Abstract:
Representative seed lot sampling is important in analyzing seed quality. This study aimed to determine whether sampling during processing and after packaging using samplers interferes in analyzing the physiological quality and health of soybean seed lots. The study was conducted with six and twelve soybean seed lots produced in the 2017/2018 and 2018/2019 growing seasons, respectively. Two sampling methods were used, according to the Rules for Seed Testing: a) sampling during processing; b) sampling of seed lots stored in bags using a sampler. Seed quality was assessed by germination percentage, accelerated aging, and tetrazolium tests to determine vigor, and seed health using the blotter test. Sampling during processing and after storage in bags differ in the expression of seed vigor and health. Seed lot homogeneity is essential to determine the difference between sampling methods. Sampling in the processing flow is more representative and may be indicated for the germination test.
Index terms:
accelerated aging; blotter test; germination; Glycine max L
Resumo:
A amostragem representativa do lote é importante na análise da qualidade das sementes. O objetivo neste trabalho foi verificar se a amostragem durante o fluxo de beneficiamento e a amostragem com o uso de caladores após o acondicionamento das sementes pode interferir na análise da qualidade fisiológica e sanitária de lotes de sementes de soja. O trabalho foi realizado com seis e doze lotes de sementes de soja produzidas nas safras 2017/2018 e 2018/2019, respectivamente. Para a amostragem dos lotes utilizaram-se dois métodos conforme descrito pelas Regras de Análises de Sementes: a) amostragem durante o fluxo de beneficiamento; b) amostragem dos lotes de sementes armazenados em “bags” com o uso de calador. Avaliou-se a qualidade das sementes pelo percentual de germinação, vigor por envelhecimento acelerado, tetrazólio e pela determinação da sanidade das sementes pelo blotter test. A amostragem durante o fluxo do beneficiamento e nos “bags” após o armazenamento, diferiu na expressão do vigor e da qualidade sanitária das sementes. A homogeneidade do lote é preponderante para verificar a diferença dos métodos de amostragem. A amostragem no fluxo do beneficiamento é mais representativa e pode ser indicada para o teste de germinação.
Termos para indexação:
envelhecimento acelerado; teste de blotter; germinação; Glycine max L
INTRODUCTION
To achieve high yields, the seeds used in planting should exhibit high quality. Seed quality includes the sum of physical, physiological, genetic, and health attributes responsible for seed performance in the field, thereby contributing to the adequate establishment of seedlings, crucial for a successful crop (Krzyzanowski et al., 2018KRZYZANOWSKI, F.C.; FRANÇA-NETO, J.B.; HENNING, A.A. A alta qualidade da semente de soja: fator importante para a produção da cultura. Londrina, 2018, 24p. ).
Seed quality can be analyzed during all the production stages, including pre-harvest, harvest, drying, processing, and storage, but seed analysis is required to commercialize lots. Analyses are conducted in laboratories, which must follow the methodologies established in the Rules for Seed Testing (Brasil, 2009KRZYZANOWSKI, F.C.; FRANÇA-NETO, J.B.; HENNING, A.A. A alta qualidade da semente de soja: fator importante para a produção da cultura. Londrina, 2018, 24p. ).
Although the laboratory follows all the necessary recommendations and precautions, sampling errors may compromise an accurate estimate of seed lot quality. If the established sampling techniques are not followed, the sample obtained does not reflect the real condition of the lot, and the uniformity, accuracy, and results will not be reproducible. The laboratory results may result in erroneous decision-making, such as the disposal of high-quality seed lots or approval of inferior ones (Castro, 2005CASTRO, O.O. Amostragem é decisiva na busca da qualidade. Seed News, n.1, p.1-5, 2005 https://seednews.com.br/artigos/1032-amostragem-e-decisiva-na-busca-da-qualidade-edicao-janeiro-2005
https://seednews.com.br/artigos/1032-amo...
).
According to ordinance 9 of June 2, 2005 (Brasil, 2005BRASIL. Instrução Normativa n° 09 de 02 de junho de 2005. Diário Oficial da República Federativa do Brasil , Poder Executivo, Brasília, DF, 2005. 16p.), seed sampling aims at obtaining a representative amount of the lot or part of it, when subdivided for analysis purposes. According to this ordinance, sampling should be manual or use samplers, depending on the species. Sampling can be carried out on seeds stored in packages or during processing. In the latter case, samples should be collected at regular intervals during processing stages.
Direct sampling during processing is used for internal quality control or to identify uncertified seeds, unlike sampling using a sampler after seeds have been packaged. The use of samplers may damage packages, demands more time, and requires trained personnel. In addition, seed movement during storage, where light or oddly shaped seeds and those with different moisture contents may settle and segregate differently in the packages, hindering the representative sampling.
Sampling during processing makes it possible to obtain an average sample with greater seed lot representativeness, since it increases the number of single samples obtained, provided they are collected at regular intervals during processing stages, as established by Ordinance 09 of June 2, 2005 (Brasil, 2005BRASIL. Instrução Normativa n° 09 de 02 de junho de 2005. Diário Oficial da República Federativa do Brasil , Poder Executivo, Brasília, DF, 2005. 16p.). Although sampling can be carried out during processing, in practice it is performed after packaging, using samplers. It is believed that there is no difference between the quality of seeds sampled during processing and with samplers. Thus, this study aimed to determine whether sampling during processing differs from that using samplers, thereby compromising the results of physiological and health analyses of soybean seed lots, according to the RAS tolerance table.
MATERIAL AND METHODS
The study was conducted at the Seed Analysis Laboratory of the Center for Agroveterinary Sciences of the University of Santa Catarina State (CAV/UDESC) in Lages, Santa Catarina state, in seed lot samples collected in the 2017/2018 and 2018/2019 growing seasons, obtained from seed producing companies in Santa Catarina.
Sampling was performed using two methods: a) in the bagging spout (after seeds were processed on a densimetric table), and b) in seeds stored and packaged in bags. Table 1 shows the number of lots sampled in each growing season, producing city, cultivars, category, representativeness, and the number of single samples obtained by each sampling method.
Seed lot, growing season, producing region, cultivars, category, representativeness and number of simple soybean samples obtained in bagging spout and after storage in bags.
Two composite samples were obtained from each lot: one in the bagging spout and the other with the aid of a sampler after storage. For sampling in bagging spout, two single samples from each bag were deviated between 10 and 30-second intervals, forming a composite sample of each lot.
The samples obtained during processing were stored for four months in the middle of the original seed lot. After the storage period in large bags, sampling was conducted with the aid of a sampler. The number of single samples was collected according to lot size. For lots weighing between 3,001 and 20,000 kg, one single sample was collected for every 500 kg, and for those above 20,000 kg, one single sample was collected for every 700 kg.
Each composite sample was homogenized and reduced to obtain the average sample (1000 grams) (Brasil, 2009BRASIL. Ministério da Agricultura, Pecuária e Abastecimento. Regras para análise de sementes. Ministério da Agricultura, Pecuária e Abastecimento. Secretaria de Defesa Agropecuária. Brasília: MAPA/ACS, 2009. 399p. https://www.gov.br/agricultura/pt-br/assuntos/insumos-agropecuarios/arquivos-publicacoes-insumos/2946_regras_analise__sementes.pdf
https://www.gov.br/agricultura/pt-br/ass...
). The samples were homogenized and reduced in the laboratory to obtain the study sample (500 g) (Brasil, 2009BRASIL. Ministério da Agricultura, Pecuária e Abastecimento. Regras para análise de sementes. Ministério da Agricultura, Pecuária e Abastecimento. Secretaria de Defesa Agropecuária. Brasília: MAPA/ACS, 2009. 399p. https://www.gov.br/agricultura/pt-br/assuntos/insumos-agropecuarios/arquivos-publicacoes-insumos/2946_regras_analise__sementes.pdf
https://www.gov.br/agricultura/pt-br/ass...
), which was subdivided into four subsamples (Coelho et al., 2010COELHO, C.; ZILIO, M.; SOUZA, C.; GUIDOLIN, A.; MIQUELLUTI, D. Características morfo-agronômicas de cultivares crioulas de feijão comum em dois anos de cultivo. Semina: Ciências Agrárias, v.31, p.1177-1186, 2010. http://dx.doi.org/10.5433/1679-0359.2010v31n4Sup1p1177
http://dx.doi.org/10.5433/1679-0359.2010...
).
The seeds were subjected to germination, accelerated aging and tetrazolium tests to determine vigor and viability, and health assessment via the blotter test.
Determination of germination percentage
The preconditioning was not performed. Seeds had an average moisture content of 12%. Seed moisture content was determined using the oven method at 105 °C (Brasil, 2009BRASIL. Ministério da Agricultura, Pecuária e Abastecimento. Regras para análise de sementes. Ministério da Agricultura, Pecuária e Abastecimento. Secretaria de Defesa Agropecuária. Brasília: MAPA/ACS, 2009. 399p. https://www.gov.br/agricultura/pt-br/assuntos/insumos-agropecuarios/arquivos-publicacoes-insumos/2946_regras_analise__sementes.pdf
https://www.gov.br/agricultura/pt-br/ass...
). The seeds were germinated on two germitest papers covered with a third sheet, wrapped in a moist roll with an amount of water 2.5 times the weight of the substrate, after being in the vertical position at 25 ± 1 °C (Brasil, 2009BRASIL. Ministério da Agricultura, Pecuária e Abastecimento. Regras para análise de sementes. Ministério da Agricultura, Pecuária e Abastecimento. Secretaria de Defesa Agropecuária. Brasília: MAPA/ACS, 2009. 399p. https://www.gov.br/agricultura/pt-br/assuntos/insumos-agropecuarios/arquivos-publicacoes-insumos/2946_regras_analise__sementes.pdf
https://www.gov.br/agricultura/pt-br/ass...
). The test was conducted with four replications of 100 seeds, each replication composed of two subsamples of 50 seeds per roll (two rolls per replication), totaling 400 seeds. Assessments of normal seedlings were carried out five and eight days after the test, as indicated in the Rules for Seed Testing (Brasil, 2009BRASIL. Ministério da Agricultura, Pecuária e Abastecimento. Regras para análise de sementes. Ministério da Agricultura, Pecuária e Abastecimento. Secretaria de Defesa Agropecuária. Brasília: MAPA/ACS, 2009. 399p. https://www.gov.br/agricultura/pt-br/assuntos/insumos-agropecuarios/arquivos-publicacoes-insumos/2946_regras_analise__sementes.pdf
https://www.gov.br/agricultura/pt-br/ass...
).
Determination of vigor by accelerated aging
A total of 400 seeds per treatment were used, arranged on a screen in plastic germination boxes (gerboxes) containing 40 mL of water without contact with the seeds, stored in an aging chamber with near saturation humidity and temperature monitored and maintained at 41 °C, for 48 hours (Marcos-Filho, 1999MARCOS-FILHO, J. Fisiologia de sementes de plantas cultivadas. 2 ed. Londrina: ABRATES, 2015. 660p.). The seeds were then sown and assessed, as described in the germination test.
Determination of vigor by the tetrazolium test
Four replications of 50 seeds were used. These were placed between two sheets of germitest paper, moistened at 2.5 times their weight, and kept at 25 °C for 16 hours (Brasil, 2009BRASIL. Ministério da Agricultura, Pecuária e Abastecimento. Regras para análise de sementes. Ministério da Agricultura, Pecuária e Abastecimento. Secretaria de Defesa Agropecuária. Brasília: MAPA/ACS, 2009. 399p. https://www.gov.br/agricultura/pt-br/assuntos/insumos-agropecuarios/arquivos-publicacoes-insumos/2946_regras_analise__sementes.pdf
https://www.gov.br/agricultura/pt-br/ass...
). After packaging, the seeds were submerged in a tetrazolium solution (0.075%) for 3 hours at 35-40 °C. The assessment was carried out according to the tissue color standards proposed by França-Neto and Krzyzanowski (2018FRANÇA-NETO, J.B.; KRZYZANOWSKI, F.C. Metodologia do teste de tetrazólio em sementes de soja. Londrina, 2018, 108p. ).
Determination of seed health by the blotter test
The seeds were arranged on sheets of moistened filter paper (blotting paper) in transparent plastic gerboxes, according to the Manual of Sanitary Seed Analysis (Brasil, 2009BRASIL. Ministério da Agricultura, Pecuária e Abastecimento. Regras para análise de sementes. Ministério da Agricultura, Pecuária e Abastecimento. Secretaria de Defesa Agropecuária. Brasília: MAPA/ACS, 2009. 399p. https://www.gov.br/agricultura/pt-br/assuntos/insumos-agropecuarios/arquivos-publicacoes-insumos/2946_regras_analise__sementes.pdf
https://www.gov.br/agricultura/pt-br/ass...
), with adaptations, described below. The paper and water used were sterilized in an oven at 120 °C for two hours and the boxes were sanitized with 1% sodium hypochlorite. The boxes were kept in a BOD chamber for 8 days, with a 12 hour photoperiod and temperature of 20 ± 2 °C. Assessments were made using a stereo microscope with 80 x magnification, with four replications of 100 seeds, and each replication consisted of five subsamples of 20 seeds per box (five boxes per replication), totaling 400 seeds.
Experimental design and data analysis
A completely randomized design was used. The results of germination and accelerated aging tests of each sample were compared using the 18.9 and 18.10 tolerance tables of the Rules for Seed Testing (Brasil, 2009BRASIL. Ministério da Agricultura, Pecuária e Abastecimento. Regras para análise de sementes. Ministério da Agricultura, Pecuária e Abastecimento. Secretaria de Defesa Agropecuária. Brasília: MAPA/ACS, 2009. 399p. https://www.gov.br/agricultura/pt-br/assuntos/insumos-agropecuarios/arquivos-publicacoes-insumos/2946_regras_analise__sementes.pdf
https://www.gov.br/agricultura/pt-br/ass...
). The results were transformed into √% arcsine. Analysis of variance was conducted and the means were compared by Tukey’s test at 5% probability. Data analysis was carried out in the R program (R Core Team, 2018R CORE TEAM. R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing, 2018.).
RESULTS AND DISCUSSION
The seed lots assessed exhibited a mean germination percentage of 91 and 90%, in the 2017/2018 and 2018/2019 growing seasons, respectively, irrespective of the sampling method (Table 2). Except for lot 1 (78% germination), sampled during processing in 2018/2019, the other lots showed a higher germination percentage than the minimum standard for soybean seed commercialization established by the Brazilian Ministry of Agriculture, Livestock and Supply (MAPA) in Ordinance no. 45 of 2013 (Brasil, 2013BRASIL. Instrução Normativa n° 45 de 17 de setembro de 2013. Diário Oficial da República Federativa do Brasil, Poder Executivo, Brasília, DF, 2013. 16p.), which is 80% for certified seeds and categories S1 and S2.
There were differences between sampling methods for lots 1 and 2, in the 2017/2018 growing season, and lots 1, 2, and 11 in 2018/2019 by Tukey test. According to RAS 10.18 tolerance table, lots 1 and 2 in the 2017/2018 growing season and lot 1 in 2018/2019 showed germination percentage out of tolerance.
However, the percentage that is out of tolerance according to RAS table 18.10 (Brasil, 2009BRASIL. Ministério da Agricultura, Pecuária e Abastecimento. Regras para análise de sementes. Ministério da Agricultura, Pecuária e Abastecimento. Secretaria de Defesa Agropecuária. Brasília: MAPA/ACS, 2009. 399p. https://www.gov.br/agricultura/pt-br/assuntos/insumos-agropecuarios/arquivos-publicacoes-insumos/2946_regras_analise__sementes.pdf
https://www.gov.br/agricultura/pt-br/ass...
) is 2% for lot 1 in the 2017/2018 growing season. For the other lots, the percentage out of tolerance is 1% (Table 2). We consider this percentage (2 and 1%) low and it would not be enough to differentiate the sampling methods. Although the Tukey test indicates a difference between the sampling methods for some lots, this difference can be considered small, due to the low coefficient of variation observed (3.6 and 2.9% for the 2017/2018 and 2018/2019 growing seasons, respectively). Thus, we consider that there are no differences between the sampling methods for seed germination.
The accelerated aging test to determine vigor showed differences between the sampling methods in lots 1, 4, and 5 in the 2017/2018 growing season and lots 7 and 8 in 2018/2019 according to the Tukey test (Table 3). For 2017/2018, lots 1 and 4 obtained a lower vigor percentage when the seeds were sampled during processing. On the other hand, for lot 5, lower vigor was observed when the seeds were sampled in the bag. In the 2017/2018 growing season, lot 7 showed lower vigor when the seeds were collected during processing, and lot 8 when sampled in the bag by Tukey test (Table 3).
The criteria used for the evaluation of seedlings in the vigor test are the same applied in the germination test, so the authors considered it pertinent to also apply RAS table 18.10. When applying table 18.10, it is observed that the vigor results for lots 1, 4, and 5 did not meet the established tolerance, in the 2017/2018 growing season. On the other hand, all lots met the RAS tolerance in the 2018/2019 growing season.
Even with differences between the methods for the result of vigor by accelerated aging, we emphasize that the sampling in the processing flow is more representative because a greater number of single samples are taken from the lot. We observed that the lower the vigor of the lots, the greater the differentiation between the sampling methods. In the 2017/2018 growing season, there was a difference between the sampling methods, a fact that was not observed in the 2018/2019 growing season, in which the vigor of all lots was higher.
The quality results verified in the 2018/2019 growing season are due to favorable climatic conditions for the development of plants in the field. A study carried out by Mathias and Coelho (2021MATHIAS, V.; COELHO, C.M.M. Correlation between vigor by accelerated aging at pre-sowing and soybean seedling emergence in the field. Semina: Ciências Agrárias , n.42, n.2, p.455-470, 2021. http://dx.doi.org/10.5433/1679-0359.2021v42n2p455
http://dx.doi.org/10.5433/1679-0359.2021...
), in the same growing season (2018/2019) and producing regions indicated that climatic conditions were favorable to produce quality seeds. In addition, even with differences, vigor is not an obligatory test for seed commercialization; in general, variations occurred due to the precision of the sampling method.
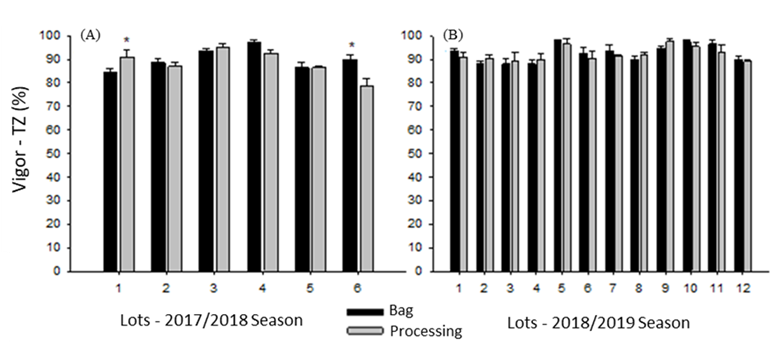
Assessment of vigor using the tetrazolium test indicated that only lots 1 and 6 from 2017/2018 exhibited differences between the sampling methods (Figure 1A).
Vigor by tetrazolium test (TZ) (A and B) in soybean seed lots sampled during processing and after storage in bags in the 2017/2018 and 2018/2019 growing seasons. *Indicates the difference between sampling methods according to Tukey’s test (p ≤ 0.05).
A health assessment was carried out to determine the effect of sampling on the incidence of the main pathogens associated with the seeds (Figure 2). There was no difference in the incidence of Fusarium spp. between sampling methods in the 2017/2018 growing season (Figure 2). On the other hand, in 2018/2019, differences were observed in lots 1, 2, 6, 9, 11, and 12 for this pathogen (Figures 2A and 2B). The highest incidence of Fusarium spp. was observed in samples collected from the bags.
Incidence of Fusarium spp. (A and B), Phomopsis sp. (C and D) and Cercosporora kikuchii (E and F) in soybean seed lots sampled during the processing and after storage in bags in the 2017/2018 and 2018/2019 growing seasons. *Indicates the difference between sampling methods according to Tukey’s test (p ≤ 0.05).
For Phomopsis sp., only samples collected during processing for lot 4 showed a higher incidence of this pathogen (2017/2018). For 2018/2019, the samples collected from bags exhibited a higher incidence, with the greatest differences observed in lots 8 and 11 (Figures 2C and 2D). For Cercospora kikuchii, a low incidence of this pathogen was observed in 2017/2018, and only the samples from lots 3 and 4 collected from bags obtained a higher pathogen incidence. However, in 2018/2019, the highest incidence was observed in samples collected during processing (Figures 2E and 2F).
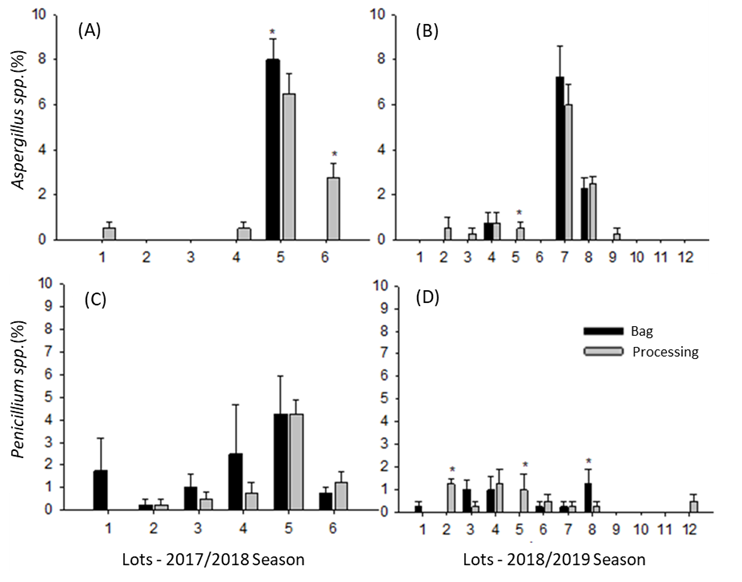
There was a low incidence of storage fungi (Penicillium sp. and Aspergillus spp.) in the growing seasons evaluated (Figure 3). In 2017/2018, Aspergillus spp. exhibited the highest incidence in lot 5 sampled in bags and lot 6 during processing. In 2018/2019, only lot 5, sampled during processing, showed a significant difference for this pathogen (Figures 3A and 3B). For Penicillium spp., only in 2018/2019 were differences observed in the incidence of this pathogen between sampling methods. Only lots 2 and 5 collected during processing displayed a higher incidence of Penicillium spp. For the samples collected in bags, only lot 8 obtained a higher incidence of this pathogen (Figures 3C and 3D).
Incidence of Aspergillus spp. (A and B) and Penicillium spp. (C and D) in soybean seed lots sampled during processing and after storage in bags in the 2017/2018 and 2018/2019 growing seasons. *Indicates the difference between sampling methods according to Tukey’s test (p ≤ 0.05).
Occurrences during the productive phase may cause disturbances and negatively affect potential seed performance. The physiological quality of seeds is related to climate stability in producing regions (Mathias et al., 2020MATHIAS, V.; COELHO, C.M.M.; ARALDI, C.G.; NERLING, D.; BORBA, P.; UARROTA, V.G. Characterization of the physiological quality of soybean seeds produced in Santa Catarina State. Semina: Ciencias Agrarias, n.41, n.1, p.49-60, 2020. http://dx.doi.org/10.5433/1679-0359.2020v41n1p49
http://dx.doi.org/10.5433/1679-0359.2020...
) and cultivation practices that directly affect seed performance and their rate of deterioration (Marcos-Filho, 2015MARCOS-FILHO, J. Fisiologia de sementes de plantas cultivadas. 2 ed. Londrina: ABRATES, 2015. 660p.). Seeds with greater vigor exhibit better performance and a high potential for establishing crop stands (Mathias et al., 2020MATHIAS, V.; COELHO, C.M.M.; ARALDI, C.G.; NERLING, D.; BORBA, P.; UARROTA, V.G. Characterization of the physiological quality of soybean seeds produced in Santa Catarina State. Semina: Ciencias Agrarias, n.41, n.1, p.49-60, 2020. http://dx.doi.org/10.5433/1679-0359.2020v41n1p49
http://dx.doi.org/10.5433/1679-0359.2020...
). Mechanical damage, moisture-related degradation, and stink bug incidence, in addition to harvesting, drying, processing, storing, and pathogens, may compromise the physiological quality of seeds (Galli et al., 2007GALLI, J., PANIZI, R., VIEIRA, R. Sobrevivência de patógenos associados a sementes de soja armazenadas durante seis meses. Revista Brasileira de Sementes, v.29, n.2, p.205-213, 2007. https://doi.org/10.1590/S0101-31222007000200027
https://doi.org/10.1590/S0101-3122200700...
; França-Neto et al., 2016FRANÇA-NETO, J.B.; KRZYZANOWSKI, F.C.; HENNING, A.A.; PÁDUA, G.P.; LORINI, I.; HENNING, F.A. Tecnologia da produção de semente de soja de alta qualidade. Londrina, 2016, 82p. ). All these factors may cause a variation in seed quality and lot heterogeneity. Lot heterogeneity may have caused the difference between sampling methods, given that the seeds from the original lot may have been produced in different fields and therefore subjected to the different soil, climate, and management factors.
The checking of lot heterogeneity by the H test, recommended by the RAS, was not performed, because at the time of sampling we assumed the lots were homogeneous. Furthermore, according to the RAS, this method is impractical as a routine analysis and should not be used for any characteristic whose occurrence is close to zero or 100% (Brasil, 2009BRASIL. Ministério da Agricultura, Pecuária e Abastecimento. Regras para análise de sementes. Ministério da Agricultura, Pecuária e Abastecimento. Secretaria de Defesa Agropecuária. Brasília: MAPA/ACS, 2009. 399p. https://www.gov.br/agricultura/pt-br/assuntos/insumos-agropecuarios/arquivos-publicacoes-insumos/2946_regras_analise__sementes.pdf
https://www.gov.br/agricultura/pt-br/ass...
). The small variation observed in the results indicates that it would not be necessary to apply the H test in this work.
PCA was applied to identify similarities between sampling methods and seed quality (Figure 4). The total variance explained by the two principal components (PC) was 61.2% (Figure 4). PC1 represented 40.9% of the total variance, with normal, dead, and abnormal seedlings in accelerated aging contributing the most to this component. PC2 accounted for 20.3% of the variance, with abnormal and normal seedlings in germination and Fusarium sp. contributing the most to this component (Figure 4). PCA indicates that sampling during processing and in the bags after storage did not interfere with the results obtained in the seed physiological and health analyses. PCA indicated a strong correlation between the presence of pathogens and the decline in physiological performance. There was a correlation between the presence of Aspergillus spp., Penicillium sp., and bacteria and abnormal seedlings in accelerated aging and dead seeds in germination and vigor tests. On the other hand, Fusarium spp. and Phomopsis sp. exhibited the highest correlation with abnormal seedlings in germination. The relation between normal seedlings and pathogens was negative, indicating that when the pathogen incidence increases, the number of normal seedlings declines in the germination and vigor tests using accelerated aging. When pathogen incidence interferes with the standard germination test results, alternative methods can be used, such as sand, seed treatment, or increasing the spacing between seeds.
Principal components analysis (PCA) of the health and physiological quality of soybean seed lots collected during processing (PF) and after storage in bags (BB) in 2017/2018 and 2018/2019 growing season. PC1 First principal component; PC2 Second principal component.
The presence of pathogens negatively affected the results of germination, viability, and seed weight when soybean cultivars were inoculated with Fusarium graminearum (Peruzzo et al., 2015PERUZZO, A.; PIOLI, R.; SALINAS, A. Effect of Fusarium graminearum Schwabe on physiological quality of soybean seeds and wheat caryopsis in Argentina. Revista Caatinga, v.28, n.3, p.1-11, 2015. https://doi.org/10.1590/1983-21252015v28n301rc
https://doi.org/10.1590/1983-21252015v28...
). A negative correlation was also observed between the incidence of Phomopsis sp. and seed viability (Raeisi et al., 2011RAEISI, S.; PUTEH, A.; SIJAM, K.; PSYQUAY A.N. Seed Quality of soybean in relation to Phomopsis seed decay in Malaysia. Asian Journal of Plant Pathology, v.5, n.1, p.28-36, 2011. https://scialert.net/abstract/?doi=ajppaj.2011.28.36
https://scialert.net/abstract/?doi=ajppa...
). The presence of Aspergillus spp. with inoculum levels of more than 50% on the seed surface reduced seed vigor (Rocha et al., 2014ROCHA, F.S.; CATÃO, H.C.R.M.; BRANDÃO A.A.; GOMES, L.A.A. Damage caused by different inoculum levels of Aspergillus ochraceus on the vigour of soybean seeds. Semina: Ciências Agrárias , v.35, n.6, p.2895-2904, 2014. http://dx.doi.org/10.5433/1679-0359.2014v35n6p2895
http://dx.doi.org/10.5433/1679-0359.2014...
). These fungi interfere in several essential physiological processes, destroying reserve organs or young tissues and damaging the seedling root or vascular system, thereby affecting water and nutrient absorption and transport, respectively.
Sampling methods can be used during processing to obtain soybean samples aimed at assessing seed quality, irrespective of cultivar or category. In this study, seed lots from different producing regions, different cultivars, and categories were evaluated (Table 1). For seed producers, sampling during processing makes it possible to collect a larger number of single samples, being more representative of the lot. The number of single samples collected during the processing flow is higher because single samples are taken from all bags at regular intervals during filling. This method also requires less time to obtain the average sample, thereby preventing the sampler from physically damaging the packaging, contributing indirectly to maintaining seed quality during storage, since damaged packages could expose the seeds to greater environmental variations and rodent attacks. Concerning inspection, the sampling procedure using samplers, as is typically done, does not interfere in germination analyses because there are no differences in results between the two methods. Studies need to be carried out with more lots, different flow sampling systems, and vigor levels.
CONCLUSIONS
There is a difference between sampling in the processing flow and with the use of samplers; however, sampling in the processing flow is more representative and may be indicated for the germination test.
ACKNOWLEDGMENTS
The authors would like to thank the PAP/UDESC/FAPESC for the financial support. The corresponding author (Coelho, C.M.M) thanks Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for productivity scholarship. The authors would like to thank the availability of seeds in the cooperatives: Cooperativa Regional Alfa (Cooperalfa); Cooperativa Regional Agropecuária de Campos Novos (Copercampos); Cooperativa Agropecuária do Celeiro Catarinense (Coperacel). This article is part of Folquini P.’s Master’s dissertation.
REFERENCES
- BRASIL. Instrução Normativa n° 45 de 17 de setembro de 2013 Diário Oficial da República Federativa do Brasil, Poder Executivo, Brasília, DF, 2013. 16p.
- BRASIL. Ministério da Agricultura, Pecuária e Abastecimento. Regras para análise de sementes Ministério da Agricultura, Pecuária e Abastecimento. Secretaria de Defesa Agropecuária. Brasília: MAPA/ACS, 2009. 399p. https://www.gov.br/agricultura/pt-br/assuntos/insumos-agropecuarios/arquivos-publicacoes-insumos/2946_regras_analise__sementes.pdf
» https://www.gov.br/agricultura/pt-br/assuntos/insumos-agropecuarios/arquivos-publicacoes-insumos/2946_regras_analise__sementes.pdf - BRASIL. Instrução Normativa n° 09 de 02 de junho de 2005 Diário Oficial da República Federativa do Brasil , Poder Executivo, Brasília, DF, 2005. 16p.
- CASTRO, O.O. Amostragem é decisiva na busca da qualidade. Seed News, n.1, p.1-5, 2005 https://seednews.com.br/artigos/1032-amostragem-e-decisiva-na-busca-da-qualidade-edicao-janeiro-2005
» https://seednews.com.br/artigos/1032-amostragem-e-decisiva-na-busca-da-qualidade-edicao-janeiro-2005 - COELHO, C.; ZILIO, M.; SOUZA, C.; GUIDOLIN, A.; MIQUELLUTI, D. Características morfo-agronômicas de cultivares crioulas de feijão comum em dois anos de cultivo. Semina: Ciências Agrárias, v.31, p.1177-1186, 2010. http://dx.doi.org/10.5433/1679-0359.2010v31n4Sup1p1177
» http://dx.doi.org/10.5433/1679-0359.2010v31n4Sup1p1177 - FRANÇA-NETO, J.B.; KRZYZANOWSKI, F.C. Metodologia do teste de tetrazólio em sementes de soja Londrina, 2018, 108p.
- FRANÇA-NETO, J.B.; KRZYZANOWSKI, F.C.; HENNING, A.A.; PÁDUA, G.P.; LORINI, I.; HENNING, F.A. Tecnologia da produção de semente de soja de alta qualidade Londrina, 2016, 82p.
- GALLI, J., PANIZI, R., VIEIRA, R. Sobrevivência de patógenos associados a sementes de soja armazenadas durante seis meses. Revista Brasileira de Sementes, v.29, n.2, p.205-213, 2007. https://doi.org/10.1590/S0101-31222007000200027
» https://doi.org/10.1590/S0101-31222007000200027 - KRZYZANOWSKI, F.C.; FRANÇA-NETO, J.B.; HENNING, A.A. A alta qualidade da semente de soja: fator importante para a produção da cultura Londrina, 2018, 24p.
- MARCOS-FILHO, J. Fisiologia de sementes de plantas cultivadas 2 ed. Londrina: ABRATES, 2015. 660p.
- MATHIAS, V.; COELHO, C.M.M. Correlation between vigor by accelerated aging at pre-sowing and soybean seedling emergence in the field. Semina: Ciências Agrárias , n.42, n.2, p.455-470, 2021. http://dx.doi.org/10.5433/1679-0359.2021v42n2p455
» http://dx.doi.org/10.5433/1679-0359.2021v42n2p455 - MATHIAS, V.; COELHO, C.M.M.; ARALDI, C.G.; NERLING, D.; BORBA, P.; UARROTA, V.G. Characterization of the physiological quality of soybean seeds produced in Santa Catarina State. Semina: Ciencias Agrarias, n.41, n.1, p.49-60, 2020. http://dx.doi.org/10.5433/1679-0359.2020v41n1p49
» http://dx.doi.org/10.5433/1679-0359.2020v41n1p49 - PERUZZO, A.; PIOLI, R.; SALINAS, A. Effect of Fusarium graminearum Schwabe on physiological quality of soybean seeds and wheat caryopsis in Argentina. Revista Caatinga, v.28, n.3, p.1-11, 2015. https://doi.org/10.1590/1983-21252015v28n301rc
» https://doi.org/10.1590/1983-21252015v28n301rc - R CORE TEAM. R: A language and environment for statistical computing Vienna: R Foundation for Statistical Computing, 2018.
- RAEISI, S.; PUTEH, A.; SIJAM, K.; PSYQUAY A.N. Seed Quality of soybean in relation to Phomopsis seed decay in Malaysia. Asian Journal of Plant Pathology, v.5, n.1, p.28-36, 2011. https://scialert.net/abstract/?doi=ajppaj.2011.28.36
» https://scialert.net/abstract/?doi=ajppaj.2011.28.36 - ROCHA, F.S.; CATÃO, H.C.R.M.; BRANDÃO A.A.; GOMES, L.A.A. Damage caused by different inoculum levels of Aspergillus ochraceus on the vigour of soybean seeds. Semina: Ciências Agrárias , v.35, n.6, p.2895-2904, 2014. http://dx.doi.org/10.5433/1679-0359.2014v35n6p2895
» http://dx.doi.org/10.5433/1679-0359.2014v35n6p2895
Publication Dates
-
Publication in this collection
22 Apr 2022 -
Date of issue
2022
History
-
Received
28 Dec 2021 -
Accepted
23 Feb 2022