitation data for the months of January, February, and April 2017. During the study period, January 2017 had the highest volume of monthly accumulated precipitation (309.2 mm), while in July 2017 there was no precipitation. In addition to this, the spring and summer seasons showed the highest monthly accumulated precipitation, followed by autumn, mainly in May 2017, whose monthly accumulated precipitation was 176 mm. Regarding the average temperature, summer had the highest average, especially in January (24.65 °C) and February (26.05 °C) in 2017, while the lowest average temperature was recorded in the winter period in July (17.79 °C) and August (16.9 °C) (Figure 4).
Accumulated monthly precipitation (mm) in the region of the marginal lagoon of the Tibagi River, Upper Paraná River Basin, municipality of Sertanópolis - PR, from January 2017 to February 2018.
2.4. Diet analysis
In laboratory adult individuals identified through sexual dimorphism had their stomachs removed and stored in 70% alcohol, their content was analyzed under a stereomicroscope or optical microscope and identified at the lowest possible taxonomic level, based on Mugnai et al. (2010)Mugnai, R., Nessimian, J.L., & Baptista, D.F., 2010. Manual de identificação de macroinvertebrados aquáticos. Rio de Janeiro: Technical Books, 176 p.. The volume (V) of each content was obtained by compression in a millimeter Petri dish, with a volume given in mm3 and later transformed into mL (Hellawell & Abel, 1971Hellawell, J.M., & Abel, R., 1971. A rapid volumetric method for the analysis of the food of fishes. J. Fish Biol. 3(1), 29-37. http://dx.doi.org/10.1111/j.1095-8649.1971.tb05903.x.
http://dx.doi.org/10.1111/j.1095-8649.19...
). Food items were grouped into broad categories: Insects (Diptera larvae, adult Hymenoptera, adult Hemiptera, Ephemeroptera larvae and Odonata larvae), Other Invertebrates (Ostracoda, Copepoda, Cladocera, Rotifera, Tecameba, Bryozoa and Gastropoda), Crustaceans (Microcrustaceans), Plants (Algae, Superior plants), Others (Fish scales, Organic detritus, Inorganic detritus and Microplastic).
The trophic guild of the species in each season was determined by the prevailing volume of food categories. If the species consumed a volume equal to or greater than 60% of plants, it was classified as herbivorous; of macroinvertebrate as invertivorous; of algae as algivorous, of detritus as detritivore and as omnivorous when the species did not present any dominant food categories in its diet. We adopted a volume value of 60% as a mean of the volume range usually found in the literature (50% to 70%) (e.g. De Mérona et al., 2001De Mérona, B., Santos, G.M., & Almeida, R.G., 2001. Short term effects of Tucuruí Dam (Amazonia, Brazil) on the trophic organization of fish communities. Environ. Biol. Fishes 60(4), 375-392. http://dx.doi.org/10.1023/A:1011033025706.
http://dx.doi.org/10.1023/A:101103302570...
; Corrêa et al., 2011Corrêa, C.E., Albrecht, M.P., & Hahn, N.S., 2011. Patterns of niche breadth and feeding overlap of the fish fauna in the seasonal Brazilian Pantanal, Cuiabá River basin. Neotrop. Ichthyol. 9(3), 637-646. http://dx.doi.org/10.1590/S1679-62252011000300017.
http://dx.doi.org/10.1590/S1679-62252011...
; Delariva et al., 2013Delariva, R.L., Hahn, N.S., & Kashiwaqui, E.A.L., 2013. Diet and trophic structure of the fish fauna in a subtropical ecosystem: impoundment effects. Neotrop. Ichthyol. 11(4), 891-904. http://dx.doi.org/10.1590/S1679-62252013000400017.
http://dx.doi.org/10.1590/S1679-62252013...
).
2.5. Data analysis
To verify differences in the diet of S. notomelas among seasons (summer, autumn, winter, and spring), a permutational variation analysis was used (PERMANOVA, Anderson et al., 2008Anderson, M.J., Gorley, R.N., & Clarke, K.R., 2008. PERMANOVA + for PRIMER: guide to software and statistical methods. Plymouth, UK: PRIMER-E.), based on a Bray-Curtis similarity matrix of volume data, log transformed (x + 1). The pseudo-F statistic resulting from this analysis was tested by the Monte Carlo method using 999 randomizations and later we identified which compartment pairs differed significantly with the pairwise adonis test. The ordering of species according to the composition of the diet was visualized using a principal coordinate analysis (PCoA) based on a Bray-Curtis dissimilarity matrix (Bray & Curtis, 1957Bray, J.R., & Curtis, J.T., 1957. An ordination of the upland forest community of southern Wisconsin. Ecol. Monogr. 27(4), 325-349. http://dx.doi.org/10.2307/1942268.
http://dx.doi.org/10.2307/1942268...
). Finally, we identified which seasonality pairs differed significantly with Tukey's post-hoc test (Tukey, 1953Tukey, J.W., 1953. The problem of multiple comparisons. Princeton: Princeton University. Unpublished report.).
Possible differences in species niche breadth between different seasons were determined through a permutational analysis of multivariate dispersions (PERMDISP; Anderson, 2004Anderson, M.J., 2004. PERMDISP: a FORTRAN computer program for permutational analysis of multivariate dispersions (for any two-factor ANOVA design) using permutation tests. New Zealand: Department of Statistics, University of Auckland.). This analysis indicates dietary variability among individuals of the same species in the sampled location, reflecting the population's niche breadth (Correa & Winemiller, 2014Correa, S., & Winemiller, K.O., 2014. Niche partitioning among frugivorous fishes in response to fluctuating resources in the Amazonian floodplain forest. Ecology 95(1), 210-224. PMid:24649660. http://dx.doi.org/10.1890/13-0393.1.
http://dx.doi.org/10.1890/13-0393.1...
). The probability values used to determine significant differences in the dispersion of the S. notomelas diet between seasons were calculated by residue permutation (999 permutations), and subsequently identified which seasonality season pairs differed significantly with Tukey's post-hoc test (Tukey, 1953Tukey, J.W., 1953. The problem of multiple comparisons. Princeton: Princeton University. Unpublished report.). All statistical analyzes were performed using the R software (R Development Core Team, 2019R Development Core Team, 2019. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing.), using the “vegan” package (Oksanen et al., 2018Oksanen, J., Blanchet, F.G., Friendly, M., Kindt, R., Legendre, P., McGlinn, D., Minchin, P.R., O’Hara, R.B., Simpson, G.L., Solymos, P., Stevens, M.H.H., Szoecs, E., & Wagner, H., 2018. Vegan: Community Ecology Package. Vienna: R Foundation for Statistical Computing.), and the graphics were constructed using the “ggplot2” package (Wickham, 2016Wickham, H., 2016. ggplot2: elegant graphics for data analysis [online]. New York: Springer-Verlag. Retrieved in 2021, July 25, from https://ggplot2.tidyverse.org
https://ggplot2.tidyverse.org...
).
3. Results
A total of 1077 individuals were captured along the study. We aimed to randomly select 30 individuals for the diet analysis for each month; however, we did not reach this amount in some months. A total of 358 stomachs of S. notomelas analyzed: 90 in autumn, 59 in spring, 119 in summer and 90 in winter. Considering all seasons, a total of 19 food resources were recorded in the species' diet, 14 items of autochthonous origin and five allochthonous (Table 1). When evaluating the food composition of S. notomelas, it was observed that in all seasons, individuals consumed predominantly aquatic resources, mainly algae, organic detritus and Tecameba (Table 1).
Volume (%) of food items identified in stomach contents of Serrapinnus notomelas in a lagoon located on the Tibagi River, Upper Paraná River Basin, in the municipality of Sertanópolis - PR.
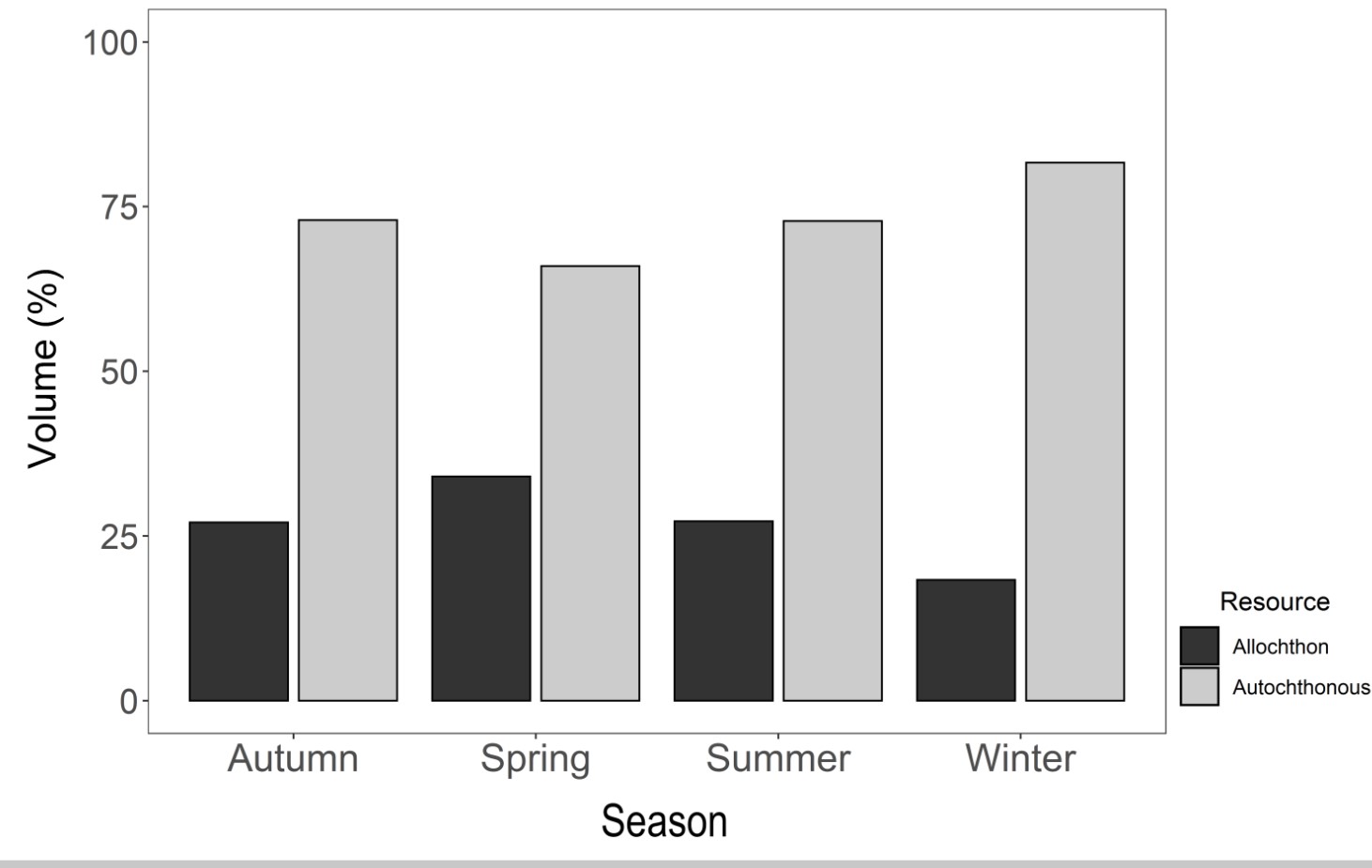
The diet of S. notomelas was predominantly composed of food resources of autochthonous origin in all seasons. The highest consumption of these resources occurred in winter (81.64%), followed by autumn and summer (72.93% and 72.78%, respectively; Figure 5). Plant items were the most abundant in the diet of S. notomelas during spring (24.30%), followed by algae (18.37%) and organic detritus. In spring, the most abundant items were plants (24.30%), followed by organic detritus (23.26%) and algae (14.23%). Of the 19 items consumed in the summer, the most abundant were algae (32.05%), inorganic detritus (15.15%) and Tecameba (11.76%). Of 17 items consumed in winter, the most abundant were algae (35.99%), organic detritus (17.95%) and plants (13.59%, Table 1).
Origin of food resources that constituted the Serrapinnus notomelas diet in the coastal zone of a marginal lagoon on the Tibagi River, Upper Paraná River Basin, sampled from February 2017 to January 2018.
PERMANOVA showed a significant difference in the composition of the diet between seasons (PERMANOVA: pseudoF(3.356) = 16.736; p < 0.05), indicating that the diet composition of S. notomelas was different between all seasons (Table 2). PCoA revealed the significance of the first two axes, which together explained 39.21%. When analyzing the axes separately, it was observed that in the first axis of the PCoA there was a segregation between the summer season (negative side) and autumn and spring (positive side). In the second axis, it was observed that the segregation occurred between the summer and autumn seasons (positive side) and spring (negative side) (Figure 6).
Results of the pair-by-pair test of the permutational multivariate analysis of variance (PERMANOVA) applied to the diet data of Serrapinnus notomelas in the autumn, spring, summer, and winter seasons.
Ordering of the first two PCoA axes for the diet of Serrapinnus notomelas in the autumn, spring, summer, and winter seasons.
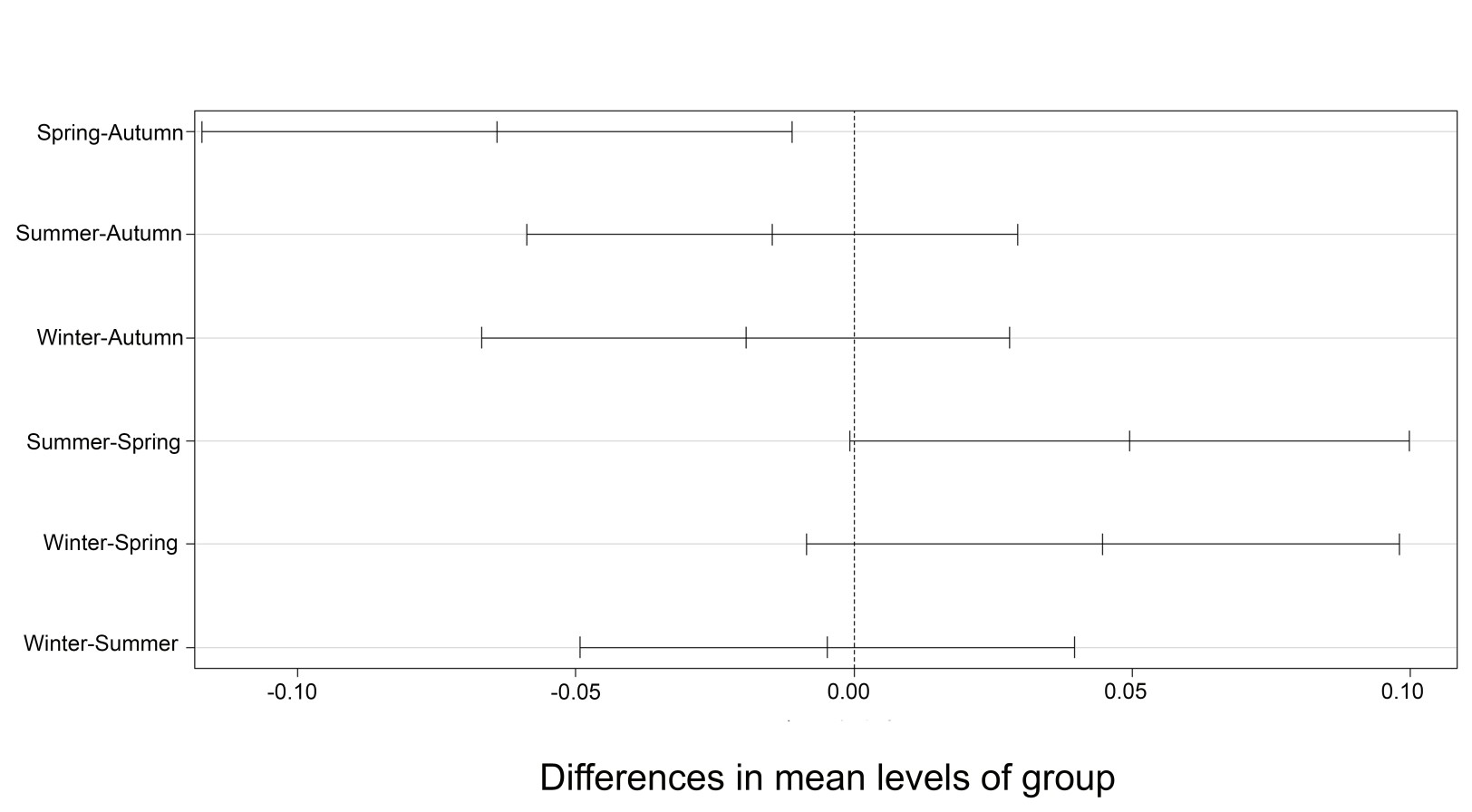
The PERMDISP results showed that only the spring and autumn seasons differed significantly from each other in relation to the variation in diet among individuals (ANOVA: F (3,353) = 3.4294; p < 0.05, Figure 7). Thus, greater variability was observed between individuals in the autumn season (Average distance to centroid - DC: 0.48), followed by summer (DC = 0.47), winter (DC = 0.46) and spring (DC = 0.42).
Tukey test results of the permutational analysis of multivariate dispersions (PERMDISP) applied to the diet data of Serrapinnus notomelas in the autumn, spring, summer, and winter seasons.
4. Discussion
Our results show that the population of Serrapinnus notomelas consumed a large volume of autochthonous resources with a highly diversified food composition in all seasons, being herein considered an omnivorous species. The presence of rooted structure in the lagoon provides foraging habitat for small fish, which can harbor communities of algae and invertebrates (Thomaz et al., 2008Thomaz, S.M., Dibble, E.D., Evangelista, L.R., Higuti, J., & Bini, L.M., 2008. Influence of aquatic macrophyte habitat complexity on invertebrate abundance and richness in tropical lagoons. Freshw. Biol. 53, 358-367. https://doi.org/10.1111/j.1365-2427.2007.01898.x.
https://doi.org/10.1111/j.1365-2427.2007...
; Carniatto et al., 2012Carniatto, N., Fugi, R., Cantanhêde, G., Gubiani, É.A., & Hahn, N.S., 2012. Effects of flooding regime and diel cycle on diet of a small sized fish associated to macrophytes. Acta Limnol. Bras. 24(4), 363-372. http://dx.doi.org/10.1590/S2179-975X2013005000007.
http://dx.doi.org/10.1590/S2179-975X2013...
), which were evidenced in the diet of S. notomelas. The seasonal change in the diet of the species was evident with significant results. In the autumn and spring seasons the most abundant resource was plants, and, in the summer and winter, it was algae.
The diet of S. notomelas was dominated by autochthonous resources in all seasons, this shows how dependent the species is on the resources provided by the lagoon itself. The absence of a complex riparian vegetation, once the lagoon is surrounded by crops, might be the main cause for the low amount of allochthonous resources (personal observation). In addition, the behavior of the species associated with vegetation (grasses of the genus Brachiaria) favored the large consumption of algae and invertebrates as the main resources in the diet of S. notomelas during all seasons (Casatti et al., 2003Casatti, L., Mendes, H.F., & Ferreira, K.M., 2003. Aquatic macrophytes as feeding site for small fishes in the Rosana reservoir, Paranapanema River, Southeastern Brazil. Braz. J. Biol. 63(2), 213-222. PMid:14509843. http://dx.doi.org/10.1590/S1519-69842003000200006.
http://dx.doi.org/10.1590/S1519-69842003...
; Pelicice et al., 2005Pelicice, F.M., Agostinho, A.A., & Thomaz, S.M., 2005. Fish assemblages associated with Egeria in a tropical reservoir: investigating the effects of plant biomass and diel period. Acta Oecol, 27, 9-16. http://dx.doi.org/10.1016/j.actao.2004.08.004.
http://dx.doi.org/10.1016/j.actao.2004.0...
; Dibble & Pelicice, 2010Dibble, E.D., & Pelicice, F.M., 2010. Influence of aquatic plant‐specific habitat on an assemblage of small neotropical floodplain fishes. Ecol. Freshwat. Fish 19(3), 381-389. http://dx.doi.org/10.1111/j.1600-0633.2010.00420.x.
http://dx.doi.org/10.1111/j.1600-0633.20...
; Carniatto et al., 2012Carniatto, N., Fugi, R., Cantanhêde, G., Gubiani, É.A., & Hahn, N.S., 2012. Effects of flooding regime and diel cycle on diet of a small sized fish associated to macrophytes. Acta Limnol. Bras. 24(4), 363-372. http://dx.doi.org/10.1590/S2179-975X2013005000007.
http://dx.doi.org/10.1590/S2179-975X2013...
). The mouth morphology of the species contributes to explain the high consumption of algae. S. notomelas have wide multicuspid teeth, playing an important role in scraping the peripheryton adhered to macrophyte stems (Hahn & Loureiro-Crippa, 2006Hahn, N.S., & Loureiro-Crippa, V.E., 2006. Estudo comparativo da dieta, hábitos alimentares e morfologia trófica de duas espécies simpátricas, de peixes de pequeno porte, associados à macrófitas aquáticas. Acta Sci. Biol. Sci. 28(4), 359-363. https://doi.org/10.4025/actascibiolsci.v28i4.167.
https://doi.org/10.4025/actascibiolsci.v...
). Animal resources mainly Tecameba and Cladoceros were also found. Thus, S. notomelas can be considered as an omnivorous species with a tendency to herbivory, due to the consumption of both invertebrados, algae and superior plants, as proposed by Minzão et al. (2004)Minzão, L.D., Brucznitsk, V.F.B., & Cardoso, L.Q.F., 2004. Sazonalidade na dieta de duas espécies de peixes do gênero Serrapinnus, em uma lagoa marginal do alto Rio Paraná. Brasília, Editora UNB, 306 p.; Brandão-Gonçalves et al. (2010)Brandão-Gonçalves, L., Oliveira, S.A.D., & Lima-Junior, S.E., 2010. Hábitos alimentares da ictiofauna do córrego Franco, Mato Grosso do Sul, Brasil. Biota Neotrop. 10(2), 21-30. http://dx.doi.org/10.1590/S1676-06032010000200001.
http://dx.doi.org/10.1590/S1676-06032010...
; Costa & Rocha (2017)Costa, I.D.D., & Rocha, V.M.D., 2017. Feeding ecology of Serrapinnus notomelas (Characiformes: Cheirodontinae) in small forest streams in the Machado River basin, Rondônia, Brazil. Acta Amazon. 47(1), 19-28. http://dx.doi.org/10.1590/1809-4392201601944.
http://dx.doi.org/10.1590/1809-439220160...
.
Many studies have evaluated differences in the species' diet between drought and flood only (Aranha et al., 1998Aranha, J.M.R., Takeuti, D.F., & Yoshimura, T., 1998. Habitat use and food partitioning of the fishes in the Mergulhão stream (coastal stream of Atlantic Forest, Brazil). Rev. Biol. Trop. 46(4), 951-959.; Esteves & Lobón-Cerviá, 2001Esteves, K.E., & Lobón-Cerviá, J., 2001. Composition and trophic structure of a fish community of a clear water Atlantic rainforest stream in southeastern Brazil. Environ. Biol. Fishes 62(4), 429-440. http://dx.doi.org/10.1023/A:1012249313341.
http://dx.doi.org/10.1023/A:101224931334...
; Lampert et al., 2003Lampert, V.R., Azevedo, M.A., & Fialho, C.B., 2003. Hábito alimentar de Mimagoniates microlepis Steindachner, 1876 (Characidae: Glandulocaudinae) do canal de ligação entre as lagoas Emboaba e Emboabinha, Rio Grande do Sul, Brasil. Comun. Mus. Cienc. Tecnol. PUCRS 16(1), 3-16.), with few records of variations in the diet of Neotropical fishes among seasons. However, it is of great importance to assess how species are feeding during the seasons of the year, as it defines with better precision periods with better feeding conditions (Barbieri & Barbieri, 1984Barbieri, G., & Barbieri, M.C., 1984. Note on nutricional dinamics of Gymnotus carap (L.) from the Lobo Reservoir. São Paulo State, Brazil. J. Fish Biol. 24(4), 351-355. http://dx.doi.org/10.1111/j.1095-8649.1984.tb04807.x.
http://dx.doi.org/10.1111/j.1095-8649.19...
; Raposo & Gurgel, 2003Raposo, R.M.G., & Gurgel, H.C.B., 2003. Variação da alimentação natural de Serrasalmus spilopleura Kner, 1860 (Pises, Serrasalmidae) em função do lunar e das estações do ano na lagoa de Extremoz, Rio Grande do Norte, Brasil. Acta Sci. Biol. Sci. 25(2), 267-272. https://doi.org/10.4025/actascianimsci.v25i2.2003.
https://doi.org/10.4025/actascianimsci.v...
).
Another factor that could explain the changes in the diet of S. notomelas between autumn and spring, is the temporal abundance of food resources. We found an increase of Rotifera, Hymenoptera and Hemiptera in the diet of S. notomelas during spring (flooding period), when there was a greater availability of food items and abundance of these invertebrates in the lake during the rainiest season (Mérona & Rankin-de-Mérona, 2004Mérona, B., & Rankin-de-Mérona, J., 2004. Food resource partitioning in a fish community of the central Amazon floodplain. Neotrop. Ichthyol. 2(2), 75-84. http://dx.doi.org/10.1590/S1679-62252004000200004.
http://dx.doi.org/10.1590/S1679-62252004...
; Balcombe et al., 2005Balcombe, S.R., Bunn, S.E., Mckenzie-Smith, F.J., & Davies, P.M., 2005. Variability of fish diets between dry and flood periods in an arid zone floodplain river. J. Fish Biol. 67(6), 1552-1567. http://dx.doi.org/10.1111/j.1095-8649.2005.00858.x.
http://dx.doi.org/10.1111/j.1095-8649.20...
; Quirino et al., 2015Quirino, B.A., Carniatto, N., Gaiotto, J.V., & Fugi, R., 2015. Seasonal variation in the use of food resources by small fishes inhabiting the littoral zone in a Neotropical floodplain lake. Aquat. Ecol. 49(4), 431-440. http://dx.doi.org/10.1007/s10452-015-9535-2.
http://dx.doi.org/10.1007/s10452-015-953...
). Also, it is a period of higher activity of insects for reproduction, feeding and flocks (Galinha & Hahn, 2004Galinha, A.B., & Hahn, N.S., 2004. Atividade de forrageamento de Triportheus spp. (Characidae, Triportheinae) utilizada como ferramenta de amostragem da entomofauna, na área do reservatório de Manso, MT. Rev. Bras. Zoociênc. 6(1)).
Autumn is characterized by the ebb period in the rivers at the studied region (Lourenço et al., 2012Lourenço, L.S., Mateus, L.A.F., & Penha, J.M., 2012. Variação espaço-temporal na distribuição e abundância de Moenkhausia sanctaefilomenae (Characiformes: Characidae) em lagoas da planície de inundação do rio Cuiabá, Pantanal, Brasil. Acta Sci. Biol. Sci. 34(1), 23-32. http://dx.doi.org/10.4025/actascibiolsci.v34i1.6809.
http://dx.doi.org/10.4025/actascibiolsci...
), and it is associated with a reduction in the feeding activity of ichthyofauna, which can be explained by the reduction of food availability (Payne, 1986Payne, A.L., 1986. The ecology of tropical lakes and rivers. New York: John Wiley & Sons.; Prejs & Prejs, 1987Prejs, A., & Prejs, K., 1987. Feeding of tropical freshwater fishes: seasonality in resource availability and resource use. Oecologia 71(3), 397-404. PMid:28312987. http://dx.doi.org/10.1007/BF00378713.
http://dx.doi.org/10.1007/BF00378713...
; Galinha & Hahn, 2004Galinha, A.B., & Hahn, N.S., 2004. Atividade de forrageamento de Triportheus spp. (Characidae, Triportheinae) utilizada como ferramenta de amostragem da entomofauna, na área do reservatório de Manso, MT. Rev. Bras. Zoociênc. 6(1)). In this season, we found a higher volume of Tecameba, Copepoda and microcrustaceans in stomach contents when compared to spring. The prevalence of these items can suffer influence of environment, as demonstrated by Takeda et al. (1990)Takeda, A.M., Shimizu, G.Y., & Higuti, J., 1990. Zoobentos de uma lagoa marginal (lagoa Fechada, rio Baia, Alto Paraná). Cienc. Cult. 42(11), 1003-1007. and Lansac-Tôha et al. (1993)Lansac-Tôha, F.A., Lima, A.F., Thomaz, S.M., & Roberto, M.C., 1993. Zooplâncton de uma planície de inundação do rio Paraná. II. Variação sazonal e influência dos níveis fluviométricos sobre a comunidade. Acta Limnol. Bras. 6, 42-55.. These findings differ from other studies that showed that S. notomelas fed on Cladocera and Copepoda in rainy seasons and Rotifers in the less rainy seasons in lakes of floodplain of the Upper Paraná River (Carniatto et al., 2012Carniatto, N., Fugi, R., Cantanhêde, G., Gubiani, É.A., & Hahn, N.S., 2012. Effects of flooding regime and diel cycle on diet of a small sized fish associated to macrophytes. Acta Limnol. Bras. 24(4), 363-372. http://dx.doi.org/10.1590/S2179-975X2013005000007.
http://dx.doi.org/10.1590/S2179-975X2013...
), or showing that the species feeds predominantly on algae in reservoirs from the Paraná River (Casatti et al., 2003Casatti, L., Mendes, H.F., & Ferreira, K.M., 2003. Aquatic macrophytes as feeding site for small fishes in the Rosana reservoir, Paranapanema River, Southeastern Brazil. Braz. J. Biol. 63(2), 213-222. PMid:14509843. http://dx.doi.org/10.1590/S1519-69842003000200006.
http://dx.doi.org/10.1590/S1519-69842003...
; Pelicice & Agostinho, 2006Pelicice, F.M., & Agostinho, A.A., 2006. Feeding ecology of fishes associated with Egeria spp. patches in a tropical reservoir, Brazil. Ecol. Freshwat. Fish 15(1), 10-19. http://dx.doi.org/10.1111/j.1600-0633.2005.00121.x.
http://dx.doi.org/10.1111/j.1600-0633.20...
; Hahn & Loureiro-Crippa, 2006Hahn, N.S., & Loureiro-Crippa, V.E., 2006. Estudo comparativo da dieta, hábitos alimentares e morfologia trófica de duas espécies simpátricas, de peixes de pequeno porte, associados à macrófitas aquáticas. Acta Sci. Biol. Sci. 28(4), 359-363. https://doi.org/10.4025/actascibiolsci.v28i4.167.
https://doi.org/10.4025/actascibiolsci.v...
). Such divergences can be favored by the difference in habitat, by the dynamics of connection and retraction of the marginal lagoon with the Tibagi river, riverside and interannual variation of precipitation among the study sites. In addition, our results highlight the importance of evaluating the S. notomelas diet, as it varies in different ecosystems and under different conditions.
Despite the large consumption of algae, plants, and detritus, which probably led to the low amplitude of the trophic niche as evidenced by Permidisp, we showed that there was a difference in the inter-individual variability in the consumption of food resources only between autumn and spring, which indicates that there was higher consumption of different resources. This inter-individual variation might have been favored by the unequal distribution of resources in aquatic environments, variation in the availability of food resources as a function of space and time (Pringle et al., 1988Pringle, C.M., Naiman, R.J., Bretschko, G., Karr, J.R., Oswood, M.W., Webster, J.R., Welcomme, R.L., & Winterbourn, M.J., 1988. Patch dynamics in lotic systems: the stream as a mosaic. J. N. Am. Benthol. Soc. 7(4), 503-524. http://dx.doi.org/10.2307/1467303.
http://dx.doi.org/10.2307/1467303...
), and connectivity and retraction of the lagoon with the main river (Deus & Petrere-Junior, 2003Deus, C.D., & Petrere-Junior, M., 2003. Seasonal diet shifts of seven fish species in an Atlantic rainforest stream in southeastern Brazil. Braz. J. Biol. 63(4), 579-588. PMid:15029369. http://dx.doi.org/10.1590/S1519-69842003000400005.
http://dx.doi.org/10.1590/S1519-69842003...
; Cunha et al., 2018Cunha, A.F., Wolff, L.L., & Hahn, N.S., 2018. Seasonal changes at population and individual levels in the diet of juvenile catfish in a Neotropical floodplain. J. Freshwat. Ecol. 33(1), 273-284. http://dx.doi.org/10.1080/02705060.2018.1442371.
http://dx.doi.org/10.1080/02705060.2018....
).
In general, we can conclude that seasonality influenced the diet of S. notomelas, showing changes in the proportion of items consumed in all seasons. We also observed a greater inter-individual variation during the autumn, indicating that there was a greater expansion of food resources by individuals, compared to other seasons. Although seasonality is not the only factor capable of promoting changes in the diet of fish, they provide relevant information about the species' capacity of adaptation, in addition to obtaining information on the provision of resources to aquatic communities in order to promote preservation and conservation of aquatic ecosystems.
Associate Editor: Ronaldo Angelini.
Acknowledgements
Our thanks to the technicians Edson Santana and Aparecido de Sousa for the help in the field work. The State University of Londrina and the UEL Zoology Museum for transportation and analysis and processing facilities. This project was partially funded by CNPq (Proc. 453850-2014, Fernando Jerep).
-
Cite as: Santiago, N.C. et al. Seasonal patterns may influence the diet of the lambari Serrapinnus notomelas (Eigenmann 1915). Acta Limnologica Brasiliensia, 2022, vol. 34, e16.
References
- Abelha, M.C.F., Agostinho, A.A., & Goulart, E., 2001. Plasticidade trófica em peixes de água doce. Acta Scientiarum 23(2), 425-434. https://doi.org/10.4025/actascibiolsci.v23i0.2696
» https://doi.org/10.4025/actascibiolsci.v23i0.2696 - Anderson, M.J., 2004. PERMDISP: a FORTRAN computer program for permutational analysis of multivariate dispersions (for any two-factor ANOVA design) using permutation tests. New Zealand: Department of Statistics, University of Auckland.
- Anderson, M.J., Gorley, R.N., & Clarke, K.R., 2008. PERMANOVA + for PRIMER: guide to software and statistical methods. Plymouth, UK: PRIMER-E.
- Aranha, J.M.R., Takeuti, D.F., & Yoshimura, T., 1998. Habitat use and food partitioning of the fishes in the Mergulhão stream (coastal stream of Atlantic Forest, Brazil). Rev. Biol. Trop. 46(4), 951-959.
- Balcombe, S.R., Bunn, S.E., Mckenzie-Smith, F.J., & Davies, P.M., 2005. Variability of fish diets between dry and flood periods in an arid zone floodplain river. J. Fish Biol. 67(6), 1552-1567. http://dx.doi.org/10.1111/j.1095-8649.2005.00858.x
» http://dx.doi.org/10.1111/j.1095-8649.2005.00858.x - Barbieri, G., & Barbieri, M.C., 1984. Note on nutricional dinamics of Gymnotus carap (L.) from the Lobo Reservoir. São Paulo State, Brazil. J. Fish Biol. 24(4), 351-355. http://dx.doi.org/10.1111/j.1095-8649.1984.tb04807.x
» http://dx.doi.org/10.1111/j.1095-8649.1984.tb04807.x - Barger, C.P., & Kitaysky, A.S., 2012. Isotopic segregation between sympatric seabird species increases with nutritional stress. Biol. Lett. 8(3), 442-445. PMid:22171022. http://dx.doi.org/10.1098/rsbl.2011.1020
» http://dx.doi.org/10.1098/rsbl.2011.1020 - Brambilla, E.M., Uieda, V.S., & Nogueira, M.G., 2018. Influence of habitat connectivity and seasonality on the ichthyofauna structure of a riverine knickzone. Iheringia Ser. Zool. 108, 1-7. http://dx.doi.org/10.1590/1678-4766e2018035
» http://dx.doi.org/10.1590/1678-4766e2018035 - Brandão-Gonçalves, L., Oliveira, S.A.D., & Lima-Junior, S.E., 2010. Hábitos alimentares da ictiofauna do córrego Franco, Mato Grosso do Sul, Brasil. Biota Neotrop. 10(2), 21-30. http://dx.doi.org/10.1590/S1676-06032010000200001
» http://dx.doi.org/10.1590/S1676-06032010000200001 - Bray, J.R., & Curtis, J.T., 1957. An ordination of the upland forest community of southern Wisconsin. Ecol. Monogr. 27(4), 325-349. http://dx.doi.org/10.2307/1942268
» http://dx.doi.org/10.2307/1942268 - Bührnheim, C.M., Carvalho, T.P., Malabarba, L.R., & Weitzman, S.W., 2008. A new genus and species of characid fish from the Amazon basin: the recognition of a relictual lineage of characid fishes (Ostariophysi: Cheirodontinae: Cheirodontini). Neotrop. Ichthyol. 6(4), 663-678. http://dx.doi.org/10.1590/S1679-62252008000400016
» http://dx.doi.org/10.1590/S1679-62252008000400016 - Carniatto, N., Fugi, R., Cantanhêde, G., Gubiani, É.A., & Hahn, N.S., 2012. Effects of flooding regime and diel cycle on diet of a small sized fish associated to macrophytes. Acta Limnol. Bras. 24(4), 363-372. http://dx.doi.org/10.1590/S2179-975X2013005000007
» http://dx.doi.org/10.1590/S2179-975X2013005000007 - Carnicer, J., Abrams, P.A., & Jordano, P., 2008. Switching behavior, coexistence and diversification: comparing empirical community‐wide evidence with theoretical predictions. Ecol. Lett. 11(8), 802-808. PMid:18445033. http://dx.doi.org/10.1111/j.1461-0248.2008.01195.x
» http://dx.doi.org/10.1111/j.1461-0248.2008.01195.x - Casatti, L., Mendes, H.F., & Ferreira, K.M., 2003. Aquatic macrophytes as feeding site for small fishes in the Rosana reservoir, Paranapanema River, Southeastern Brazil. Braz. J. Biol. 63(2), 213-222. PMid:14509843. http://dx.doi.org/10.1590/S1519-69842003000200006
» http://dx.doi.org/10.1590/S1519-69842003000200006 - Corrêa, C.E., Albrecht, M.P., & Hahn, N.S., 2011. Patterns of niche breadth and feeding overlap of the fish fauna in the seasonal Brazilian Pantanal, Cuiabá River basin. Neotrop. Ichthyol. 9(3), 637-646. http://dx.doi.org/10.1590/S1679-62252011000300017
» http://dx.doi.org/10.1590/S1679-62252011000300017 - Corrêa, C.E., Petry, A.C., & Hahn, N.S., 2009. Influência do ciclo hidrológico na dieta e estrutura trófica da ictiofauna do rio Cuiabá, Pantanal Mato-Grossense. Iheringia Ser. Zool. 99(4), 456-463. http://dx.doi.org/10.1590/S0073-47212009000400018
» http://dx.doi.org/10.1590/S0073-47212009000400018 - Correa, S., & Winemiller, K.O., 2014. Niche partitioning among frugivorous fishes in response to fluctuating resources in the Amazonian floodplain forest. Ecology 95(1), 210-224. PMid:24649660. http://dx.doi.org/10.1890/13-0393.1
» http://dx.doi.org/10.1890/13-0393.1 - Costa, I.D.D., & Rocha, V.M.D., 2017. Feeding ecology of Serrapinnus notomelas (Characiformes: Cheirodontinae) in small forest streams in the Machado River basin, Rondônia, Brazil. Acta Amazon. 47(1), 19-28. http://dx.doi.org/10.1590/1809-4392201601944
» http://dx.doi.org/10.1590/1809-4392201601944 - Cunha, A.F., Wolff, L.L., & Hahn, N.S., 2018. Seasonal changes at population and individual levels in the diet of juvenile catfish in a Neotropical floodplain. J. Freshwat. Ecol. 33(1), 273-284. http://dx.doi.org/10.1080/02705060.2018.1442371
» http://dx.doi.org/10.1080/02705060.2018.1442371 - De Mérona, B., Santos, G.M., & Almeida, R.G., 2001. Short term effects of Tucuruí Dam (Amazonia, Brazil) on the trophic organization of fish communities. Environ. Biol. Fishes 60(4), 375-392. http://dx.doi.org/10.1023/A:1011033025706
» http://dx.doi.org/10.1023/A:1011033025706 - Delariva, R.L., Hahn, N.S., & Kashiwaqui, E.A.L., 2013. Diet and trophic structure of the fish fauna in a subtropical ecosystem: impoundment effects. Neotrop. Ichthyol. 11(4), 891-904. http://dx.doi.org/10.1590/S1679-62252013000400017
» http://dx.doi.org/10.1590/S1679-62252013000400017 - Deus, C.D., & Petrere-Junior, M., 2003. Seasonal diet shifts of seven fish species in an Atlantic rainforest stream in southeastern Brazil. Braz. J. Biol. 63(4), 579-588. PMid:15029369. http://dx.doi.org/10.1590/S1519-69842003000400005
» http://dx.doi.org/10.1590/S1519-69842003000400005 - Dias, T.S., & Fialho, C.B., 2009. Biologia alimentar de quatro espécies simpátricas de Cheirodontinae (Characiformes, Characidae) do rio Ceará Mirim, Rio Grande do Norte. Iheringia Ser. Zool. 99(3), 242-248. http://dx.doi.org/10.1590/S0073-47212009000300003
» http://dx.doi.org/10.1590/S0073-47212009000300003 - Dibble, E.D., & Pelicice, F.M., 2010. Influence of aquatic plant‐specific habitat on an assemblage of small neotropical floodplain fishes. Ecol. Freshwat. Fish 19(3), 381-389. http://dx.doi.org/10.1111/j.1600-0633.2010.00420.x
» http://dx.doi.org/10.1111/j.1600-0633.2010.00420.x - Esteves, K.E., & Lobón-Cerviá, J., 2001. Composition and trophic structure of a fish community of a clear water Atlantic rainforest stream in southeastern Brazil. Environ. Biol. Fishes 62(4), 429-440. http://dx.doi.org/10.1023/A:1012249313341
» http://dx.doi.org/10.1023/A:1012249313341 - Ferrareze, M., & Nogueira, M.G., 2011. Importance of lateral lagoons for the ichthyofauna in a large tropical reservoir. Braz. J. Biol. 71(4), 807-820. http://dx.doi.org/10.1590/S1519-69842011000500002
» http://dx.doi.org/10.1590/S1519-69842011000500002 - Galinha, A.B., & Hahn, N.S., 2004. Atividade de forrageamento de Triportheus spp. (Characidae, Triportheinae) utilizada como ferramenta de amostragem da entomofauna, na área do reservatório de Manso, MT. Rev. Bras. Zoociênc. 6(1)
- Gomes, L.C., Bulla, C.K., Agostinho, A.A., Vasconcelos, L.P., & Miranda, L.E., 2012. Fish assemblage dynamics in a Neotropical floodplain relative to aquatic macrophytes and the homogenizing effect of a flood pulse. Hydrobiologia 685(1), 97-107. http://dx.doi.org/10.1007/s10750-011-0870-6
» http://dx.doi.org/10.1007/s10750-011-0870-6 - Goulding, M., 1980. The fishes and the forest: exploration in Amazonian Natural History. Berkeley: University of California, 280 p. http://dx.doi.org/10.1525/9780520316133
» http://dx.doi.org/10.1525/9780520316133 - Graça, W.J., & Pavanelli, C.S., 2007. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: EDUEM, 241 p.
- Hahn, N.S., & Loureiro-Crippa, V.E., 2006. Estudo comparativo da dieta, hábitos alimentares e morfologia trófica de duas espécies simpátricas, de peixes de pequeno porte, associados à macrófitas aquáticas. Acta Sci. Biol. Sci. 28(4), 359-363. https://doi.org/10.4025/actascibiolsci.v28i4.167
» https://doi.org/10.4025/actascibiolsci.v28i4.167 - Hellawell, J.M., & Abel, R., 1971. A rapid volumetric method for the analysis of the food of fishes. J. Fish Biol. 3(1), 29-37. http://dx.doi.org/10.1111/j.1095-8649.1971.tb05903.x
» http://dx.doi.org/10.1111/j.1095-8649.1971.tb05903.x - Henry, R., Panarelli, E.A., Casanova, S.M.C., Granado, D.C., Mortari, R.C., & Abra, J., 2011. Plankton richness and abundance in several different hydrological situations in lakes lateral to a river: a case study in the mouth zone of a tributary into a tropical Reservoir. Oecol. Aust. 15(3), 537-558. http://dx.doi.org/10.4257/oeco.2011.1503.08
» http://dx.doi.org/10.4257/oeco.2011.1503.08 - Lampert, V.R., Azevedo, M.A., & Fialho, C.B., 2003. Hábito alimentar de Mimagoniates microlepis Steindachner, 1876 (Characidae: Glandulocaudinae) do canal de ligação entre as lagoas Emboaba e Emboabinha, Rio Grande do Sul, Brasil. Comun. Mus. Cienc. Tecnol. PUCRS 16(1), 3-16.
- Lansac-Tôha, F.A., Lima, A.F., Thomaz, S.M., & Roberto, M.C., 1993. Zooplâncton de uma planície de inundação do rio Paraná. II. Variação sazonal e influência dos níveis fluviométricos sobre a comunidade. Acta Limnol. Bras. 6, 42-55.
- Lévêque, C., Oberdorff, T., Paugy, D., Stiassny, M.L.J., & Tedesco, P.A., 2008. Global diversity of fish (Pisces) in freshwater. Hydrobiologia 595(1), 545-567. http://dx.doi.org/10.1007/s10750-007-9034-0
» http://dx.doi.org/10.1007/s10750-007-9034-0 - Lourenço, L.S., Mateus, L.A.F., & Penha, J.M., 2012. Variação espaço-temporal na distribuição e abundância de Moenkhausia sanctaefilomenae (Characiformes: Characidae) em lagoas da planície de inundação do rio Cuiabá, Pantanal, Brasil. Acta Sci. Biol. Sci. 34(1), 23-32. http://dx.doi.org/10.4025/actascibiolsci.v34i1.6809
» http://dx.doi.org/10.4025/actascibiolsci.v34i1.6809 - Maack, R., 1981. Geografia física do Estado do Paraná. Rio de Janeiro: Livraria José Olympio Editora.
- Malabarba, L.R., 2003. Subfamily Cheirodontinae In: Reis, R.E., Kullander, S.O. & Ferraris-Jr, C.J., eds. Check list of the freshwater fishes of South and Central America. Porto Alegre: Edipucrs, 215-221.
- Mérona, B., & Rankin-de-Mérona, J., 2004. Food resource partitioning in a fish community of the central Amazon floodplain. Neotrop. Ichthyol. 2(2), 75-84. http://dx.doi.org/10.1590/S1679-62252004000200004
» http://dx.doi.org/10.1590/S1679-62252004000200004 - Minzão, L.D., Brucznitsk, V.F.B., & Cardoso, L.Q.F., 2004. Sazonalidade na dieta de duas espécies de peixes do gênero Serrapinnus, em uma lagoa marginal do alto Rio Paraná. Brasília, Editora UNB, 306 p.
- Mugnai, R., Nessimian, J.L., & Baptista, D.F., 2010. Manual de identificação de macroinvertebrados aquáticos. Rio de Janeiro: Technical Books, 176 p.
- Neiffer, D.L., & Stamper, M.A., 2009. Fish sedation, analgesia, anesthesia, and euthanasia: considerations, methods, and types of drugs. ILAR J. 50(4), 343-360. PMid:19949251. http://dx.doi.org/10.1093/ilar.50.4.343
» http://dx.doi.org/10.1093/ilar.50.4.343 - O’Callaghan, M.J., Hannah, D.M., Boomer, I., Williams, M., & Sadler, J.P., 2013. Responses to river inundation pressures control prey selection of riparian beetles. PLoS One 8(4), e61866. PMid:23613958. http://dx.doi.org/10.1371/journal.pone.0061866
» http://dx.doi.org/10.1371/journal.pone.0061866 - Oksanen, J., Blanchet, F.G., Friendly, M., Kindt, R., Legendre, P., McGlinn, D., Minchin, P.R., O’Hara, R.B., Simpson, G.L., Solymos, P., Stevens, M.H.H., Szoecs, E., & Wagner, H., 2018. Vegan: Community Ecology Package. Vienna: R Foundation for Statistical Computing.
- Oliveira, J.F., Costa, R.S., Novaes, J.L.C., Rebouças, L.G.F., Morais-Segundo, A.L.N., & Peretti, D., 2016. Efeito da seca e da variação espacial na abundância de indivíduos nas guildas tróficas da ictiofauna em um reservatório no Semiárido Brasileiro. Bol. Inst. Pesca 42(1), 51-64. http://dx.doi.org/10.20950/1678-2305.2016v42n1p51
» http://dx.doi.org/10.20950/1678-2305.2016v42n1p51 - Payne, A.L., 1986. The ecology of tropical lakes and rivers. New York: John Wiley & Sons.
- Pelicice, F.M., & Agostinho, A.A., 2006. Feeding ecology of fishes associated with Egeria spp. patches in a tropical reservoir, Brazil. Ecol. Freshwat. Fish 15(1), 10-19. http://dx.doi.org/10.1111/j.1600-0633.2005.00121.x
» http://dx.doi.org/10.1111/j.1600-0633.2005.00121.x - Pelicice, F.M., Agostinho, A.A., & Thomaz, S.M., 2005. Fish assemblages associated with Egeria in a tropical reservoir: investigating the effects of plant biomass and diel period. Acta Oecol, 27, 9-16. http://dx.doi.org/10.1016/j.actao.2004.08.004
» http://dx.doi.org/10.1016/j.actao.2004.08.004 - Piana, P.A., Gomes, L.C., & Cortez, E.M., 2006. Factors influencing Serrapinnus notomelas (Characiformes: Characidae) populations in upper Paraná river floodplain lagoons. Neotrop. Ichthyol. 4(1), 81-86. http://dx.doi.org/10.1590/S1679-62252006000100008
» http://dx.doi.org/10.1590/S1679-62252006000100008 - Prejs, A., & Prejs, K., 1987. Feeding of tropical freshwater fishes: seasonality in resource availability and resource use. Oecologia 71(3), 397-404. PMid:28312987. http://dx.doi.org/10.1007/BF00378713
» http://dx.doi.org/10.1007/BF00378713 - Pringle, C.M., Naiman, R.J., Bretschko, G., Karr, J.R., Oswood, M.W., Webster, J.R., Welcomme, R.L., & Winterbourn, M.J., 1988. Patch dynamics in lotic systems: the stream as a mosaic. J. N. Am. Benthol. Soc. 7(4), 503-524. http://dx.doi.org/10.2307/1467303
» http://dx.doi.org/10.2307/1467303 - Quirino, B.A., Carniatto, N., Gaiotto, J.V., & Fugi, R., 2015. Seasonal variation in the use of food resources by small fishes inhabiting the littoral zone in a Neotropical floodplain lake. Aquat. Ecol. 49(4), 431-440. http://dx.doi.org/10.1007/s10452-015-9535-2
» http://dx.doi.org/10.1007/s10452-015-9535-2 - R Development Core Team, 2019. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing.
- Raposo, R.M.G., & Gurgel, H.C.B., 2003. Variação da alimentação natural de Serrasalmus spilopleura Kner, 1860 (Pises, Serrasalmidae) em função do lunar e das estações do ano na lagoa de Extremoz, Rio Grande do Norte, Brasil. Acta Sci. Biol. Sci. 25(2), 267-272. https://doi.org/10.4025/actascianimsci.v25i2.2003
» https://doi.org/10.4025/actascianimsci.v25i2.2003 - Souza, A.E.F., Oliveira, J.F., Peretti, D., Fernandes, R., Costa, R.S., & Novaes, J.L.C., 2017. Effects of a supraseasonal drought on the ecological attributes of Plagioscion squamosissimus (Heckel, 1840) (Pisces, Sciaenidae) in a Brazilian Reservoir. ScientificWorldJournal 2017, 5930516. PMid:28326431. http://dx.doi.org/10.1155/2017/5930516
» http://dx.doi.org/10.1155/2017/5930516 - Súarez, Y.R., Valério, S.B., Tondato, K.K., Ximenes, L.Q.L., & Felipe, T.R.A., 2007. Determinantes ambientais da ocorrência de espécies de peixes em riachos de cabeceira da bacia do rio Ivinhema, Alto Rio Paraná. Acta Sci. Biol. Sci. 19(2), 145-150.
- Suzuki, H.I., Pelicice, F.M., Luiz, E.A., Latini, J.D., & Agostinho, A.A., 2004. Reproductive strategies of the fish community of the upper Paraná River floodplain. In: Agostinho, A.A., Rodrigues, L., Gomes, L.C., Thomaz, S.M., & Miranda, L.E., eds. Structure and functioning of the Paraná River and its floodplain: LTER – Site 6 (PELD – Sítio 6). Maringá: Eduem, 125-130.
- Takeda, A.M., Shimizu, G.Y., & Higuti, J., 1990. Zoobentos de uma lagoa marginal (lagoa Fechada, rio Baia, Alto Paraná). Cienc. Cult. 42(11), 1003-1007.
- Thomaz, S.M., Dibble, E.D., Evangelista, L.R., Higuti, J., & Bini, L.M., 2008. Influence of aquatic macrophyte habitat complexity on invertebrate abundance and richness in tropical lagoons. Freshw. Biol. 53, 358-367. https://doi.org/10.1111/j.1365-2427.2007.01898.x
» https://doi.org/10.1111/j.1365-2427.2007.01898.x - Tukey, J.W., 1953. The problem of multiple comparisons. Princeton: Princeton University. Unpublished report.
- Wickham, H., 2016. ggplot2: elegant graphics for data analysis [online]. New York: Springer-Verlag. Retrieved in 2021, July 25, from https://ggplot2.tidyverse.org
» https://ggplot2.tidyverse.org - Zavala-Camin, L.A., 1996. Introdução aos estudos sobre alimentação natural em peixes. Maringá: EDUEM, 129 p.
Publication Dates
-
Publication in this collection
10 June 2022 -
Date of issue
2022
History
-
Received
25 July 2021 -
Accepted
20 May 2022