Abstract
Objective
To investigate the association of the rs10885122G>T polymorphism in the ADRA2A gene in a Euro-Brazilian sample of healthy (controls) and type 2 diabetic (T2D) subjects.
Subjects and methods
We used fluorescent probes (TaqMan) to genotype 241 subjects, that is, 121 healthy and 120 T2D subjects, who were classified based on the Brazilian Diabetes Association (2013) and American Diabetes Association (2014) criteria.
Results
The genotype and allele frequencies showed no significant (P > 0.05) difference between the two studied groups. The minor allele (T) frequencies (95%CI) for rs10885122 were 19% (14-24%) and 20% (15-26%) for healthy and T2D groups, respectively. Carriers of the T allele (genotypes GT+TT) were significantly associated (P = 0.016) with approximately a 7-kg body weight reduction compared with the genotype GG, which was only found in the T2D group.
Conclusion
The rs10885122G>T polymorphism of the ADRA2A gene was not associated with T2D in Euro-Brazilians, and carriers of the T allele had lower body weight in the presence of T2D. Arch Endocrinol Metab. 2015;59(1):29-33
Type 2 diabetes mellitus; ADRA2A polymorphism; case-controlled study; SNP
INTRODUCTION
Type 2 diabetes mellitus (T2D) accounts for 90-95% of those with
diabetes mellitus (DM, diabetes) and is characterized by
insulin resistance and relative insulin deficiency (11 ADA. Diagnosis and classification of diabetes mellitus. Diabetes
Care. 2014;37 Suppl 1:S81-90.,22 SBD. Diretrizes da Sociedade Brasileira de Diabetes 2012–2013.
Barueri, São Paulo: Guanabara Koogan; 2013. p. 388.). T2D is a polygenic disease,
and several single nucleotide polymorphisms (SNPs) have been associated with risk or
protection regarding this disease and its complications (33 Malandrino N, Smith RJ. Personalized medicine in diabetes. Clin
Chem. 2011;57(2):231-40.
4 Marchetti P, Syed F, Suleiman M, Bugliani M, Marselli L. From
genotype to human beta cell phenotype and beyond. Islets.
2012;4(5):323-32.-55 Scheen AJ, Paquot N. [Type 2 diabetes: journey in the heart of a
complex disease]. Rev Med Liege. 2012;67(5-6):326-31.).
The effects of norepinephrine on pancreatic b-cells are primarily mediated by
adrenoceptor alpha 2A, which is encoded by the ADRA2A gene (HGNC:
281; OMIM: 104210) (66 Straub SG, Sharp GW. Evolving insights regarding mechanisms for the
inhibition of insulin release by norepinephrine and heterotrimeric G proteins.
Am J Physiol Cell Physiol. 2012;302(12):C1687-98.). Since norepinephrine
inhibits both of the major pathways by which glucose induces biphasic insulin
secretion and adrenoceptor alpha 2A is the responsible for these effects, genetic
variations of the ADRA2A gene may be candidates for T2D
susceptibility (77 Rodriguez-Pena MS, Collins R, Woodard C, Spiegel AM. Decreased
insulin content and secretion in RIN 1046-38 cells overexpressing alpha
2-adrenergic receptors. Endocrine. 1997;7(2):255-60.
8 Sharp GW. Mechanisms of inhibition of insulin release. Am J Physiol.
1996;271(6 Pt 1):C1781-99.
9 Straub SG, Sharp GW. Glucose-stimulated signaling pathways in
biphasic insulin secretion. Diabetes Metab Res Rev.
2002;18(6):451-63.
10 Angel I, Niddam R, Langer SZ. Involvement of alpha-2 adrenergic
receptor subtypes in hyperglycemia. J Pharmacol Exp Ther.
1990;254(3):877-82.
11 Niddam R, Angel I, Bidet S, Langer SZ. Pharmacological
characterization of alpha-2 adrenergic receptor subtype involved in the release
of insulin from isolated rat pancreatic islets. J Pharmacol Exp Ther.
1990;254(3):883-7.-1212 Peterhoff M, Sieg A, Brede M, Chao CM, Hein L, Ullrich S. Inhibition
of insulin secretion via distinct signaling pathways in alpha2-adrenoceptor
knockout mice. Eur J Endocrinol. 2003;149(4):343-50.).
Several genetic variations in the human ADRA2A gene or in its
flanking region are related to decreased insulin secretion and increased T2D risk
(1313 Michel MC, Plogmann C, Philipp T, Brodde OE. Functional correlates
of alpha(2A)-adrenoceptor gene polymorphism in the HANE study. Nephrology,
Dialysis, Transplantation. 1999;14(11):2657-63.
14 Boesgaard TW, Grarup N, Jorgensen T, Borch-Johnsen K, Hansen T,
Pedersen O. Variants at DGKB/TMEM195, ADRA2A, GLIS3 and C2CD4B loci are
associated with reduced glucose-stimulated beta cell function in middle-aged
Danish people. Diabetologia. 2010;53(8):1647-55.-1515 Rosengren AH, Jokubka R, Tojjar D, Granhall C, Hansson O, Li DQ, et
al. Overexpression of alpha2A-adrenergic receptors contributes to type 2
diabetes. Science. 2010;327(5962):217-20.). Rosengren and cols. (1515 Rosengren AH, Jokubka R, Tojjar D, Granhall C, Hansson O, Li DQ, et
al. Overexpression of alpha2A-adrenergic receptors contributes to type 2
diabetes. Science. 2010;327(5962):217-20.),
using a tagging SNP approach, identified several polymorphisms in the coding or
flanking regions of the ADRA2A gene. In particular, a SNP,
rs10885122, which is located 0.2 Mb from the ADRA2A gene in a
non-coding DNA region, was associated with different regulatory expression of nearby
or distant genes (1616 Billings LK, Florez JC. The genetics of type 2 diabetes: what have
we learned from GWAS? Ann N Y Acad Sci. 2010;1212:59-77.
17 Dupuis J, Langenberg C, Prokopenko I, Saxena R, Soranzo N, Jackson
AU, et al. New genetic loci implicated in fasting glucose homeostasis and their
impact on type 2 diabetes risk. Nat Genet. 2010;42(2):105-16.-1818 Hindorff LA, Sethupathy P, Junkins HA, Ramos EM, Mehta JP, Collins
FS, et al. Potential etiologic and functional implications of genome-wide
association loci for human diseases and traits. Proc Natl Acad Sci U S A.
2009;106(23):9362-7.). The rs10885122 polymorphism was identified as associated
with fasting glucose level and reduced glucose-stimulated insulin release, but not
with T2D (1414 Boesgaard TW, Grarup N, Jorgensen T, Borch-Johnsen K, Hansen T,
Pedersen O. Variants at DGKB/TMEM195, ADRA2A, GLIS3 and C2CD4B loci are
associated with reduced glucose-stimulated beta cell function in middle-aged
Danish people. Diabetologia. 2010;53(8):1647-55.,1717 Dupuis J, Langenberg C, Prokopenko I, Saxena R, Soranzo N, Jackson
AU, et al. New genetic loci implicated in fasting glucose homeostasis and their
impact on type 2 diabetes risk. Nat Genet. 2010;42(2):105-16.,1919 Talmud PJ, Cooper JA, Gaunt T, Holmes MV, Shah S, Palmen J, et al.
Variants of ADRA2A are associated with fasting glucose, blood pressure, body
mass index and type 2 diabetes risk: meta-analysis of four prospective studies.
Diabetologia. 2011;54(7):1710-9.).
In this study, we investigated the association of the rs10885122 polymorphism of the ADRA2A gene in a sample of Euro-Brazilians with and without T2D.
SUBJECTS AND METHODS
Subjects
A total of 241 unrelated Euro-Brazilian subjects, matched by sex, were investigated. Healthy control (n = 121) and T2D (n = 120) subjects were classified according to the criteria of the American Diabetes Association 2014 (ADA) and Brazilian Diabetes Association 2013 (SBD) (11 ADA. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37 Suppl 1:S81-90.,22 SBD. Diretrizes da Sociedade Brasileira de Diabetes 2012–2013. Barueri, São Paulo: Guanabara Koogan; 2013. p. 388.). The control group was recruited among the patients of the Clinical Hospital of Federal University of Parana (HC-UFPR) blood bank in Curitiba City, Parana State, Brazil. T2D patients were recruited from HC-UFPR. Subjects with overt kidney disease or other severe diabetic complications were excluded from this study.
The Federal University of Parana Ethics Committee approved this research (CAAE 05335612.4.0000.0102).
Laboratory data and genotyping
Biochemical parameters were determined by routine laboratory methods (Abbott Diagnostics) in an automated system with reagents, calibrators, and controls provided by the manufacturer (Architect Ci8200, Abbott Diagnostics). The 1,5-anhydroglucitol level was measured enzymatically (GlycoMark, Inc). Glycated hemoglobin was measured by immunoturbidimetry (Architect, Abbott Diagnostics).
DNA from blood samples was extracted using a salting out technique (2020 Barroso I. Genetics of Type 2 diabetes. Diabet Med. 2005;22(5):517-35.), and the concentrations were normalized to 20 ng/µL for the assays (NanoDrop, ThermoScientific). Only DNA samples with absorbance ratios (280/260) between 1.8 to 2.0 were used in this study. The rs10885122 polymorphism was genotyped using fluorescent probes (TaqMan®, Life Technologies; code C_175459_10) in the real-time PCR StepOnePlus™ System (Life Technologies) with all reagents supplied by Life Technologies. The reaction mixture (6 µL final volume) contained 3.0 µL of Master Mix (DNA polymerase, Mg2+, buffer, additives), 0.3 µL of SNP Genotyping Assay (40X), 1.7 µL ultra-pure water, and 1.0 µL of genomic DNA (20 ng/µL). The cycle sequencing conditions were: 1 cycle of 30 s at 60ºC (pre-PCR), 1 cycle for 10 min at 95ºC, and 55 cycles of 15 s at 95ºC, followed by 60ºC for 60 s, and 1 final cycle of 30 s at 60ºC (final extension). All genotypes were analyzed by the StepOnePlus software (TaqMan® Genotyper Software version 1.0, Life Technologies) and using a minimum quality threshold of 95% in all analyses.
Statistical analysis
Normality was tested with the Kolmogorov-Smirnov test. The comparison of parameters with normal distribution was performed by the Student’s t-test for independent samples or Mann-Whitney U test for data with a non-normal distribution. Categorical variables were compared by chi-square test. Allele frequencies and Hardy-Weinberg equilibrium (HW) were evaluated by chi-square test (http://ihg.gsf.de/cgi-bin/hw/hwa1.pl).
Statistical analyses were performed with the software Statistica for windows version 8.0 (StatSoft Inc, Tulsa, OK, USA). A probability lower than 5% (P < 0.05) was considered significant.
RESULTS
The anthropometric and clinical characteristics of the studied subjects are shown in table 1. The T2D showed high frequency of family history for diabetes (61.7%) and hypertension (66%). The T2D subjects were older, heavier, and more hypertensive than the control subjects. The mean values for HbA1C (7.9%) and median value for 1,5-anhydroglucitol (8.5 µg/mL) demonstrated that the T2D group showed poor glycemic control. The lipid profile for the T2D group showed serum concentrations similar to triglycerides and total cholesterol, as well as HDL-cholesterol and LDL-cholesterol levels that were significantly lower than those in the healthy subjects. This pattern was expected since antilipemic drugs are commonly used, particularly in high doses, in T2D patients. Kidney function, which was monitored by creatinine and urea, did not show any indication of overt kidney disease, since the included subjects are in the reference range for these parameters. However, albumin was significantly reduced in the T2D group but without clinical significance.
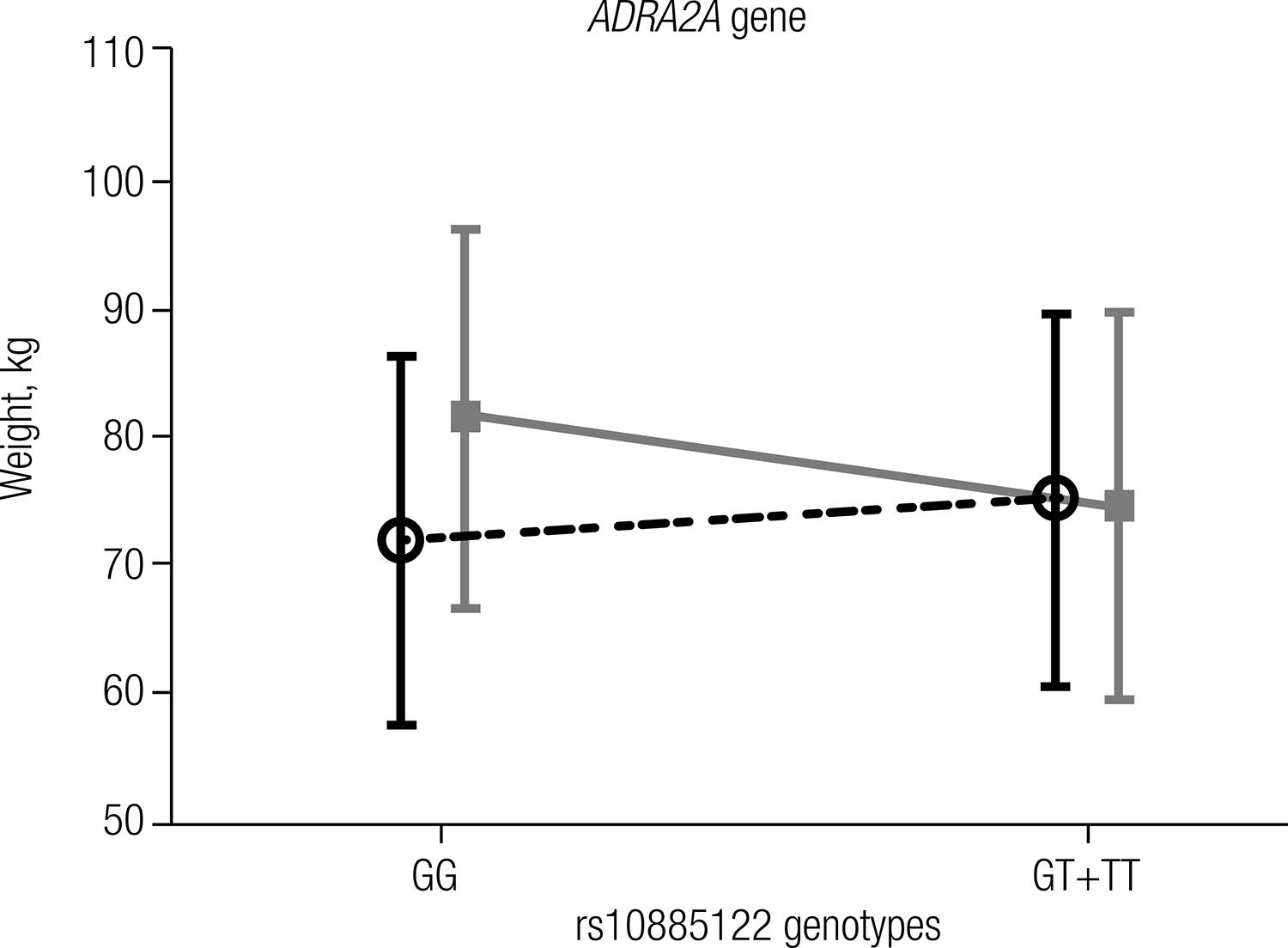
Both studied groups were in Hardy-Weinberg equilibrium (P > 0.05), and comparisons of genotype (P = 0.891) and allele (P = 0.698) frequencies showed no significant differences between the groups (Table 2). A positive and significant correlation between weight and the genotypes of rs10885122 was observed only in the T2D group (r = 0.277; P = 0.006). The association of the rs10885122 polymorphism of the ADRA2A genotypes in the dominant model (GG vs. GT+TT) with weight is shown in figure 1. The genotypes with the T allele (GT+TT) were significantly associated (P = 0.016) with weight reduction by approximately 7 kg compared with the genotype GG, which was only found in the T2D group.
DISCUSSION
T2D is a burdensome epidemic that is highly associated with genetic susceptibility (2020 Barroso I. Genetics of Type 2 diabetes. Diabet Med. 2005;22(5):517-35.). Few studies were available that compared the association of SNPs with T2D in the Brazilian population. In our study, we revealed that the characteristics of the T2D group indicated poor glycemic control, as demonstrated by the concentration of the biomarkers HbA1C (> 7%) and 1,5-anhydroglucitol (< 10 µg/mL) (2121 Kim WJ, Park CY. 1,5-Anhydroglucitol in diabetes mellitus. Endocrine. 2013;43(1):33-40.,2222 Viana LV, Leitao CB, Kramer CK, Zucatti AT, Jezini DL, Felicio J, et al. Poor glycaemic control in Brazilian patients with type 2 diabetes attending the public healthcare system: a cross-sectional study. BMJ Open. 2013;3(9):e003336.). In addition, the minor but significant reduction in serum albumin concentration associated with high frequency of hypertension in the T2D group could suggest an increase in albumin loss (microalbuminuria) by the kidney (2323 UKPDS. UK Prospective Diabetes Study (UKPDS). X. Urinary albumin excretion over 3 years in diet-treated type 2, (non-insulin-dependent) diabetic patients, and association with hypertension, hyperglycaemia and hypertriglyceridaemia. Diabetologia. 1993;36(10):1021-9.,2424 Nelson RG, Bennett PH, Beck GJ, Tan M, Knowler WC, Mitch WE, et al. Development and progression of renal disease in Pima Indians with non-insulin-dependent diabetes mellitus. Diabetic Renal Disease Study Group. N Engl J Med. 1996;335(22):1636-42.).
Polymorphisms of the ADRA2A gene have been primarily studied by meta-analysis (2525 Chen X, Liu L, He W, Lu Y, Ma D, Du T, et al. Association of the ADRA2A polymorphisms with the risk of type 2 diabetes: A meta-analysis. Clin Biochem. 2013;46(9):722-6.). The G allele of the ADRA2A rs10885122 polymorphism was described as a risk factor associated with higher fasting glucose and reduced insulin secretion in non-diabetic subjects (1414 Boesgaard TW, Grarup N, Jorgensen T, Borch-Johnsen K, Hansen T, Pedersen O. Variants at DGKB/TMEM195, ADRA2A, GLIS3 and C2CD4B loci are associated with reduced glucose-stimulated beta cell function in middle-aged Danish people. Diabetologia. 2010;53(8):1647-55.,1717 Dupuis J, Langenberg C, Prokopenko I, Saxena R, Soranzo N, Jackson AU, et al. New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat Genet. 2010;42(2):105-16.,2626 Wagner R, Dudziak K, Herzberg-Schafer SA, Machicao F, Stefan N, Staiger H, et al. Glucose-raising genetic variants in MADD and ADCY5 impair conversion of proinsulin to insulin. PloS One. 2011;6(8):e23639.,2727 Renstrom F, Shungin D, Johansson I, Florez JC, Hallmans G, Hu FB, et al. Genetic predisposition to long-term nondiabetic deteriorations in glucose homeostasis: Ten-year follow-up of the GLACIER study. Diabetes. 2011;60(1):345-54.). In contrast, others studies did not find an association of this polymorphism with T2D in European Caucasians (1717 Dupuis J, Langenberg C, Prokopenko I, Saxena R, Soranzo N, Jackson AU, et al. New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat Genet. 2010;42(2):105-16.,1919 Talmud PJ, Cooper JA, Gaunt T, Holmes MV, Shah S, Palmen J, et al. Variants of ADRA2A are associated with fasting glucose, blood pressure, body mass index and type 2 diabetes risk: meta-analysis of four prospective studies. Diabetologia. 2011;54(7):1710-9.,2525 Chen X, Liu L, He W, Lu Y, Ma D, Du T, et al. Association of the ADRA2A polymorphisms with the risk of type 2 diabetes: A meta-analysis. Clin Biochem. 2013;46(9):722-6.), Japanese (2828 Fujita H, Hara K, Shojima N, Horikoshi M, Iwata M, Hirota Y, et al. Variations with modest effects have an important role in the genetic background of type 2 diabetes and diabetes-related traits. J Hum Genet. 2012;57(12):776-9.), Chinese (2525 Chen X, Liu L, He W, Lu Y, Ma D, Du T, et al. Association of the ADRA2A polymorphisms with the risk of type 2 diabetes: A meta-analysis. Clin Biochem. 2013;46(9):722-6.), and several other ethnicities (2929 Florez JC, Jablonski KA, McAteer JB, Franks PW, Mason CC, Mather K, et al. Effects of genetic variants previously associated with fasting glucose and insulin in the Diabetes Prevention Program. PloS One. 2012;7(9):e44424.). We showed that the ADRA2A rs10885122 SNP in the Euro-Brazilian population was not associated with T2D. The minor allele frequencies (T allele) of rs10885122 observed in this study were similar (close to the 95%CI) to those of different ethnicities that are shown in table 2. Chinese and African Americans showed significantly lower and higher T allele frequencies, respectively (Table 2).
The lack of consistent association of the ADRA2A variant with glycemic traits in the present report is similar to the results of others studies, including studies by Talmud and cols. (1919 Talmud PJ, Cooper JA, Gaunt T, Holmes MV, Shah S, Palmen J, et al. Variants of ADRA2A are associated with fasting glucose, blood pressure, body mass index and type 2 diabetes risk: meta-analysis of four prospective studies. Diabetologia. 2011;54(7):1710-9.) involving 1,307 diabetic subjects and by Florez and cols. (2929 Florez JC, Jablonski KA, McAteer JB, Franks PW, Mason CC, Mather K, et al. Effects of genetic variants previously associated with fasting glucose and insulin in the Diabetes Prevention Program. PloS One. 2012;7(9):e44424.) involving 1,307 subjects with prediabetes. In addition, the meta-analysis of glucose and insulin-related traits consortium (MAGIC) study (1717 Dupuis J, Langenberg C, Prokopenko I, Saxena R, Soranzo N, Jackson AU, et al. New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat Genet. 2010;42(2):105-16.) and four UK studies (1919 Talmud PJ, Cooper JA, Gaunt T, Holmes MV, Shah S, Palmen J, et al. Variants of ADRA2A are associated with fasting glucose, blood pressure, body mass index and type 2 diabetes risk: meta-analysis of four prospective studies. Diabetologia. 2011;54(7):1710-9.), comprised of over 118,000 and 17,000 individuals, respectively, also did not find an association of rs10885122 with T2D. Although, one of the limitations of our study was the relatively small sample size, our results were comparable and consistent with these works.
Dupuis and cols. (1717 Dupuis J, Langenberg C, Prokopenko I, Saxena R, Soranzo N, Jackson AU, et al. New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat Genet. 2010;42(2):105-16.) showed an association of the rs10885122 T allele with lower fasting glucose in subjects with normal glucose tolerance. On the other hand, the same authors found an association with higher fasting glucose concentration for the G allele of this polymorphism, and Boesgaard and cols. (1414 Boesgaard TW, Grarup N, Jorgensen T, Borch-Johnsen K, Hansen T, Pedersen O. Variants at DGKB/TMEM195, ADRA2A, GLIS3 and C2CD4B loci are associated with reduced glucose-stimulated beta cell function in middle-aged Danish people. Diabetologia. 2010;53(8):1647-55.) described a reduction in glucose-stimulated insulin release for this allele. In our study, the T allele was associated with a reduction of weight in the T2D group (Figure 1). We hypothesized that T2D patients that are carriers of the T allele could have better weight control and consequently improved glycemic control, which is consistent with the findings of Dupuis and cols. (1717 Dupuis J, Langenberg C, Prokopenko I, Saxena R, Soranzo N, Jackson AU, et al. New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat Genet. 2010;42(2):105-16.). The mechanisms that explain the effects of the rs10885122 SNP of the ADRA2A gene on weight or glucose concentration are currently unknown.
In conclusion, the rs10885122 polymorphism of the ADRA2A gene was not associated with T2D in the studied Euro-Brazilian population. T2D patients that are carriers of the T allele of rs10885122 was significantly associated with lower weight compared with those who carried the G allele.
Acknowledgments
we would like to thank Dr. M. G. Yates for reading the manuscript and for his suggestions. CNPq and Araucaria Foundation supported this project. No potential conflicts of interest relevant to this article were reported.
REFERENCES
-
1ADA. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37 Suppl 1:S81-90.
-
2SBD. Diretrizes da Sociedade Brasileira de Diabetes 2012–2013. Barueri, São Paulo: Guanabara Koogan; 2013. p. 388.
-
3Malandrino N, Smith RJ. Personalized medicine in diabetes. Clin Chem. 2011;57(2):231-40.
-
4Marchetti P, Syed F, Suleiman M, Bugliani M, Marselli L. From genotype to human beta cell phenotype and beyond. Islets. 2012;4(5):323-32.
-
5Scheen AJ, Paquot N. [Type 2 diabetes: journey in the heart of a complex disease]. Rev Med Liege. 2012;67(5-6):326-31.
-
6Straub SG, Sharp GW. Evolving insights regarding mechanisms for the inhibition of insulin release by norepinephrine and heterotrimeric G proteins. Am J Physiol Cell Physiol. 2012;302(12):C1687-98.
-
7Rodriguez-Pena MS, Collins R, Woodard C, Spiegel AM. Decreased insulin content and secretion in RIN 1046-38 cells overexpressing alpha 2-adrenergic receptors. Endocrine. 1997;7(2):255-60.
-
8Sharp GW. Mechanisms of inhibition of insulin release. Am J Physiol. 1996;271(6 Pt 1):C1781-99.
-
9Straub SG, Sharp GW. Glucose-stimulated signaling pathways in biphasic insulin secretion. Diabetes Metab Res Rev. 2002;18(6):451-63.
-
10Angel I, Niddam R, Langer SZ. Involvement of alpha-2 adrenergic receptor subtypes in hyperglycemia. J Pharmacol Exp Ther. 1990;254(3):877-82.
-
11Niddam R, Angel I, Bidet S, Langer SZ. Pharmacological characterization of alpha-2 adrenergic receptor subtype involved in the release of insulin from isolated rat pancreatic islets. J Pharmacol Exp Ther. 1990;254(3):883-7.
-
12Peterhoff M, Sieg A, Brede M, Chao CM, Hein L, Ullrich S. Inhibition of insulin secretion via distinct signaling pathways in alpha2-adrenoceptor knockout mice. Eur J Endocrinol. 2003;149(4):343-50.
-
13Michel MC, Plogmann C, Philipp T, Brodde OE. Functional correlates of alpha(2A)-adrenoceptor gene polymorphism in the HANE study. Nephrology, Dialysis, Transplantation. 1999;14(11):2657-63.
-
14Boesgaard TW, Grarup N, Jorgensen T, Borch-Johnsen K, Hansen T, Pedersen O. Variants at DGKB/TMEM195, ADRA2A, GLIS3 and C2CD4B loci are associated with reduced glucose-stimulated beta cell function in middle-aged Danish people. Diabetologia. 2010;53(8):1647-55.
-
15Rosengren AH, Jokubka R, Tojjar D, Granhall C, Hansson O, Li DQ, et al. Overexpression of alpha2A-adrenergic receptors contributes to type 2 diabetes. Science. 2010;327(5962):217-20.
-
16Billings LK, Florez JC. The genetics of type 2 diabetes: what have we learned from GWAS? Ann N Y Acad Sci. 2010;1212:59-77.
-
17Dupuis J, Langenberg C, Prokopenko I, Saxena R, Soranzo N, Jackson AU, et al. New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat Genet. 2010;42(2):105-16.
-
18Hindorff LA, Sethupathy P, Junkins HA, Ramos EM, Mehta JP, Collins FS, et al. Potential etiologic and functional implications of genome-wide association loci for human diseases and traits. Proc Natl Acad Sci U S A. 2009;106(23):9362-7.
-
19Talmud PJ, Cooper JA, Gaunt T, Holmes MV, Shah S, Palmen J, et al. Variants of ADRA2A are associated with fasting glucose, blood pressure, body mass index and type 2 diabetes risk: meta-analysis of four prospective studies. Diabetologia. 2011;54(7):1710-9.
-
20Barroso I. Genetics of Type 2 diabetes. Diabet Med. 2005;22(5):517-35.
-
21Kim WJ, Park CY. 1,5-Anhydroglucitol in diabetes mellitus. Endocrine. 2013;43(1):33-40.
-
22Viana LV, Leitao CB, Kramer CK, Zucatti AT, Jezini DL, Felicio J, et al. Poor glycaemic control in Brazilian patients with type 2 diabetes attending the public healthcare system: a cross-sectional study. BMJ Open. 2013;3(9):e003336.
-
23UKPDS. UK Prospective Diabetes Study (UKPDS). X. Urinary albumin excretion over 3 years in diet-treated type 2, (non-insulin-dependent) diabetic patients, and association with hypertension, hyperglycaemia and hypertriglyceridaemia. Diabetologia. 1993;36(10):1021-9.
-
24Nelson RG, Bennett PH, Beck GJ, Tan M, Knowler WC, Mitch WE, et al. Development and progression of renal disease in Pima Indians with non-insulin-dependent diabetes mellitus. Diabetic Renal Disease Study Group. N Engl J Med. 1996;335(22):1636-42.
-
25Chen X, Liu L, He W, Lu Y, Ma D, Du T, et al. Association of the ADRA2A polymorphisms with the risk of type 2 diabetes: A meta-analysis. Clin Biochem. 2013;46(9):722-6.
-
26Wagner R, Dudziak K, Herzberg-Schafer SA, Machicao F, Stefan N, Staiger H, et al. Glucose-raising genetic variants in MADD and ADCY5 impair conversion of proinsulin to insulin. PloS One. 2011;6(8):e23639.
-
27Renstrom F, Shungin D, Johansson I, Florez JC, Hallmans G, Hu FB, et al. Genetic predisposition to long-term nondiabetic deteriorations in glucose homeostasis: Ten-year follow-up of the GLACIER study. Diabetes. 2011;60(1):345-54.
-
28Fujita H, Hara K, Shojima N, Horikoshi M, Iwata M, Hirota Y, et al. Variations with modest effects have an important role in the genetic background of type 2 diabetes and diabetes-related traits. J Hum Genet. 2012;57(12):776-9.
-
29Florez JC, Jablonski KA, McAteer JB, Franks PW, Mason CC, Mather K, et al. Effects of genetic variants previously associated with fasting glucose and insulin in the Diabetes Prevention Program. PloS One. 2012;7(9):e44424.
-
30Rees SD, Hydrie MZ, O’Hare JP, Kumar S, Shera AS, Basit A, et al. Effects of 16 genetic variants on fasting glucose and type 2 diabetes in South Asians: ADCY5 and GLIS3 variants may predispose to type 2 diabetes. PloS One. 2011;6(9):e24710.
-
31Hu C, Zhang R, Wang C, Wang J, Ma X, Hou X, et al. Variants from GIPR, TCF7L2, DGKB, MADD, CRY2, GLIS3, PROX1, SLC30A8 and IGF1 are associated with glucose metabolism in the Chinese. PloS One. 2010;5(11):e15542.
-
32Liu C, Li H, Qi L, Loos RJ, Qi Q, Lu L, et al. Variants in GLIS3 and CRY2 are associated with type 2 diabetes and impaired fasting glucose in Chinese Hans. PloS One. 2011;6(6):e21464.
Publication Dates
-
Publication in this collection
Feb 2015
History
-
Received
20 Mar 2014 -
Accepted
10 Oct 2014