Abstract:
Aim
Positive relationships between occupancy and abundance are often found for different groups of organisms and ecosystem types. However, to our knowledge, no study has sought to assess this relationship considering a context in which a particular mechanism is the most likely explanation. In this study, in addition to the positive relationship with abundance, we tested the hypothesis that occupancy of zooplankton species should be greater during the flood season because in this period the hydrological connectivity is greater than in the dry season, facilitating passive dispersal between floodplain environments.
Methods
Our study was carried out at 52 sites - including lakes and rivers - on the Araguaia River floodplain. We used an analysis of covariance to test the effects of abundance and hydrological period on zooplankton occupancy.
Results
We found, as expected, a positive relationship between occupancy and abundance of zooplankton species in each hydrological period. Our results also indicated that, with the increase in abundance, species occupancies were higher in the flood than in the dry season.
Conclusions
The positive effect of the flood on zooplankton occupancy can be explained by the increase in water level that increases the hydrological connectivity and the potential for plankton passive dispersal.
Keywords:
Araguaia river; spatial distribution; flood pulse; hydrological connectivity; mechanisms
Resumo:
Objetivo
Relações positivas entre distribuição regional e abundância são frequentemente encontradas para diferentes grupos de organismos e tipos de ecossistemas. No entanto, até onde sabemos, nenhum estudo buscou avaliar essa relação considerando um contexto no qual um determinado mecanismo fosse a explicação mais provável. Neste estudo, além da relação positiva com a abundância, nós testamos a hipótese de que a distribuição regional de espécies zooplanctônicas deveria ser maior durante o período de cheia uma vez que neste período a conectividade hidrológica é maior que no período de seca, facilitando a dispersão passiva entre os ambientes de uma planície de inundação.
Métodos
Nosso estudo foi realizado em 52 locais - incluindo lagoas e rios - na planície de inundação do rio Araguaia. Nós utilizamos uma análise de covariância para testar os efeitos da abundância e do período hidrológico sobre a distribuição regional.
Resultados
Encontramos, como esperado, uma relação positiva entre distribuição regional e abundância de espécies zooplanctônicas em cada período hidrológico. Nossos resultados também indicaram que, com o aumento da abundância, as distribuições regionais das espécies foram maiores no período de cheia.
Conclusões
O efeito positivo da cheia sobre a distribuição regional pode ser explicado pelo aumento do nível de água, que aumenta a conectividade hidrológica e o potencial de dispersão passiva do plâncton.
Palavras-chave:
rio Araguaia; distribuição espacial; pulso de inundação; conectividade hidrológica; mecanismos
1. Introduction
More abundant species generally occur in a greater number of local communities (i.e., they have higher occupancies) than less abundant ones. This positive relationship between occupancy and local abundance is one of the most ubiquitous patterns in ecology (Gaston et al., 2000Gaston, K.J., Blackburn, T.M., Greenwood, J.J., Gregory, R.D., Quinn, R.M., & Lawton, J.H., 2000. Abundance-occupancy relationships. J. Appl. Ecol. 37(s1), 39-59. http://dx.doi.org/10.1046/j.1365-2664.2000.00485.x.
http://dx.doi.org/10.1046/j.1365-2664.20...
; Gaston & Blackburn, 2003Gaston, K.J., & Blackburn, T.M., 2003. Dispersal and the interspecific abundance‐occupancy relationship in British birds. Glob. Ecol. Biogeogr. 12(5), 373-379. http://dx.doi.org/10.1046/j.1466-822X.2003.00054.x.
http://dx.doi.org/10.1046/j.1466-822X.20...
; Borregaard & Rahbek, 2010Borregaard, M.K., & Rahbek, C., 2010. Causality of the relationship between geographic distribution and species abundance. Q. Rev. Biol. 85(1), 3-25. http://dx.doi.org/10.1086/650265.
http://dx.doi.org/10.1086/650265...
). Different mechanisms can generate this relationship. For example, species that use widely distributed resources tend to have higher abundances and occupancies (Hanski et al., 1993Hanski, I., Kouki, J., & Halkka, A., (1993). Three explanations of the positive relationship between distribution and abundance of species. In: Ricklefs, R.E., & Schluter, D. eds. Species diversity in ecological communities: historical and geographical perspectives. Chicago: University of Chicago Press, 108-116.; Ten Caten et al., 2022Ten Caten, C., Holian, L., Dallas, T., & Pither, J., 2022. Weak but consistent abundance-occupancy relationships across taxa, space and time. Glob. Ecol. Biogeogr. 31(5), 968-977. http://dx.doi.org/10.1111/geb.13472.
http://dx.doi.org/10.1111/geb.13472...
). Occupancy and abundance would also increase with the niche breadth and dispersal capacity of the species (Gaston et al., 1997Gaston, K.J., Blackburn, T.M., & Lawton, J.H., 1997. Interspecific abundance-range size relationships: an appraisal of mechanisms. J. Anim. Ecol. 66(4), 579. http://dx.doi.org/10.2307/5951.
http://dx.doi.org/10.2307/5951...
; Ten Caten et al., 2022Ten Caten, C., Holian, L., Dallas, T., & Pither, J., 2022. Weak but consistent abundance-occupancy relationships across taxa, space and time. Glob. Ecol. Biogeogr. 31(5), 968-977. http://dx.doi.org/10.1111/geb.13472.
http://dx.doi.org/10.1111/geb.13472...
). However, the positive relationship between occupancy and abundance could emerge from sampling artifacts as less abundant species are more difficult to sample (Gaston et al., 1997Gaston, K.J., Blackburn, T.M., & Lawton, J.H., 1997. Interspecific abundance-range size relationships: an appraisal of mechanisms. J. Anim. Ecol. 66(4), 579. http://dx.doi.org/10.2307/5951.
http://dx.doi.org/10.2307/5951...
; Ten Caten et al., 2022Ten Caten, C., Holian, L., Dallas, T., & Pither, J., 2022. Weak but consistent abundance-occupancy relationships across taxa, space and time. Glob. Ecol. Biogeogr. 31(5), 968-977. http://dx.doi.org/10.1111/geb.13472.
http://dx.doi.org/10.1111/geb.13472...
). Finally, other factors such as habitat heterogeneity and biotic interactions can influence the strength of observed occupancy-abundance relationships (Holt et al., 2004Holt, A.R., Warren, P.H., & Gaston, K.J., 2004. The importance of habitat heterogeneity, biotic interactions and dispersal in abundance-occupancy relationships. J. Anim. Ecol. 73(5), 841-851. http://dx.doi.org/10.1111/j.0021-8790.2004.00862.x.
http://dx.doi.org/10.1111/j.0021-8790.20...
).
Studies on the relationship between occupancy and abundance are variable in methodological terms. Even the choice of the response variable (abundance or occupancy) and, consequently, of the explanatory variable, can vary between studies (Gaston et al., 2000Gaston, K.J., Blackburn, T.M., Greenwood, J.J., Gregory, R.D., Quinn, R.M., & Lawton, J.H., 2000. Abundance-occupancy relationships. J. Appl. Ecol. 37(s1), 39-59. http://dx.doi.org/10.1046/j.1365-2664.2000.00485.x.
http://dx.doi.org/10.1046/j.1365-2664.20...
). Also, there is a variation in how models are specified. For example, in analyzes that consider occupancy as a response variable, species-specific attributes (e.g., dispersal ability and niche breadth), in addition to abundance, can be used as explanatory variables in multiple regression models (Gaston & Blackburn, 2003Gaston, K.J., & Blackburn, T.M., 2003. Dispersal and the interspecific abundance‐occupancy relationship in British birds. Glob. Ecol. Biogeogr. 12(5), 373-379. http://dx.doi.org/10.1046/j.1466-822X.2003.00054.x.
http://dx.doi.org/10.1046/j.1466-822X.20...
; Webb et al., 2009Webb, T.J., Tyler, E.H.M., & Somerfield, P.J., 2009. Life history mediates large-scale population ecology in marine benthic taxa. Mar. Ecol. Prog. Ser. 396, 293-306. http://dx.doi.org/10.3354/meps08253.
http://dx.doi.org/10.3354/meps08253...
). There are also examples of studies that analyze the relationship between occupancy and abundance and, later, the relationship between each of these variables and species-specific traits (e.g., Siqueira et al., 2009Siqueira, T., Bini, L.M., Cianciaruso, M.V., Roque, F.O., & Trivinho-Strixino, S., 2009. The role of niche measures in explaining the abundance-distribution relationship in tropical lotic chironomids. Hydrobiologia 636(1), 163-172. http://dx.doi.org/10.1007/s10750-009-9945-z.
http://dx.doi.org/10.1007/s10750-009-994...
; Vilmi et al., 2019Vilmi, A., Tolonen, K.T., Karjalainen, S.M., & Heino, J., 2019. Niche position drives interspecific variation in occupancy and abundance in a highly-connected lake system. Ecol. Indic. 99, 159-166. http://dx.doi.org/10.1016/j.ecolind.2018.12.029.
http://dx.doi.org/10.1016/j.ecolind.2018...
). However, no study - to the best of our knowledge - has sought to assess the relationship between occupancy and abundance considering a context in which a particular mechanism (related to the environmental characteristics) to explain the relationship is more likely. For example, water level fluctuations in floodplain systems can alter hydrological connectivity and, therefore, the ease with which organisms can disperse between the different environments of these systems. Specifically, during flood periods, the increase in water level can increase the connectivity among aquatic ecosystems (Thomaz et al., 2007Thomaz, S.M., Bini, L.M., & Bozelli, R.L., 2007. Floods increase similarity among aquatic habitats in river-floodplain systems. Hydrobiologia 579(1), 1-13. http://dx.doi.org/10.1007/s10750-006-0285-y.
http://dx.doi.org/10.1007/s10750-006-028...
; Bozelli et al., 2015Bozelli, R.L., Thomaz, S.M., Padial, A.A., Lopes, P.M., & Bini, L.M., 2015. Floods decrease zooplankton beta diversity and environmental heterogeneity in an Amazonian floodplain system. Hydrobiologia 753(1), 233-241. http://dx.doi.org/10.1007/s10750-015-2209-1.
http://dx.doi.org/10.1007/s10750-015-220...
). As the water level rises, the transport of plankton may be more intense (e.g., Bonecker et al., 2005Bonecker, C.C., Costa, C.L.D., Velho, L.F.M., & Lansac-Tôha, F.A., 2005. Diversity and abundance of the planktonic rotifers in different environments of the Upper Paraná River floodplain (Paraná State - Mato Grosso do Sul State, Brazil). In: Herzig, A., Gulati, R.D., & Jersabek, C.D., May, L., eds. Rotifera X. Dordrecht: Springer, 405-414. http://dx.doi.org/10.1007/1-4020-4408-9_42.
http://dx.doi.org/10.1007/1-4020-4408-9_...
) and, therefore, it is expected that the species will have a higher occupancy. On the other hand, environments may be hydrologically less favorable for planktonic communities during flood periods, decreasing their abundance (Bozelli, 1992Bozelli, R.L., 1992. Composition of the zooplankton community of Batata and Mussurá Lakes and of the Trombetas River, State of Pará, Brazil. Amazoniana. Limnologia Oecol. Regionalis Systematis Fluminis Amazonas 12(2), 239-261.).
Here, we aimed to test the relationship between occupancy and abundance of zooplankton species in the dry and flood seasons in the Araguaia River floodplain. Specifically, we tested whether, in addition to a relationship with abundance, the regional distribution (occupancy) of zooplankton species was higher during the flood season than during the dry season. This expectation is justified considering that the hydrological connectivity among the floodplain environments increases during the flood season. Thus, compared to the dry season, when the environments are more isolated, the species could reach (mainly by drift) a larger number of sites during the flood season.
2. Methods
We used a dataset obtained by Vieira (2008)Vieira, L.C.G., 2008. Padrões ecológicos da comunidade zooplanctônica na planície de inundação do rio Araguaia [Tese de doutorado em Ciências Ambientais]. Goiânia: Programa de Pós-graduação em Ecologia e Evolução, Universidade Federal de Goiás. for this study. Data on zooplankton species densities (individuals/m3) were obtained from 52 sites distributed in 30 environments (22 lakes with two sampling sites - one in the deepest zone and other near the shoreline of the lake - and 8 rivers, including two sampling sites in the Vermelho River and six sampling sites in the main stem of the Araguaia River (Figure 1), between the municipalities of Aruanã and São Miguel do Araguaia (Goiás, Brazil). The same sites were sampled during the flood (January) and dry (July) periods of 2006 (for details of the field and laboratory work, see Vieira, 2008Vieira, L.C.G., 2008. Padrões ecológicos da comunidade zooplanctônica na planície de inundação do rio Araguaia [Tese de doutorado em Ciências Ambientais]. Goiânia: Programa de Pós-graduação em Ecologia e Evolução, Universidade Federal de Goiás.). The monthly average flows registered during these months (January and July) were typical of the flood and dry periods in the Araguaia River floodplain (ca. 2507 m3/s and 519 m3/s, respectively; data from the National Agency of Waters near the city of Nova Crixás, state of Goiás, Brazil).
Sampling locations in the Araguaia River floodplain. The same environments (lakes or rivers) were sampled in two periods (January 2006 and July 2006). Two sites were sampled in each lake.
For each hydrological period, we calculated the occupancy by dividing the number of occurrences of each species (i.e., number of sites in which the species was present) by the total number of sites (52). The local mean abundance of each species consisted of the mean density (individuals/m3) considering only the sites with densities greater than zero. Prior to the analyzes, occupancy and abundance data were transformed using the logit and logarithmic transformations, respectively. These transformations were necessary to satisfy the assumption of normality of the residuals. Finally, to test the relationship between occupancy and the explanatory variables (mean abundance and hydrological period), we used an analysis of covariance (ANCOVA). The hydrological period - with two levels (flood and dry) - was the categorical factor tested, whereas the mean abundance was the quantitative variable (covariate) in our ANCOVA model. All analyses were performed in the R environment (R Core Team, 2020R Core Team, 2020. R: a language and environment for statistical computing (Online). Vienna: R Foundation for Statistical Computing. Retrieved in 2022, August 24, from https://www.R-project.org/
https://www.R-project.org/...
; version 4.0.2) using the package car (Fox & Weisberg, 2019Fox, J., & Weisberg, S., 2019. An R companion to applied regression (3rd ed.). Thousand Oaks: Sage.).
3. Results
The numbers of taxa with densities greater than zero in the flood and dry seasons were equal to 145 and 111, respectively (considering a total of 190 taxa in both periods). Young copepods (Diaptomidae and Cyclopidae) and Bdelloidea, treated as taxonomic units in our analyzes, showed the highest occupancies (i.e., presence in more than 90% of the sites) in each of the periods studied. In addition to the young forms, the species with the highest occupancies (> 70%) in the flood season were Bosminopsis deitersi Richard (1895), Lecane curvicornis Murray (1913), Ceriodaphnia cornuta Sars (1885), Moina minuta Hansen (1899) and Platyias quadricornis Ehrenberg (1832). For the dry season, the species with the highest occupancies (> 70%) were Moina minuta and Bosmina hagmanni Stingelin (1904).
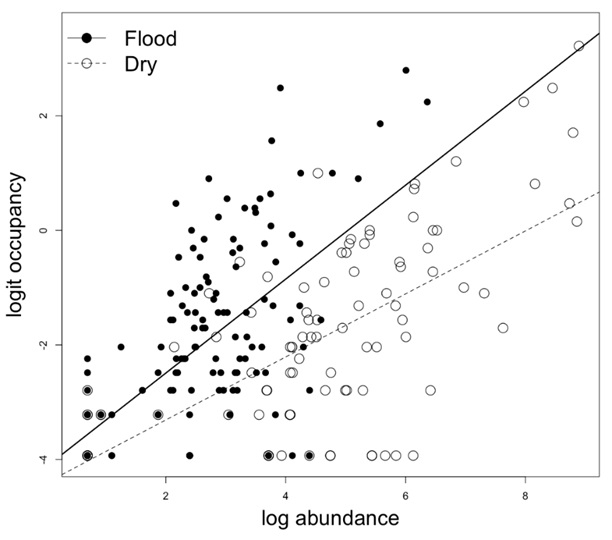
In each hydrological period, we found a positive relationship between occupancy and abundance of zooplankton taxa (F1,143 = 94.6, R2 = 0.40 for the flood season and F1,109 = 107.8, R2 = 0.50 for the dry season; Figure 2). In addition, the results of the analysis of covariance (Table 1) indicated that the slopes (b) of the relationship between occupancy and abundance differed between the flood (b = 0.820 ± 0.084 SE) and dry (b = 0.550 ± 0.053 SE) seasons. The lack of parallelism, as evidenced by the significant interaction between abundance and hydrological period, did not allow the direct test of our main hypothesis. However, we found that, with increasing abundance, the occupancy was progressively higher during the flood season than during the dry season (Figure 2). We also performed the analyses with only the taxa that occurred in both hydrological periods (83); however, the results were qualitatively the same.
Relationship between occupancy and abundance of zooplanktonic taxa in the Araguaia River floodplain in two hydrological periods (flood and dry seasons; January 2006 and July 2006, respectively).
Results of the analysis of covariance (ANCOVA) evaluating the relationship between zooplankton occupancy in the Araguaia River floodplain and the explanatory variables (logarithm of abundance and hydrological period).
4. Discussion
We found a positive relationship between occupancy and abundance in the two seasons. However, when compared to the dry season and as abundance increased, the zooplankton taxa progressively showed higher occupancy during the flood season.
The positive relationship between occupancy and abundance has been verified for several groups of organisms, but especially for terrestrial vertebrates and insects (Holt et al., 2002Holt, A.R., Gaston, K.J., & He, F., 2002. Occupancy-abundance relationships and spatial distribution: a review. Basic Appl. Ecol. 3(1), 1-13. http://dx.doi.org/10.1078/1439-1791-00083.
http://dx.doi.org/10.1078/1439-1791-0008...
; Blackburn et al., 2006Blackburn, T.M., Cassey, P., & Gaston, K.J., 2006. Variations on a theme: sources of heterogeneity in the form of the interspecific relationship between abundance and distribution. J. Anim. Ecol. 75(6), 1426-1439. PMid:17032375. http://dx.doi.org/10.1111/j.1365-2656.2006.01167.x.
http://dx.doi.org/10.1111/j.1365-2656.20...
). In this context, our results suggest that the relationship can be generalized to the zooplankton community (see also O'Brien et al., 2004O'Brien, W.J., Barfield, M., Bettez, N.D., Gettel, G.M., Hershey, A.E., McDonald, M.E., Miller, M.C., Mooers, H., Pastor, J., Richards, C., & Schuldt, J., 2004. Physical, chemical, and biotic effects on arctic zooplankton communities and diversity. Limnol. Oceanogr. 49(4 Part 2), 1250-1261. http://dx.doi.org/10.4319/lo.2004.49.4_part_2.1250.
http://dx.doi.org/10.4319/lo.2004.49.4_p...
; Ten Caten et al., 2022Ten Caten, C., Holian, L., Dallas, T., & Pither, J., 2022. Weak but consistent abundance-occupancy relationships across taxa, space and time. Glob. Ecol. Biogeogr. 31(5), 968-977. http://dx.doi.org/10.1111/geb.13472.
http://dx.doi.org/10.1111/geb.13472...
). However, our results are also consistent with those obtained by Ten Caten et al. (2022)Ten Caten, C., Holian, L., Dallas, T., & Pither, J., 2022. Weak but consistent abundance-occupancy relationships across taxa, space and time. Glob. Ecol. Biogeogr. 31(5), 968-977. http://dx.doi.org/10.1111/geb.13472.
http://dx.doi.org/10.1111/geb.13472...
by demonstrating that, despite the ubiquity of the relationship (including over time, as also demonstrated in our study), a substantial part of the variation in occupancy was not explained by abundance (as indicated by the coefficients of non-determination: 1-R2 = 0.60 for the flood season and 1-R2 = 0.50 for the dry season; see also Blackburn et al., 2006Blackburn, T.M., Cassey, P., & Gaston, K.J., 2006. Variations on a theme: sources of heterogeneity in the form of the interspecific relationship between abundance and distribution. J. Anim. Ecol. 75(6), 1426-1439. PMid:17032375. http://dx.doi.org/10.1111/j.1365-2656.2006.01167.x.
http://dx.doi.org/10.1111/j.1365-2656.20...
).
Our results do not allow us to point out the main mechanism that can explain the positive relationship between occupancy and abundance. For example, according to Gaston et al. (1997)Gaston, K.J., Blackburn, T.M., & Lawton, J.H., 1997. Interspecific abundance-range size relationships: an appraisal of mechanisms. J. Anim. Ecol. 66(4), 579. http://dx.doi.org/10.2307/5951.
http://dx.doi.org/10.2307/5951...
and Holt et al. (2004)Holt, A.R., Warren, P.H., & Gaston, K.J., 2004. The importance of habitat heterogeneity, biotic interactions and dispersal in abundance-occupancy relationships. J. Anim. Ecol. 73(5), 841-851. http://dx.doi.org/10.1111/j.0021-8790.2004.00862.x.
http://dx.doi.org/10.1111/j.0021-8790.20...
, the metapopulation dynamics hypothesis predicts that more abundant species simultaneously have lower extinction rates and higher colonization rates, resulting in higher occupancy. Furthermore, according to the rescue effect, there is a decrease in the probability of local extinction with the increase in the number of habitats colonized (Hanski, 1991Hanski, I., 1991. Single-species metapopulation dynamics: concepts, models and observations. Biol. J. Linn. Soc. Lond. 42(1-2), 17-38. http://dx.doi.org/10.1111/j.1095-8312.1991.tb00549.x.
http://dx.doi.org/10.1111/j.1095-8312.19...
; Gaston et al., 1997Gaston, K.J., Blackburn, T.M., & Lawton, J.H., 1997. Interspecific abundance-range size relationships: an appraisal of mechanisms. J. Anim. Ecol. 66(4), 579. http://dx.doi.org/10.2307/5951.
http://dx.doi.org/10.2307/5951...
; Holt et al., 2004Holt, A.R., Warren, P.H., & Gaston, K.J., 2004. The importance of habitat heterogeneity, biotic interactions and dispersal in abundance-occupancy relationships. J. Anim. Ecol. 73(5), 841-851. http://dx.doi.org/10.1111/j.0021-8790.2004.00862.x.
http://dx.doi.org/10.1111/j.0021-8790.20...
). On the other hand, according to the niche breadth hypothesis, the relationship between occupancy and abundance would emerge because there are positive relationships between these variables and niche breadth (Brown, 1984Brown, J.H., 1984. On the relationship between abundance and distribution of species. Am. Nat. 124(2), 255-279. http://dx.doi.org/10.1086/284267.
http://dx.doi.org/10.1086/284267...
; Gaston et al., 1997Gaston, K.J., Blackburn, T.M., & Lawton, J.H., 1997. Interspecific abundance-range size relationships: an appraisal of mechanisms. J. Anim. Ecol. 66(4), 579. http://dx.doi.org/10.2307/5951.
http://dx.doi.org/10.2307/5951...
). Finally, species can be more abundant and widely distributed when they exploit resources that are also common and widely distributed (Hanski et al., 1993Hanski, I., Kouki, J., & Halkka, A., (1993). Three explanations of the positive relationship between distribution and abundance of species. In: Ricklefs, R.E., & Schluter, D. eds. Species diversity in ecological communities: historical and geographical perspectives. Chicago: University of Chicago Press, 108-116.; Holt et al., 2004Holt, A.R., Warren, P.H., & Gaston, K.J., 2004. The importance of habitat heterogeneity, biotic interactions and dispersal in abundance-occupancy relationships. J. Anim. Ecol. 73(5), 841-851. http://dx.doi.org/10.1111/j.0021-8790.2004.00862.x.
http://dx.doi.org/10.1111/j.0021-8790.20...
). Thus, the three mechanisms could explain the positive relationship between occupancy and abundance in each period (flood or dry season). In short, species-specific characteristics (including niche position and breadth, as demonstrated for other aquatic organisms, e.g., Siqueira et al., 2009Siqueira, T., Bini, L.M., Cianciaruso, M.V., Roque, F.O., & Trivinho-Strixino, S., 2009. The role of niche measures in explaining the abundance-distribution relationship in tropical lotic chironomids. Hydrobiologia 636(1), 163-172. http://dx.doi.org/10.1007/s10750-009-9945-z.
http://dx.doi.org/10.1007/s10750-009-994...
; Rocha et al., 2018Rocha, M.P., Bini, L.M., Siqueira, T., Hjort, J., Grönroos, M., Lindholm, M., Karjalainen, S.M., & Heino, J., 2018. Predicting occupancy and abundance by niche position, niche breadth and body size in stream organisms. Oecologia 186(1), 205-216. PMid:29090405. http://dx.doi.org/10.1007/s00442-017-3988-z.
http://dx.doi.org/10.1007/s00442-017-398...
) can be important predictors of occupancy. For example, O'Brien et al. (2004)O'Brien, W.J., Barfield, M., Bettez, N.D., Gettel, G.M., Hershey, A.E., McDonald, M.E., Miller, M.C., Mooers, H., Pastor, J., Richards, C., & Schuldt, J., 2004. Physical, chemical, and biotic effects on arctic zooplankton communities and diversity. Limnol. Oceanogr. 49(4 Part 2), 1250-1261. http://dx.doi.org/10.4319/lo.2004.49.4_part_2.1250.
http://dx.doi.org/10.4319/lo.2004.49.4_p...
, in a study carried out in 104 lakes in northern North America, hypothesized that the most abundant zooplankton species (and with higher occupancy) were those least vulnerable to fish predation.
After statistically controlling for the effect of abundance (using ANCOVA), however, we observed that the species tended to have higher occupancy during the flood season as compared to the dry season (especially at high abundance). Thus, although species-specific characteristics may explain why some species have higher occupancy than others, our results indicate that the flood pulse (Junk et al., 1989Junk, W.J., Bayley, P.B., & Sparks, R.E., 1989. The flood pulse concept in river-floodplain systems. Can. Spec. Publ. Fish. Aquat. Sci. 106(1), 110-127.) is fundamental for the occupation of habitats. Probably, the positive effect of flood on the occupancy can be attributed to the increase in water level, which, in turn, simultaneously increased the hydrological connectivity, the similarity of habitats, and the transport of plankton among the different environments in the Araguaia River floodplain (see Havel & Shurin, 2004Havel, J.E., & Shurin, J.B., 2004. Mechanisms, effects, and scales of dispersal in freshwater zooplankton. Limnol. Oceanogr. 49(4 Part 2), 1229-1238. http://dx.doi.org/10.4319/lo.2004.49.4_part_2.1229.
http://dx.doi.org/10.4319/lo.2004.49.4_p...
for a review of freshwater zooplankton dispersal). It is interesting also to note that despite the commonly negative effect of water velocity (or a positive effect of water residence time) on zooplankton abundance (e.g., Baranyi et al., 2002Baranyi, C., Hein, T., Holarek, C., Keckeis, S., & Schiemer, F., 2002. Zooplankton biomass and community structure in a Danube River floodplain system: effects of hydrology. Freshw. Biol. 47(3), 473-482. http://dx.doi.org/10.1046/j.1365-2427.2002.00822.x.
http://dx.doi.org/10.1046/j.1365-2427.20...
; but see Ning et al., 2013Ning, N.S., Gawne, B., Cook, R.A., & Nielsen, D.L., 2013. Zooplankton dynamics in response to the transition from drought to flooding in four Murray-Darling Basin rivers affected by differing levels of flow regulation. Hydrobiologia 702(1), 45-62. http://dx.doi.org/10.1007/s10750-012-1306-7.
http://dx.doi.org/10.1007/s10750-012-130...
for different results), our results show that the occupancies of the taxa were higher in the flood than in the dry season. Our hypothesis of the synergistic effect of species-specific characteristics (e.g., abundance, niche breadth, dispersal ability) and system connectivity (increasing passive dispersal rates) is reinforced if we consider that the difference in occupancy between the seasons increased with abundance (see the significant interaction between abundance and period, as demonstrated by ANCOVA).
The comparison of the relationships between occupancy and abundance between periods of lower and higher connectivity (dry and flood, respectively), as performed in our study, is similar to that performed by Foggo et al. (2007)Foggo, A., Bilton, D.T., & Rundle, S.D., 2007. Do developmental mode and dispersal shape abundance-occupancy relationships in marine macroinvertebrates? J. Anim. Ecol. 76(4), 695-702. PMid:17584375. http://dx.doi.org/10.1111/j.1365-2656.2007.01245.x.
http://dx.doi.org/10.1111/j.1365-2656.20...
. These authors compare the relationships between occupancy and abundance after classifying 362 taxa of marine macroinvertebrates collected in the British Isles into planktonic and non-planktonic organisms. Their results clearly demonstrated that, after controlling for abundance, the occupancy was greater for planktonic organisms. Our results also demonstrate the duality of the flood effect on the dynamics of zooplankton species in the floodplain. Although the flood causes a reduction in species abundance (as observed by Vieira, 2008Vieira, L.C.G., 2008. Padrões ecológicos da comunidade zooplanctônica na planície de inundação do rio Araguaia [Tese de doutorado em Ciências Ambientais]. Goiânia: Programa de Pós-graduação em Ecologia e Evolução, Universidade Federal de Goiás. for the Araguaia River floodplain), in general, occupancies were higher during this period. This observation can be linked to the increase in habitat similarity frequently observed during floods (Thomaz et al., 2007Thomaz, S.M., Bini, L.M., & Bozelli, R.L., 2007. Floods increase similarity among aquatic habitats in river-floodplain systems. Hydrobiologia 579(1), 1-13. http://dx.doi.org/10.1007/s10750-006-0285-y.
http://dx.doi.org/10.1007/s10750-006-028...
). For instance, an experiment conducted by Holt et al. (2004)Holt, A.R., Warren, P.H., & Gaston, K.J., 2004. The importance of habitat heterogeneity, biotic interactions and dispersal in abundance-occupancy relationships. J. Anim. Ecol. 73(5), 841-851. http://dx.doi.org/10.1111/j.0021-8790.2004.00862.x.
http://dx.doi.org/10.1111/j.0021-8790.20...
with protists and bacteria showed that some species achieved higher occupancies in homogeneous than heterogeneous environments, despite having similar abundances in both habitat conditions.
In conclusion, our study suggests that system characteristics, in terms of facilitating the dispersal among habitats, in addition to species-specific characteristics (as demonstrated in other studies; e.g., Foggo et al., 2007Foggo, A., Bilton, D.T., & Rundle, S.D., 2007. Do developmental mode and dispersal shape abundance-occupancy relationships in marine macroinvertebrates? J. Anim. Ecol. 76(4), 695-702. PMid:17584375. http://dx.doi.org/10.1111/j.1365-2656.2007.01245.x.
http://dx.doi.org/10.1111/j.1365-2656.20...
), are key to explaining the distribution of species in floodplains. Considering the increasing human interference in these ecosystems (Pelicice et al., 2021Pelicice, F.M., Agostinho, A.A., Akama, A., Andrade Filho, J.D., Azevedo-Santos, V.M., Barbosa, M.V.M., Bini, L.M., Brito, M.F.G., Anjos Candeiro, C.R., Caramaschi, E.P., Carvalho, P., Carvalho, R.A., Castello, L., Chagas, D.B., Chamon, C.C., Colli, G.R., Daga, V.S., Dias, M.S., Diniz Filho, J.A.F., Fearnside, P., Melo Ferreira, W., Garcia, D.A.Z., Krolow, T.K., Kruger, R.F., Latrubesse, E.M., Lima Junior, D.P., de Fátima Lolis, S., Lopes, F.A.C., Loyola, R.D., Magalhães, A.L.B., Malvasio, A., De Marco Junior, P., Martins, P.R., Mazzoni, R., Nabout, J.C., Orsi, M.L., Padial, A.A., Pereira, H.R., Pereira, T.N.A., Perônico, P.B., Petrere Junior, M., Pinheiro, R.T., Pires, E.F., Pompeu, P.S., Portelinha, T.C.G., Sano, E.E., Santos, V.L.M., Shimabukuro, P.H.F., Silva, I.G., Souza, L.B.E., Tejerina-Garro, F.L., Campos Telles, M.P., Teresa, F.B., Thomaz, S.M., Tonella, L.H., Vieira, L.C.G., Vitule, J.R.S., & Zuanon, J., 2021. Large-scale Degradation of the Tocantins-Araguaia River Basin. Environ. Manage. 68(4), 445-452. PMid:34341867. http://dx.doi.org/10.1007/s00267-021-01513-7.
http://dx.doi.org/10.1007/s00267-021-015...
), we also speculate that changes in the hydrological regime (e.g., caused by dams or climate change) may reduce the connectivity of the system and, therefore, the ability of planktonic species to occupy the floodplain environments.
Acknowledgements
L.M.B. and L.C.G.V. receive continuous grants and research scholarships from CNPq. This work was developed in the context of the Institutos Nacionais de Ciência e Tecnologia (INCT) in Ecologia, Evolução e Conservação da Biodiversidade (EECBio), supported by MCTIC/CNPq (proc. 465610/2014-5) and Fundação de Amparo à Pesquisa do Estado de Goiás (FAPEG).
-
Cite as: Santos, K.N.J. et al. The relationship between zooplankton occupancy and abundance in a floodplain is mediated by the hydrological regime. Acta Limnologica Brasiliensia, 2022, vol. 34, e29.
References
- Baranyi, C., Hein, T., Holarek, C., Keckeis, S., & Schiemer, F., 2002. Zooplankton biomass and community structure in a Danube River floodplain system: effects of hydrology. Freshw. Biol. 47(3), 473-482. http://dx.doi.org/10.1046/j.1365-2427.2002.00822.x
» http://dx.doi.org/10.1046/j.1365-2427.2002.00822.x - Blackburn, T.M., Cassey, P., & Gaston, K.J., 2006. Variations on a theme: sources of heterogeneity in the form of the interspecific relationship between abundance and distribution. J. Anim. Ecol. 75(6), 1426-1439. PMid:17032375. http://dx.doi.org/10.1111/j.1365-2656.2006.01167.x
» http://dx.doi.org/10.1111/j.1365-2656.2006.01167.x - Bonecker, C.C., Costa, C.L.D., Velho, L.F.M., & Lansac-Tôha, F.A., 2005. Diversity and abundance of the planktonic rotifers in different environments of the Upper Paraná River floodplain (Paraná State - Mato Grosso do Sul State, Brazil). In: Herzig, A., Gulati, R.D., & Jersabek, C.D., May, L., eds. Rotifera X. Dordrecht: Springer, 405-414. http://dx.doi.org/10.1007/1-4020-4408-9_42
» http://dx.doi.org/10.1007/1-4020-4408-9_42 - Borregaard, M.K., & Rahbek, C., 2010. Causality of the relationship between geographic distribution and species abundance. Q. Rev. Biol. 85(1), 3-25. http://dx.doi.org/10.1086/650265
» http://dx.doi.org/10.1086/650265 - Bozelli, R.L., 1992. Composition of the zooplankton community of Batata and Mussurá Lakes and of the Trombetas River, State of Pará, Brazil. Amazoniana. Limnologia Oecol. Regionalis Systematis Fluminis Amazonas 12(2), 239-261.
- Bozelli, R.L., Thomaz, S.M., Padial, A.A., Lopes, P.M., & Bini, L.M., 2015. Floods decrease zooplankton beta diversity and environmental heterogeneity in an Amazonian floodplain system. Hydrobiologia 753(1), 233-241. http://dx.doi.org/10.1007/s10750-015-2209-1
» http://dx.doi.org/10.1007/s10750-015-2209-1 - Brown, J.H., 1984. On the relationship between abundance and distribution of species. Am. Nat. 124(2), 255-279. http://dx.doi.org/10.1086/284267
» http://dx.doi.org/10.1086/284267 - Foggo, A., Bilton, D.T., & Rundle, S.D., 2007. Do developmental mode and dispersal shape abundance-occupancy relationships in marine macroinvertebrates? J. Anim. Ecol. 76(4), 695-702. PMid:17584375. http://dx.doi.org/10.1111/j.1365-2656.2007.01245.x
» http://dx.doi.org/10.1111/j.1365-2656.2007.01245.x - Fox, J., & Weisberg, S., 2019. An R companion to applied regression (3rd ed.). Thousand Oaks: Sage.
- Gaston, K.J., Blackburn, T.M., & Lawton, J.H., 1997. Interspecific abundance-range size relationships: an appraisal of mechanisms. J. Anim. Ecol. 66(4), 579. http://dx.doi.org/10.2307/5951
» http://dx.doi.org/10.2307/5951 - Gaston, K.J., & Blackburn, T.M., 2003. Dispersal and the interspecific abundance‐occupancy relationship in British birds. Glob. Ecol. Biogeogr. 12(5), 373-379. http://dx.doi.org/10.1046/j.1466-822X.2003.00054.x
» http://dx.doi.org/10.1046/j.1466-822X.2003.00054.x - Gaston, K.J., Blackburn, T.M., Greenwood, J.J., Gregory, R.D., Quinn, R.M., & Lawton, J.H., 2000. Abundance-occupancy relationships. J. Appl. Ecol. 37(s1), 39-59. http://dx.doi.org/10.1046/j.1365-2664.2000.00485.x
» http://dx.doi.org/10.1046/j.1365-2664.2000.00485.x - Hanski, I., 1991. Single-species metapopulation dynamics: concepts, models and observations. Biol. J. Linn. Soc. Lond. 42(1-2), 17-38. http://dx.doi.org/10.1111/j.1095-8312.1991.tb00549.x
» http://dx.doi.org/10.1111/j.1095-8312.1991.tb00549.x - Hanski, I., Kouki, J., & Halkka, A., (1993). Three explanations of the positive relationship between distribution and abundance of species. In: Ricklefs, R.E., & Schluter, D. eds. Species diversity in ecological communities: historical and geographical perspectives. Chicago: University of Chicago Press, 108-116.
- Havel, J.E., & Shurin, J.B., 2004. Mechanisms, effects, and scales of dispersal in freshwater zooplankton. Limnol. Oceanogr. 49(4 Part 2), 1229-1238. http://dx.doi.org/10.4319/lo.2004.49.4_part_2.1229
» http://dx.doi.org/10.4319/lo.2004.49.4_part_2.1229 - Holt, A.R., Gaston, K.J., & He, F., 2002. Occupancy-abundance relationships and spatial distribution: a review. Basic Appl. Ecol. 3(1), 1-13. http://dx.doi.org/10.1078/1439-1791-00083
» http://dx.doi.org/10.1078/1439-1791-00083 - Holt, A.R., Warren, P.H., & Gaston, K.J., 2004. The importance of habitat heterogeneity, biotic interactions and dispersal in abundance-occupancy relationships. J. Anim. Ecol. 73(5), 841-851. http://dx.doi.org/10.1111/j.0021-8790.2004.00862.x
» http://dx.doi.org/10.1111/j.0021-8790.2004.00862.x - Junk, W.J., Bayley, P.B., & Sparks, R.E., 1989. The flood pulse concept in river-floodplain systems. Can. Spec. Publ. Fish. Aquat. Sci. 106(1), 110-127.
- Ning, N.S., Gawne, B., Cook, R.A., & Nielsen, D.L., 2013. Zooplankton dynamics in response to the transition from drought to flooding in four Murray-Darling Basin rivers affected by differing levels of flow regulation. Hydrobiologia 702(1), 45-62. http://dx.doi.org/10.1007/s10750-012-1306-7
» http://dx.doi.org/10.1007/s10750-012-1306-7 - O'Brien, W.J., Barfield, M., Bettez, N.D., Gettel, G.M., Hershey, A.E., McDonald, M.E., Miller, M.C., Mooers, H., Pastor, J., Richards, C., & Schuldt, J., 2004. Physical, chemical, and biotic effects on arctic zooplankton communities and diversity. Limnol. Oceanogr. 49(4 Part 2), 1250-1261. http://dx.doi.org/10.4319/lo.2004.49.4_part_2.1250
» http://dx.doi.org/10.4319/lo.2004.49.4_part_2.1250 - Pelicice, F.M., Agostinho, A.A., Akama, A., Andrade Filho, J.D., Azevedo-Santos, V.M., Barbosa, M.V.M., Bini, L.M., Brito, M.F.G., Anjos Candeiro, C.R., Caramaschi, E.P., Carvalho, P., Carvalho, R.A., Castello, L., Chagas, D.B., Chamon, C.C., Colli, G.R., Daga, V.S., Dias, M.S., Diniz Filho, J.A.F., Fearnside, P., Melo Ferreira, W., Garcia, D.A.Z., Krolow, T.K., Kruger, R.F., Latrubesse, E.M., Lima Junior, D.P., de Fátima Lolis, S., Lopes, F.A.C., Loyola, R.D., Magalhães, A.L.B., Malvasio, A., De Marco Junior, P., Martins, P.R., Mazzoni, R., Nabout, J.C., Orsi, M.L., Padial, A.A., Pereira, H.R., Pereira, T.N.A., Perônico, P.B., Petrere Junior, M., Pinheiro, R.T., Pires, E.F., Pompeu, P.S., Portelinha, T.C.G., Sano, E.E., Santos, V.L.M., Shimabukuro, P.H.F., Silva, I.G., Souza, L.B.E., Tejerina-Garro, F.L., Campos Telles, M.P., Teresa, F.B., Thomaz, S.M., Tonella, L.H., Vieira, L.C.G., Vitule, J.R.S., & Zuanon, J., 2021. Large-scale Degradation of the Tocantins-Araguaia River Basin. Environ. Manage. 68(4), 445-452. PMid:34341867. http://dx.doi.org/10.1007/s00267-021-01513-7
» http://dx.doi.org/10.1007/s00267-021-01513-7 - R Core Team, 2020. R: a language and environment for statistical computing (Online). Vienna: R Foundation for Statistical Computing. Retrieved in 2022, August 24, from https://www.R-project.org/
» https://www.R-project.org/ - Rocha, M.P., Bini, L.M., Siqueira, T., Hjort, J., Grönroos, M., Lindholm, M., Karjalainen, S.M., & Heino, J., 2018. Predicting occupancy and abundance by niche position, niche breadth and body size in stream organisms. Oecologia 186(1), 205-216. PMid:29090405. http://dx.doi.org/10.1007/s00442-017-3988-z
» http://dx.doi.org/10.1007/s00442-017-3988-z - Siqueira, T., Bini, L.M., Cianciaruso, M.V., Roque, F.O., & Trivinho-Strixino, S., 2009. The role of niche measures in explaining the abundance-distribution relationship in tropical lotic chironomids. Hydrobiologia 636(1), 163-172. http://dx.doi.org/10.1007/s10750-009-9945-z
» http://dx.doi.org/10.1007/s10750-009-9945-z - Ten Caten, C., Holian, L., Dallas, T., & Pither, J., 2022. Weak but consistent abundance-occupancy relationships across taxa, space and time. Glob. Ecol. Biogeogr. 31(5), 968-977. http://dx.doi.org/10.1111/geb.13472
» http://dx.doi.org/10.1111/geb.13472 - Thomaz, S.M., Bini, L.M., & Bozelli, R.L., 2007. Floods increase similarity among aquatic habitats in river-floodplain systems. Hydrobiologia 579(1), 1-13. http://dx.doi.org/10.1007/s10750-006-0285-y
» http://dx.doi.org/10.1007/s10750-006-0285-y - Vieira, L.C.G., 2008. Padrões ecológicos da comunidade zooplanctônica na planície de inundação do rio Araguaia [Tese de doutorado em Ciências Ambientais]. Goiânia: Programa de Pós-graduação em Ecologia e Evolução, Universidade Federal de Goiás.
- Vilmi, A., Tolonen, K.T., Karjalainen, S.M., & Heino, J., 2019. Niche position drives interspecific variation in occupancy and abundance in a highly-connected lake system. Ecol. Indic. 99, 159-166. http://dx.doi.org/10.1016/j.ecolind.2018.12.029
» http://dx.doi.org/10.1016/j.ecolind.2018.12.029 - Webb, T.J., Tyler, E.H.M., & Somerfield, P.J., 2009. Life history mediates large-scale population ecology in marine benthic taxa. Mar. Ecol. Prog. Ser. 396, 293-306. http://dx.doi.org/10.3354/meps08253
» http://dx.doi.org/10.3354/meps08253
Edited by
Publication Dates
-
Publication in this collection
21 Nov 2022 -
Date of issue
2022
History
-
Received
24 Aug 2022 -
Accepted
01 Nov 2022