Abstract
Objective
Patients with incidental nonfunctioning adrenal adenoma are associated with increased risk of obesity, impaired glucose tolerance and dyslipidemia. We aimed to investigate the relationship between thyroid function, serum lipids and insulin resistance in patients with nonfunctioning adrenal incidentaloma.
Subjects and methods
Forty patients who had diagnosed as adrenal incidentaloma (AI) in our department were included in the study. Serum free triiodothyronine (fT3), free thyroxine (fT4), thyroid stimulating hormone (TSH), anti-thyroperoxidase antibody (anti-TPO Ab) and anti-thyroglobulin antibody (anti-Tg Ab), lipid profile, hs-CRP, fasting insulin levels were measured and insulin resistance calculated by HOMA-IR. Thyroid volume (TV) was assessed.
Results
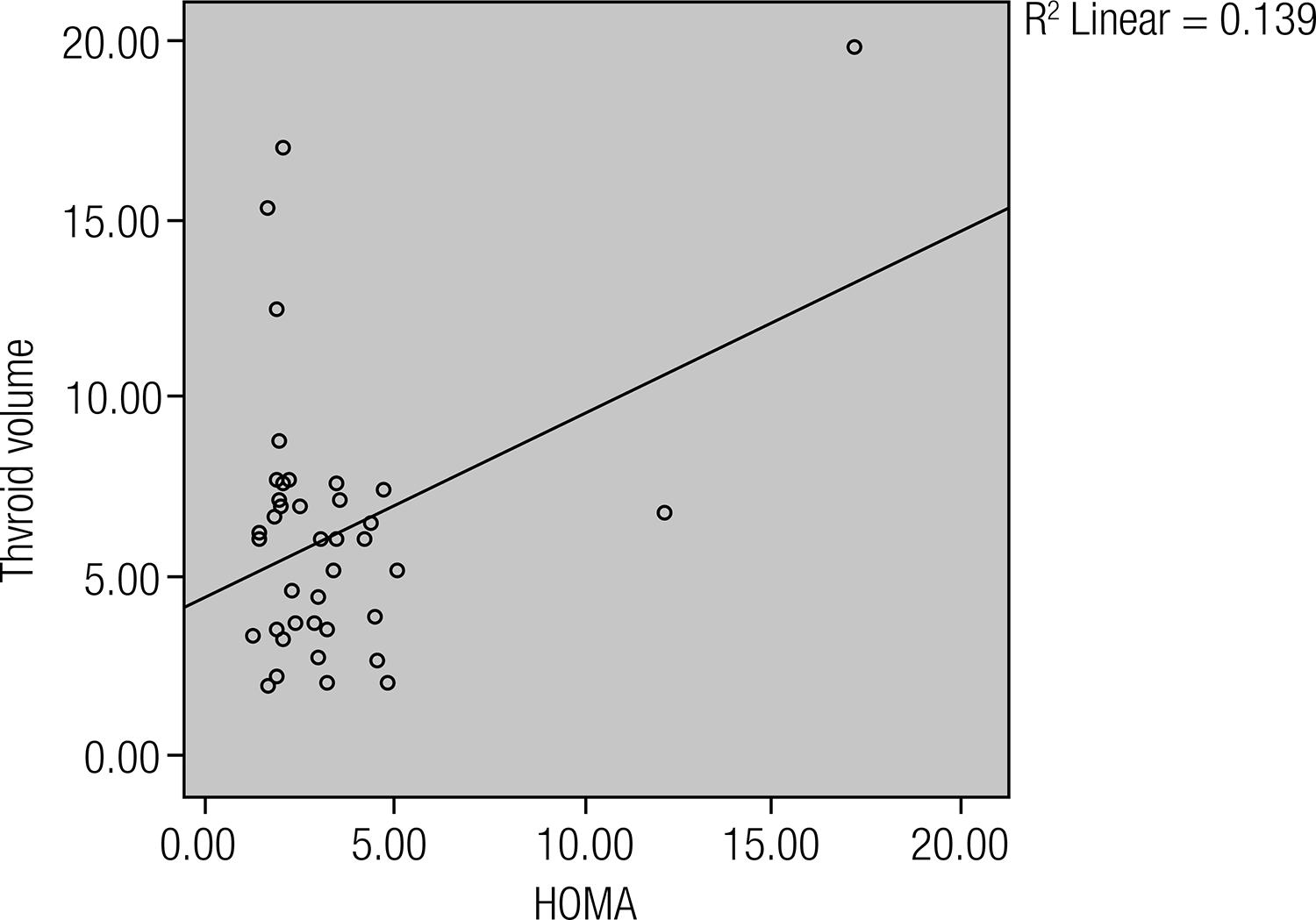
None of the patients showed specific signs and symptoms of hormone excess. TV, TSH and fT3 levels in the patient and control groups did not differ significantly (p > 0.05). The serum fT4, anti-TG Ab, anti-TPO Ab levels in the patient group were significantly higher than in the control group (p = 0.013, p < 0.0001, p = 0.016 respectively). The HOMA-IR, hs-CRP and HDL cholesterol levels in the AI patients were significantly higher than the control group (p = 0.034, p = 0.041, p = 0.002, respectively). Statistically significant relationship was found between HOMA-IR and thyroid volume (r = 0.373, p = 0.018), fT4 (r = 0.382, p = 0.015), hs-CRP (r = 0.512, p = 0.001), HDL cholesterol (r = 0,351 p = 0.026) in AI patients. There were significant correlation between anti-TG Ab, anti-TPO Ab and TSH levels in AI patients (r = 0.431 p = 0.006, r = 0.402 p = 0.012).
Conclusions
Patients with nonfunctioning adrenal incidentaloma have several metabolic disturbances. At the same time autoimmune thyroid disorders are more frequent in nonfunctioning adrenal incidentaloma patient so that thyroid functions must be evaluated in those patients. Arch Endocrinol Metab. 2015;59(1):42-6
Nonfunctioning adrenal incidentaloma; thyroid; autoimmunity; insulin resistance
INTRODUCTION
The term adrenal incidentaloma (AI) is usually defined as an adrenal mass
unexpectedly detected through an imaging procedure performed for reasons a prior
unrelated to adrenal dysfunction or suspected dysfunction. The presence of an AI has
been associated with an increased incidence of several cardiovascular risk factors.
In fact, patients with AI can show a high prevalence of obesity, hypertension,
diabetes mellitus, glucose intolerance and dyslipidemia (11 Mansmann G, Lau J, Balk E, Rothberg M, Miyachi Y, Bornstein SR.
Clinically inapparent adrenal mass: update in diagnosis and management. Endocr
Rev. 2004;25:309-40.
2 Mantero F, Terzolo M, Arnaldi G, Osella G, Masini AM, Alì A, et al.
A survey on adrenal incidentaloma in Italy. Study Group on Adrenal Tumors of the
Italian Society of Endocrinology. J Clin Endocrinol Metab.
2000;85(2):637-44.
3 Ambrosi B, Peverelli S, Passini E, Re T, Ferrario R, Colombo P, et
al. Abnormalities of endocrine function in patients with clinically silent
adrenal masses. Eur J Endocrinol. 1995;132(4):422-8.-44 Grumbach MM, Biller BM, Braunstein GD, Campbell KK, Carney JA,
Godley PA, et al. Management of the clinically inapparent adrenal mass
(‘incidentaloma’). Ann Intern Med. 2003;138(5):424-9.).
These abnormalities, typical of overt Cushing syndrome (CS), are more frequent in
patients with subclinical Cushing syndrome (SCS); nevertheless, they can be also
found in patients with apparently nonfunctioning adrenal masses (55 Terzolo M, Pia A, Alı A, Osella G, Reimondo G, Bovio S, et al.
Adrenal incidentaloma: a new cause of the metabolic syndrome? J Clin Endocrinol
Metab. 2002 Mar;87(3):998-1003.). Patients with non-functioning AI exhibit
elevated levels of D-dimers, interleukin-6 (IL-6), adiponectin, resistin, tumor
necrosis factor (TNF)-alpha and monocyte chemoattractant protein 1 (MCP-1) (66 Yener S, Comlekci A, Akinci B, Secil M, Demir T, Ertilav S, et al.
Non-functioning adrenal incidentalomas are associated with elevated D-dimer
levels. J Endocrinol Invest. 2009;32(4):338-43.,77 Ermetici F, Malavazos AE, Corbetta S, Morricone L, Dall’Asta C,
Corsi MM, et al. Adipokine levels and cardiovascular risk in patients with
adrenal incidentaloma. Metabolism. 2007;56:686-92.).
Thyroid hormones influence carbohydrate and lipid metabolism, even in the euthyroid state. They regulate hepatic gluconeogenesis, lipogenesis and lipolysis. Also, thyroid hormones modulate mRNA and protein expression of the glucose transporter 4, AMP activated protein kinase, and acetyl CoA carboxylase in skeletal muscle (88 Crunkhorn S, Patti ME. Links between thyroid hormone action, oxidative metabolism, and diabetes risk? Thyroid. 2008;18:227-37.). Recently, there have been reports suggesting an association between low thyroid hormone levels and insulin resistance and/or metabolic syndrome in euthyroid subjects. Ross and cols. found that insulin resistance; total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol and triglycerides were significantly associated with free T4 level in euthyroid subjects (99 Ross A, Stephan J, Bakker L, Links TP, Gans R, Wollfenbuttel HR. Thyroid function is associated with components of the metabolic syndrome in subjects eutiroid. J Clin Endocrinol Metab. 2007;92(2):491-6.). Garduño-Garcia and cols. reported a significant association between fT4 levels and high-density lipoprotein cholesterol, insulin and HOMA-IR (1010 Garduño-Garcia Jde J, Alvirde-Garcia U, López-Carrasco G, Padilla Mendoza ME, Mehta R, Arellano-Campos O, et al. TSH and free thyroxine concentrations are associated with differing metabolic markers in euthyroid subjects. Eur J Endocrinol. 2010;163(2):273-8.).
The routine use of abdominal imaging procedures have significantly increased the
incidental finding of adrenal masses. Many patients with incidental nonfunctioning
adrenal adenoma have increased risk of obesity, impaired glucose tolerance and
dyslipidemia (1111 Kolan&ska K, Owecki M, Nikisch E, Sowinski J. High prevalence of
obesity in patients with non-functioning adrenal incidentalomas. Neuro
Endocrinol Lett. 2010;31(3):418-22.
12 Midorikawa S, Sanada H, Hashimoto S, Suzuki T, Watanabe T. The
improvement of insulin resistance in patients with adrenal incidentaloma by
surgical resection. Clin Endocrinol (Oxf). 2001;54(6):797-804.-1313 Bernini G, Moretti A, Iacconi P, Miccoli P, Nami R, Lucani B, et al.
Anthropometric, haemodynamic, humoral and hormonal evaluation in patients with
incidental adrenocortical adenomas before and after surgery. Eur J Endocrinol.
2003 Feb;148(2):213-9.). We aimed to investigate the relationship between thyroid
function, serum lipids and insulin resistance in patients with nonfunctioning
adrenal incidentaloma.
MATERIALS AND METHODS
Patients and controls
Forty patients with consecutively diagnosed AI in our department were included in the study. None of the patients with AI showed specific signs and/or symptoms of hormone excess and none were receiving hormonal therapy. Screening tests to rule out catecholamine, mineralocorticoid as well as glucocorticoid overproduction were negative in all patients. None of them had symptoms of overt endocrinopathy or other disease that could influence results of the evaluations. Patients with a history of acute coronary syndrome, heart failure, pulmonary embolism, stroke, cardiomyopathy, diabetes mellitus, infectious diseases and hepatic or renal disorders, pregnancy were excluded from the study.
The control group consisted of 61 healthy volunteers. Control group was selected among healthy subjects with normal adrenal imaging that matches with patients according to age, gender and body mass index (BMI). All patients and controls underwent medical history and physical examination. The study protocol was approved by the ethics committee of our hospital and all the participants provided written informed consent.
Clinical and biochemical evaluation
All patients with AI and controls underwent anthropometric measurements and biochemical screening. The waist/hip ratio and body mass index (BMI) were calculated. Fasting blood samples were obtained in the morning between 08:00 and 11:00 hours. Serum high-density lipoprotein cholesterol (HDL-C), total cholesterol (TC), triglycerides levels by spectrophotometric assay (Advia 2400, Siemens Healthcare Diagnostics Inc., Tarrytown USA). The fasting serum insulin level was measured using the chemiluminescent immunoassay method (Advia Centaur XP, Siemens Healthcare Diagnostics Inc., Tarrytown, USA). The estimate of insulin resistance was calculated using the HOMA-IR index. TSH, fT3 and fT4 were measured via chemiluminescent microparticle immunoassay (Abbott, Architect i2000, Abbott Laboratories Diagnostics Division, IL, USA). Anti-thyroglobulin antibody (anti-Tg Ab) and anti-thyroperoxidase antibody (anti-TPO Ab) were measured via chemiluminescent competitive immunoassay (Siemens, Advia centaur XP). Reference limits were as follows: fT3: 1.71-3.71 pg/mL; fT4: 0.7-1.48 ng/dL, TSH: 0.35-4.94 µIU/mL, anti-Tg Ab: 0-60 U/mL, anti-TPO Ab: 0-57 U/mL. Anti-Tg Ab and anti-TPO Ab concentrations > 60 IU/mL and > 57 IU/mL, respectively, were considered positive.
Endocrine evaluation
None of the patients with AI showed specific signs and/or symptoms of hormone excess and none were receiving hormonal therapy. All patients underwent the following endocrine workup aimed to study the hypothalamic-pituitary-adrenal axis. Serum cortisol, and plasma corticotrophin (ACTH) were determined in basal condition and measurement of the 24-h excretion of urinary-free cortisol (UFC) was performed. All patients underwent an overnight 1-mg dexamethasone (DXM) test. The suppression was adequate when morning cortisol fell below 1.8 µg/dL. If inadequate, a two-day low-dose DXM suppression test was performed (2 mg, four times a day for 2 days). Urinary metanephrine, and normetanephrine excretion to exclude the presence of pheochromocytoma and the upright plasma aldosterone to plasma renin activity ratio to exclude primary aldosteronism, were performed. In control subjects, measurement of serum cortisol in the morning, measurement of 24-h UFC excretion and overnight low-dose dexamethasone suppression test was performed. Normal ranges of serum and urinary cortisol and plasma ACTH were determined as previously reported (1414 Terzolo M, Osella G, Alì A, Borretta G, Cesario F, Paccotti P, et al. Subclinical Cushing’s syndrome in adrenal incidentaloma. Clin Endocrinol (Oxf). 1998;48(1):89-97.).
Thyroid volume (TV) was assessed using a high resolution ultrasound machine (Hitachi, Japan; EUB 7000) with a 6-14 megahertz high frequency linear transducer. Thyroid volumes were calculated by multiplication of three diameters.
Statistical analysis
The statistical analysis was performed with the SPSS statistical software (version 18; SPSS, Chicago, IL, USA). Normality of the variables was tested by Kolmogorov-Smirnov test. Differences between groups were analyzed by one way analysis of variance or Mann-Whitney U-test when appropriate. Frequencies were analyzed by χ2. Correlations between variables were performed using Spearman’s rho correlation coefficient. Results are expressed as mean ± SD. A probability value of 0.05 was considered to be statistically significant.
RESULTS
The study included 40 AI patients (mean age of 55.6 ± 10.6 years) and 61 controls (mean age of 53.9 ± 6 years). The general characteristics of the patients and controls are presented in the table 1. The mean adrenal mass size was 3.0 ± 0.7 cm. None of the patients showed specific signs and symptoms of hormone excess. All adrenal incidentalomas were unilateral. None of the patients with AI underwent adrenalectomy and followed-up with serial CT or magnetic resonance imaging (MRI) and endocrine evaluation was planned.
There weren’t any significant differences in age, gender, BMI, triglyceride, fasting blood glucose between the patient and control groups. The total cholesterol, LDL cholesterol were significantly higher in the controls than in the AI patients (p = 0.044, p = 0.001). HDL cholesterol were significantly higher in the AI patients (p = 0.041). HOMA-IR and hs-CRP values were higher in the AI patients than that in the controls (3.3 ± 2.9 vs. 1.9 ± 1.2, p = 0.002 and 1.8 ± 0.8 m/L vs. 1.3 ± 1 mg/L, p = 0.034 respectively) (Figure 1).
The mean thyroid volume was 6.38 ± 3.90 mL was in AI patients while 6.81 ± 3.55 mL in healty controls (p = 0.58). AI patients had higher prevalence of positive anti-Tg Ab than control group (52.5 vs. 11.7%). The divergence was statistically significant (p < 0.0001). The prevalence of subjects with positive anti-TPO Ab in patients and control group were 51.3 and 27.6%, respectively. It was significantly higher in AI patients (p = 0.016).
The TSH and fT3 levels in the patient and control groups did not differ significantly (TSH: 1.8722 ± 1.4482 µIU/mL vs. 1.6412 ± 0.9949 µIU/ mL, p = 0.868; fT3: 2.90 ± 0.5 pg/mL vs. 2.89 ± 0.4 pg/mL, p = 0.928). The serum fT4 levels in the patient group were significantly higher than in the control group (fT4: 1.22 ± 0.5 ng/dL vs. 1.05 ± 0.2 ng/dL, p = 0.013) (Figure 1). The thyroid test of the AI patients and controls were presented in the table 2.
Statistically significant relationship was found between HOMA-IR and thyroid volume (r = 0.373, p = 0.018) (Figure 2), hs-CRP (r = 0.512, p = 0.001) (Figure 3), fT4 (r = 0.382, p = 0.015), HDL cholesterol (r = 0,351 p = 0.026) in AI patients. And also there was statistically significant relationship between fT4 and HDL cholesterol (r = 0.366, p = 0.02) in AI patients. The correlation between anti-TG Ab, anti-TPO Ab and TSH levels were also statistically significant in AI patients (r = 0.431 p = 0.006, r = 0.402 p = 0.012).
There was not a statistically significant relationship between TSH, fT4, fT3, hs-CRP, HOMA-IR, fasting glucose, lipid parameters, BMI and morning cortisol levels, 1-mg overnight dexamethasone suppression test results.
DISCUSSION
The widespread usage of imaging tools increased the frequency of adrenal neoplasms
(1515 Gross MD, Korobkin M, Bou-Assaly W, Rubello D.
Incidentally-discovered adrenal masses. Discov Med.
2010;9(44):24-33.). These incidentally discovered
adrenal masses are mostly benign and asymptomatic and are often considered as
non-functional tumors. Patients with clinically inactive adrenal adenomas as a group
exhibit insulin resistance and a variety of metabolic disturbances and
manifestations of the metabolic syndrome (55 Terzolo M, Pia A, Alı A, Osella G, Reimondo G, Bovio S, et al.
Adrenal incidentaloma: a new cause of the metabolic syndrome? J Clin Endocrinol
Metab. 2002 Mar;87(3):998-1003.,1616 Grumbach MM, Biller BM, Braunstein GD, Campbell KK, Carney JA,
Godley PA, et al. Management of the clinically inapparent adrenal mass
(“incidentaloma”). Ann Intern Med. 2003;138(5):424-9.
17 Kloos RT, Gross MD, Francis IR, Korobkin M, Shapiro B. Incidentally
discovered adrenal masses. Endocr Rev. 1995;16(4):460-84.-1818 Terzolo M, Bovio S, Reimondo G, Pia A, Osella G, Borretta G, et al.
Subclinical Cushing’s syndrome in adrenal incidentalomas. Endocrinol Metab Clin
North Am. 2005;34(2):423-39, x.). The present data allow them to hypothesize that the subtle
autonomous cortisol secretion of these adrenal adenomas may cause an acquired
condition of insulin resistance and leading to an increased incidence of several
cardiovascular risk factors. In a multi-institutional survey performed in Italy
which collected 1,004 patients with AI, the prevalence of arterial hypertension,
diabetes mellitus type 2 and obesity were 41%, 10% and 28%, respectively (1919 Mantero F, Terzolo M, Arnaldi G, Osella G, Masini AM, Alì A, et al.
A survey on adrenal incidentaloma in Italy. Study Group on Adrenal Tumors of the
Italian Society of Endocrinology. J Clin Endocrinol Metab.
2000;85(2):637-44.). Yener and cols. showed that carotid intima
media thickness (CIMT) and HOMA-IR were increased in non-functioning adrenal
incidentaloma patients and also morning cortisol was independently associated with
HOMA levels in AI patients (2020 Yener S1, Genc S, Akinci B, Secil M, Demir T, Comlekci A, et al.
Carotid intima media thickness is increased and associated with morning cortisol
in subjects with non-functioning adrenal incidentaloma. Endocrine.
2009;35(3):365-70.).
Insulin resistance generally considered to induce disturbed glucose tolerance, dislipoproteinemia, hypertension and play a key role in the development of atherosclerotic diseases. Midorikawa and cols. had demonstrated an incidence of insulin resistance, disturbed glucose tolerance and elevated blood pressure in patients with non-functioning adrenal adenoma and subsequent improvement in these metabolic states after removal of the tumors (2121 Midorikawa S, Sanada H, Hashimoto S, Suzuki T, Watanabe T. The improvement of insulin resistance in patients with adrenal incidentaloma by surgical resection. Clin Endocrinol (Oxf). 2001;54(6):797-804.). Fernández-Real and cols. showed that a high prevalence of disturbed glucose tolerance was found among patients with incidental non-functional adrenal adenomas however lower than that described for Cushing’s syndrome but higher than expected (2222 Fernández-Real JM, Engel WR, Simó R, Salinas I, Webb SM. Study of glucose tolerance in consecutive patients harbouring incidental adrenal tumours. Study Group of Incidental Adrenal Adenoma. Clin Endocrinol (Oxf). 1998;49(1):53-61.).
In this study, we showed that subjects with non-functioning AI have increased HOMA-IR, HDL cholesterol, hs-CRP, fT4 when compared with healthy controls. We found a significant positive correlation between HOMA-IR, thyroid volume, fT4, hs-CRP and HDL cholesterol. Statistically significant relationship was found between fT4 and HDL cholesterol. On the contrary there were not a relation between cortisol levels and fasting glucose levels, HOMA-IR, lipid parameters.
Those unknown steroid hormones that we could not detected in AI patients with today’s laboratory tests might be the reason of high levels of fT4. Even within the euthyroid range, circulating free T4 may contribute to the development of insulin resistance in nonfunctional adrenal masses. Although thyroid function tests were within the normal range but statistical significant high fT4 levels might be responsible for those symptoms in nonfunctioning AI patients.
Both anti-TPO Ab and anti-Tg Ab s were more frequent in AI patients than in the healthy controls, even in AI patients who don’t have clinical thyroid disease. Explanation for the high incidence of thyroid autoimmunity in AI is open to speculation. It is still a subject of discussion as to whether AI is an independent risk factor for these thyroid abnormalities or whether this is a co-incidental finding. Our data suggest that all patients with AI should be screened for thyroid function and thyroid specific autoantibodies even without evidence of overt thyroid dysfunction, as it is known that those patients with anti-thyroperoxidase and anti-thyroglobulin antibodies are likely to develop thyroid dysfunction later in life.
In conclusion, patients with adrenal incidentaloma might have several metabolic disturbances. The present study is the first in the literature to evaluate thyroid function, thyroid volume and metabolic parameters together in nonfunctioning adrenal incidentalomas. We demonstrated that fT4 level has a positive correlation with HOMA-IR and HDL cholesterol levels in nonfunctioning adrenal incidentaloma patients who were euthyroid. Subtle abnormalities in thyroid hormone secretion may be occurring in adrenal incidentalomas. Further prospective studies should be performed to determine the clinical significance of these findings in adrenal incidentaloma patients.
REFERENCES
-
1Mansmann G, Lau J, Balk E, Rothberg M, Miyachi Y, Bornstein SR. Clinically inapparent adrenal mass: update in diagnosis and management. Endocr Rev. 2004;25:309-40.
-
2Mantero F, Terzolo M, Arnaldi G, Osella G, Masini AM, Alì A, et al. A survey on adrenal incidentaloma in Italy. Study Group on Adrenal Tumors of the Italian Society of Endocrinology. J Clin Endocrinol Metab. 2000;85(2):637-44.
-
3Ambrosi B, Peverelli S, Passini E, Re T, Ferrario R, Colombo P, et al. Abnormalities of endocrine function in patients with clinically silent adrenal masses. Eur J Endocrinol. 1995;132(4):422-8.
-
4Grumbach MM, Biller BM, Braunstein GD, Campbell KK, Carney JA, Godley PA, et al. Management of the clinically inapparent adrenal mass (‘incidentaloma’). Ann Intern Med. 2003;138(5):424-9.
-
5Terzolo M, Pia A, Alı A, Osella G, Reimondo G, Bovio S, et al. Adrenal incidentaloma: a new cause of the metabolic syndrome? J Clin Endocrinol Metab. 2002 Mar;87(3):998-1003.
-
6Yener S, Comlekci A, Akinci B, Secil M, Demir T, Ertilav S, et al. Non-functioning adrenal incidentalomas are associated with elevated D-dimer levels. J Endocrinol Invest. 2009;32(4):338-43.
-
7Ermetici F, Malavazos AE, Corbetta S, Morricone L, Dall’Asta C, Corsi MM, et al. Adipokine levels and cardiovascular risk in patients with adrenal incidentaloma. Metabolism. 2007;56:686-92.
-
8Crunkhorn S, Patti ME. Links between thyroid hormone action, oxidative metabolism, and diabetes risk? Thyroid. 2008;18:227-37.
-
9Ross A, Stephan J, Bakker L, Links TP, Gans R, Wollfenbuttel HR. Thyroid function is associated with components of the metabolic syndrome in subjects eutiroid. J Clin Endocrinol Metab. 2007;92(2):491-6.
-
10Garduño-Garcia Jde J, Alvirde-Garcia U, López-Carrasco G, Padilla Mendoza ME, Mehta R, Arellano-Campos O, et al. TSH and free thyroxine concentrations are associated with differing metabolic markers in euthyroid subjects. Eur J Endocrinol. 2010;163(2):273-8.
-
11Kolan&ska K, Owecki M, Nikisch E, Sowinski J. High prevalence of obesity in patients with non-functioning adrenal incidentalomas. Neuro Endocrinol Lett. 2010;31(3):418-22.
-
12Midorikawa S, Sanada H, Hashimoto S, Suzuki T, Watanabe T. The improvement of insulin resistance in patients with adrenal incidentaloma by surgical resection. Clin Endocrinol (Oxf). 2001;54(6):797-804.
-
13Bernini G, Moretti A, Iacconi P, Miccoli P, Nami R, Lucani B, et al. Anthropometric, haemodynamic, humoral and hormonal evaluation in patients with incidental adrenocortical adenomas before and after surgery. Eur J Endocrinol. 2003 Feb;148(2):213-9.
-
14Terzolo M, Osella G, Alì A, Borretta G, Cesario F, Paccotti P, et al. Subclinical Cushing’s syndrome in adrenal incidentaloma. Clin Endocrinol (Oxf). 1998;48(1):89-97.
-
15Gross MD, Korobkin M, Bou-Assaly W, Rubello D. Incidentally-discovered adrenal masses. Discov Med. 2010;9(44):24-33.
-
16Grumbach MM, Biller BM, Braunstein GD, Campbell KK, Carney JA, Godley PA, et al. Management of the clinically inapparent adrenal mass (“incidentaloma”). Ann Intern Med. 2003;138(5):424-9.
-
17Kloos RT, Gross MD, Francis IR, Korobkin M, Shapiro B. Incidentally discovered adrenal masses. Endocr Rev. 1995;16(4):460-84.
-
18Terzolo M, Bovio S, Reimondo G, Pia A, Osella G, Borretta G, et al. Subclinical Cushing’s syndrome in adrenal incidentalomas. Endocrinol Metab Clin North Am. 2005;34(2):423-39, x.
-
19Mantero F, Terzolo M, Arnaldi G, Osella G, Masini AM, Alì A, et al. A survey on adrenal incidentaloma in Italy. Study Group on Adrenal Tumors of the Italian Society of Endocrinology. J Clin Endocrinol Metab. 2000;85(2):637-44.
-
20Yener S1, Genc S, Akinci B, Secil M, Demir T, Comlekci A, et al. Carotid intima media thickness is increased and associated with morning cortisol in subjects with non-functioning adrenal incidentaloma. Endocrine. 2009;35(3):365-70.
-
21Midorikawa S, Sanada H, Hashimoto S, Suzuki T, Watanabe T. The improvement of insulin resistance in patients with adrenal incidentaloma by surgical resection. Clin Endocrinol (Oxf). 2001;54(6):797-804.
-
22Fernández-Real JM, Engel WR, Simó R, Salinas I, Webb SM. Study of glucose tolerance in consecutive patients harbouring incidental adrenal tumours. Study Group of Incidental Adrenal Adenoma. Clin Endocrinol (Oxf). 1998;49(1):53-61.
Publication Dates
-
Publication in this collection
Feb 2015
History
-
Received
15 May 2014 -
Accepted
10 Oct 2014