Abstract
Introduction
In addition to their role in regulation of the hypothalamic-pituitary-adrenal-axis, corticotropin-releasing factor (CRF) and its related peptides, the urocortins, are important mediators of physiological and pathophysiological processes of the central nervous, cardiovascular, gastrointestinal, immune, endocrine, reproductive, and skin systems. Altered regulation of CRF-mediated adaptive responses to various stressful stimuli disrupts healthy function and might confer vulnerability to several disorders, including depression and anxiety.
Methodology
This narrative review was conducted through search and analysis of studies retrieved from online databases using a snowball method.
Results
This review covers aspects beginning with the discovery of CRF, CRF binding protein and their actions via interaction with CRF receptors type 1 and type 2. These are surface plasma membrane receptors, activation of which is associated with conformational changes and interaction with a variety of G-proteins and signaling pathways. We also reviewed the pharmacology and mechanisms of the receptor signaling modulatory activity of these receptors.
Conclusion
This review compiles and presents knowledge regarding the CRFergic system, including CRF related peptides, CRF binding protein, and CRF receptors, as well as some evidence that is potentially indicative of the biological roles of these entities in several physiological and pathophysiological processes.
Corticotropin-releasing factor; corticotropin-releasing hormone; CRF; anxiety; depression; stress
Corticotropin-releasing factor (CRF) and stress
The concept of stress is widely known in biomedical research. However, due to its popularization, the term “stress” has been used indiscriminately to define cause, processes, and responses.11. McEwen BS, Wingfield JC. The concept of allostasis in biology and biomedicine. Horm Behav. 2003;43:2-15. There is a distinct temporal dynamic that characterizes the stress response: 1) a rapid, stimulatory, initial phase involving activation of the sympatho-adrenomedullary (SAM) and hypothalamic-pituitary-adrenal (HPA) axes, which is accompanied by behavioral changes resembling the ‘fight or flight’ response (this phase of the response increases energy expenditure and heart function, shuts down the immune system, and boosts the body to action22. Grammatopoulos DK, Ourailidou S. CRH receptor signalling: potential roles in Pathophysiology. Curr Mol Pharmacol. 2017;10:296-310.
3. Meerlo P, Sgoifo A, De Boer SF, Koolhaas JM. Long-lasting consequences of a social conflict in rats: behavior during the interaction predicts subsequent changes in daily rhythms of heart rate, temperature, and activity. Behav Neurosci. 1999;113:1283-90.
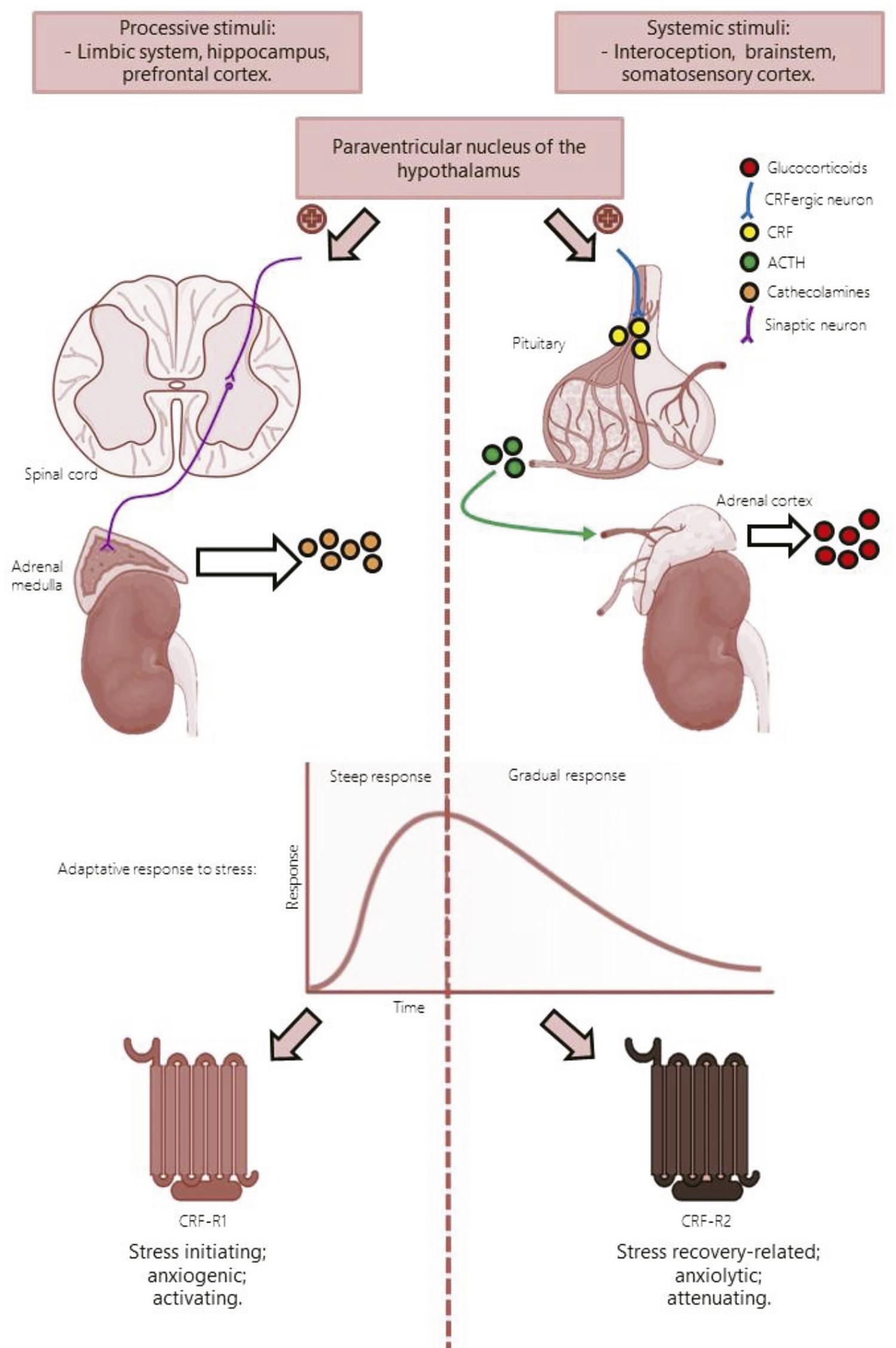
4. Sgoifo A, Costoli T, Meerlo P, Buwalda B, Pico’-Alfonso MA, De Boer S, et al. Individual differences in cardiovascular response to social challenge. Neurosci Biobehav Rev. 2005;29:59-66. - 55. Koolhaas JM, Meerlo P, de Boer SF, Strubbe JH, Bohus B. The temporal dynamics of the stress response. Neurosci Biobehav Rev. 1997;21:775-82. ); and 2) a second phase, responsible for switching off this increased activation, which is a lasting adaptation and restoration process.22. Grammatopoulos DK, Ourailidou S. CRH receptor signalling: potential roles in Pathophysiology. Curr Mol Pharmacol. 2017;10:296-310. , 66. Sapolsky RM. The influence of social hierarchy on primate health. Science. 2005;308:648-52. The main regulatory mechanism known to take place at this time is the glucocorticoid negative feedback, which acts on the HPA-axis ( Figure 1 ). Whenever this switch control is unbalanced, there might be a prolonged or absent activating step of the stress response leading to maladaptive consequences and potential harm.77. de Kloet ER, Joëls M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6:463-75. In the case of persistent release of glucocorticoids, the resources to maintain the internal physiology (i.e. “allostasis”) are overused and a diverse range of different kinds of damage affect several body systems, especially in vulnerable individuals.88. Fava GA, McEwen BS, Guidi J, Gostoli S, Offidani E, Sonino N. Clinical characterization of allostatic overload. Psychoneuroendocrinology. 2019;108:94-101.
- Both emotional and systemic stimuli have the capacity to induce a stress response in the paraventricular nucleus of the hypothalamus. A rapid response occurs through the SAM axis, culminating in release of catecholamines from the adrenal medulla. The second response is usually gradual, since the final product is released minutes after the presence of a stressor. From the paraventricular nucleus, CRFergic neurons projecting to the pituitary gland release CRF, stimulating production and release of ACTH in the bloodstream. Once it reaches the adrenal cortex, ACTH stimulates production and release of glucocorticoids. CRF receptors have different expression patterns and roles in response to stress. CRFR1 participates in the initial phases of the stress response, at a faster and more intense rate when compared to CRFR2, triggering anxiety behaviors. In contrast, CRFR2 activity is mainly involved in a later phase, opposing the activating role of CRFR1, thereby generating a stress-attenuating response. ACTH = adrenocorticotropic hormone; CRF = corticotropin-releasing factor; CRFR1 = corticotropin-releasing factor receptor type 1; CRFR2 = corticotropin-releasing factor receptor type 2; HPA = hypothalamus-pituitary-adrenal; SAM = sympatho-adrenomedullary.
Beyond the sympathetic and HPA axis components (i.e. neurotransmitters and hormones), several other molecules are involved in the adaptive stress response.99. Joëls M, Baram TZ. The neuro-symphony of stress. Nat Rev Neurosci. 2009;10:459-66. CRF has emerged as a central molecule capable of integrating and orchestrating such responses, adapted to mastering challenging situations. In this sense, CRF was rapidly identified as the initiator of both neuroendocrine and sympathetic stress responses.1010. Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and β-endorphin. Science. 1981;213:1394-7.
11. Deussing JM, Chen A. The corticotropin-releasing factor family: physiology of the stress response. Physiol Rev. 2018;98:2225-86. - 1212. Brown MR, Fisher LA. Corticotropin-releasing factor: effects on the autonomic nervous system and visceral systems. Fed Proc. 1985;44:243-8. Moreover, CRF is also recognized as the link between stress and psychiatric disorders, since exposure to major stressful events or recurrent exposure to mild stressors are risk factors for development of adverse conditions such as depression, anxiety, and substance abuse disorders.77. de Kloet ER, Joëls M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6:463-75. , 1313. McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87:873-904. , 1414. Miczek KA, Yap JJ, Covington III HE. Social stress, therapeutics and drug abuse: Preclinical models of escalated and depressed intake. Pharmacol Ther. 2008;120:102-28. CRF was termed “corticotropin-releasing factor” (also referred as “corticotropin-releasing hormone”) because of its ability to stimulate release of the adrenocorticotropic hormone (ACTH) from the pituitary.1010. Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and β-endorphin. Science. 1981;213:1394-7. However, this neuropeptide also has additional functions beyond the single one described in its name: CRF acts as a hormone and a neuromodulator in multiple brain areas, interacting with distinct neurotransmitter systems.1515. Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol. 2004;44:525-57. , 1616. Hauger RL, Risbrough V, Oakley RH, Olivares-Reyes JA, Dautzenberg FM. Role of CRF receptor signaling in stress vulnerability, anxiety, and depression. Ann N Y Acad Sci. 2009;1179:120-43. More than thirty years on from its discovery, extensive efforts have been made to uncover the physiological and pathophysiological significance of CRF. Dysregulation of the HPA-axis and CRF signaling has been associated with the etiology of many human diseases that seem to be related to exposure to stress and maladaptive stress responses (e.g. depression, anxiety, anorexia, obesity, inflammatory diseases, and Alzheimer’s disease).1717. Arborelius L, Owens MJ, Plotsky PM, Nemeroff CB. The role of corticotropin-releasing factor in depression and anxiety disorders. J Endocrinol. 1999;160:1-12.
18. Bailer U, Kaye W. A review of neuropeptide and neuroendocrine dysregulation in anorexia and bulimia nervosa. Curr Drug Targets CNS Neurol Disord. 2003;2:53-9.
19. Behan DP, Khongsaly O, Owens MJ, Chung HD, Nemeroff CB, De Souza EB. Corticotropin-releasing factor (CRF), CRF-binding protein (CRF-BP), and CRF/CRF-BP complex in Alzheimer’s disease and control postmortem human brain. J Neurochem. 1997;68:2053-60.
20. Jessop DS, Harbuz MS, Lightman SL. CRH in chronic inflammatory stress. Peptides. 2001;22:803-7. - 2121. Richard D, Huang Q, Timofeeva E. The corticotropin-releasing hormone system in the regulation of energy balance in obesity. Int J Obes Relat Metab Disord. 2000;24 Suppl 2:S36-9.
The family of CRF receptors and their ligands
The CRFergic system comprises a family of molecules whose members include CRF, urocortin (UCN), corticotropin-releasing factor receptor type 1 (CRFR1), corticotropin-releasing factor receptor type 2 (CRFR2), and the corticotropin-releasing factor binding protein (CRFBP).2222. Hsu SY, Hsueh AJW. Human stresscopin and stresscopin-related peptide are selective ligands for the type 2 corticotropin-releasing hormone receptor. Nat Med. 2001;7:605-11.
23. Lewis K, Li C, Perrin MH, Blount A, Kunitake K, Donaldson C, et al. Identification of urocortin III, an additional member of the corticotropin-releasing factor (CRF) family with high affinity for the CRF2 receptor. Proc Natl Acad Sci. 2001;98:7570-5.
24. Reyes TM, Lewis K, Perrin MH, Kunitake KS, Vaughan J, Arias CA, et al. Urocortin II: a member of the corticotropin-releasing factor (CRF) neuropeptide family that is selectively bound by type 2 CRF receptors. Proc Natl Acad Sci. 2001;98:2843-8. - 2525. Vaughan J, Donaldson C, Bittencourt J, Perrin MH, Lewis K, Sutton S, et al. Urocortin, a mammalian neuropeptide related to fish urotensin I and to corticotropin-releasing factor. Nature. 1995;378:287-92. Both CRF and CRF-like molecules bind to these CRF receptors (CRFRs). The first CRF receptor discovered, CRFR1, was initially identified in rats, mice, and humans in 1993.2626. Chang CP, Pearse RV, O’Connell S, Rosenfeld MG. Identification of a seven transmembrane helix receptor for corticotropin-releasing factor and sauvagine in mammalian brain. Neuron. 1993;11:1187-95.
27. Chen R, Lewis KA, Perrin MH, Vale WW. Expression cloning of a human corticotropin-releasing-factor receptor. Proc Natl Acad Sci U S A. 1993;90:8967-71. - 2828. Vita N, Laurent P, Lefort S, Chalon P, Lelias J-M, Kaghad M, et al. Primary structure and functional expression of mouse pituitary and human brain corticotrophin releasing factor receptors. FEBS Lett. 1993;335:1-5. The second CRF receptor, CRFR2, was cloned and identified as an alternative spliced form of CRFR1.2929. Lovenberg TW, Liaw CW, Grigoriadis DE, Clevenger W, Chalmers DT, Souza EBD, et al. Cloning and characterization of a functionally distinct corticotropin-releasing factor receptor subtype from rat brain. Proc Natl Acad Sci. 1995;92:836-40. , 3030. Perrin M, Donaldson C, Chen R, Blount A, Berggren T, Bilezikjian L, et al. Identification of a second corticotropin-releasing factor receptor gene and characterization of a cDNA expressed in heart. Proc Natl Acad Sci U S A. 1995;92:2969-73. However, the numbers of functional CRFRs and differential splicing subtypes vary depending on the body tissues and taxa involved. The importance of the CRFergic system ranges from its involvement in the accelerated metamorphosis in response to pond-drying in some amphibians to intrauterine fetal stress syndromes in humans.3131. Denver RJ. Acceleration of anuran amphibian metamorphosis by corticotropin-releasing hormone-like peptides. Gen Comp Endocrinol. 1993;91:38-51. , 3232. Smith R. Alterations in the hypothalamic pituitary adrenal axis during pregnancy and the placental clock that determines the length of parturition. J Reprod Immunol. 1998;39:215-20. CRF and UCNs may share a common ancestral origin in a precursor peptide related to helping organisms deal with environmental changes during their developing stages.3333. Denver RJ. Evolution of the corticotropin-releasing hormone signaling system and its role in stress-induced phenotypic plasticity. Ann N Y Acad Sci. 1999;897:46-53. This significance can, in part, explain how this system presents such a wide range of actions over multiple physiological functions.3434. Hillhouse EW, Grammatopoulos DK. The molecular mechanisms underlying the regulation of the biological activity of corticotropin-releasing hormone receptors: implications for physiology and pathophysiology. Endocr Rev. 2006;27:260-86.
The role of CRFR1, along with CRF, in modulation of HPA axis activity, as well as in stress-induced behavior and cognitive alterations, has been extensively studied. Some thought was given to the therapeutic potential of CRFR antagonists to treat stress-related psychiatric disorders. In the first decade of the current century, several preclinical experiments and clinical trials were conducted for a wide range of stress-related conditions. Unfortunately, many candidate-drugs, designed as CRFR1 antagonists, have failed in double-blind, placebo-controlled trials.3535. Spierling SR, Zorrilla EP. Don’t stress about CRF: assessing the translational failures of CRF1antagonists. Psychopharmacology (Berl). 2017;234:1467-81.Table 1 shows a list of registered clinical trials designed to evaluate the effects of CRFergic drugs under various clinical conditions. It is worth noting that few of these studies were registered for treatment of psychiatric conditions. Some studies resulted in drug withdrawal, while others remain to ascertain drug safety as aids in diagnosis rather than treatment of conditions.
Due to the high prevalence of studies focusing on CRFR1, the specific roles of CRFR2, CRFBP, and UCNs remain to be established. Although there is no consensus yet, it is presumed that CRF interacts with CRFR1 to mediate the initial reaction to stress, whereas UCNs and CRFR2 interactions are thought to be related to modulation of a post-stressor presentation phase3636. Heinrichs SC, Koob GF. Corticotropin-releasing factor in brain: a role in activation, arousal, and affect regulation. J Pharmacol Exp Ther. 2004;311:427-40. ( Figure 1 ). This review aims to clarify CRF signaling, specifically with relation to CRFRs and their binding protein, and how the mechanisms that underlie the cellular responses to CRF and UCNs might be implicated in responses to stress. Briefly, we review data from pharmacology and behavioral neuroscience that are most closely associated with this topic.
It has been described that the CRF peptide is expressed in the paraventricular nucleus of the hypothalamus, in the central nucleus of the amygdala, and in hindbrain regions of the central nervous system (CNS). In the periphery, CRF has been found expressed in the gut, skin, and adrenal glands.1515. Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol. 2004;44:525-57. Meanwhile, the UCNs exhibit differing degrees of structural homology to the CRF protein and homology between regions where they are expressed. There are three of these CRF-like peptides, often named urocortins: stresscopin-related peptide-I and -II (or UCN-I and UCN-II, respectively), and stresscopin (or UCN-III).3434. Hillhouse EW, Grammatopoulos DK. The molecular mechanisms underlying the regulation of the biological activity of corticotropin-releasing hormone receptors: implications for physiology and pathophysiology. Endocr Rev. 2006;27:260-86. Each UCN presents a specific pattern of distribution in different brain structures. Several terminal fields of UCN-III fibers are expressed in regions with high levels of CRFR2, suggesting that UCN-III is an endogenous ligand for CRFR2.3434. Hillhouse EW, Grammatopoulos DK. The molecular mechanisms underlying the regulation of the biological activity of corticotropin-releasing hormone receptors: implications for physiology and pathophysiology. Endocr Rev. 2006;27:260-86. , 3737. Li C, Vaughan J, Sawchenko PE, Vale WW. Urocortin III-immunoreactive projections in rat brain: partial overlap with sites of type 2 corticotropin-releasing factor receptor expression. J Neurosci. 2002;22:991-1001. Apparently, the affinity between ligands and receptors obeys a certain pattern that resembles their brain region distribution, which is a possible explanation for part of their biological significance. CRF has a tenfold greater affinity for CRFR1 than for CRFR2. Thus, CRF and UCN-I would be unselective agonists for CRFR1, whereas UCN-II and UCN-III appear to be selective for CRFR2.1515. Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol. 2004;44:525-57. , 3434. Hillhouse EW, Grammatopoulos DK. The molecular mechanisms underlying the regulation of the biological activity of corticotropin-releasing hormone receptors: implications for physiology and pathophysiology. Endocr Rev. 2006;27:260-86. CRF cognate receptors exhibit distinct distribution patterns in the CNS and peripheral tissues. In general, CRFR1 represents the major CRF receptor in the brain, while CRFR2 is predominantly expressed outside the CNS.3838. Hiroi N, Wong ML, Licinio J, Park C, Young M, Gold PW, et al. Expression of corticotropin releasing hormone receptors type I and type II mRNA in suicide victims and controls. Mol Psychiatry. 2001;6:540-6. It seems that CRFR1 has only one known functional splice variant, the CRFR1α, which is expressed in the brain.3434. Hillhouse EW, Grammatopoulos DK. The molecular mechanisms underlying the regulation of the biological activity of corticotropin-releasing hormone receptors: implications for physiology and pathophysiology. Endocr Rev. 2006;27:260-86. The CRFR1β isoform and its subtypes are originated by differential splicing of the CRFR1 gene.1515. Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol. 2004;44:525-57. They have been detected in human and rodent tissues, although several of these isoforms have been shown to be nonfunctional.1515. Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol. 2004;44:525-57. In mammals, three known CRFR2 splicing variants are found: CRFR2α and CRFR2β are expressed in both humans and rodents, while CRFR2γ has only been isolated in human limbic regions.3434. Hillhouse EW, Grammatopoulos DK. The molecular mechanisms underlying the regulation of the biological activity of corticotropin-releasing hormone receptors: implications for physiology and pathophysiology. Endocr Rev. 2006;27:260-86. , 3939. Kostich WA, Chen A, Sperle K, Largent BL. Molecular identification and analysis of a novel human corticotropin-releasing factor (CRF) receptor: the CRF2γ receptor. Mol Endocrinol. 1998;12:1077-85.
CRFR1 is expressed in the cerebral cortex, cerebellum, olfactory bulb, medial septum, hippocampus, amygdala, pituitary, and peripheral tissues.4040. Van Pett K, Viau V, Bittencourt JC, Chan RKW, Li H-Y, Arias C, et al. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol. 2000;428:191-212. Studies using genetic knockout mice shed some light on the importance of this CRF receptor subtype. CRFR1-deficient mice display decreased levels of behaviors related to anxiety and dysfunctional HPA-axis response.4141. Contarino A, Heinrichs SC, Gold LH. Understanding corticotropin releasing factor neurobiology: contributions from mutant mice. Neuropeptides. 1999;33:1-12. In regard to CRFR2 distribution and function, it is highly expressed in the lateral septum, medial and posterior bed nucleus of the stria terminalis, ventral medial hypothalamic nuclei, olfactory bulb, dorsal raphe nuclei, and peripheral tissues.4040. Van Pett K, Viau V, Bittencourt JC, Chan RKW, Li H-Y, Arias C, et al. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol. 2000;428:191-212. Alternatively, compared to CRFR1-deficient mice, CRFR2-deficient animals display increased levels of behaviors related to anxiety, with accelerated HPA-axis response and impaired coping behaviors.4242. Bale TL, Contarino A, Smith GW, Chan R, Gold LH, Sawchenko PE, et al. Mice deficient for corticotropin-releasing hormone receptor-2 display anxiety-like behaviour and are hypersensitive to stress. Nat Genet. 2000;24:410-4. , 4343. Coste SC, Heard AD, Phillips TJ, Stenzel-Poore MP. Corticotropin-releasing factor receptor type 2-deficient mice display impaired coping behaviors during stress. Genes Brain Behav. 2006;5:131-8. Pharmacological manipulation of CRFR2 yielded conflicting evidence about its role in anxiety related behaviors.1515. Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol. 2004;44:525-57. In this sense, the role of CRFR2 in the endocrine response to stress is still under debate, with attempts to determine its biological function pointing to a prominent behavioral significance (i.e. anxiolytic or reducing arousal), probably mediated by its activation in other brain structures apart from the HPA-axis components.4444. Pelleymounter MA, Joppa M, Ling N, Foster AC. Pharmacological evidence supporting a role for central corticotropin-releasing factor(2) receptors in behavioral, but not endocrine, response to environmental stress. J Pharmacol Exp Ther. 2002;302:145-52.
Corticotropin-releasing factor binding protein is a 37 kDa protein expressed predominantly in the brainstem, amygdala, bed nucleus of the stria terminalis, ventral premammillary and dorsomedial nuclei of the hypothalamus, and cerebral cortex.4545. Behan DP, De Souza EB, Lowry PJ, Potter E, Sawchenko P, Vale WW. Corticotropin releasing factor (CRF) binding protein: a novel regulator of CRF and related peptides. Front Neuroendocrinol. 1995;16:362-82. , 4646. Potter E, Behan DP, Linton EA, Lowry PJ, Sawchenko PE, Vale WW. The central distribution of a corticotropin-releasing factor (CRF)-binding protein predicts multiple sites and modes of interaction with CRF. Proc Natl Acad Sci U S A. 1992;89:4192-6. It binds to CRF with an affinity equal to or greater than the CRFRs, which led researchers to consider it as an important modulator of CRF and its receptors.4747. Sutton SW, Behan DP, Lahrichi SL, Kaiser R, Corrigan A, Lowry P, et al. Ligand requirements of the human corticotropin-releasing factor-binding protein. Endocrinology. 1995;136:1097-102. Clinical and preclinical studies have demonstrated the importance of CRFBP in stress-related psychiatric illness, including anxiety, depression, and substance abuse disorders.4848. Albrechet-Souza L, Hwa LS, Han X, Zhang EY, DeBold JF, Miczek KA. Corticotropin releasing factor binding protein and CRF2 receptors in the ventral tegmental area: modulation of ethanol binge drinking in C57BL/6J mice. Alcohol Clin Exp Res. 2015;39:1609-18.
49. Binder EB, Owens MJ, Liu W, Deveau TC, Rush AJ, Trivedi MH, et al. Association of polymorphisms in genes regulating the corticotropin-releasing factor system with antidepressant treatment response. Arch Gen Psychiatry. 2010;67:369-79.
50. Enoch MA, Shen PH, Ducci F, Yuan Q, Liu J, White KV, et al. Common genetic origins for EEG, alcoholism and anxiety: the role of CRH-BP. PLoS One. 2008;3:e3620. - 5151. Haass-Koffler CL, Henry AT, Melkus G, Simms JA, Naemmuddin M, Nielsen CK, et al. Defining the role of corticotropin releasing factor binding protein in alcohol consumption. Transl Psychiatry. 2016;6:e953. We recently demonstrated that stress-impaired social behavior is recovered by a CRFBP antagonist, CRF6-33, microinjected into the bed nucleus of the stria terminalis.5252. Vasconcelos M, Stein DJ, Albrechet-Souza L, Miczek KA, de Almeida RMM. Recovery of stress-impaired social behavior by an antagonist of the CRF binding protein, CRF6-33, in the bed nucleus of the stria terminalis of male rats. Behav Brain Res. 2019;357-358:104-10.
Unfortunately, CRFBP has received less attention than CRF and its receptors. Multiple hypotheses have been proposed to explain the biological significance of CRFBP.5353. Westphal NJ, Seasholtz AF. CRH-BP: the regulation and function of a phylogenetically conserved binding protein. Front Biosci. 2006;11:1878-91. Along with its initial characterization, it has been proposed that CRFBP is a CRF-sequestering molecule, promoting antagonism of CRFR1. This hypothesis was based on experiments that demonstrated recombinant CRFBP interfering with the binding of CRF to a CRF antibody.5454. Cortright DN, Nicoletti A, Seasholtz AF. Molecular and biochemical characterization of the mouse brain corticotropin-releasing hormone-binding protein. Mol Cell Endocrinol. 1995;111:147-57. , 5555. Potter E, Behan DP, Fischer WH, Linton EA, Lowry PJ, Vale WW. Cloning and characterization of the cDNAs for human and rat corticotropin releasing factor-binding proteins. Nature. 1991;349:423-6. Other studies proposed a potential facilitating role for CRFBP in CRFR2 function: this second hypothesis was largely based on studies conducted in the ventral tegmental area.4848. Albrechet-Souza L, Hwa LS, Han X, Zhang EY, DeBold JF, Miczek KA. Corticotropin releasing factor binding protein and CRF2 receptors in the ventral tegmental area: modulation of ethanol binge drinking in C57BL/6J mice. Alcohol Clin Exp Res. 2015;39:1609-18. , 5656. Ungless MA, Singh V, Crowder TL, Yaka R, Ron D, Bonci A. Corticotropin-releasing factor requires CRF binding protein to potentiate NMDA receptors via CRF receptor 2 in dopamine neurons. Neuron. 2003;39:401-7. , 5757. Wang B, You Z-B, Rice KC, Wise RA. Stress-induced relapse to cocaine seeking: roles for the CRF2 receptor and CRF-binding protein in the ventral tegmental area of the rat. Psychopharmacology (Berl). 2007;193:283-94. Additionally, some studies suggest that CRFBP may have signaling properties independent of CRFRs5858. Chan RKW, Vale WW, Sawchenko PE. Paradoxical activational effects of a corticotropin-releasing factor-binding protein “ligand inhibitor” in rat brain. Neuroscience. 2000;101:115-29. or may act as an escort protein to traffic CRFR2α from organelles to the cell surface.5959. Slater PG, Cerda CA, Pereira LA, Andrés ME, Gysling K. CRF binding protein facilitates the presence of CRF type 2α receptor on the cell surface. Proc Natl Acad Sci. 2016;113:4075-80. It seems that the role and mechanism of action of CRFBP vary depending on several factors, including brain region, cell type, and CRF receptor subtype.
Still, it remains to be determined whether abnormalities in expression of CRFRs or CRFBP contribute to the pathogenesis of diseases, or whether it is a trait secondary to disease onset and initiation of stress. To date, no abnormalities in the expression or function of these receptors and binding protein have been directly observed in human diseases.3434. Hillhouse EW, Grammatopoulos DK. The molecular mechanisms underlying the regulation of the biological activity of corticotropin-releasing hormone receptors: implications for physiology and pathophysiology. Endocr Rev. 2006;27:260-86.
CRF as a neuromodulator, hormone, and neuropeptide
CRF is considered a neuromodulator rather than a neurotransmitter, since concentrations higher than 1 mM are required to induce direct depolarization of neurons. Thus, in the physiological nanomolar range (up to 250 nM), low CRF concentrations are not sufficient to disturb neuronal resting states.1111. Deussing JM, Chen A. The corticotropin-releasing factor family: physiology of the stress response. Physiol Rev. 2018;98:2225-86. The neuromodulatory effects of CRF seem to be related to excitatory transmission, neuronal plasticity, and consequent synaptic efficacy. In cortical layers, such as in the olfactory bulb, CRFergic projections innervate new neurons to stabilize and promote new synapses, thus consolidating the shaping of new circuits.6060. Garcia I, Quast KB, Huang L, Herman AM, Selever J, Deussing JM, et al. Local CRH signaling promotes synaptogenesis and circuit integration of adult-born neurons. Dev Cell. 2014;30:645-59. In the hippocampus, CRF induces spontaneous action potentials while it also shortens after-hyperpolarization; these effects are linked to the facilitation of signal propagation.6161. Aldenhoff JB, Gruol DL, Rivier J, Vale W, Siggins GR. Corticotropin releasing factor decreases postburst hyperpolarizations and excites hippocampal neurons. Science. 1983;221:875-7. , 6262. Wang HL, Tsai LY, Lee EH. Corticotropin-releasing factor produces a protein synthesis–dependent long-lasting potentiation in dentate gyrus neurons. J Neurophysiol. 2000;83:343-9. Microinjection of CRF into the hippocampus improves context-dependent fear conditioning, and this effect can be prevented by CRFR1 antagonism or protein kinase inhibition (using Astressin and KN-62, respectively).6363. Blank T, Nijholt I, Eckart K, Spiess J. Priming of long-term potentiation in mouse hippocampus by corticotropin-releasing factor and acute stress: implications for hippocampus-dependent learning. J Neurosci. 2002;22:3788-94. Knowledge about CRF-induced plasticity effects extends beyond the hippocampus, including brain regions such as the amygdala,6464. Fu Y, Pollandt S, Liu J, Krishnan B, Genzer K, Orozco-Cabal L, et al. Long-term potentiation (LTP) in the central amygdala (CeA) is enhanced after prolonged withdrawal from chronic cocaine and requires CRF 1 receptors. J Neurophysiol. 2007;97:937-41. , 6565. Krishnan B, Centeno M, Pollandt S, Fu Y, Genzer K, Liu J, et al. Dopamine receptor mechanisms mediate corticotropin-releasing factor-induced long-term potentiation in the rat amygdala following cocaine withdrawal. Eur J Neurosci. 2010;31:1027-42. lateral septum,6666. Liu J, Yu B, Orozco-Cabal L, Grigoriadis DE, Rivier J, Vale WW, et al. Chronic cocaine administration switches corticotropin-releasing factor2 receptor-mediated depression to facilitation of glutamatergic transmission in the lateral septum. J Neurosci. 2005;25:577-83. bed nucleus of the stria terminalis,6767. Henckens MJAG, Printz Y, Shamgar U, Dine J, Lebow M, Drori Y, et al. CRF receptor type 2 neurons in the posterior bed nucleus of the stria terminalis critically contribute to stress recovery. Mol Psychiatry. 2017;22:1691-700. and brainstem.5656. Ungless MA, Singh V, Crowder TL, Yaka R, Ron D, Bonci A. Corticotropin-releasing factor requires CRF binding protein to potentiate NMDA receptors via CRF receptor 2 in dopamine neurons. Neuron. 2003;39:401-7. , 6868. Valentino RJ, Foote SL, Aston-Jones G. Corticotropin-releasing factor activates noradrenergic neurons of the locus coeruleus. Brain Res. 1983;270:363-7. In the locus coeruleus and ventral tegmental area, CRF administration leads to dose-dependent increases in excitatory noradrenergic and dopaminergic transmission.5656. Ungless MA, Singh V, Crowder TL, Yaka R, Ron D, Bonci A. Corticotropin-releasing factor requires CRF binding protein to potentiate NMDA receptors via CRF receptor 2 in dopamine neurons. Neuron. 2003;39:401-7. , 6868. Valentino RJ, Foote SL, Aston-Jones G. Corticotropin-releasing factor activates noradrenergic neurons of the locus coeruleus. Brain Res. 1983;270:363-7. CRFergic excitatory activity induced by stress seems to promote pruning and rearrangement of the dendritic tree.6969. Chen Y, Andres AL, Frotscher M, Baram TZ. Tuning synaptic transmission in the hippocampus by stress: the CRH system. Front Cell Neurosci. 2012;6:13. Again, these effects are dose-dependent, since high levels of CRF induce retraction of thin spines, preventing their remodeling, with consequent harm to learning and memory formation.7070. Chen Y, Dube CM, Rice CJ, Baram TZ. Rapid loss of dendritic spines after stress snvolves derangement of spine dynamics by corticotropin-releasing hormone. J Neurosci. 2008;28:2903-11.
The excitatory properties of CRF play a pivotal role in neurodevelopment. Activation of CRFR1 promotes neuronal differentiation and projection elongation in cultured hippocampal cells.7171. Inda C, Bonfiglio JJ, dos Santos Claro PA, Senin SA, Armando NG, Deussing JM, et al. cAMP-dependent cell differentiation triggered by activated CRHR1 in hippocampal neuronal cells. Sci Rep. 2017;7:1944. Accordingly, CRF provides protection for neural stem cells against excessive glucocorticoid of maternal origin, while deletion of the CRF gene results in compromised proliferation and enhanced apoptosis during neurogenesis.7272. Koutmani Y, Politis PK, Elkouris M, Agrogiannis G, Kemerli M, Patsouris E, et al. Corticotropin-releasing hormone exerts direct effects on neuronal progenitor cells: implications for neuroprotection. Mol Psychiatry. 2013;18:300-7. In later developmental stages, the CRF function appears to be quite the opposite in response to stress. For instance, CRFR1 activation mediates stress-induced loss of apical dendritic spines in the hippocampus with associated impairments of spatial memory.7373. Chen Y, Rex CS, Rice CJ, Dubé CM, Gall CM, Lynch G, et al. Correlated memory defects and hippocampal dendritic spine loss after acute stress involve corticotropin-releasing hormone signaling. Proc Natl Acad Sci U S A. 2010;107:13123-8. Whereas, CRFR2 UCN-II-activation induces nerve growth factor production in astrocytes, promoting synapse formation in cultured hippocampal cells.7474. Zheng Y, Zhang Y-M, Ni X. Urocortin 2 but not urocortin 3 promotes the synaptic formation in hipppocampal neurons via induction of NGF production by astrocytes. Endocrinology. 2016;157:1200-10. In terms of a broader CRFergic circuit effect, there are few studies extending these findings to other brain structures. Nevertheless, it was demonstrated that exposure to stressors or stress-induced c-Fos activation in the nucleus incertus impaired long-term potentiation in a hippocampal-medial prefrontal cortex circuit.7575. Rajkumar R, Wu Y, Farooq U, Tan WH, Dawe GS. Stress activates the nucleus incertus and modulates plasticity in the hippocampo-medial prefrontal cortical pathway. Brain Res Bull. 2016;120:83-9. Opposite effects can be observed in studies focusing on regions such as the amygdala and bed nucleus of the stria terminalis.6565. Krishnan B, Centeno M, Pollandt S, Fu Y, Genzer K, Liu J, et al. Dopamine receptor mechanisms mediate corticotropin-releasing factor-induced long-term potentiation in the rat amygdala following cocaine withdrawal. Eur J Neurosci. 2010;31:1027-42. , 7676. Francesconi W, Berton F, Koob GF, Sanna PP. Intrinsic neuronal plasticity in the juxtacapsular nucleus of the bed nuclei of the stria terminalis (jcBNST). Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1347-55. , 7777. Pollandt S, Liu J, Orozco-Cabal L, Grigoriadis DE, Vale WW, Gallagher JP, et al. Cocaine withdrawal enhances long-term potentiation induced by corticotropin-releasing factor at central amygdala glutamatergic synapses via CRF, NMDA receptors and PKA. Eur J Neurosci. 2006;24:1733-43. Chronic exposure to stressors or administration of UCNs seem to reinforce long-term potentiation and excitability in the basolateral amygdala.7878. Chen L, Li S, Cai J, Wei T-J, Liu L-Y, Zhao H-Y, et al. Activation of CRF/CRFR1 signaling in the basolateral nucleus of the amygdala contributes to chronic forced swim-induced depressive-like behaviors in rats. Behav Brain Res. 2018;338:134-42. , 7979. Rainnie DG, Bergeron R, Sajdyk TJ, Patil M, Gehlert DR, Shekhar A. Corticotrophin releasing factor-induced synaptic plasticity in the amygdala translates stress into emotional disorders. J Neurosci. 2004;24:3471-9. Although contrasting, these plasticity effects could be abolished by administration of CRFR1 antagonists (e.g. Antalarmin and NBI27914).7575. Rajkumar R, Wu Y, Farooq U, Tan WH, Dawe GS. Stress activates the nucleus incertus and modulates plasticity in the hippocampo-medial prefrontal cortical pathway. Brain Res Bull. 2016;120:83-9. , 7878. Chen L, Li S, Cai J, Wei T-J, Liu L-Y, Zhao H-Y, et al. Activation of CRF/CRFR1 signaling in the basolateral nucleus of the amygdala contributes to chronic forced swim-induced depressive-like behaviors in rats. Behav Brain Res. 2018;338:134-42.
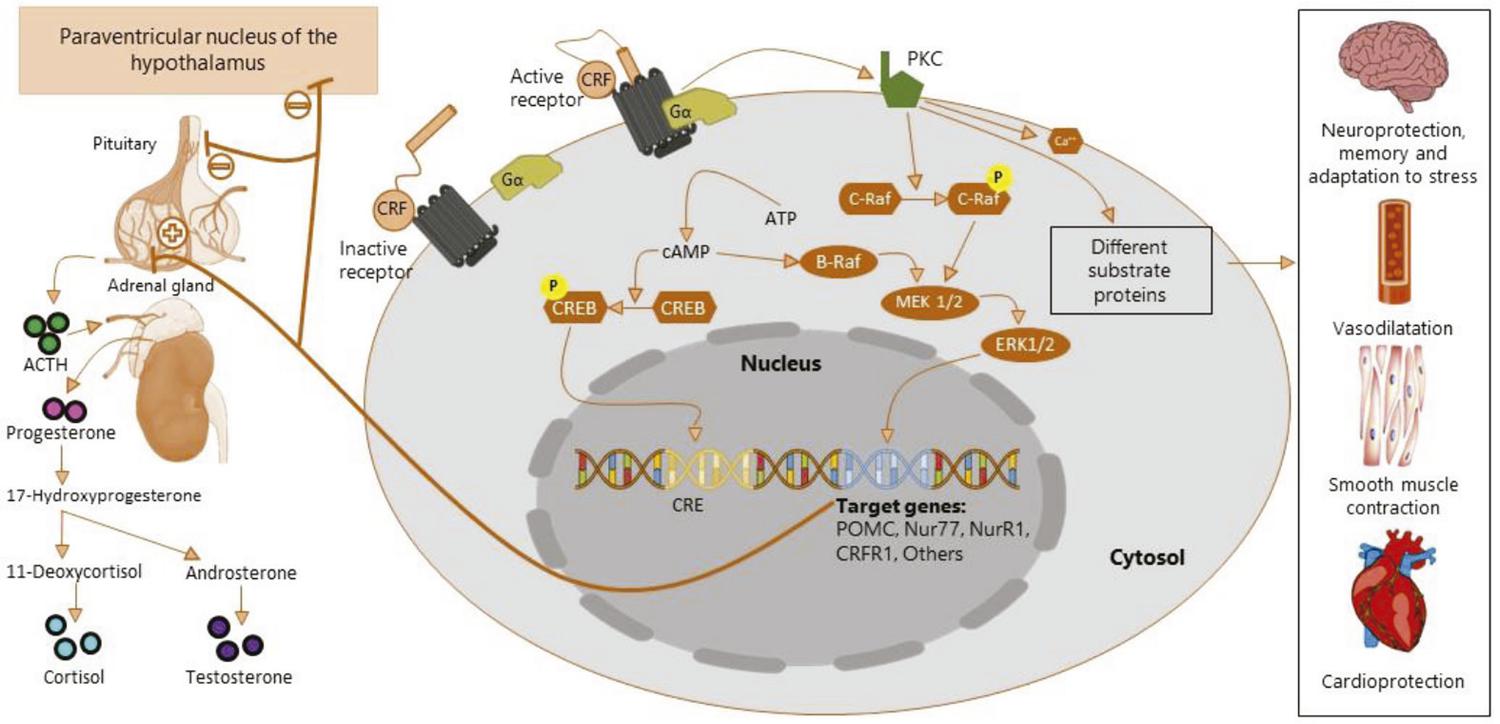
In the HPA-axis, CRF activates CRFR1 on pituitary corticotropes to stimulate release of ACTH, which activates receptors in the adrenal gland cortex to stimulate synthesis and release of glucocorticoids.3636. Heinrichs SC, Koob GF. Corticotropin-releasing factor in brain: a role in activation, arousal, and affect regulation. J Pharmacol Exp Ther. 2004;311:427-40. This response is shut down by a negative-feedback system: CRF production and release from the hypothalamus is inhibited, ceasing the stress response.2525. Vaughan J, Donaldson C, Bittencourt J, Perrin MH, Lewis K, Sutton S, et al. Urocortin, a mammalian neuropeptide related to fish urotensin I and to corticotropin-releasing factor. Nature. 1995;378:287-92. , 8080. Rivier C, Rivier J, Mormede P, Vale W. Studies of the nature of the interaction between vasopressin and corticotropin-releasing factor on adrenocorticotropin release in the rat. Endocrinology. 1984;115:882-6. Aside from induction of expression of proopiomelanocortin (POMC) genes and release of β-endorphins, activation of CRFR1 under stressful conditions induces upregulation of expression of the CRFR1 gene in the hypothalamus, hippocampus, and prefrontal cortex8181. Branson KL, Grigoriadis DE, Lorang MT, Baram TZ. Corticotropin-releasing hormone (CRH) downregulates the function of its receptor (CRF1) and induces CRF1 expression in hippocampal and cortical regions of the immature rat brain. Exp Neurol. 2002;176:75-86. ( Figure 2 ). More detailed information of the role of CRF in the HPA-axis is given in subsequent subsections in this review.
This figure depicts alternate signaling pathways activated after agonist binding to CRFR1 and CRFR2. When CRF or UCN binds to the receptor, the N-terminal segment integrates the receptor transmembrane domains. This process enables activation of the receptor and binding with G-proteins. This complex catalyzes conversion from ATP to cAMP, which is involved in both activation of the mitogen-activated protein kinase/extracellular-regulated kinase (MAPK/ERK) pathways and in phosphorylation of CREB transcription factor. Both pathways will ultimately lead to activation of transcription of target genes (POMC, Nur77, NurR1, CRFR1, and others) and transcription of the CRE gene respectively. POMC leads to release of ACTH by the pituitary gland. This process stimulates conversion of progesterone into cortisol. Activation of PKC (Inositol triphosphate-PKC) leads to phosphorylation of C-Raf protein, which is also involved in activation of transcription of target genes. Intracellular Ca2+ release and other different substrate proteins are also activated by PKC, triggering another set of CRF physiological responses in the CNS, cardiovascular system, and muscular system. ACTH = adrenocorticotropic hormone; ATP = adenosine triphosphate; cAMP = cyclic adenosine monophosphate; CNS = central nervous system; CRE = cAMP response element; CREB = cAMP response-element binding protein; CRF = corticotropin-releasing factor; CRFR1 = corticotropin-releasing factor receptor type 1; CRFR2 = corticotropin-releasing factor receptor type 2; PKC = protein kinase C; POMC = proopiomelanocortin; UCN: urocortin.
Since hyperactivation of the neuroimmune system was recognized as a powerful element in development of neuropsychiatric disorders,8282. Lehmann ML, Cooper HA, Maric D, Herkenham M. Social defeat induces depressive-like states and microglial activation without involvement of peripheral macrophages. J Neuroinflammation. 2016;13:224. , 8383. Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: implications for health. Nat Rev Immunol. 2005;5:243-51. CRF has also been implicated as one of the modulators of the intricate association between the immune system, stress, and mood disorders.8484. Tsigos C, Chrousos GP. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. J Psychosom Res. 2002;53:865-71. Beyond neurotransmission, CRF signals to neighboring glial cells affecting inflammatory responses. Activated microglial cells express high levels of CRFR18585. Wang W, Ji P, Dow KE. Corticotropin‐releasing hormone induces proliferation and TNF‐α release in cultured rat microglia via MAP kinase signalling pathways. J Neurochem. 2003;84:189-95. and therefore CRF can stimulate microglia to release signal molecules (e.g. cytokines, chemokines) that influence brain function and disease states.8585. Wang W, Ji P, Dow KE. Corticotropin‐releasing hormone induces proliferation and TNF‐α release in cultured rat microglia via MAP kinase signalling pathways. J Neurochem. 2003;84:189-95. , 8686. Stevens SL, Shaw TE, Dykhuizen E, Lessov NS, Hill JK, Wurst W, et al. Reduced cerebral injury in CRH-R1 deficient mice after focal ischemia: a potential link to microglia and atrocytes that express CRH-R1. J Cereb Blood Flow Metab. 2003;23:1151-9. In turn, cytokines exert rapid effects over the paraventricular nucleus, stimulating CRF release and promoting the stress response.8787. Uehara A, Gottschall PE, Dahl RR, Arimura A. Interleukin-1 stimulates ACTH release by an indirect action which requires endogenous corticotropin releasing factor. Endocrinology. 1987;121:1580-2.
88. Naitoh Y, Fukata J, Tominaga T, Nakai Y, Tamai S, Mori K, et al. Interleukin-6 stimulates the secretion of adrenocorticotropic hormone in conscious, freely-moving rats. Biochem Biophys Res Commun. 1988;155:1459-63. - 8989. Berkenbosch F, Van Oers J, Del Rey A, Tilders F, Besedovsky H. Corticotropin-releasing factor-producing neurons in the rat activated by interleukin-1. Science. 1987;238:524-6. Other studies have found evidence of the role of CRF in the immune system through regulation of the nuclear factor kappa B (NF-κB) pathway. For instance, CRF-induced upregulation of this transcription factor also promotes pituitary POMC gene expression,9090. Karalis KP, Venihaki M, Zhao J, van Vlerken LE, Chandras C. NF-κB participates in the corticotropin-releasing, hormone-induced regulation of the pituitary proopiomelanocortin gene. J Biol Chem. 2004;279:10837-40. while its inhibition results in CRF-mediated neuroprotection.9191. Lezoualc’h F, Engert S, Berning B, Behl C. Corticotropin-releasing hormone-mediated neuroprotection against oxidative stress is associated with the increased release of non-amyloidogenic amyloid β precursor protein and with the suppression of nuclear factor-κB. Mol Endocrinol. 2000;14:147-59. In sum, CRF appears to have several modes of action: 1) as a hormone, orchestrating the neuroendocrine response to stress; 2) as a neuromodulator, potentiating signal transduction and inducing arousal-like responses during the stress experience; and 3) performing other functions like immunosuppression (via glucocorticoid secretion) or boosting inflammation (via direct actions on glial cells).
Mechanisms of CRF receptor signaling
The CRFRs belong to the family of G protein-coupled receptors (GPCRs), also known as seven-transmembrane domain receptors.9292. Lagerström MC, Schiöth HB. Structural diversity of G protein-coupled receptors and significance for drug discovery. Nat Rev Drug Discov. 2008;7:339-57. The CRFRs are part of the class B or secretin receptor family of GPCRs, a small family of receptors that are activated by peptide ligands.9393. Hoare SRJ. Mechanisms of peptide and nonpeptide ligand binding to Class B G-protein-coupled receptors. Drug Discov Today. 2005;10:417-27. Therefore, CRFR1 and CRFR2 are responsible for detecting molecules outside the cell (in this case, CRF and CRF-like molecules), then activating internal signal transduction pathways and, ultimately, cellular responses. Coupling of CRFRs and G proteins helps to stabilize the receptor and confers an active state of high ligand affinity.9494. Grammatopoulos DK. Insights into mechanisms of corticotropin-releasing hormone receptor signal transduction. Br J Pharmacol. 2012;166:85-97. The initial studies of the effects of CRF inducing pituitary ACTH release demonstrated that this response takes place through activation of Gαs/cyclic adenosine monophosphate (cAMP).9595. Millan MA, Samra AB, Wynn PC, Catt KJ, Aguilera G. Receptors and actions of corticotropin-releasing hormone in the primate pituitary gland. J Clin Endocrinol Metab. 1987;64:1036-41. Both CRFRs act to activate primarily adenylyl/cyclase and cAMP signaling pathways,2727. Chen R, Lewis KA, Perrin MH, Vale WW. Expression cloning of a human corticotropin-releasing-factor receptor. Proc Natl Acad Sci U S A. 1993;90:8967-71. , 2929. Lovenberg TW, Liaw CW, Grigoriadis DE, Clevenger W, Chalmers DT, Souza EBD, et al. Cloning and characterization of a functionally distinct corticotropin-releasing factor receptor subtype from rat brain. Proc Natl Acad Sci. 1995;92:836-40. through activation of Gαs.3434. Hillhouse EW, Grammatopoulos DK. The molecular mechanisms underlying the regulation of the biological activity of corticotropin-releasing hormone receptors: implications for physiology and pathophysiology. Endocr Rev. 2006;27:260-86. However, other studies also demonstrated that CRFRs are highly promiscuous (in fact several GPCRs seem to be) and can activate other types of Gα besides Gαs.1111. Deussing JM, Chen A. The corticotropin-releasing factor family: physiology of the stress response. Physiol Rev. 2018;98:2225-86. It seems that the patterns of activation of signaling pathways are unique to each tissue where the CRFRs are expressed. The mechanisms underlying this diversity are not well understood. It is possible that even the type of agonist bound to the CRFR might be involved in receptor/G-protein coupling selectivity.
Activation of the cAMP/protein kinase A (PKA) signaling pathway engenders both cytoplasmic events (acute post translational changes in target proteins) and nuclear events (regulation of genetic transcription through cAMP response element-binding [CREB] proteins).9696. Shaywitz AJ, Greenberg ME. CREB: a stimulus-induced transcription factor activated by a diverse array of extracellular signals. Annu Rev Biochem. 1999;68:821-61. , 9797. Taskén K, Aandahl EM. Localized effects of cAMP mediated by distinct routes of protein kinase A. Physiol Rev. 2004;84:137-67. In the HPA-axis, activation of the cAMP pathway via CRF induces expression of Nur77 and Nurr1 messenger ribonucleic acids (mRNAs), as well as transcription of the NurRE site, leading to transcriptional activation of the POMC gene.9898. Murphy EP, Conneely OM. Neuroendocrine regulation of the hypothalamic pituitary adrenal axis by the nurr1/nur77 subfamily of nuclear receptors. Mol Endocrinol. 1997;11:39-47. Moreover, specific activation of cAMP via CRFR1 induces transcription of several responsive genes related to attenuation of CRF signaling properties that constitute several mechanisms that promote the HPA-axis negative feedback.9999. Peeters PJ, Göhlmann HW, Van den Wyngaert I, Swagemakers SM, Bijnens L, Kass SU, et al. Transcriptional response to corticotropin-releasing factor in AtT-20 cells. Mol Pharmacol. 2004;66:1083-92. Other effects that have been identified as activated by CRFR1 via CRF and UCN-I binding include expression of several enzymes belonging to the cortisol synthesis pathway.100100. Sirianni R, Mayhew BA, Carr BR, Parker CR, Rainey WE. Corticotropin-releasing hormone (CRH) and urocortin act through type 1 CRH receptors to stimulate dehydroepiandrosterone sulfate production in human fetal adrenal cells. J Clin Endocrinol Metab. 2005;90:5393-400. As previously mentioned, CRF signaling also promotes self-upregulation of CRFR1 gene transcription9999. Peeters PJ, Göhlmann HW, Van den Wyngaert I, Swagemakers SM, Bijnens L, Kass SU, et al. Transcriptional response to corticotropin-releasing factor in AtT-20 cells. Mol Pharmacol. 2004;66:1083-92. , 100100. Sirianni R, Mayhew BA, Carr BR, Parker CR, Rainey WE. Corticotropin-releasing hormone (CRH) and urocortin act through type 1 CRH receptors to stimulate dehydroepiandrosterone sulfate production in human fetal adrenal cells. J Clin Endocrinol Metab. 2005;90:5393-400. ( Figure 2 ).
There are also cAMP-independent signaling pathways activated by CRFRs. MAPK/ERK cascades can be activated by CRFR1 and CRFR2 when activated by specific agonists.3434. Hillhouse EW, Grammatopoulos DK. The molecular mechanisms underlying the regulation of the biological activity of corticotropin-releasing hormone receptors: implications for physiology and pathophysiology. Endocr Rev. 2006;27:260-86. These enzymes constitute the pathways that mediate physiological functions of CRF and CRFRs, such as cardioprotection against injury-induced hypoperfusion,101101. Brar BK, Jonassen AK, Egorina EM, Chen A, Negro A, Perrin MH, et al. Urocortin-II and urocortin-III are cardioprotective against ischemia reperfusion injury: an essential endogenous cardioprotective role for corticotropin releasing factor receptor type 2 in the murine heart. Endocrinology. 2004;145:24-35; discussion 21-23. behavioral and memory adaptation to stress,102102. Sananbenesi F, Fischer A, Schrick C, Spiess J, Radulovic J. Mitogen-activated protein kinase signaling in the hippocampus and its modulation by corticotropin-releasing factor receptor 2: a possible link between stress and fear memory. J Neurosci. 2003;23:11436-43. neuroprotection,103103. Pedersen WA, Wan R, Zhang P, Mattson MP. Urocortin, but not urocortin II, protects cultured hippocampal neurons from oxidative and excitotoxic cell death via corticotropin-releasing hormone receptor type I. J Neurosci. 2002;22:404-12. vasodilatation,104104. Kageyama K, Furukawa K-I, Miki I, Terui K, Motomura S, Suda T. Vasodilative effects of urocortin II via protein kinase A and a mitogen-activated protein kinase in rat thoracic aorta. J Cardiovasc Pharmacol. 2003;42:561-5. and smooth muscle contractility.105105. Grammatopoulos DK, Dai Y, Randeva HS, Levine MA, Karteris E, Easton AJ, et al. A novel spliced variant of the type 1 corticotropin-releasing hormone receptor with a deletion in the seventh transmembrane domain present in the human pregnant term myometrium and fetal membranes. Mol Endocrinol. 1999;13:2189-202. ERK and p38 MAPK can also participate in transcription of Nur factors and consequent induction of POMC secretion106106. Kovalovsky D, Refojo D, Liberman AC, Hochbaum D, Pereda MP, Coso OA, et al. Activation and induction of NUR77/NURR1 in corticotrophs by CRH/cAMP: involvement of calcium, protein kinase A, and MAPK pathways. Mol Endocrinol. 2002;16:1638-51. ( Figure 2 ). Additionally, it has been demonstrated that CRFRs can also signal through induction of protein kinase C (PKC), protein kinase B (PKB/ATK), important second messengers such as Ca2, nitric oxide synthase, guanylyl cyclase, prostaglandins, Fas, and Fas-ligand.106106. Kovalovsky D, Refojo D, Liberman AC, Hochbaum D, Pereda MP, Coso OA, et al. Activation and induction of NUR77/NURR1 in corticotrophs by CRH/cAMP: involvement of calcium, protein kinase A, and MAPK pathways. Mol Endocrinol. 2002;16:1638-51.
107. Cantarella G, Lempereur L, Lombardo G, Chiarenza A, Pafumi C, Zappalà G, et al. Divergent effects of corticotropin releasing hormone on endothelial cell nitric oxide synthase are associated with different expression of CRH type 1 and 2 receptors. Br J Pharmacol. 2001;134:837-44.
108. Dermitzaki E, Tsatsanis C, Gravanis A, Margioris AN. Corticotropin-releasing hormone induces Fas ligand production and apoptosis in PC12 cells via activation of p38 mitogen-activated protein kinase. J Biol Chem. 2002;277:12280-7.
109. Grammatopoulos DK, Hillhouse EW. Basal and interleukin-1beta-stimulated prostaglandin production from cultured human myometrial cells: differential regulation by corticotropin-releasing hormone. J Clin Endocrinol Metab. 1999;84:2204-11.
110. Kiang JG. Corticotropin-releasing factor-like peptides increase cytosolic [Ca2+] in human epidermoid A-431 cells. Eur J Pharmacol. 1997;329:237-244. - 111111. Kostic TS, Andric SA, Stojilkovic SS. Spontaneous and receptor-controlled soluble guanylyl cyclase activity in anterior pituitary cells. Mol Endocrinol. 2001;15:1010-22. It is reasonable to think that the vast range of signaling modes built-in to the CRFergic system demonstrate its importance, specially mediating the CRF biological effects throughout several biological tissues.
Regulatory mechanisms of CRF receptor signaling
Knowledge of the cellular mechanisms that regulate the effects of CRF and CRF-like molecules on their cell-targets is still unsatisfactory. However, GPCR-mediated signaling can rapidly be attenuated by protein kinase phosphorylation and consequent interaction with arrestins. This interaction leads to receptor desensitization, G-protein uncoupling and subsequent receptor internalization.112112. Claing A, Laporte SA, Caron MG, Lefkowitz RJ. Endocytosis of G protein-coupled receptors: roles of G protein-coupled receptor kinases and beta-arrestin proteins. Prog Neurobiol. 2002;66:61-79. , 113113. Freedman NJ, Lefkowitz RJ. Desensitization of G protein-coupled receptors. Recent Prog Horm Res. 1996;51:319-351; discussion 352-3. Moreover, studies have demonstrated that exposure to high levels of CRF desensitizes the CRFR1 signaling response.5555. Potter E, Behan DP, Fischer WH, Linton EA, Lowry PJ, Vale WW. Cloning and characterization of the cDNAs for human and rat corticotropin releasing factor-binding proteins. Nature. 1991;349:423-6. , 114114. Dieterich KD, Grigoriadis DE, De Souza EB. Homologous desensitization of human corticotropin-releasing factor1 receptor in stable transfected mouse fibroblast cells. Brain Res. 1996;710:287-92.
115. Hauger RL, Smith RD, Braun S, Dautzenberg FM, Catt KJ. Rapid agonist-induced phosphorylation of the human CRF receptor, type 1: a potential mechanism for homologous desensitization. Biochem Biophys Res Commun. 2000;268:572-6. - 116116. Teli T, Markovic D, Levine MA, Hillhouse EW, Grammatopoulos DK. Regulation of corticotropin-releasing hormone receptor type 1alpha signaling: structural determinants for G protein-coupled receptor kinase-mediated phosphorylation and agonist-mediated desensitization. Mol Endocrinol. 2005;19:474-90. The coupling of a GPCR with an arrestin initiates two processes: 1) receptor membrane uncoupling and 2) internalization. Both of these processes are involved in cell desensitization and normalization of cell responses. Possibly, in the case of CRF, once an agonist binds to a receptor, a β-arrestin is translocated from the cytosol to the plasma membrane close to CRFR1 to conduct receptor internalization.117117. Rasmussen TN, Novak I, Nielsen SM. Internalization of the human CRF receptor 1 is independent of classical phosphorylation sites and of beta-arrestin 1 recruitment. Eur J Biochem. 2004;271:4366-74. There is some evidence suggesting that the process occurs with the involvement of clathrin-coated vesicles.117117. Rasmussen TN, Novak I, Nielsen SM. Internalization of the human CRF receptor 1 is independent of classical phosphorylation sites and of beta-arrestin 1 recruitment. Eur J Biochem. 2004;271:4366-74. , 118118. Perry SJ, Junger S, Kohout TA, Hoare SRJ, Struthers RS, Grigoriadis DE, et al. Distinct conformations of the corticotropin releasing factor type 1 receptor adopted following agonist and antagonist binding are differentially regulated. J Biol Chem. 2005;280:11560-8. This receptor internalization mechanism is not completely understood for CRFR1 and a β-arrestin-independent mechanism has also been proposed.117117. Rasmussen TN, Novak I, Nielsen SM. Internalization of the human CRF receptor 1 is independent of classical phosphorylation sites and of beta-arrestin 1 recruitment. Eur J Biochem. 2004;271:4366-74. It is highly probable that after internalization, CRFRs are trapped into lysosomes for degradation or stored in intracellular compartments, being available for future redirection to the membrane.119119. Shukla AK, Xiao K, Lefkowitz RJ. Emerging paradigms of β-arrestin-dependent seven transmembrane receptor signaling. Trends Biochem Sci. 2011;36:457-69.
The activity of CRFRs is susceptible to intracellular mechanisms that rapidly attenuate signaling output and prevent cell overstimulation. Efforts to unravel the plethora of CRFR biological actions are recent, and what can be inferred is related to what is known about the GPCR family. Usually, such processes of signaling cessation begin with receptor phosphorylation by second messenger-activated protein kinases (PKs), followed by recruitment of arrestin family proteins, leading to receptor desensitization.9494. Grammatopoulos DK. Insights into mechanisms of corticotropin-releasing hormone receptor signal transduction. Br J Pharmacol. 2012;166:85-97. The main PKs identified as regulators of CRFR1 phosphorylation and desensitization is the family of G protein-coupled receptor kinases (GRK).116116. Teli T, Markovic D, Levine MA, Hillhouse EW, Grammatopoulos DK. Regulation of corticotropin-releasing hormone receptor type 1alpha signaling: structural determinants for G protein-coupled receptor kinase-mediated phosphorylation and agonist-mediated desensitization. Mol Endocrinol. 2005;19:474-90. Once a GPCR has been phosphorylated by a GRK, it binds with high affinity to β-arrestins. This might result in uncoupling of the receptor to the G-protein with subsequent receptor inactivation. Alternatively, the β-arrestins serve as an adaptor protein to couple the receptor to clathrin-coated pits with subsequent receptor endocytosis. This process of endocytosis culminates in either sequestration or degradation of the receptor and the final outcome appears to be dependent on the cell type, the concentration of the agonist, and the duration of the presence of these ligands at the binding site.120120. Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Signaling through G-protein-linked cell-surface receptors. In: Molecular biology of the cell. New York: Garland Science; 2002.
Recently, other proteins that can regulate CRFRs have been discovered: these are called GPCR-interaction proteins (GIP).1111. Deussing JM, Chen A. The corticotropin-releasing factor family: physiology of the stress response. Physiol Rev. 2018;98:2225-86. One example of a GIP seems to be CRFBP itself, a protein capable of acting in different ways with respect to CRF and UCNs and their interactions with CRFRs.121121. Seasholtz AF, Valverde RA, Denver RJ. Corticotropin-releasing hormone-binding protein: biochemistry and function from fishes to mammals. J Endocrinol. 2002;175:89-97. , 122122. Slater PG, Yarur HE, Gysling K. Corticotropin-releasing factor receptors and their interacting proteins: functional consequences. Mol Pharmacol. 2016;90:627-32. The second class of molecules that interact with the CRFergic system components are the receptor activity modifying proteins (RAMP).123123. Hay DL, Walker CS, Gingell JJ, Ladds G, Reynolds CA, Poyner DR. Receptor activity-modifying proteins; multifunctional G protein-coupled receptor accessory proteins. Biochem Soc Trans. 2016;44:568-73. For instance, the interaction between CRFR1 and receptor activity modifying proteins 2 (RAMP-2) promotes their expression on the cell surface, modulates G protein coupling to CRFR1, and ultimately regulates the receptor signaling and desensitization processes.124124. Moeser AJ, Klok CV, Ryan KA, Wooten JG, Little D, Cook VL, et al. Stress signaling pathways activated by weaning mediate intestinal dysfunction in the pig. Am J Physiol Gastrointest Liver Physiol. 2007;292:G173-81. It has been demonstrated that CRFRs, as well as other GPCRs, form dimers.125125. Kamal M, Jockers R. Biological Significance of GPCR Heteromerization in the Neuro-Endocrine System. Front Endocrinol. 2011;2. Homodimerization is mainly reported for CRFR1,126126. Rutz C, Renner A, Alken M, Schulz K, Beyermann M, Wiesner B, et al. The corticotropin-releasing factor receptor type 2a contains an N-terminal pseudo signal peptide. J Biol Chem. 2006;281:24910-21. , 127127. Teichmann A, Rutz C, Kreuchwig A, Krause G, Wiesner B, Schülein R. The pseudo signal peptide of the corticotropin-releasing factor receptor Type 2A prevents receptor oligomerization. J Biol Chem. 2012;287:27265-74. while heterodimerization between CRFR1 and CRFR2 is a mechanism that increases CRFR2 cell surface expression.128128. Hasdemir B, Mahajan S, Oses-Prieto J, Chand S, Woolley M, Burlingame A, et al. Actin cytoskeleton-dependent regulation of corticotropin-releasing factor receptor heteromers. Mol Biol Cell. 2017;28:2386-99. As will be discussed in the next subsection, this is an important process during the entire stress response. Finally, CRFR1 can form heterodimers with other classes of GPCRs (e.g. orexin129129. Navarro G, Quiroz C, Moreno-Delgado D, Sierakowiak A, McDowell K, Moreno E, et al. Orexin-corticotropin-releasing factor receptor heteromers in the ventral tegmental area as targets for cocaine. J Neurosci. 2015;35:6639-53. and vasopressin130130. Murat B, Devost D, Andrés M, Mion J, Boulay V, Corbani M, et al. V1b and CRHR1 receptor heterodimerization mediates synergistic biological actions of vasopressin and CRH. Mol Endocrinol. 2012;26:502-20. ). These abilities of CRFRs to form dimers along with interaction with GIPs extend the diversity of their functioning and regulatory mechanisms.1111. Deussing JM, Chen A. The corticotropin-releasing factor family: physiology of the stress response. Physiol Rev. 2018;98:2225-86. Malfunctions in these modulatory processes are often linked to neurological disturbances and psychiatric disorders. Dysfunctional CRF activity may underlie anxiety-like responses by G-protein-coupled CRF receptor signaling.131131. Hauger RL, Risbrough V, Brauns O, Dautzenberg FM. Corticotropin releasing factor (CRF) receptor signaling in the central nervous system: new molecular targets. CNS Neurol Disord Drug Targets. 2006;5:453-79. Exploring the activation of metabotropic receptors and the modulatory role of their second messenger system could be ideal for understanding pathological states and may also shed light on the biological basis of vulnerability and resilience to stress.
CRF signaling and individual reactivity to stress
Although activation of the stress response is critical for survival, rapid counterregulation of this response is equally important for reestablishing normal functioning and emotional state after a threat subsides. Not all individuals experiencing stress develop depressive or anxiety states, which suggests that the response to stress is, to a significant extent, determined by individual vulnerability or resilience. “Resilience” can be described as a function determined by thresholds that characterize the boundary at which internal or external disturbances activate the stress response and a second threshold related to how quickly and effectively these stress responses can be shut down in response to disappearance of the stress event.131131. Hauger RL, Risbrough V, Brauns O, Dautzenberg FM. Corticotropin releasing factor (CRF) receptor signaling in the central nervous system: new molecular targets. CNS Neurol Disord Drug Targets. 2006;5:453-79.
It is possible that the modus operandi of the CRFergic system in a particular individual influences that individual’s ability to cope with a stressor. This might be true, since CRF signaling is directly linked to initiation and to cessation of the stress response ( Figure 1 ). Clinical and preclinical studies suggest that persistent dysfunctions of central CRF neurotransmission, possibly engendered by CRF hypersecretion or CRF receptor dynamics, contribute to the etiology of anxiety, depression-related illnesses and stress adjustment disorders. Genetic abnormalities, exposure to stress during developmental stages, exposure to traumatic events, or unpredictable stress at any stage can modulate an individual’s resilience to stress.131131. Hauger RL, Risbrough V, Brauns O, Dautzenberg FM. Corticotropin releasing factor (CRF) receptor signaling in the central nervous system: new molecular targets. CNS Neurol Disord Drug Targets. 2006;5:453-79. In this sense, adversity in life is expected to modulate coping responses as well as how the CRFergic system is activated in response to a stressor. Several studies highlight the relationship between adopting a passive strategy to cope with stress and a consequent association with vulnerability to development of affective disorders in humans.132132. Billings AG, Moos RH. Coping, stress, and social resources among adults with unipolar depression. J Pers Soc Psychol. 1984;46:877-91. , 133133. Vinberg M, Froekjaer VG, Kessing LV. Coping styles in healthy individuals at risk of affective disorder. J Nerv Ment Dis. 2010;198:39-44. In experimental social stress paradigms, submissive individuals tend to develop a depressive-like endocrine and behavioral profile. In contrast, a proactive strategy is associated with resilience in both basic and clinical research settings.134134. Wood SK, Zhang X-Y, Reyes BAS, Lee CS, Van Bockstaele EJ, Valentino RJ. Cellular adaptations of dorsal raphe serotonin neurons associated with the development of active coping in response to social stress. Biol Psychiatry. 2013;73:1087-94. , 135135. Wood SK, Walker HE, Valentino RJ, Bhatnagar S. Individual differences in reactivity to social stress predict susceptibility and resilience to a depressive phenotype: role of corticotropin-releasing factor. Endocrinology. 2010;151:1795-805. Chronic administration of a CRFR1 antagonist (NBI-30775) promoted changes in the behavioral response to stress in the rat resident-intruder social defeat model, inducing shifts in coping strategies from a passive to an active style.136136. Wood SK, McFadden KV, Grigoriadis D, Bhatnagar S, Valentino RJ. Depressive and cardiovascular disease comorbidity in a rat model of social stress: a putative role for corticotropin-releasing factor. Psychopharmacology (Berl). 2012;222:325-36. In animal models, NBI-30775 administration also prevents depressive-like symptoms induced by exposure to repeated social stress, represented by decreased immobility in the forced swim test and adrenal hypertrophy.136136. Wood SK, McFadden KV, Grigoriadis D, Bhatnagar S, Valentino RJ. Depressive and cardiovascular disease comorbidity in a rat model of social stress: a putative role for corticotropin-releasing factor. Psychopharmacology (Berl). 2012;222:325-36. Later, these effects were reproduced with additional characterization of CRFRs ultrastructural distribution.134134. Wood SK, Zhang X-Y, Reyes BAS, Lee CS, Van Bockstaele EJ, Valentino RJ. Cellular adaptations of dorsal raphe serotonin neurons associated with the development of active coping in response to social stress. Biol Psychiatry. 2013;73:1087-94. Understanding the cellular adaptations that enable active coping responses to stress may provide critical insight into mechanisms that promote resilience.
CRF signaling is involved in the modulating process of monoaminergic activity implicated in affective states. Within the dorsal raphe nuclei (DRN), CRF has opposing effects on serotonergic neuronal activity, a dual CRF action that is influenced by prior exposure to stress. For example, a single exposure to forced swim stress qualitatively shifts the rat serotonergic DRN response to CRF from inhibition to excitation.137137. Waselus M, Nazzaro C, Valentino RJ, Van Bockstaele EJ. Stress-induced redistribution of corticotropin-releasing factor receptor subtypes in the dorsal raphe nucleus. Biol Psychiatry. 2009;66:76-83. Repeated exposure to social stress also promotes dynamic changes in this neuronal subpopulation in rats that adopt an active coping strategy.134134. Wood SK, Zhang X-Y, Reyes BAS, Lee CS, Van Bockstaele EJ, Valentino RJ. Cellular adaptations of dorsal raphe serotonin neurons associated with the development of active coping in response to social stress. Biol Psychiatry. 2013;73:1087-94. Furthermore, a cellular redistribution of CRFRs underlies this effect such that CRFR1 became internalized, while CRFR2 was recruited to the plasma membrane, leading to a shift in the types of response of serotonergic neurons to CRF, from inhibition to a CRFR2-mediated excitation.134134. Wood SK, Zhang X-Y, Reyes BAS, Lee CS, Van Bockstaele EJ, Valentino RJ. Cellular adaptations of dorsal raphe serotonin neurons associated with the development of active coping in response to social stress. Biol Psychiatry. 2013;73:1087-94. Thus, CRFR1 internalization in response to stressful threatening stimuli seems to be related to development of resilience, inducing cessation of the stress response at the appropriate time, while recruitment of CRFR2 resumes the stress experience, at least in the affective state.
Unfortunately, preclinical studies that address the role of CRFR2 in stress and anxiety did not attain a reliable level of certainty. As mentioned earlier, pharmacological manipulation of CRFR2 produces conflicting results regarding anxiety-like behaviors and stress responses in animal models. Some authors suggest that the behavioral results produced by manipulation of CRFR2 can be largely influenced by cell type, neuroanatomic substrate, and peculiarities of the experimental procedures applied to obtain the evidence.131131. Hauger RL, Risbrough V, Brauns O, Dautzenberg FM. Corticotropin releasing factor (CRF) receptor signaling in the central nervous system: new molecular targets. CNS Neurol Disord Drug Targets. 2006;5:453-79. It is possible that CRFR2 activation counteracts the anxiety effects caused by CRFR1 activation, but in order to observe CRFR2 effects it would be necessary to achieve better control of the response time and even an initial CRFR1 activation.
Conclusion
The CRF family’s involvement with the ability to cope with a stressor might be founded on a simplistic dual process of CRFR functioning. CRFR1 is thought to be the subtype through which CRF primarily initiates its HPA axis response to stress. The consequences of CRFR1 signaling amplify the stress response throughout the brain and trigger their own negative feedback. CRFR1 activation in the forebrain, hippocampus, and/or amygdala is linked to expression of anxiety-like behaviors, indicating that CRFR1 signaling might be required to initiate associated defensive responses interpreted as protective anxiety behaviors. Complementarily, a restorative process of the stress response has been attributed to UCNs, mainly UCN-II and III, that act as agonists of CRFR2.2222. Hsu SY, Hsueh AJW. Human stresscopin and stresscopin-related peptide are selective ligands for the type 2 corticotropin-releasing hormone receptor. Nat Med. 2001;7:605-11. , 2424. Reyes TM, Lewis K, Perrin MH, Kunitake KS, Vaughan J, Arias CA, et al. Urocortin II: a member of the corticotropin-releasing factor (CRF) neuropeptide family that is selectively bound by type 2 CRF receptors. Proc Natl Acad Sci. 2001;98:2843-8. CRFR2 signaling might be involved in reduction of CRFR1-induced activation, reduction of anxiety responses, and restoration of cellular processes. The physiological state in which an individual can manage these two responses seems to be linked to that individual’s ability to maintain health in adversity.
The evidence compiled by reviewing the literature in the present study demonstrates the ability of CRF to activate the stress response, constituting an initial defense of homeostasis.1010. Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and β-endorphin. Science. 1981;213:1394-7. Cumulative evidence supports the role of a CRFergic dysfunction in the pathogenesis of mood and stress adjustment disorders, driving the development of better strategies to discover and develop more suitable therapies for stress-related disorders.
Acknowledgements
This study received financial support from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
References
-
1McEwen BS, Wingfield JC. The concept of allostasis in biology and biomedicine. Horm Behav. 2003;43:2-15.
-
2Grammatopoulos DK, Ourailidou S. CRH receptor signalling: potential roles in Pathophysiology. Curr Mol Pharmacol. 2017;10:296-310.
-
3Meerlo P, Sgoifo A, De Boer SF, Koolhaas JM. Long-lasting consequences of a social conflict in rats: behavior during the interaction predicts subsequent changes in daily rhythms of heart rate, temperature, and activity. Behav Neurosci. 1999;113:1283-90.
-
4Sgoifo A, Costoli T, Meerlo P, Buwalda B, Pico’-Alfonso MA, De Boer S, et al. Individual differences in cardiovascular response to social challenge. Neurosci Biobehav Rev. 2005;29:59-66.
-
5Koolhaas JM, Meerlo P, de Boer SF, Strubbe JH, Bohus B. The temporal dynamics of the stress response. Neurosci Biobehav Rev. 1997;21:775-82.
-
6Sapolsky RM. The influence of social hierarchy on primate health. Science. 2005;308:648-52.
-
7de Kloet ER, Joëls M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6:463-75.
-
8Fava GA, McEwen BS, Guidi J, Gostoli S, Offidani E, Sonino N. Clinical characterization of allostatic overload. Psychoneuroendocrinology. 2019;108:94-101.
-
9Joëls M, Baram TZ. The neuro-symphony of stress. Nat Rev Neurosci. 2009;10:459-66.
-
10Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and β-endorphin. Science. 1981;213:1394-7.
-
11Deussing JM, Chen A. The corticotropin-releasing factor family: physiology of the stress response. Physiol Rev. 2018;98:2225-86.
-
12Brown MR, Fisher LA. Corticotropin-releasing factor: effects on the autonomic nervous system and visceral systems. Fed Proc. 1985;44:243-8.
-
13McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87:873-904.
-
14Miczek KA, Yap JJ, Covington III HE. Social stress, therapeutics and drug abuse: Preclinical models of escalated and depressed intake. Pharmacol Ther. 2008;120:102-28.
-
15Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol. 2004;44:525-57.
-
16Hauger RL, Risbrough V, Oakley RH, Olivares-Reyes JA, Dautzenberg FM. Role of CRF receptor signaling in stress vulnerability, anxiety, and depression. Ann N Y Acad Sci. 2009;1179:120-43.
-
17Arborelius L, Owens MJ, Plotsky PM, Nemeroff CB. The role of corticotropin-releasing factor in depression and anxiety disorders. J Endocrinol. 1999;160:1-12.
-
18Bailer U, Kaye W. A review of neuropeptide and neuroendocrine dysregulation in anorexia and bulimia nervosa. Curr Drug Targets CNS Neurol Disord. 2003;2:53-9.
-
19Behan DP, Khongsaly O, Owens MJ, Chung HD, Nemeroff CB, De Souza EB. Corticotropin-releasing factor (CRF), CRF-binding protein (CRF-BP), and CRF/CRF-BP complex in Alzheimer’s disease and control postmortem human brain. J Neurochem. 1997;68:2053-60.
-
20Jessop DS, Harbuz MS, Lightman SL. CRH in chronic inflammatory stress. Peptides. 2001;22:803-7.
-
21Richard D, Huang Q, Timofeeva E. The corticotropin-releasing hormone system in the regulation of energy balance in obesity. Int J Obes Relat Metab Disord. 2000;24 Suppl 2:S36-9.
-
22Hsu SY, Hsueh AJW. Human stresscopin and stresscopin-related peptide are selective ligands for the type 2 corticotropin-releasing hormone receptor. Nat Med. 2001;7:605-11.
-
23Lewis K, Li C, Perrin MH, Blount A, Kunitake K, Donaldson C, et al. Identification of urocortin III, an additional member of the corticotropin-releasing factor (CRF) family with high affinity for the CRF2 receptor. Proc Natl Acad Sci. 2001;98:7570-5.
-
24Reyes TM, Lewis K, Perrin MH, Kunitake KS, Vaughan J, Arias CA, et al. Urocortin II: a member of the corticotropin-releasing factor (CRF) neuropeptide family that is selectively bound by type 2 CRF receptors. Proc Natl Acad Sci. 2001;98:2843-8.
-
25Vaughan J, Donaldson C, Bittencourt J, Perrin MH, Lewis K, Sutton S, et al. Urocortin, a mammalian neuropeptide related to fish urotensin I and to corticotropin-releasing factor. Nature. 1995;378:287-92.
-
26Chang CP, Pearse RV, O’Connell S, Rosenfeld MG. Identification of a seven transmembrane helix receptor for corticotropin-releasing factor and sauvagine in mammalian brain. Neuron. 1993;11:1187-95.
-
27Chen R, Lewis KA, Perrin MH, Vale WW. Expression cloning of a human corticotropin-releasing-factor receptor. Proc Natl Acad Sci U S A. 1993;90:8967-71.
-
28Vita N, Laurent P, Lefort S, Chalon P, Lelias J-M, Kaghad M, et al. Primary structure and functional expression of mouse pituitary and human brain corticotrophin releasing factor receptors. FEBS Lett. 1993;335:1-5.
-
29Lovenberg TW, Liaw CW, Grigoriadis DE, Clevenger W, Chalmers DT, Souza EBD, et al. Cloning and characterization of a functionally distinct corticotropin-releasing factor receptor subtype from rat brain. Proc Natl Acad Sci. 1995;92:836-40.
-
30Perrin M, Donaldson C, Chen R, Blount A, Berggren T, Bilezikjian L, et al. Identification of a second corticotropin-releasing factor receptor gene and characterization of a cDNA expressed in heart. Proc Natl Acad Sci U S A. 1995;92:2969-73.
-
31Denver RJ. Acceleration of anuran amphibian metamorphosis by corticotropin-releasing hormone-like peptides. Gen Comp Endocrinol. 1993;91:38-51.
-
32Smith R. Alterations in the hypothalamic pituitary adrenal axis during pregnancy and the placental clock that determines the length of parturition. J Reprod Immunol. 1998;39:215-20.
-
33Denver RJ. Evolution of the corticotropin-releasing hormone signaling system and its role in stress-induced phenotypic plasticity. Ann N Y Acad Sci. 1999;897:46-53.
-
34Hillhouse EW, Grammatopoulos DK. The molecular mechanisms underlying the regulation of the biological activity of corticotropin-releasing hormone receptors: implications for physiology and pathophysiology. Endocr Rev. 2006;27:260-86.
-
35Spierling SR, Zorrilla EP. Don’t stress about CRF: assessing the translational failures of CRF1antagonists. Psychopharmacology (Berl). 2017;234:1467-81.
-
36Heinrichs SC, Koob GF. Corticotropin-releasing factor in brain: a role in activation, arousal, and affect regulation. J Pharmacol Exp Ther. 2004;311:427-40.
-
37Li C, Vaughan J, Sawchenko PE, Vale WW. Urocortin III-immunoreactive projections in rat brain: partial overlap with sites of type 2 corticotropin-releasing factor receptor expression. J Neurosci. 2002;22:991-1001.
-
38Hiroi N, Wong ML, Licinio J, Park C, Young M, Gold PW, et al. Expression of corticotropin releasing hormone receptors type I and type II mRNA in suicide victims and controls. Mol Psychiatry. 2001;6:540-6.
-
39Kostich WA, Chen A, Sperle K, Largent BL. Molecular identification and analysis of a novel human corticotropin-releasing factor (CRF) receptor: the CRF2γ receptor. Mol Endocrinol. 1998;12:1077-85.
-
40Van Pett K, Viau V, Bittencourt JC, Chan RKW, Li H-Y, Arias C, et al. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol. 2000;428:191-212.
-
41Contarino A, Heinrichs SC, Gold LH. Understanding corticotropin releasing factor neurobiology: contributions from mutant mice. Neuropeptides. 1999;33:1-12.
-
42Bale TL, Contarino A, Smith GW, Chan R, Gold LH, Sawchenko PE, et al. Mice deficient for corticotropin-releasing hormone receptor-2 display anxiety-like behaviour and are hypersensitive to stress. Nat Genet. 2000;24:410-4.
-
43Coste SC, Heard AD, Phillips TJ, Stenzel-Poore MP. Corticotropin-releasing factor receptor type 2-deficient mice display impaired coping behaviors during stress. Genes Brain Behav. 2006;5:131-8.
-
44Pelleymounter MA, Joppa M, Ling N, Foster AC. Pharmacological evidence supporting a role for central corticotropin-releasing factor(2) receptors in behavioral, but not endocrine, response to environmental stress. J Pharmacol Exp Ther. 2002;302:145-52.
-
45Behan DP, De Souza EB, Lowry PJ, Potter E, Sawchenko P, Vale WW. Corticotropin releasing factor (CRF) binding protein: a novel regulator of CRF and related peptides. Front Neuroendocrinol. 1995;16:362-82.
-
46Potter E, Behan DP, Linton EA, Lowry PJ, Sawchenko PE, Vale WW. The central distribution of a corticotropin-releasing factor (CRF)-binding protein predicts multiple sites and modes of interaction with CRF. Proc Natl Acad Sci U S A. 1992;89:4192-6.
-
47Sutton SW, Behan DP, Lahrichi SL, Kaiser R, Corrigan A, Lowry P, et al. Ligand requirements of the human corticotropin-releasing factor-binding protein. Endocrinology. 1995;136:1097-102.
-
48Albrechet-Souza L, Hwa LS, Han X, Zhang EY, DeBold JF, Miczek KA. Corticotropin releasing factor binding protein and CRF2 receptors in the ventral tegmental area: modulation of ethanol binge drinking in C57BL/6J mice. Alcohol Clin Exp Res. 2015;39:1609-18.
-
49Binder EB, Owens MJ, Liu W, Deveau TC, Rush AJ, Trivedi MH, et al. Association of polymorphisms in genes regulating the corticotropin-releasing factor system with antidepressant treatment response. Arch Gen Psychiatry. 2010;67:369-79.
-
50Enoch MA, Shen PH, Ducci F, Yuan Q, Liu J, White KV, et al. Common genetic origins for EEG, alcoholism and anxiety: the role of CRH-BP. PLoS One. 2008;3:e3620.
-
51Haass-Koffler CL, Henry AT, Melkus G, Simms JA, Naemmuddin M, Nielsen CK, et al. Defining the role of corticotropin releasing factor binding protein in alcohol consumption. Transl Psychiatry. 2016;6:e953.
-
52Vasconcelos M, Stein DJ, Albrechet-Souza L, Miczek KA, de Almeida RMM. Recovery of stress-impaired social behavior by an antagonist of the CRF binding protein, CRF6-33, in the bed nucleus of the stria terminalis of male rats. Behav Brain Res. 2019;357-358:104-10.
-
53Westphal NJ, Seasholtz AF. CRH-BP: the regulation and function of a phylogenetically conserved binding protein. Front Biosci. 2006;11:1878-91.
-
54Cortright DN, Nicoletti A, Seasholtz AF. Molecular and biochemical characterization of the mouse brain corticotropin-releasing hormone-binding protein. Mol Cell Endocrinol. 1995;111:147-57.
-
55Potter E, Behan DP, Fischer WH, Linton EA, Lowry PJ, Vale WW. Cloning and characterization of the cDNAs for human and rat corticotropin releasing factor-binding proteins. Nature. 1991;349:423-6.
-
56Ungless MA, Singh V, Crowder TL, Yaka R, Ron D, Bonci A. Corticotropin-releasing factor requires CRF binding protein to potentiate NMDA receptors via CRF receptor 2 in dopamine neurons. Neuron. 2003;39:401-7.
-
57Wang B, You Z-B, Rice KC, Wise RA. Stress-induced relapse to cocaine seeking: roles for the CRF2 receptor and CRF-binding protein in the ventral tegmental area of the rat. Psychopharmacology (Berl). 2007;193:283-94.
-
58Chan RKW, Vale WW, Sawchenko PE. Paradoxical activational effects of a corticotropin-releasing factor-binding protein “ligand inhibitor” in rat brain. Neuroscience. 2000;101:115-29.
-
59Slater PG, Cerda CA, Pereira LA, Andrés ME, Gysling K. CRF binding protein facilitates the presence of CRF type 2α receptor on the cell surface. Proc Natl Acad Sci. 2016;113:4075-80.
-
60Garcia I, Quast KB, Huang L, Herman AM, Selever J, Deussing JM, et al. Local CRH signaling promotes synaptogenesis and circuit integration of adult-born neurons. Dev Cell. 2014;30:645-59.
-
61Aldenhoff JB, Gruol DL, Rivier J, Vale W, Siggins GR. Corticotropin releasing factor decreases postburst hyperpolarizations and excites hippocampal neurons. Science. 1983;221:875-7.
-
62Wang HL, Tsai LY, Lee EH. Corticotropin-releasing factor produces a protein synthesis–dependent long-lasting potentiation in dentate gyrus neurons. J Neurophysiol. 2000;83:343-9.
-
63Blank T, Nijholt I, Eckart K, Spiess J. Priming of long-term potentiation in mouse hippocampus by corticotropin-releasing factor and acute stress: implications for hippocampus-dependent learning. J Neurosci. 2002;22:3788-94.
-
64Fu Y, Pollandt S, Liu J, Krishnan B, Genzer K, Orozco-Cabal L, et al. Long-term potentiation (LTP) in the central amygdala (CeA) is enhanced after prolonged withdrawal from chronic cocaine and requires CRF 1 receptors. J Neurophysiol. 2007;97:937-41.
-
65Krishnan B, Centeno M, Pollandt S, Fu Y, Genzer K, Liu J, et al. Dopamine receptor mechanisms mediate corticotropin-releasing factor-induced long-term potentiation in the rat amygdala following cocaine withdrawal. Eur J Neurosci. 2010;31:1027-42.
-
66Liu J, Yu B, Orozco-Cabal L, Grigoriadis DE, Rivier J, Vale WW, et al. Chronic cocaine administration switches corticotropin-releasing factor2 receptor-mediated depression to facilitation of glutamatergic transmission in the lateral septum. J Neurosci. 2005;25:577-83.
-
67Henckens MJAG, Printz Y, Shamgar U, Dine J, Lebow M, Drori Y, et al. CRF receptor type 2 neurons in the posterior bed nucleus of the stria terminalis critically contribute to stress recovery. Mol Psychiatry. 2017;22:1691-700.
-
68Valentino RJ, Foote SL, Aston-Jones G. Corticotropin-releasing factor activates noradrenergic neurons of the locus coeruleus. Brain Res. 1983;270:363-7.
-
69Chen Y, Andres AL, Frotscher M, Baram TZ. Tuning synaptic transmission in the hippocampus by stress: the CRH system. Front Cell Neurosci. 2012;6:13.
-
70Chen Y, Dube CM, Rice CJ, Baram TZ. Rapid loss of dendritic spines after stress snvolves derangement of spine dynamics by corticotropin-releasing hormone. J Neurosci. 2008;28:2903-11.
-
71Inda C, Bonfiglio JJ, dos Santos Claro PA, Senin SA, Armando NG, Deussing JM, et al. cAMP-dependent cell differentiation triggered by activated CRHR1 in hippocampal neuronal cells. Sci Rep. 2017;7:1944.
-
72Koutmani Y, Politis PK, Elkouris M, Agrogiannis G, Kemerli M, Patsouris E, et al. Corticotropin-releasing hormone exerts direct effects on neuronal progenitor cells: implications for neuroprotection. Mol Psychiatry. 2013;18:300-7.
-
73Chen Y, Rex CS, Rice CJ, Dubé CM, Gall CM, Lynch G, et al. Correlated memory defects and hippocampal dendritic spine loss after acute stress involve corticotropin-releasing hormone signaling. Proc Natl Acad Sci U S A. 2010;107:13123-8.
-
74Zheng Y, Zhang Y-M, Ni X. Urocortin 2 but not urocortin 3 promotes the synaptic formation in hipppocampal neurons via induction of NGF production by astrocytes. Endocrinology. 2016;157:1200-10.
-
75Rajkumar R, Wu Y, Farooq U, Tan WH, Dawe GS. Stress activates the nucleus incertus and modulates plasticity in the hippocampo-medial prefrontal cortical pathway. Brain Res Bull. 2016;120:83-9.
-
76Francesconi W, Berton F, Koob GF, Sanna PP. Intrinsic neuronal plasticity in the juxtacapsular nucleus of the bed nuclei of the stria terminalis (jcBNST). Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1347-55.
-
77Pollandt S, Liu J, Orozco-Cabal L, Grigoriadis DE, Vale WW, Gallagher JP, et al. Cocaine withdrawal enhances long-term potentiation induced by corticotropin-releasing factor at central amygdala glutamatergic synapses via CRF, NMDA receptors and PKA. Eur J Neurosci. 2006;24:1733-43.
-
78Chen L, Li S, Cai J, Wei T-J, Liu L-Y, Zhao H-Y, et al. Activation of CRF/CRFR1 signaling in the basolateral nucleus of the amygdala contributes to chronic forced swim-induced depressive-like behaviors in rats. Behav Brain Res. 2018;338:134-42.
-
79Rainnie DG, Bergeron R, Sajdyk TJ, Patil M, Gehlert DR, Shekhar A. Corticotrophin releasing factor-induced synaptic plasticity in the amygdala translates stress into emotional disorders. J Neurosci. 2004;24:3471-9.
-
80Rivier C, Rivier J, Mormede P, Vale W. Studies of the nature of the interaction between vasopressin and corticotropin-releasing factor on adrenocorticotropin release in the rat. Endocrinology. 1984;115:882-6.
-
81Branson KL, Grigoriadis DE, Lorang MT, Baram TZ. Corticotropin-releasing hormone (CRH) downregulates the function of its receptor (CRF1) and induces CRF1 expression in hippocampal and cortical regions of the immature rat brain. Exp Neurol. 2002;176:75-86.
-
82Lehmann ML, Cooper HA, Maric D, Herkenham M. Social defeat induces depressive-like states and microglial activation without involvement of peripheral macrophages. J Neuroinflammation. 2016;13:224.
-
83Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: implications for health. Nat Rev Immunol. 2005;5:243-51.
-
84Tsigos C, Chrousos GP. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. J Psychosom Res. 2002;53:865-71.
-
85Wang W, Ji P, Dow KE. Corticotropin‐releasing hormone induces proliferation and TNF‐α release in cultured rat microglia via MAP kinase signalling pathways. J Neurochem. 2003;84:189-95.
-
86Stevens SL, Shaw TE, Dykhuizen E, Lessov NS, Hill JK, Wurst W, et al. Reduced cerebral injury in CRH-R1 deficient mice after focal ischemia: a potential link to microglia and atrocytes that express CRH-R1. J Cereb Blood Flow Metab. 2003;23:1151-9.
-
87Uehara A, Gottschall PE, Dahl RR, Arimura A. Interleukin-1 stimulates ACTH release by an indirect action which requires endogenous corticotropin releasing factor. Endocrinology. 1987;121:1580-2.
-
88Naitoh Y, Fukata J, Tominaga T, Nakai Y, Tamai S, Mori K, et al. Interleukin-6 stimulates the secretion of adrenocorticotropic hormone in conscious, freely-moving rats. Biochem Biophys Res Commun. 1988;155:1459-63.
-
89Berkenbosch F, Van Oers J, Del Rey A, Tilders F, Besedovsky H. Corticotropin-releasing factor-producing neurons in the rat activated by interleukin-1. Science. 1987;238:524-6.
-
90Karalis KP, Venihaki M, Zhao J, van Vlerken LE, Chandras C. NF-κB participates in the corticotropin-releasing, hormone-induced regulation of the pituitary proopiomelanocortin gene. J Biol Chem. 2004;279:10837-40.
-
91Lezoualc’h F, Engert S, Berning B, Behl C. Corticotropin-releasing hormone-mediated neuroprotection against oxidative stress is associated with the increased release of non-amyloidogenic amyloid β precursor protein and with the suppression of nuclear factor-κB. Mol Endocrinol. 2000;14:147-59.
-
92Lagerström MC, Schiöth HB. Structural diversity of G protein-coupled receptors and significance for drug discovery. Nat Rev Drug Discov. 2008;7:339-57.
-
93Hoare SRJ. Mechanisms of peptide and nonpeptide ligand binding to Class B G-protein-coupled receptors. Drug Discov Today. 2005;10:417-27.
-
94Grammatopoulos DK. Insights into mechanisms of corticotropin-releasing hormone receptor signal transduction. Br J Pharmacol. 2012;166:85-97.
-
95Millan MA, Samra AB, Wynn PC, Catt KJ, Aguilera G. Receptors and actions of corticotropin-releasing hormone in the primate pituitary gland. J Clin Endocrinol Metab. 1987;64:1036-41.
-
96Shaywitz AJ, Greenberg ME. CREB: a stimulus-induced transcription factor activated by a diverse array of extracellular signals. Annu Rev Biochem. 1999;68:821-61.
-
97Taskén K, Aandahl EM. Localized effects of cAMP mediated by distinct routes of protein kinase A. Physiol Rev. 2004;84:137-67.
-
98Murphy EP, Conneely OM. Neuroendocrine regulation of the hypothalamic pituitary adrenal axis by the nurr1/nur77 subfamily of nuclear receptors. Mol Endocrinol. 1997;11:39-47.
-
99Peeters PJ, Göhlmann HW, Van den Wyngaert I, Swagemakers SM, Bijnens L, Kass SU, et al. Transcriptional response to corticotropin-releasing factor in AtT-20 cells. Mol Pharmacol. 2004;66:1083-92.
-
100Sirianni R, Mayhew BA, Carr BR, Parker CR, Rainey WE. Corticotropin-releasing hormone (CRH) and urocortin act through type 1 CRH receptors to stimulate dehydroepiandrosterone sulfate production in human fetal adrenal cells. J Clin Endocrinol Metab. 2005;90:5393-400.
-
101Brar BK, Jonassen AK, Egorina EM, Chen A, Negro A, Perrin MH, et al. Urocortin-II and urocortin-III are cardioprotective against ischemia reperfusion injury: an essential endogenous cardioprotective role for corticotropin releasing factor receptor type 2 in the murine heart. Endocrinology. 2004;145:24-35; discussion 21-23.
-
102Sananbenesi F, Fischer A, Schrick C, Spiess J, Radulovic J. Mitogen-activated protein kinase signaling in the hippocampus and its modulation by corticotropin-releasing factor receptor 2: a possible link between stress and fear memory. J Neurosci. 2003;23:11436-43.
-
103Pedersen WA, Wan R, Zhang P, Mattson MP. Urocortin, but not urocortin II, protects cultured hippocampal neurons from oxidative and excitotoxic cell death via corticotropin-releasing hormone receptor type I. J Neurosci. 2002;22:404-12.
-
104Kageyama K, Furukawa K-I, Miki I, Terui K, Motomura S, Suda T. Vasodilative effects of urocortin II via protein kinase A and a mitogen-activated protein kinase in rat thoracic aorta. J Cardiovasc Pharmacol. 2003;42:561-5.
-
105Grammatopoulos DK, Dai Y, Randeva HS, Levine MA, Karteris E, Easton AJ, et al. A novel spliced variant of the type 1 corticotropin-releasing hormone receptor with a deletion in the seventh transmembrane domain present in the human pregnant term myometrium and fetal membranes. Mol Endocrinol. 1999;13:2189-202.
-
106Kovalovsky D, Refojo D, Liberman AC, Hochbaum D, Pereda MP, Coso OA, et al. Activation and induction of NUR77/NURR1 in corticotrophs by CRH/cAMP: involvement of calcium, protein kinase A, and MAPK pathways. Mol Endocrinol. 2002;16:1638-51.
-
107Cantarella G, Lempereur L, Lombardo G, Chiarenza A, Pafumi C, Zappalà G, et al. Divergent effects of corticotropin releasing hormone on endothelial cell nitric oxide synthase are associated with different expression of CRH type 1 and 2 receptors. Br J Pharmacol. 2001;134:837-44.
-
108Dermitzaki E, Tsatsanis C, Gravanis A, Margioris AN. Corticotropin-releasing hormone induces Fas ligand production and apoptosis in PC12 cells via activation of p38 mitogen-activated protein kinase. J Biol Chem. 2002;277:12280-7.
-
109Grammatopoulos DK, Hillhouse EW. Basal and interleukin-1beta-stimulated prostaglandin production from cultured human myometrial cells: differential regulation by corticotropin-releasing hormone. J Clin Endocrinol Metab. 1999;84:2204-11.
-
110Kiang JG. Corticotropin-releasing factor-like peptides increase cytosolic [Ca2+] in human epidermoid A-431 cells. Eur J Pharmacol. 1997;329:237-244.
-
111Kostic TS, Andric SA, Stojilkovic SS. Spontaneous and receptor-controlled soluble guanylyl cyclase activity in anterior pituitary cells. Mol Endocrinol. 2001;15:1010-22.
-
112Claing A, Laporte SA, Caron MG, Lefkowitz RJ. Endocytosis of G protein-coupled receptors: roles of G protein-coupled receptor kinases and beta-arrestin proteins. Prog Neurobiol. 2002;66:61-79.
-
113Freedman NJ, Lefkowitz RJ. Desensitization of G protein-coupled receptors. Recent Prog Horm Res. 1996;51:319-351; discussion 352-3.
-
114Dieterich KD, Grigoriadis DE, De Souza EB. Homologous desensitization of human corticotropin-releasing factor1 receptor in stable transfected mouse fibroblast cells. Brain Res. 1996;710:287-92.
-
115Hauger RL, Smith RD, Braun S, Dautzenberg FM, Catt KJ. Rapid agonist-induced phosphorylation of the human CRF receptor, type 1: a potential mechanism for homologous desensitization. Biochem Biophys Res Commun. 2000;268:572-6.
-
116Teli T, Markovic D, Levine MA, Hillhouse EW, Grammatopoulos DK. Regulation of corticotropin-releasing hormone receptor type 1alpha signaling: structural determinants for G protein-coupled receptor kinase-mediated phosphorylation and agonist-mediated desensitization. Mol Endocrinol. 2005;19:474-90.
-
117Rasmussen TN, Novak I, Nielsen SM. Internalization of the human CRF receptor 1 is independent of classical phosphorylation sites and of beta-arrestin 1 recruitment. Eur J Biochem. 2004;271:4366-74.
-
118Perry SJ, Junger S, Kohout TA, Hoare SRJ, Struthers RS, Grigoriadis DE, et al. Distinct conformations of the corticotropin releasing factor type 1 receptor adopted following agonist and antagonist binding are differentially regulated. J Biol Chem. 2005;280:11560-8.
-
119Shukla AK, Xiao K, Lefkowitz RJ. Emerging paradigms of β-arrestin-dependent seven transmembrane receptor signaling. Trends Biochem Sci. 2011;36:457-69.
-
120Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Signaling through G-protein-linked cell-surface receptors. In: Molecular biology of the cell. New York: Garland Science; 2002.
-
121Seasholtz AF, Valverde RA, Denver RJ. Corticotropin-releasing hormone-binding protein: biochemistry and function from fishes to mammals. J Endocrinol. 2002;175:89-97.
-
122Slater PG, Yarur HE, Gysling K. Corticotropin-releasing factor receptors and their interacting proteins: functional consequences. Mol Pharmacol. 2016;90:627-32.
-
123Hay DL, Walker CS, Gingell JJ, Ladds G, Reynolds CA, Poyner DR. Receptor activity-modifying proteins; multifunctional G protein-coupled receptor accessory proteins. Biochem Soc Trans. 2016;44:568-73.
-
124Moeser AJ, Klok CV, Ryan KA, Wooten JG, Little D, Cook VL, et al. Stress signaling pathways activated by weaning mediate intestinal dysfunction in the pig. Am J Physiol Gastrointest Liver Physiol. 2007;292:G173-81.
-
125Kamal M, Jockers R. Biological Significance of GPCR Heteromerization in the Neuro-Endocrine System. Front Endocrinol. 2011;2.
-
126Rutz C, Renner A, Alken M, Schulz K, Beyermann M, Wiesner B, et al. The corticotropin-releasing factor receptor type 2a contains an N-terminal pseudo signal peptide. J Biol Chem. 2006;281:24910-21.
-
127Teichmann A, Rutz C, Kreuchwig A, Krause G, Wiesner B, Schülein R. The pseudo signal peptide of the corticotropin-releasing factor receptor Type 2A prevents receptor oligomerization. J Biol Chem. 2012;287:27265-74.
-
128Hasdemir B, Mahajan S, Oses-Prieto J, Chand S, Woolley M, Burlingame A, et al. Actin cytoskeleton-dependent regulation of corticotropin-releasing factor receptor heteromers. Mol Biol Cell. 2017;28:2386-99.
-
129Navarro G, Quiroz C, Moreno-Delgado D, Sierakowiak A, McDowell K, Moreno E, et al. Orexin-corticotropin-releasing factor receptor heteromers in the ventral tegmental area as targets for cocaine. J Neurosci. 2015;35:6639-53.
-
130Murat B, Devost D, Andrés M, Mion J, Boulay V, Corbani M, et al. V1b and CRHR1 receptor heterodimerization mediates synergistic biological actions of vasopressin and CRH. Mol Endocrinol. 2012;26:502-20.
-
131Hauger RL, Risbrough V, Brauns O, Dautzenberg FM. Corticotropin releasing factor (CRF) receptor signaling in the central nervous system: new molecular targets. CNS Neurol Disord Drug Targets. 2006;5:453-79.
-
132Billings AG, Moos RH. Coping, stress, and social resources among adults with unipolar depression. J Pers Soc Psychol. 1984;46:877-91.
-
133Vinberg M, Froekjaer VG, Kessing LV. Coping styles in healthy individuals at risk of affective disorder. J Nerv Ment Dis. 2010;198:39-44.
-
134Wood SK, Zhang X-Y, Reyes BAS, Lee CS, Van Bockstaele EJ, Valentino RJ. Cellular adaptations of dorsal raphe serotonin neurons associated with the development of active coping in response to social stress. Biol Psychiatry. 2013;73:1087-94.
-
135Wood SK, Walker HE, Valentino RJ, Bhatnagar S. Individual differences in reactivity to social stress predict susceptibility and resilience to a depressive phenotype: role of corticotropin-releasing factor. Endocrinology. 2010;151:1795-805.
-
136Wood SK, McFadden KV, Grigoriadis D, Bhatnagar S, Valentino RJ. Depressive and cardiovascular disease comorbidity in a rat model of social stress: a putative role for corticotropin-releasing factor. Psychopharmacology (Berl). 2012;222:325-36.
-
137Waselus M, Nazzaro C, Valentino RJ, Van Bockstaele EJ. Stress-induced redistribution of corticotropin-releasing factor receptor subtypes in the dorsal raphe nucleus. Biol Psychiatry. 2009;66:76-83.
-
This manuscript was based on the first author’s academic dissertation, titled “Estresse por derrota social intermitente em ratos Wistar machos: revisão e modulação farmacológica experimental do sistema CRF,” presented at Instituto de Psicologia, Universidade Federal do Rio Grande do Sul, in 2018, as partial fulfillment of the requirements for a doctoral degree in Psychology.
Publication Dates
-
Publication in this collection
17 July 2020 -
Date of issue
Apr-Jun 2020
History
-
Received
09 Apr 2019 -
Accepted
25 Sept 2019