Abstract
Objective
To determine the frequency of indication of the GH suppression test and pituitary magnetic resonance imaging (MRI) in patients with clinical suspicion of acromegaly with GH concentrations > 0.4 µg/L despite normal serum IGF-1.
Subjects and methods
A total of 160 patients with clinical suspicion of acromegaly with normal IGF-1 were studied.
Results
Basal GH > 0.4 µg/L was observed in 70/88 women (79.5%). Nadir GH > 0.4 µg/L was found in 21/70 women (30%) and these patients were submitted to MRI, which revealed a microadenoma in 2/21 women (9.5%). In these two women, IGF-1 continued to be normal in subsequent measurements and no clinical progression has been observed so far (time of follow-up until now 4 years). Basal GH > 0.4 µg/L was seen in 33/72 men (45.8%). Nadir GH was < 0.4 µg/L in all of them.
Conclusions
In patients with clinical suspicion of acromegaly, concern over GH concentration in the presence of normal IGF-1 results in the unwarranted complementary investigation in many cases, and even in possible equivocal diagnoses. It is only in exceptional cases that normal IGF-1 should not rule out acromegaly. Arch Endocrinol Metab. 2015;59(1):54-8
Acromegaly; laboratory diagnosis; serum IGF-1; basal GH
INTRODUCTION
Acromegaly is an insidious disease which prevalence might be underestimated (11 Schneider HJ, Sievers C, Saller B, Wittchen HU, Stalla GK. High
prevalence of biochemical acromegaly in primary care patients with elevated
IGF-1 levels. Clin Endocrinol (Oxf). 2008;69:432-5.
2 Rosario PW. Frequency of acromegaly in adults with diabetes or
glucose intolerance and estimated prevalence in the general population.
Pituitary. 2011; 14:217-21.-33 Rosario PW, Calsolari MR. Screening for acromegaly by application of
a simple questionnaire evaluating the enlargement of extremities in adult
patients seen at primary health care units. Pituitary.
2012;15:179-83.). If
untreated, acromegaly is associated with high morbidity and mortality; however, when
diagnosed and treated adequately, control of the tumor and of hormone hypersecretion
is possible in most cases, a fact highlighting the importance of an early diagnosis
of the disease.
In the case of clinical suspicion of acromegaly, investigation is traditionally
started by the measurement of IGF-1 combined with basal GH (44 Giustina A, Barkan A, Casanueva FF, Cavagnini F, Frohman L, Ho K, et
al. Criteria for cure of acromegaly: a consensus statement. J Clin Endocrinol
Metab. 2000;85:526-9.
5 Trainer PJ. Acromegaly – consensus, what consensus? J Clin
Endocrinol Metab. 2002;87:3534-6.
6 Levy A. Pituitary disease: presentation, diagnosis, and management.
J Neurol Neurosurg Psychiatry. 2004;75 Suppl 3:iii47-52.
7 Pokrajac-Simeunovic A, Trainer PJ. Pitfalls in the diagnosis of
acromegaly. Horm Res. 2004;62:74-8.
8 Lim EM, Pullan P; Growth Hormone Research Society; Pituitary
Society. Biochemical assessment and long-term monitoring in patients with
acromegaly: statement from a joint consensus conference of the Growth Hormone
Research Society and the Pituitary Society. Clin Biochem Rev.
2005;26:41-3.
9 Tzanela M. Dynamic tests and basal values for defining active
acromegaly. Neuroendocrinology. 2006;83:200-4.
10 Ayuk J, Sheppard MC. Growth hormone and its disorders. Postgrad Med
J. 2006;82:24-30.
11 Scacchi M, Cavagnini F. Acromegaly. Pituitary.
2006;9:297-303.
12 Kalavalapalli S, Reid H, Kane J, Buckler H, Trainer P, Heald AH.
Silent growth hormone secreting pituitary adenomas: IGF-1 is not sufficient to
exclude growth hormone excess. Ann Clin Biochem. 2007;
44:89-93.
13 Giustina A, Barkan A, Chanson P, Grossman A, Hoffman A, Ghigo E, et
al. Guidelines for the treatment of growth hormone excess and growth hormone
deficiency in adults. J Endocrinol Invest. 2008;31:820-38.
14 Cordero RA, Barkan AL. Current diagnosis of acromegaly. Rev Endocr
Metab Disord. 2008;9:13-9.
15 Chanson P, Salenave S. Acromegaly. Orphanet J Rare Dis.
2008;3:17.
16 Cazabat L, Souberbielle JC, Chanson P. Dynamic tests for the
diagnosis and assessment of treatment efficacy in acromegaly. Pituitary.
2008;11:129-39.
17 Chanson P, Bertherat J, Beckers A, Bihan H, Brue T, Caron P, et al.
French consensus on the management of acromegaly. Ann Endocrinol (Paris).
2009;70:92-106.
18 Cozzi R, Baldelli R, Colao A, Lasio G, Zini M, Attanasio R; Italian
Association of Clinical Endocrinologists (AME). AME Position Statement on
clinical management of acromegaly. J Endocrinol Invest.
2009;32:2-25.
19 Chanson P, Salenave S, Kamenicky P, Cazabat L, Young J. Pituitary
tumours: acromegaly. Best Pract Res Clin Endocrinol Metab.
2009;23:555-74.
20 Barkan A, Bronstein MD, Bruno OD, Cob A, Espinosa-de-los-Monteros
AL, Gadelha MR, et al. Management of acromegaly in Latin America: expert panel
recommendations. Pituitary. 2010;13: 168-75.
21 Reddy R, Hope S, Wass J. Acromegaly. BMJ.
2010;341:c4189.
22 Vieira Neto L, Abucham J, Araujo LA, Boguszewski CL, Bronstein MD,
Czepielewski M, et al. Recommendations of Neuroendocrinology Department from
Brazilian Society of Endocrinology and Metabolism for diagnosis and treatment of
acromegaly in Brazil. Arq Bras Endocrinol Metabol.
2011;55:91-105.-2323 d’Herbomez M, Bauters C, Cortet-Rudelli C, Dewailly D, Docao C,
Wémeau JL. Biomarkers in endocrinology. Presse Med.
2014;43:40-56.). The disease is
ruled out if IGF-1 is normal and basal GH is less than 0.4 µg/L. Thus, diagnosis
cannot be ruled out in patients with normal IGF-1 but basal GH > 0.4 µg/L. In
this situation, investigation is based on GH suppression test after oral glucose
overload (44 Giustina A, Barkan A, Casanueva FF, Cavagnini F, Frohman L, Ho K, et
al. Criteria for cure of acromegaly: a consensus statement. J Clin Endocrinol
Metab. 2000;85:526-9.
5 Trainer PJ. Acromegaly – consensus, what consensus? J Clin
Endocrinol Metab. 2002;87:3534-6.
6 Levy A. Pituitary disease: presentation, diagnosis, and management.
J Neurol Neurosurg Psychiatry. 2004;75 Suppl 3:iii47-52.
7 Pokrajac-Simeunovic A, Trainer PJ. Pitfalls in the diagnosis of
acromegaly. Horm Res. 2004;62:74-8.
8 Lim EM, Pullan P; Growth Hormone Research Society; Pituitary
Society. Biochemical assessment and long-term monitoring in patients with
acromegaly: statement from a joint consensus conference of the Growth Hormone
Research Society and the Pituitary Society. Clin Biochem Rev.
2005;26:41-3.
9 Tzanela M. Dynamic tests and basal values for defining active
acromegaly. Neuroendocrinology. 2006;83:200-4.
10 Ayuk J, Sheppard MC. Growth hormone and its disorders. Postgrad Med
J. 2006;82:24-30.
11 Scacchi M, Cavagnini F. Acromegaly. Pituitary.
2006;9:297-303.
12 Kalavalapalli S, Reid H, Kane J, Buckler H, Trainer P, Heald AH.
Silent growth hormone secreting pituitary adenomas: IGF-1 is not sufficient to
exclude growth hormone excess. Ann Clin Biochem. 2007;
44:89-93.
13 Giustina A, Barkan A, Chanson P, Grossman A, Hoffman A, Ghigo E, et
al. Guidelines for the treatment of growth hormone excess and growth hormone
deficiency in adults. J Endocrinol Invest. 2008;31:820-38.
14 Cordero RA, Barkan AL. Current diagnosis of acromegaly. Rev Endocr
Metab Disord. 2008;9:13-9.
15 Chanson P, Salenave S. Acromegaly. Orphanet J Rare Dis.
2008;3:17.
16 Cazabat L, Souberbielle JC, Chanson P. Dynamic tests for the
diagnosis and assessment of treatment efficacy in acromegaly. Pituitary.
2008;11:129-39.
17 Chanson P, Bertherat J, Beckers A, Bihan H, Brue T, Caron P, et al.
French consensus on the management of acromegaly. Ann Endocrinol (Paris).
2009;70:92-106.
18 Cozzi R, Baldelli R, Colao A, Lasio G, Zini M, Attanasio R; Italian
Association of Clinical Endocrinologists (AME). AME Position Statement on
clinical management of acromegaly. J Endocrinol Invest.
2009;32:2-25.
19 Chanson P, Salenave S, Kamenicky P, Cazabat L, Young J. Pituitary
tumours: acromegaly. Best Pract Res Clin Endocrinol Metab.
2009;23:555-74.
20 Barkan A, Bronstein MD, Bruno OD, Cob A, Espinosa-de-los-Monteros
AL, Gadelha MR, et al. Management of acromegaly in Latin America: expert panel
recommendations. Pituitary. 2010;13: 168-75.
21 Reddy R, Hope S, Wass J. Acromegaly. BMJ.
2010;341:c4189.
22 Vieira Neto L, Abucham J, Araujo LA, Boguszewski CL, Bronstein MD,
Czepielewski M, et al. Recommendations of Neuroendocrinology Department from
Brazilian Society of Endocrinology and Metabolism for diagnosis and treatment of
acromegaly in Brazil. Arq Bras Endocrinol Metabol.
2011;55:91-105.-2323 d’Herbomez M, Bauters C, Cortet-Rudelli C, Dewailly D, Docao C,
Wémeau JL. Biomarkers in endocrinology. Presse Med.
2014;43:40-56.). This strategy is justified by the analytical and normative
limitations related to this hormone, conditions that interfere in IGF-1 measurements
(reducing it), and cases of acromegaly with normal IGF-1 (55 Trainer PJ. Acromegaly – consensus, what consensus? J Clin
Endocrinol Metab. 2002;87:3534-6.,88 Lim EM, Pullan P; Growth Hormone Research Society; Pituitary
Society. Biochemical assessment and long-term monitoring in patients with
acromegaly: statement from a joint consensus conference of the Growth Hormone
Research Society and the Pituitary Society. Clin Biochem Rev.
2005;26:41-3.,1212 Kalavalapalli S, Reid H, Kane J, Buckler H, Trainer P, Heald AH.
Silent growth hormone secreting pituitary adenomas: IGF-1 is not sufficient to
exclude growth hormone excess. Ann Clin Biochem. 2007;
44:89-93.,1717 Chanson P, Bertherat J, Beckers A, Bihan H, Brue T, Caron P, et al.
French consensus on the management of acromegaly. Ann Endocrinol (Paris).
2009;70:92-106.,1818 Cozzi R, Baldelli R, Colao A, Lasio G, Zini M, Attanasio R; Italian
Association of Clinical Endocrinologists (AME). AME Position Statement on
clinical management of acromegaly. J Endocrinol Invest.
2009;32:2-25.,2020 Barkan A, Bronstein MD, Bruno OD, Cob A, Espinosa-de-los-Monteros
AL, Gadelha MR, et al. Management of acromegaly in Latin America: expert panel
recommendations. Pituitary. 2010;13: 168-75.,2323 d’Herbomez M, Bauters C, Cortet-Rudelli C, Dewailly D, Docao C,
Wémeau JL. Biomarkers in endocrinology. Presse Med.
2014;43:40-56.,2424 Petersenn S, Buchfelder M, Reincke M, Strasburger CM, Franz H,
Lohmann R, et al.; Participants of the German Acromegaly Register. Results of
surgical and somatostatin analog therapies and their combination in acromegaly:
a retrospective analysis of the German Acromegaly Register. Eur J Endocrinol.
2008;159: 525-32.). Although the determination of GH concentration increases
the sensitivity of patient testing, it is important to evaluate the consequences of
attributing a cut-off for GH concentrations when IGF-1 is normal, i.e., to consider
how many subjects are additionally submitted to the GH suppression test and
pituitary imaging methods that may lead to an equivocal diagnosis. We emphasize
that, except for patients with a typical phenotype or pituitary adenoma, the
“clinical suspicion” of acromegaly is prone to the subjective impression of each
physician. Therefore, the true probability of the disease among patients submitted
to laboratory investigation is variable, with consequences on the predictive value
of the tests.
Using current assays and excluding known causes of GH suppression failure, nadir GH
> 0.4 µg/L after oral glucose overload is indicative of autonomous GH secretion
(55 Trainer PJ. Acromegaly – consensus, what consensus? J Clin
Endocrinol Metab. 2002;87:3534-6.,77 Pokrajac-Simeunovic A, Trainer PJ. Pitfalls in the diagnosis of
acromegaly. Horm Res. 2004;62:74-8.,88 Lim EM, Pullan P; Growth Hormone Research Society; Pituitary
Society. Biochemical assessment and long-term monitoring in patients with
acromegaly: statement from a joint consensus conference of the Growth Hormone
Research Society and the Pituitary Society. Clin Biochem Rev.
2005;26:41-3.,1111 Scacchi M, Cavagnini F. Acromegaly. Pituitary.
2006;9:297-303.,1414 Cordero RA, Barkan AL. Current diagnosis of acromegaly. Rev Endocr
Metab Disord. 2008;9:13-9.
15 Chanson P, Salenave S. Acromegaly. Orphanet J Rare Dis.
2008;3:17.
16 Cazabat L, Souberbielle JC, Chanson P. Dynamic tests for the
diagnosis and assessment of treatment efficacy in acromegaly. Pituitary.
2008;11:129-39.-1717 Chanson P, Bertherat J, Beckers A, Bihan H, Brue T, Caron P, et al.
French consensus on the management of acromegaly. Ann Endocrinol (Paris).
2009;70:92-106.,1919 Chanson P, Salenave S, Kamenicky P, Cazabat L, Young J. Pituitary
tumours: acromegaly. Best Pract Res Clin Endocrinol Metab.
2009;23:555-74.,2222 Vieira Neto L, Abucham J, Araujo LA, Boguszewski CL, Bronstein MD,
Czepielewski M, et al. Recommendations of Neuroendocrinology Department from
Brazilian Society of Endocrinology and Metabolism for diagnosis and treatment of
acromegaly in Brazil. Arq Bras Endocrinol Metabol.
2011;55:91-105.,2323 d’Herbomez M, Bauters C, Cortet-Rudelli C, Dewailly D, Docao C,
Wémeau JL. Biomarkers in endocrinology. Presse Med.
2014;43:40-56.,2525 Melmed S, Casanueva F, Cavagnini F, Chanson P, Frohman LA, Gaillard
R, et al. Consensus statement: medical management of acromegaly. Eur J
Endocrinol. 2005;153:737-40.
26 Melmed S. Medical progress: acromegaly. N Engl J Med.
2006;355:2558-73.
27 Arellano S, Aguilar P, Domínguez B, Espinosa-de-Los-Monteros AL,
González Virla B, Sosa E, et al. Segundo Consenso Nacional de Acromegalia:
recomendaciones para su diagnóstico, tratamiento y seguimiento. Rev Endocrinol
Nutr. 2007;15:S7-16.
28 Melmed S, Colao A, Barkan A, Molitch M, Grossman AB, Kleinberg D, et
al. Guidelines for acromegaly management: an update. J Clin Endocrinol Metab.
2009;94:1509-17.
29 Giustina A, Chanson P, Bronstein MD, Klibanski A, Lamberts S,
Casanueva FF, et al.; Acromegaly Consensus Group. A consensus on criteria for
cure of acromegaly. J Clin Endocrinol Metab. 2010;95:3141-8.
30 Katznelson L, Atkinson JL, Cook DM, Ezzat SZ, Hamrahian AH, Miller
KK; American Association of Clinical Endocrinologists. American Association of
Clinical Endocrinologists medical guidelines for clinical practice for the
diagnosis and treatment of acromegaly – 2011 update. Endocr Pract.
2011;17:1-44.
31 Melmed S, Casanueva FF, Klibanski A, Bronstein MD, Chanson P,
Lamberts SW, et al. A consensus on the diagnosis and treatment of acromegaly
complications. Pituitary. 2013;16:294-302.-3232 Cordido F, García Arnés JA, Marazuela Aspiroz M, Torres Vela E;
grupo de Neuroendocrinología de la Sociedad Española de Endocrinología y
Nutrición. Practical guidelines for diagnosis and treatment of acromegaly.
Endocrinol Nutr. 2013;60:457.e1-457.e15.).
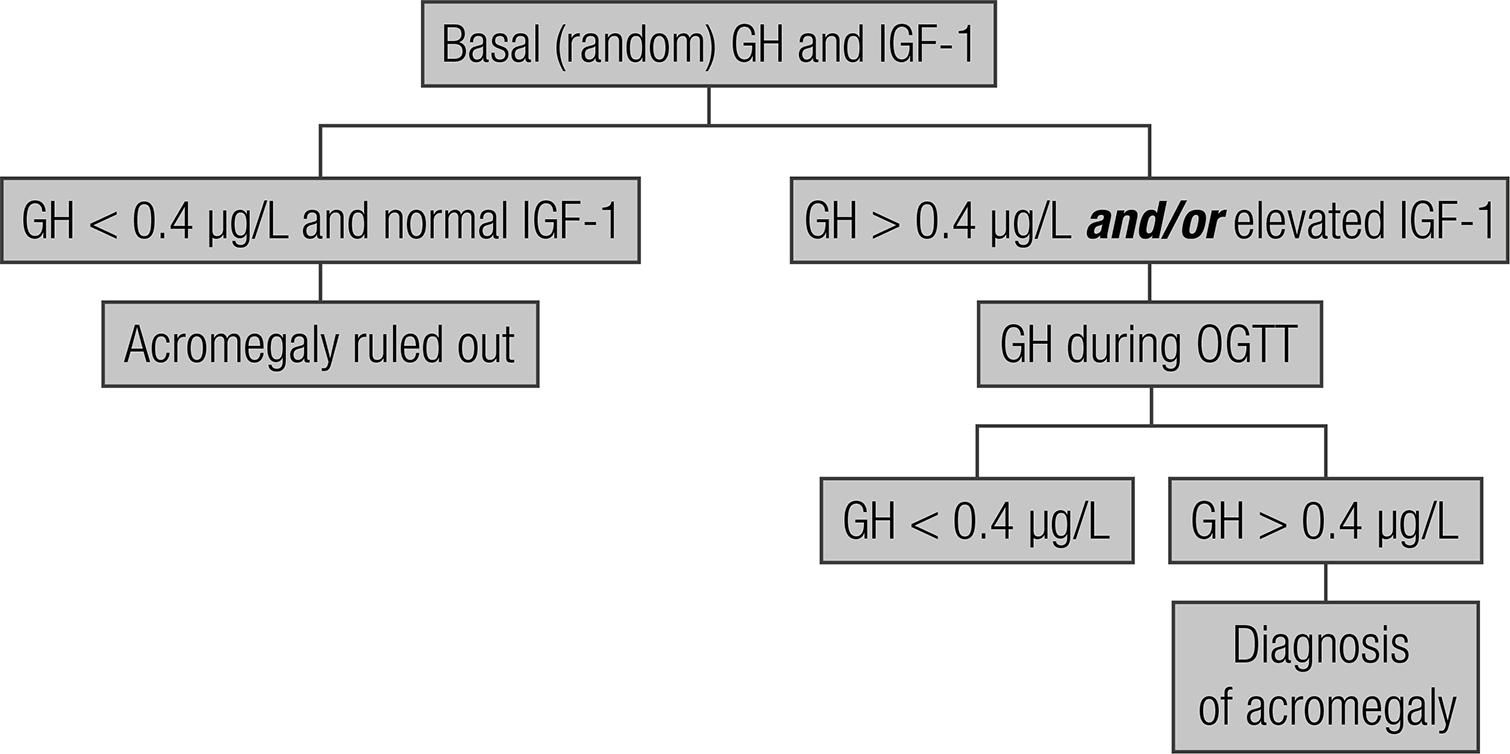
Using one of the algorithm traditionally recommended for the investigation of patients with clinical suspicion of acromegaly (Figure 1), the objective of this prospective study was to determine the frequency of indication of the GH suppression test and pituitary MRI and the probability of an equivocal diagnosis when GH concentrations > 0.4 µg/L despite normal serum IGF-1 are taken into consideration.
Algorithm of laboratory investigation of patients with a clinical suspicion of acromegaly (44 Giustina A, Barkan A, Casanueva FF, Cavagnini F, Frohman L, Ho K, et al. Criteria for cure of acromegaly: a consensus statement. J Clin Endocrinol Metab. 2000;85:526-9.
5 Trainer PJ. Acromegaly – consensus, what consensus? J Clin Endocrinol Metab. 2002;87:3534-6.
6 Levy A. Pituitary disease: presentation, diagnosis, and management. J Neurol Neurosurg Psychiatry. 2004;75 Suppl 3:iii47-52.
7 Pokrajac-Simeunovic A, Trainer PJ. Pitfalls in the diagnosis of acromegaly. Horm Res. 2004;62:74-8.
8 Lim EM, Pullan P; Growth Hormone Research Society; Pituitary Society. Biochemical assessment and long-term monitoring in patients with acromegaly: statement from a joint consensus conference of the Growth Hormone Research Society and the Pituitary Society. Clin Biochem Rev. 2005;26:41-3.
9 Tzanela M. Dynamic tests and basal values for defining active acromegaly. Neuroendocrinology. 2006;83:200-4.
10 Ayuk J, Sheppard MC. Growth hormone and its disorders. Postgrad Med J. 2006;82:24-30.
11 Scacchi M, Cavagnini F. Acromegaly. Pituitary. 2006;9:297-303.
12 Kalavalapalli S, Reid H, Kane J, Buckler H, Trainer P, Heald AH. Silent growth hormone secreting pituitary adenomas: IGF-1 is not sufficient to exclude growth hormone excess. Ann Clin Biochem. 2007; 44:89-93.
13 Giustina A, Barkan A, Chanson P, Grossman A, Hoffman A, Ghigo E, et al. Guidelines for the treatment of growth hormone excess and growth hormone deficiency in adults. J Endocrinol Invest. 2008;31:820-38.
14 Cordero RA, Barkan AL. Current diagnosis of acromegaly. Rev Endocr Metab Disord. 2008;9:13-9.
15 Chanson P, Salenave S. Acromegaly. Orphanet J Rare Dis. 2008;3:17.
16 Cazabat L, Souberbielle JC, Chanson P. Dynamic tests for the diagnosis and assessment of treatment efficacy in acromegaly. Pituitary. 2008;11:129-39.
17 Chanson P, Bertherat J, Beckers A, Bihan H, Brue T, Caron P, et al. French consensus on the management of acromegaly. Ann Endocrinol (Paris). 2009;70:92-106.
18 Cozzi R, Baldelli R, Colao A, Lasio G, Zini M, Attanasio R; Italian Association of Clinical Endocrinologists (AME). AME Position Statement on clinical management of acromegaly. J Endocrinol Invest. 2009;32:2-25.
19 Chanson P, Salenave S, Kamenicky P, Cazabat L, Young J. Pituitary tumours: acromegaly. Best Pract Res Clin Endocrinol Metab. 2009;23:555-74.
20 Barkan A, Bronstein MD, Bruno OD, Cob A, Espinosa-de-los-Monteros AL, Gadelha MR, et al. Management of acromegaly in Latin America: expert panel recommendations. Pituitary. 2010;13: 168-75.
21 Reddy R, Hope S, Wass J. Acromegaly. BMJ. 2010;341:c4189.
22 Vieira Neto L, Abucham J, Araujo LA, Boguszewski CL, Bronstein MD, Czepielewski M, et al. Recommendations of Neuroendocrinology Department from Brazilian Society of Endocrinology and Metabolism for diagnosis and treatment of acromegaly in Brazil. Arq Bras Endocrinol Metabol. 2011;55:91-105.-2323 d’Herbomez M, Bauters C, Cortet-Rudelli C, Dewailly D, Docao C, Wémeau JL. Biomarkers in endocrinology. Presse Med. 2014;43:40-56.).
SUBJECTS AND METHODS
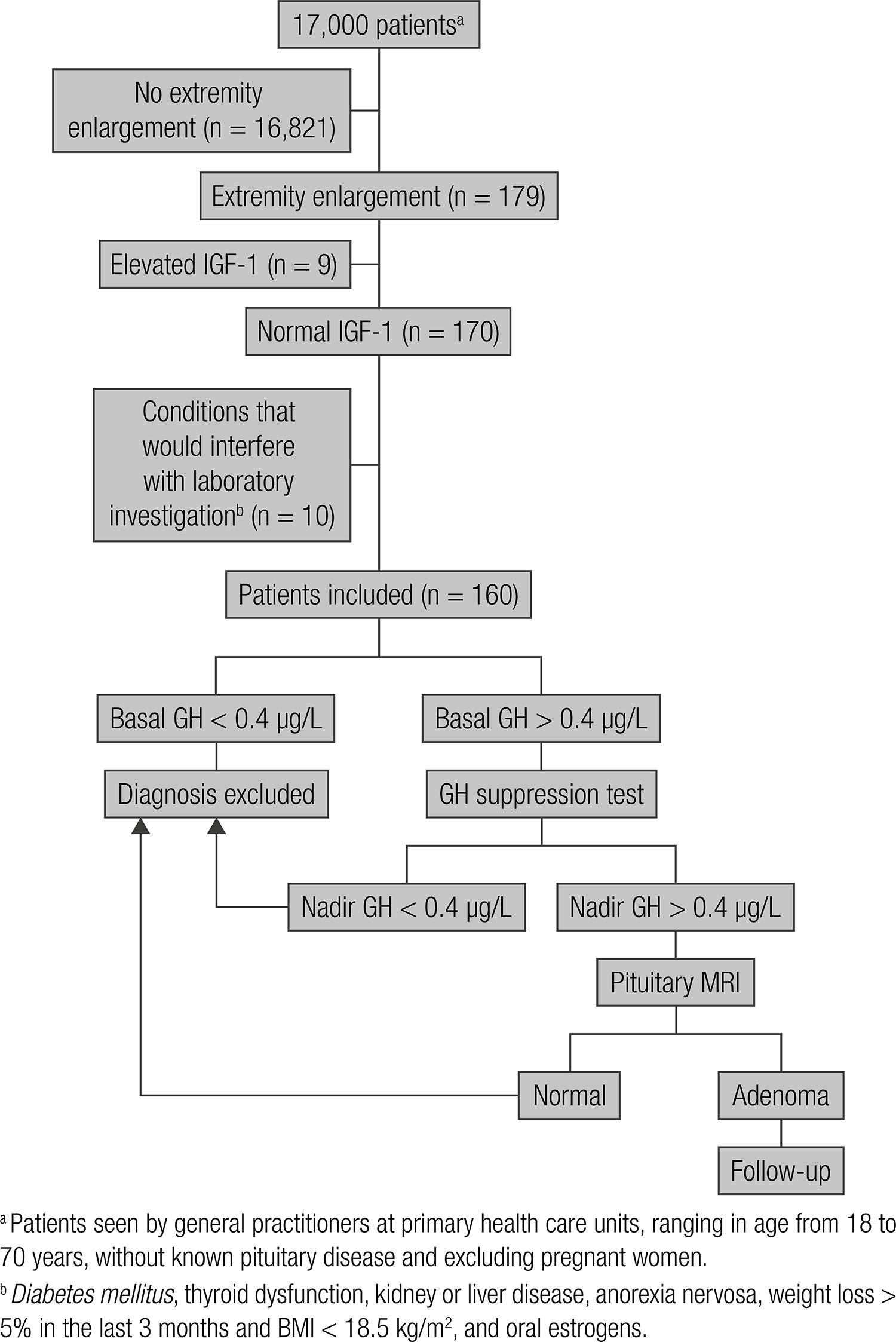
The study and its respective protocol (Figure 2) were approved by the Ethics Committee and informed consent was obtained from all subjects. The study was prospective.
Among the patients seen by general practitioners between July and December 2010 at the nine primary health care units of the city of Belo Horizonte (one per Sanitary District), subjects of both genders ranging in age from 18 to 70 years without known pituitary disease, and excluding pregnant women, were initially evaluated (n = 17,000). The medical records of these patients were analyzed and personal interviews were conducted, including the application of a questionnaire for the detection of extremity enlargement (33 Rosario PW, Calsolari MR. Screening for acromegaly by application of a simple questionnaire evaluating the enlargement of extremities in adult patients seen at primary health care units. Pituitary. 2012;15:179-83.). The items of the questionnaire were: has your shoe size increased over the last 5 years? did you have to change your wedding ring or ring over the last 5 years because it became tight? The interviews were conducted and the questionnaires were applied by nursing students enrolled in the School of Nursing (33 Rosario PW, Calsolari MR. Screening for acromegaly by application of a simple questionnaire evaluating the enlargement of extremities in adult patients seen at primary health care units. Pituitary. 2012;15:179-83.).
One hundred seventy nine patients (1.05%) responded positively to at least one of the items of the questionnaire. Specifically for this study, considering its objective, only patients with normal IGF-1 were included (n = 170). To avoid interference with the laboratory investigation, patients with diabetes mellitus, thyroid dysfunction, kidney or liver disease, anorexia nervosa, weight loss > 5% in the last 3 months and BMI < 18.5 kg/m2, and women receiving oral estrogens were excluded (n = 10). Thus, the sample consisted of 160 patients who reported enlarged extremities (clinical suspicious of acromegaly), but had normal IGF-1 and presented no conditions that would interfere with laboratory investigation.
Basal GH was measured in the same sample as IGF-1. The latter was normal in all
patients (inclusion criteria). When GH levels were > 0.4 µg/L, a new measurement
was obtained during an oral glucose tolerance test (OGTT) (GH before and 30, 60, 90
and 120 min after the oral administration of 75 g anhydrous glucose). The samples
were collected in the morning after an approximately 10-h fast and the subject
rested for 20 min before and during the OGTT. Patients with nadir GH > 0.4 µg/L
(55 Trainer PJ. Acromegaly – consensus, what consensus? J Clin
Endocrinol Metab. 2002;87:3534-6.,77 Pokrajac-Simeunovic A, Trainer PJ. Pitfalls in the diagnosis of
acromegaly. Horm Res. 2004;62:74-8.,88 Lim EM, Pullan P; Growth Hormone Research Society; Pituitary
Society. Biochemical assessment and long-term monitoring in patients with
acromegaly: statement from a joint consensus conference of the Growth Hormone
Research Society and the Pituitary Society. Clin Biochem Rev.
2005;26:41-3.,1111 Scacchi M, Cavagnini F. Acromegaly. Pituitary.
2006;9:297-303.,1414 Cordero RA, Barkan AL. Current diagnosis of acromegaly. Rev Endocr
Metab Disord. 2008;9:13-9.
15 Chanson P, Salenave S. Acromegaly. Orphanet J Rare Dis.
2008;3:17.
16 Cazabat L, Souberbielle JC, Chanson P. Dynamic tests for the
diagnosis and assessment of treatment efficacy in acromegaly. Pituitary.
2008;11:129-39.-1717 Chanson P, Bertherat J, Beckers A, Bihan H, Brue T, Caron P, et al.
French consensus on the management of acromegaly. Ann Endocrinol (Paris).
2009;70:92-106.,1919 Chanson P, Salenave S, Kamenicky P, Cazabat L, Young J. Pituitary
tumours: acromegaly. Best Pract Res Clin Endocrinol Metab.
2009;23:555-74.,2222 Vieira Neto L, Abucham J, Araujo LA, Boguszewski CL, Bronstein MD,
Czepielewski M, et al. Recommendations of Neuroendocrinology Department from
Brazilian Society of Endocrinology and Metabolism for diagnosis and treatment of
acromegaly in Brazil. Arq Bras Endocrinol Metabol.
2011;55:91-105.,2323 d’Herbomez M, Bauters C, Cortet-Rudelli C, Dewailly D, Docao C,
Wémeau JL. Biomarkers in endocrinology. Presse Med.
2014;43:40-56.,2525 Melmed S, Casanueva F, Cavagnini F, Chanson P, Frohman LA, Gaillard
R, et al. Consensus statement: medical management of acromegaly. Eur J
Endocrinol. 2005;153:737-40.
26 Melmed S. Medical progress: acromegaly. N Engl J Med.
2006;355:2558-73.
27 Arellano S, Aguilar P, Domínguez B, Espinosa-de-Los-Monteros AL,
González Virla B, Sosa E, et al. Segundo Consenso Nacional de Acromegalia:
recomendaciones para su diagnóstico, tratamiento y seguimiento. Rev Endocrinol
Nutr. 2007;15:S7-16.
28 Melmed S, Colao A, Barkan A, Molitch M, Grossman AB, Kleinberg D, et
al. Guidelines for acromegaly management: an update. J Clin Endocrinol Metab.
2009;94:1509-17.
29 Giustina A, Chanson P, Bronstein MD, Klibanski A, Lamberts S,
Casanueva FF, et al.; Acromegaly Consensus Group. A consensus on criteria for
cure of acromegaly. J Clin Endocrinol Metab. 2010;95:3141-8.
30 Katznelson L, Atkinson JL, Cook DM, Ezzat SZ, Hamrahian AH, Miller
KK; American Association of Clinical Endocrinologists. American Association of
Clinical Endocrinologists medical guidelines for clinical practice for the
diagnosis and treatment of acromegaly – 2011 update. Endocr Pract.
2011;17:1-44.
31 Melmed S, Casanueva FF, Klibanski A, Bronstein MD, Chanson P,
Lamberts SW, et al. A consensus on the diagnosis and treatment of acromegaly
complications. Pituitary. 2013;16:294-302.-3232 Cordido F, García Arnés JA, Marazuela Aspiroz M, Torres Vela E;
grupo de Neuroendocrinología de la Sociedad Española de Endocrinología y
Nutrición. Practical guidelines for diagnosis and treatment of acromegaly.
Endocrinol Nutr. 2013;60:457.e1-457.e15.) were submitted to MRI of the pituitary
using gadolinium as contrast agent.
GH was measured by chemiluminescence assay (Immulite, Diagnostic Products Corporation, Los Angeles, CA). The analytical and functional sensitivities of the assay were 0.01 μg/L and 0.05 µg/L, respectively; with interassay coefficient of variation < 6.6% at concentrations between 0.3 and 17 μg/L. The standard provided by the kit was calibrated against the World Health Organization (WHO) 2nd International Standard (IS) 98/574. The results are expressed as µg/L. IGF-1 was measured by immunochemiluminescent assay (Immulite 2000, Diagnostic Products Corporation, Los Angeles, CA). The analytical sensitivity of the assay was 25 μg/L, with intra- and interassay coefficients of variation < 8%. Previously established reference values stratified by sex and age based on a sample of 1,000 subjects rigorously selected in the same town where the study was conducted were used (3333 Rosario PW. Normal values of serum IGF-1 in adults: results from a Brazilian population. Arq Bras Endocrinol Metabol. 2010; 4:477-81.).
RESULTS
Women (n = 88)
Basal GH > 0.4 µg/L was observed in 70/88 women (79.5%), who were submitted to the OGTT because of this result. Nadir GH > 0.4 µg/L was found in 21/70 women (30%) (Table 1). These patients were therefore submitted to pituitary MRI, which revealed a lesion compatible with microadenoma (hypointense nodule measuring 4 and 6 mm in diameter and showing no contrast enhancement after the administration of gadolinium) in 2/21 women (9.5%). These two women continue under follow-up (at intervals of 6 months). IGF-1 levels have remained within normal range in subsequent measurements and no progression of clinical manifestations has been observed so far. In addition, reassessment of GH suppression after OGTT, performed after 36 months, still resulted in GH nadir between 0.4 µg/L and 1 µg/L (0.6 and 0.7 µg/L, respectively). The time of follow-up, until now, is 4 years.
Interestingly, nadir GH > 0.4 µg/L on OGTT and indication of pituitary MRI was observed in 14/42 (31%) young or young adult women (age < 50 years) and in 7/46 (15.2%) women ≥ 50 years of age.
Men (n = 72)
Basal GH > 0.4 µg/L was seen in 33/72 men (45.8%), who were submitted to the OGTT because of this finding. Nadir GH was < 0.4 µg/L in all of them, terminating the investigation.
DISCUSSION
The objective of this study, in agreement with the selection criteria and protocol
used, was to evaluate the diagnostic workout results in patients with clinical
suspicion of acromegaly, with normal serum IGF-1 but with GH concentrations > 0.4
µg/L (44 Giustina A, Barkan A, Casanueva FF, Cavagnini F, Frohman L, Ho K, et
al. Criteria for cure of acromegaly: a consensus statement. J Clin Endocrinol
Metab. 2000;85:526-9.
5 Trainer PJ. Acromegaly – consensus, what consensus? J Clin
Endocrinol Metab. 2002;87:3534-6.
6 Levy A. Pituitary disease: presentation, diagnosis, and management.
J Neurol Neurosurg Psychiatry. 2004;75 Suppl 3:iii47-52.
7 Pokrajac-Simeunovic A, Trainer PJ. Pitfalls in the diagnosis of
acromegaly. Horm Res. 2004;62:74-8.
8 Lim EM, Pullan P; Growth Hormone Research Society; Pituitary
Society. Biochemical assessment and long-term monitoring in patients with
acromegaly: statement from a joint consensus conference of the Growth Hormone
Research Society and the Pituitary Society. Clin Biochem Rev.
2005;26:41-3.
9 Tzanela M. Dynamic tests and basal values for defining active
acromegaly. Neuroendocrinology. 2006;83:200-4.
10 Ayuk J, Sheppard MC. Growth hormone and its disorders. Postgrad Med
J. 2006;82:24-30.
11 Scacchi M, Cavagnini F. Acromegaly. Pituitary.
2006;9:297-303.
12 Kalavalapalli S, Reid H, Kane J, Buckler H, Trainer P, Heald AH.
Silent growth hormone secreting pituitary adenomas: IGF-1 is not sufficient to
exclude growth hormone excess. Ann Clin Biochem. 2007;
44:89-93.
13 Giustina A, Barkan A, Chanson P, Grossman A, Hoffman A, Ghigo E, et
al. Guidelines for the treatment of growth hormone excess and growth hormone
deficiency in adults. J Endocrinol Invest. 2008;31:820-38.
14 Cordero RA, Barkan AL. Current diagnosis of acromegaly. Rev Endocr
Metab Disord. 2008;9:13-9.
15 Chanson P, Salenave S. Acromegaly. Orphanet J Rare Dis.
2008;3:17.
16 Cazabat L, Souberbielle JC, Chanson P. Dynamic tests for the
diagnosis and assessment of treatment efficacy in acromegaly. Pituitary.
2008;11:129-39.
17 Chanson P, Bertherat J, Beckers A, Bihan H, Brue T, Caron P, et al.
French consensus on the management of acromegaly. Ann Endocrinol (Paris).
2009;70:92-106.
18 Cozzi R, Baldelli R, Colao A, Lasio G, Zini M, Attanasio R; Italian
Association of Clinical Endocrinologists (AME). AME Position Statement on
clinical management of acromegaly. J Endocrinol Invest.
2009;32:2-25.
19 Chanson P, Salenave S, Kamenicky P, Cazabat L, Young J. Pituitary
tumours: acromegaly. Best Pract Res Clin Endocrinol Metab.
2009;23:555-74.
20 Barkan A, Bronstein MD, Bruno OD, Cob A, Espinosa-de-los-Monteros
AL, Gadelha MR, et al. Management of acromegaly in Latin America: expert panel
recommendations. Pituitary. 2010;13: 168-75.
21 Reddy R, Hope S, Wass J. Acromegaly. BMJ.
2010;341:c4189.
22 Vieira Neto L, Abucham J, Araujo LA, Boguszewski CL, Bronstein MD,
Czepielewski M, et al. Recommendations of Neuroendocrinology Department from
Brazilian Society of Endocrinology and Metabolism for diagnosis and treatment of
acromegaly in Brazil. Arq Bras Endocrinol Metabol.
2011;55:91-105.-2323 d’Herbomez M, Bauters C, Cortet-Rudelli C, Dewailly D, Docao C,
Wémeau JL. Biomarkers in endocrinology. Presse Med.
2014;43:40-56.). It was not our objective, and the results cannot be used, to define
the role of GH (basal or after OGTT) in the diagnostic confirmation of patients with
elevated IGF-1. Therefore, this aspect will not be discussed here.
Serum IGF-1 is an excellent marker of GH secretion and is therefore highly sensitive
in detecting hypersecretion of this hormone. However, concern exists if acromegaly
is ruled out based only on serum normal IGF-1 value (44 Giustina A, Barkan A, Casanueva FF, Cavagnini F, Frohman L, Ho K, et
al. Criteria for cure of acromegaly: a consensus statement. J Clin Endocrinol
Metab. 2000;85:526-9.
5 Trainer PJ. Acromegaly – consensus, what consensus? J Clin
Endocrinol Metab. 2002;87:3534-6.
6 Levy A. Pituitary disease: presentation, diagnosis, and management.
J Neurol Neurosurg Psychiatry. 2004;75 Suppl 3:iii47-52.
7 Pokrajac-Simeunovic A, Trainer PJ. Pitfalls in the diagnosis of
acromegaly. Horm Res. 2004;62:74-8.
8 Lim EM, Pullan P; Growth Hormone Research Society; Pituitary
Society. Biochemical assessment and long-term monitoring in patients with
acromegaly: statement from a joint consensus conference of the Growth Hormone
Research Society and the Pituitary Society. Clin Biochem Rev.
2005;26:41-3.
9 Tzanela M. Dynamic tests and basal values for defining active
acromegaly. Neuroendocrinology. 2006;83:200-4.
10 Ayuk J, Sheppard MC. Growth hormone and its disorders. Postgrad Med
J. 2006;82:24-30.
11 Scacchi M, Cavagnini F. Acromegaly. Pituitary.
2006;9:297-303.
12 Kalavalapalli S, Reid H, Kane J, Buckler H, Trainer P, Heald AH.
Silent growth hormone secreting pituitary adenomas: IGF-1 is not sufficient to
exclude growth hormone excess. Ann Clin Biochem. 2007;
44:89-93.
13 Giustina A, Barkan A, Chanson P, Grossman A, Hoffman A, Ghigo E, et
al. Guidelines for the treatment of growth hormone excess and growth hormone
deficiency in adults. J Endocrinol Invest. 2008;31:820-38.
14 Cordero RA, Barkan AL. Current diagnosis of acromegaly. Rev Endocr
Metab Disord. 2008;9:13-9.
15 Chanson P, Salenave S. Acromegaly. Orphanet J Rare Dis.
2008;3:17.
16 Cazabat L, Souberbielle JC, Chanson P. Dynamic tests for the
diagnosis and assessment of treatment efficacy in acromegaly. Pituitary.
2008;11:129-39.
17 Chanson P, Bertherat J, Beckers A, Bihan H, Brue T, Caron P, et al.
French consensus on the management of acromegaly. Ann Endocrinol (Paris).
2009;70:92-106.
18 Cozzi R, Baldelli R, Colao A, Lasio G, Zini M, Attanasio R; Italian
Association of Clinical Endocrinologists (AME). AME Position Statement on
clinical management of acromegaly. J Endocrinol Invest.
2009;32:2-25.
19 Chanson P, Salenave S, Kamenicky P, Cazabat L, Young J. Pituitary
tumours: acromegaly. Best Pract Res Clin Endocrinol Metab.
2009;23:555-74.
20 Barkan A, Bronstein MD, Bruno OD, Cob A, Espinosa-de-los-Monteros
AL, Gadelha MR, et al. Management of acromegaly in Latin America: expert panel
recommendations. Pituitary. 2010;13: 168-75.
21 Reddy R, Hope S, Wass J. Acromegaly. BMJ.
2010;341:c4189.
22 Vieira Neto L, Abucham J, Araujo LA, Boguszewski CL, Bronstein MD,
Czepielewski M, et al. Recommendations of Neuroendocrinology Department from
Brazilian Society of Endocrinology and Metabolism for diagnosis and treatment of
acromegaly in Brazil. Arq Bras Endocrinol Metabol.
2011;55:91-105.-2323 d’Herbomez M, Bauters C, Cortet-Rudelli C, Dewailly D, Docao C,
Wémeau JL. Biomarkers in endocrinology. Presse Med.
2014;43:40-56.), mainly because of
analytical problems and the lack of adequate normal reference values for many assays
(55 Trainer PJ. Acromegaly – consensus, what consensus? J Clin
Endocrinol Metab. 2002;87:3534-6.,88 Lim EM, Pullan P; Growth Hormone Research Society; Pituitary
Society. Biochemical assessment and long-term monitoring in patients with
acromegaly: statement from a joint consensus conference of the Growth Hormone
Research Society and the Pituitary Society. Clin Biochem Rev.
2005;26:41-3.,1717 Chanson P, Bertherat J, Beckers A, Bihan H, Brue T, Caron P, et al.
French consensus on the management of acromegaly. Ann Endocrinol (Paris).
2009;70:92-106.,1818 Cozzi R, Baldelli R, Colao A, Lasio G, Zini M, Attanasio R; Italian
Association of Clinical Endocrinologists (AME). AME Position Statement on
clinical management of acromegaly. J Endocrinol Invest.
2009;32:2-25.,2020 Barkan A, Bronstein MD, Bruno OD, Cob A, Espinosa-de-los-Monteros
AL, Gadelha MR, et al. Management of acromegaly in Latin America: expert panel
recommendations. Pituitary. 2010;13: 168-75.,2323 d’Herbomez M, Bauters C, Cortet-Rudelli C, Dewailly D, Docao C,
Wémeau JL. Biomarkers in endocrinology. Presse Med.
2014;43:40-56.,3333 Rosario PW. Normal values of serum IGF-1 in adults: results from a
Brazilian population. Arq Bras Endocrinol Metabol. 2010;
4:477-81.),
even in the absence of clinical conditions that interfere (reduce) with serum IGF-1.
Therefore, many authors also recommend the measurement of basal GH to rule out the
disease (44 Giustina A, Barkan A, Casanueva FF, Cavagnini F, Frohman L, Ho K, et
al. Criteria for cure of acromegaly: a consensus statement. J Clin Endocrinol
Metab. 2000;85:526-9.
5 Trainer PJ. Acromegaly – consensus, what consensus? J Clin
Endocrinol Metab. 2002;87:3534-6.
6 Levy A. Pituitary disease: presentation, diagnosis, and management.
J Neurol Neurosurg Psychiatry. 2004;75 Suppl 3:iii47-52.
7 Pokrajac-Simeunovic A, Trainer PJ. Pitfalls in the diagnosis of
acromegaly. Horm Res. 2004;62:74-8.
8 Lim EM, Pullan P; Growth Hormone Research Society; Pituitary
Society. Biochemical assessment and long-term monitoring in patients with
acromegaly: statement from a joint consensus conference of the Growth Hormone
Research Society and the Pituitary Society. Clin Biochem Rev.
2005;26:41-3.
9 Tzanela M. Dynamic tests and basal values for defining active
acromegaly. Neuroendocrinology. 2006;83:200-4.
10 Ayuk J, Sheppard MC. Growth hormone and its disorders. Postgrad Med
J. 2006;82:24-30.
11 Scacchi M, Cavagnini F. Acromegaly. Pituitary.
2006;9:297-303.
12 Kalavalapalli S, Reid H, Kane J, Buckler H, Trainer P, Heald AH.
Silent growth hormone secreting pituitary adenomas: IGF-1 is not sufficient to
exclude growth hormone excess. Ann Clin Biochem. 2007;
44:89-93.
13 Giustina A, Barkan A, Chanson P, Grossman A, Hoffman A, Ghigo E, et
al. Guidelines for the treatment of growth hormone excess and growth hormone
deficiency in adults. J Endocrinol Invest. 2008;31:820-38.
14 Cordero RA, Barkan AL. Current diagnosis of acromegaly. Rev Endocr
Metab Disord. 2008;9:13-9.
15 Chanson P, Salenave S. Acromegaly. Orphanet J Rare Dis.
2008;3:17.
16 Cazabat L, Souberbielle JC, Chanson P. Dynamic tests for the
diagnosis and assessment of treatment efficacy in acromegaly. Pituitary.
2008;11:129-39.
17 Chanson P, Bertherat J, Beckers A, Bihan H, Brue T, Caron P, et al.
French consensus on the management of acromegaly. Ann Endocrinol (Paris).
2009;70:92-106.
18 Cozzi R, Baldelli R, Colao A, Lasio G, Zini M, Attanasio R; Italian
Association of Clinical Endocrinologists (AME). AME Position Statement on
clinical management of acromegaly. J Endocrinol Invest.
2009;32:2-25.
19 Chanson P, Salenave S, Kamenicky P, Cazabat L, Young J. Pituitary
tumours: acromegaly. Best Pract Res Clin Endocrinol Metab.
2009;23:555-74.
20 Barkan A, Bronstein MD, Bruno OD, Cob A, Espinosa-de-los-Monteros
AL, Gadelha MR, et al. Management of acromegaly in Latin America: expert panel
recommendations. Pituitary. 2010;13: 168-75.
21 Reddy R, Hope S, Wass J. Acromegaly. BMJ.
2010;341:c4189.
22 Vieira Neto L, Abucham J, Araujo LA, Boguszewski CL, Bronstein MD,
Czepielewski M, et al. Recommendations of Neuroendocrinology Department from
Brazilian Society of Endocrinology and Metabolism for diagnosis and treatment of
acromegaly in Brazil. Arq Bras Endocrinol Metabol.
2011;55:91-105.-2323 d’Herbomez M, Bauters C, Cortet-Rudelli C, Dewailly D, Docao C,
Wémeau JL. Biomarkers in endocrinology. Presse Med.
2014;43:40-56.). The present study showed that not ruling out acromegaly diagnosis
when serum IGF-1 is normal but GH is > 0.4 µg/L (44 Giustina A, Barkan A, Casanueva FF, Cavagnini F, Frohman L, Ho K, et
al. Criteria for cure of acromegaly: a consensus statement. J Clin Endocrinol
Metab. 2000;85:526-9.
5 Trainer PJ. Acromegaly – consensus, what consensus? J Clin
Endocrinol Metab. 2002;87:3534-6.
6 Levy A. Pituitary disease: presentation, diagnosis, and management.
J Neurol Neurosurg Psychiatry. 2004;75 Suppl 3:iii47-52.
7 Pokrajac-Simeunovic A, Trainer PJ. Pitfalls in the diagnosis of
acromegaly. Horm Res. 2004;62:74-8.
8 Lim EM, Pullan P; Growth Hormone Research Society; Pituitary
Society. Biochemical assessment and long-term monitoring in patients with
acromegaly: statement from a joint consensus conference of the Growth Hormone
Research Society and the Pituitary Society. Clin Biochem Rev.
2005;26:41-3.
9 Tzanela M. Dynamic tests and basal values for defining active
acromegaly. Neuroendocrinology. 2006;83:200-4.
10 Ayuk J, Sheppard MC. Growth hormone and its disorders. Postgrad Med
J. 2006;82:24-30.
11 Scacchi M, Cavagnini F. Acromegaly. Pituitary.
2006;9:297-303.
12 Kalavalapalli S, Reid H, Kane J, Buckler H, Trainer P, Heald AH.
Silent growth hormone secreting pituitary adenomas: IGF-1 is not sufficient to
exclude growth hormone excess. Ann Clin Biochem. 2007;
44:89-93.
13 Giustina A, Barkan A, Chanson P, Grossman A, Hoffman A, Ghigo E, et
al. Guidelines for the treatment of growth hormone excess and growth hormone
deficiency in adults. J Endocrinol Invest. 2008;31:820-38.
14 Cordero RA, Barkan AL. Current diagnosis of acromegaly. Rev Endocr
Metab Disord. 2008;9:13-9.
15 Chanson P, Salenave S. Acromegaly. Orphanet J Rare Dis.
2008;3:17.
16 Cazabat L, Souberbielle JC, Chanson P. Dynamic tests for the
diagnosis and assessment of treatment efficacy in acromegaly. Pituitary.
2008;11:129-39.
17 Chanson P, Bertherat J, Beckers A, Bihan H, Brue T, Caron P, et al.
French consensus on the management of acromegaly. Ann Endocrinol (Paris).
2009;70:92-106.
18 Cozzi R, Baldelli R, Colao A, Lasio G, Zini M, Attanasio R; Italian
Association of Clinical Endocrinologists (AME). AME Position Statement on
clinical management of acromegaly. J Endocrinol Invest.
2009;32:2-25.
19 Chanson P, Salenave S, Kamenicky P, Cazabat L, Young J. Pituitary
tumours: acromegaly. Best Pract Res Clin Endocrinol Metab.
2009;23:555-74.
20 Barkan A, Bronstein MD, Bruno OD, Cob A, Espinosa-de-los-Monteros
AL, Gadelha MR, et al. Management of acromegaly in Latin America: expert panel
recommendations. Pituitary. 2010;13: 168-75.
21 Reddy R, Hope S, Wass J. Acromegaly. BMJ.
2010;341:c4189.
22 Vieira Neto L, Abucham J, Araujo LA, Boguszewski CL, Bronstein MD,
Czepielewski M, et al. Recommendations of Neuroendocrinology Department from
Brazilian Society of Endocrinology and Metabolism for diagnosis and treatment of
acromegaly in Brazil. Arq Bras Endocrinol Metabol.
2011;55:91-105.-2323 d’Herbomez M, Bauters C, Cortet-Rudelli C, Dewailly D, Docao C,
Wémeau JL. Biomarkers in endocrinology. Presse Med.
2014;43:40-56.) lead often to the
indication of GH suppression test, and less frenquently to MRI, which are expensive
procedures and have the risk of providing an equivocal diagnosis, particularly in
women.
The present results do not seem to be overestimated. First, because conditions that
could increase serum GH concentrations were rigorously excluded. In this respect,
the use of oral contraceptives is common among women and discontinuation for a
sufficient period of time is not always advised or possible before laboratory
investigation. As a consequence, the frequency of GH > 0.4 µg/L may be even
higher than the observed in the present study. Secondly, the finding of lesions
compatible with microadenoma upon MRI is also not unexpected since these lesions can
be detected in more than 10% of the adult population (3434 Chambers EF, Turski PA, LaMasters D, Newton TH. Regions of low
density in the contrast-enhanced pituitary gland: normal and pathologic
processes. Radiology. 1982;144:109-13.,3535 Hall WA, Luciano MG, Doppman JL, Patronas NJ, Oldfield EH. Pituitary
magnetic resonance imaging in normal human volunteers: occult adenomas in the
general population. Ann Intern Med. 1994;120:817-20.). Thirdly, the
current consensus is that nadir GH levels > 0.4 µg/L after OGTT are considered to
be altered (55 Trainer PJ. Acromegaly – consensus, what consensus? J Clin
Endocrinol Metab. 2002;87:3534-6.,77 Pokrajac-Simeunovic A, Trainer PJ. Pitfalls in the diagnosis of
acromegaly. Horm Res. 2004;62:74-8.,88 Lim EM, Pullan P; Growth Hormone Research Society; Pituitary
Society. Biochemical assessment and long-term monitoring in patients with
acromegaly: statement from a joint consensus conference of the Growth Hormone
Research Society and the Pituitary Society. Clin Biochem Rev.
2005;26:41-3.,1111 Scacchi M, Cavagnini F. Acromegaly. Pituitary.
2006;9:297-303.,1414 Cordero RA, Barkan AL. Current diagnosis of acromegaly. Rev Endocr
Metab Disord. 2008;9:13-9.
15 Chanson P, Salenave S. Acromegaly. Orphanet J Rare Dis.
2008;3:17.
16 Cazabat L, Souberbielle JC, Chanson P. Dynamic tests for the
diagnosis and assessment of treatment efficacy in acromegaly. Pituitary.
2008;11:129-39.-1717 Chanson P, Bertherat J, Beckers A, Bihan H, Brue T, Caron P, et al.
French consensus on the management of acromegaly. Ann Endocrinol (Paris).
2009;70:92-106.,1919 Chanson P, Salenave S, Kamenicky P, Cazabat L, Young J. Pituitary
tumours: acromegaly. Best Pract Res Clin Endocrinol Metab.
2009;23:555-74.,2222 Vieira Neto L, Abucham J, Araujo LA, Boguszewski CL, Bronstein MD,
Czepielewski M, et al. Recommendations of Neuroendocrinology Department from
Brazilian Society of Endocrinology and Metabolism for diagnosis and treatment of
acromegaly in Brazil. Arq Bras Endocrinol Metabol.
2011;55:91-105.,2323 d’Herbomez M, Bauters C, Cortet-Rudelli C, Dewailly D, Docao C,
Wémeau JL. Biomarkers in endocrinology. Presse Med.
2014;43:40-56.,2525 Melmed S, Casanueva F, Cavagnini F, Chanson P, Frohman LA, Gaillard
R, et al. Consensus statement: medical management of acromegaly. Eur J
Endocrinol. 2005;153:737-40.
26 Melmed S. Medical progress: acromegaly. N Engl J Med.
2006;355:2558-73.
27 Arellano S, Aguilar P, Domínguez B, Espinosa-de-Los-Monteros AL,
González Virla B, Sosa E, et al. Segundo Consenso Nacional de Acromegalia:
recomendaciones para su diagnóstico, tratamiento y seguimiento. Rev Endocrinol
Nutr. 2007;15:S7-16.
28 Melmed S, Colao A, Barkan A, Molitch M, Grossman AB, Kleinberg D, et
al. Guidelines for acromegaly management: an update. J Clin Endocrinol Metab.
2009;94:1509-17.
29 Giustina A, Chanson P, Bronstein MD, Klibanski A, Lamberts S,
Casanueva FF, et al.; Acromegaly Consensus Group. A consensus on criteria for
cure of acromegaly. J Clin Endocrinol Metab. 2010;95:3141-8.
30 Katznelson L, Atkinson JL, Cook DM, Ezzat SZ, Hamrahian AH, Miller
KK; American Association of Clinical Endocrinologists. American Association of
Clinical Endocrinologists medical guidelines for clinical practice for the
diagnosis and treatment of acromegaly – 2011 update. Endocr Pract.
2011;17:1-44.
31 Melmed S, Casanueva FF, Klibanski A, Bronstein MD, Chanson P,
Lamberts SW, et al. A consensus on the diagnosis and treatment of acromegaly
complications. Pituitary. 2013;16:294-302.-3232 Cordido F, García Arnés JA, Marazuela Aspiroz M, Torres Vela E;
grupo de Neuroendocrinología de la Sociedad Española de Endocrinología y
Nutrición. Practical guidelines for diagnosis and treatment of acromegaly.
Endocrinol Nutr. 2013;60:457.e1-457.e15.). Some authors recommend even lower cut-off
values such as 0.3 µg/L (55 Trainer PJ. Acromegaly – consensus, what consensus? J Clin
Endocrinol Metab. 2002;87:3534-6.,88 Lim EM, Pullan P; Growth Hormone Research Society; Pituitary
Society. Biochemical assessment and long-term monitoring in patients with
acromegaly: statement from a joint consensus conference of the Growth Hormone
Research Society and the Pituitary Society. Clin Biochem Rev.
2005;26:41-3.,1111 Scacchi M, Cavagnini F. Acromegaly. Pituitary.
2006;9:297-303.,1515 Chanson P, Salenave S. Acromegaly. Orphanet J Rare Dis.
2008;3:17.
16 Cazabat L, Souberbielle JC, Chanson P. Dynamic tests for the
diagnosis and assessment of treatment efficacy in acromegaly. Pituitary.
2008;11:129-39.-1717 Chanson P, Bertherat J, Beckers A, Bihan H, Brue T, Caron P, et al.
French consensus on the management of acromegaly. Ann Endocrinol (Paris).
2009;70:92-106.,1919 Chanson P, Salenave S, Kamenicky P, Cazabat L, Young J. Pituitary
tumours: acromegaly. Best Pract Res Clin Endocrinol Metab.
2009;23:555-74.,2323 d’Herbomez M, Bauters C, Cortet-Rudelli C, Dewailly D, Docao C,
Wémeau JL. Biomarkers in endocrinology. Presse Med.
2014;43:40-56.,2626 Melmed S. Medical progress: acromegaly. N Engl J Med.
2006;355:2558-73.),
0.25 µg/L (77 Pokrajac-Simeunovic A, Trainer PJ. Pitfalls in the diagnosis of
acromegaly. Horm Res. 2004;62:74-8.), and 0.2 µg/L (1414 Cordero RA, Barkan AL. Current diagnosis of acromegaly. Rev Endocr
Metab Disord. 2008;9:13-9.).
As only patients with clinical suspicion of acromegaly based on a specific questionnaire for the detection of extremity enlargement (33 Rosario PW, Calsolari MR. Screening for acromegaly by application of a simple questionnaire evaluating the enlargement of extremities in adult patients seen at primary health care units. Pituitary. 2012;15:179-83.) were included in this study, one can not argue that the tests were performed in subjects without clinical indication or that in clinical practice the investigation of cases with GH > 0.4 µg/L and normal IGF-1 would provide different results, i.e., leading frequently to the diagnosis of acromegaly. We emphasize that an earlier diagnosis of acromegaly requires increasing the number of suspicious and investigated cases, which tends to reduce the positive predictive value of the tests.
In view of the importance of diagnosing acromegaly and not delaying the diagnosis, as well as the existence (although uncommon) of cases with normal serum IGF-1 (1212 Kalavalapalli S, Reid H, Kane J, Buckler H, Trainer P, Heald AH. Silent growth hormone secreting pituitary adenomas: IGF-1 is not sufficient to exclude growth hormone excess. Ann Clin Biochem. 2007; 44:89-93.,2424 Petersenn S, Buchfelder M, Reincke M, Strasburger CM, Franz H, Lohmann R, et al.; Participants of the German Acromegaly Register. Results of surgical and somatostatin analog therapies and their combination in acromegaly: a retrospective analysis of the German Acromegaly Register. Eur J Endocrinol. 2008;159: 525-32.), in some special situations it is reasonable not to rule out the disease based only on IGF-1 levels within the normal range (Table 2), but the assessment should be complemented by the measurement of GH. However, in most cases GH measurement would not be necessary to rule out the disease.
In conclusion, in patients with clinical suspicion of acromegaly, the use of the
recommended algorithm shown in Figure 1 (44 Giustina A, Barkan A, Casanueva FF, Cavagnini F, Frohman L, Ho K, et
al. Criteria for cure of acromegaly: a consensus statement. J Clin Endocrinol
Metab. 2000;85:526-9.
5 Trainer PJ. Acromegaly – consensus, what consensus? J Clin
Endocrinol Metab. 2002;87:3534-6.
6 Levy A. Pituitary disease: presentation, diagnosis, and management.
J Neurol Neurosurg Psychiatry. 2004;75 Suppl 3:iii47-52.
7 Pokrajac-Simeunovic A, Trainer PJ. Pitfalls in the diagnosis of
acromegaly. Horm Res. 2004;62:74-8.
8 Lim EM, Pullan P; Growth Hormone Research Society; Pituitary
Society. Biochemical assessment and long-term monitoring in patients with
acromegaly: statement from a joint consensus conference of the Growth Hormone
Research Society and the Pituitary Society. Clin Biochem Rev.
2005;26:41-3.
9 Tzanela M. Dynamic tests and basal values for defining active
acromegaly. Neuroendocrinology. 2006;83:200-4.
10 Ayuk J, Sheppard MC. Growth hormone and its disorders. Postgrad Med
J. 2006;82:24-30.
11 Scacchi M, Cavagnini F. Acromegaly. Pituitary.
2006;9:297-303.
12 Kalavalapalli S, Reid H, Kane J, Buckler H, Trainer P, Heald AH.
Silent growth hormone secreting pituitary adenomas: IGF-1 is not sufficient to
exclude growth hormone excess. Ann Clin Biochem. 2007;
44:89-93.
13 Giustina A, Barkan A, Chanson P, Grossman A, Hoffman A, Ghigo E, et
al. Guidelines for the treatment of growth hormone excess and growth hormone
deficiency in adults. J Endocrinol Invest. 2008;31:820-38.
14 Cordero RA, Barkan AL. Current diagnosis of acromegaly. Rev Endocr
Metab Disord. 2008;9:13-9.
15 Chanson P, Salenave S. Acromegaly. Orphanet J Rare Dis.
2008;3:17.
16 Cazabat L, Souberbielle JC, Chanson P. Dynamic tests for the
diagnosis and assessment of treatment efficacy in acromegaly. Pituitary.
2008;11:129-39.
17 Chanson P, Bertherat J, Beckers A, Bihan H, Brue T, Caron P, et al.
French consensus on the management of acromegaly. Ann Endocrinol (Paris).
2009;70:92-106.
18 Cozzi R, Baldelli R, Colao A, Lasio G, Zini M, Attanasio R; Italian
Association of Clinical Endocrinologists (AME). AME Position Statement on
clinical management of acromegaly. J Endocrinol Invest.
2009;32:2-25.
19 Chanson P, Salenave S, Kamenicky P, Cazabat L, Young J. Pituitary
tumours: acromegaly. Best Pract Res Clin Endocrinol Metab.
2009;23:555-74.
20 Barkan A, Bronstein MD, Bruno OD, Cob A, Espinosa-de-los-Monteros
AL, Gadelha MR, et al. Management of acromegaly in Latin America: expert panel
recommendations. Pituitary. 2010;13: 168-75.
21 Reddy R, Hope S, Wass J. Acromegaly. BMJ.
2010;341:c4189.
22 Vieira Neto L, Abucham J, Araujo LA, Boguszewski CL, Bronstein MD,
Czepielewski M, et al. Recommendations of Neuroendocrinology Department from
Brazilian Society of Endocrinology and Metabolism for diagnosis and treatment of
acromegaly in Brazil. Arq Bras Endocrinol Metabol.
2011;55:91-105.-2323 d’Herbomez M, Bauters C, Cortet-Rudelli C, Dewailly D, Docao C,
Wémeau JL. Biomarkers in endocrinology. Presse Med.
2014;43:40-56.),
which also takes into consideration GH concentrations > 0.4 µg/L in the presence
of normal serum IGF-1 as diagnostic, results in the unwarranted complementary
investigation in many cases and even in possible equivocal diagnoses (the latter in
women). It is only in exceptional cases that normal serum IGF-1 should not rule out
acromegaly (Table 2).
REFERENCES
-
1Schneider HJ, Sievers C, Saller B, Wittchen HU, Stalla GK. High prevalence of biochemical acromegaly in primary care patients with elevated IGF-1 levels. Clin Endocrinol (Oxf). 2008;69:432-5.
-
2Rosario PW. Frequency of acromegaly in adults with diabetes or glucose intolerance and estimated prevalence in the general population. Pituitary. 2011; 14:217-21.
-
3Rosario PW, Calsolari MR. Screening for acromegaly by application of a simple questionnaire evaluating the enlargement of extremities in adult patients seen at primary health care units. Pituitary. 2012;15:179-83.
-
4Giustina A, Barkan A, Casanueva FF, Cavagnini F, Frohman L, Ho K, et al. Criteria for cure of acromegaly: a consensus statement. J Clin Endocrinol Metab. 2000;85:526-9.
-
5Trainer PJ. Acromegaly – consensus, what consensus? J Clin Endocrinol Metab. 2002;87:3534-6.
-
6Levy A. Pituitary disease: presentation, diagnosis, and management. J Neurol Neurosurg Psychiatry. 2004;75 Suppl 3:iii47-52.
-
7Pokrajac-Simeunovic A, Trainer PJ. Pitfalls in the diagnosis of acromegaly. Horm Res. 2004;62:74-8.
-
8Lim EM, Pullan P; Growth Hormone Research Society; Pituitary Society. Biochemical assessment and long-term monitoring in patients with acromegaly: statement from a joint consensus conference of the Growth Hormone Research Society and the Pituitary Society. Clin Biochem Rev. 2005;26:41-3.
-
9Tzanela M. Dynamic tests and basal values for defining active acromegaly. Neuroendocrinology. 2006;83:200-4.
-
10Ayuk J, Sheppard MC. Growth hormone and its disorders. Postgrad Med J. 2006;82:24-30.
-
11Scacchi M, Cavagnini F. Acromegaly. Pituitary. 2006;9:297-303.
-
12Kalavalapalli S, Reid H, Kane J, Buckler H, Trainer P, Heald AH. Silent growth hormone secreting pituitary adenomas: IGF-1 is not sufficient to exclude growth hormone excess. Ann Clin Biochem. 2007; 44:89-93.
-
13Giustina A, Barkan A, Chanson P, Grossman A, Hoffman A, Ghigo E, et al. Guidelines for the treatment of growth hormone excess and growth hormone deficiency in adults. J Endocrinol Invest. 2008;31:820-38.
-
14Cordero RA, Barkan AL. Current diagnosis of acromegaly. Rev Endocr Metab Disord. 2008;9:13-9.
-
15Chanson P, Salenave S. Acromegaly. Orphanet J Rare Dis. 2008;3:17.
-
16Cazabat L, Souberbielle JC, Chanson P. Dynamic tests for the diagnosis and assessment of treatment efficacy in acromegaly. Pituitary. 2008;11:129-39.
-
17Chanson P, Bertherat J, Beckers A, Bihan H, Brue T, Caron P, et al. French consensus on the management of acromegaly. Ann Endocrinol (Paris). 2009;70:92-106.
-
18Cozzi R, Baldelli R, Colao A, Lasio G, Zini M, Attanasio R; Italian Association of Clinical Endocrinologists (AME). AME Position Statement on clinical management of acromegaly. J Endocrinol Invest. 2009;32:2-25.
-
19Chanson P, Salenave S, Kamenicky P, Cazabat L, Young J. Pituitary tumours: acromegaly. Best Pract Res Clin Endocrinol Metab. 2009;23:555-74.
-
20Barkan A, Bronstein MD, Bruno OD, Cob A, Espinosa-de-los-Monteros AL, Gadelha MR, et al. Management of acromegaly in Latin America: expert panel recommendations. Pituitary. 2010;13: 168-75.
-
21Reddy R, Hope S, Wass J. Acromegaly. BMJ. 2010;341:c4189.
-
22Vieira Neto L, Abucham J, Araujo LA, Boguszewski CL, Bronstein MD, Czepielewski M, et al. Recommendations of Neuroendocrinology Department from Brazilian Society of Endocrinology and Metabolism for diagnosis and treatment of acromegaly in Brazil. Arq Bras Endocrinol Metabol. 2011;55:91-105.
-
23d’Herbomez M, Bauters C, Cortet-Rudelli C, Dewailly D, Docao C, Wémeau JL. Biomarkers in endocrinology. Presse Med. 2014;43:40-56.
-
24Petersenn S, Buchfelder M, Reincke M, Strasburger CM, Franz H, Lohmann R, et al.; Participants of the German Acromegaly Register. Results of surgical and somatostatin analog therapies and their combination in acromegaly: a retrospective analysis of the German Acromegaly Register. Eur J Endocrinol. 2008;159: 525-32.
-
25Melmed S, Casanueva F, Cavagnini F, Chanson P, Frohman LA, Gaillard R, et al. Consensus statement: medical management of acromegaly. Eur J Endocrinol. 2005;153:737-40.
-
26Melmed S. Medical progress: acromegaly. N Engl J Med. 2006;355:2558-73.
-
27Arellano S, Aguilar P, Domínguez B, Espinosa-de-Los-Monteros AL, González Virla B, Sosa E, et al. Segundo Consenso Nacional de Acromegalia: recomendaciones para su diagnóstico, tratamiento y seguimiento. Rev Endocrinol Nutr. 2007;15:S7-16.
-
28Melmed S, Colao A, Barkan A, Molitch M, Grossman AB, Kleinberg D, et al. Guidelines for acromegaly management: an update. J Clin Endocrinol Metab. 2009;94:1509-17.
-
29Giustina A, Chanson P, Bronstein MD, Klibanski A, Lamberts S, Casanueva FF, et al.; Acromegaly Consensus Group. A consensus on criteria for cure of acromegaly. J Clin Endocrinol Metab. 2010;95:3141-8.
-
30Katznelson L, Atkinson JL, Cook DM, Ezzat SZ, Hamrahian AH, Miller KK; American Association of Clinical Endocrinologists. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the diagnosis and treatment of acromegaly – 2011 update. Endocr Pract. 2011;17:1-44.
-
31Melmed S, Casanueva FF, Klibanski A, Bronstein MD, Chanson P, Lamberts SW, et al. A consensus on the diagnosis and treatment of acromegaly complications. Pituitary. 2013;16:294-302.
-
32Cordido F, García Arnés JA, Marazuela Aspiroz M, Torres Vela E; grupo de Neuroendocrinología de la Sociedad Española de Endocrinología y Nutrición. Practical guidelines for diagnosis and treatment of acromegaly. Endocrinol Nutr. 2013;60:457.e1-457.e15.
-
33Rosario PW. Normal values of serum IGF-1 in adults: results from a Brazilian population. Arq Bras Endocrinol Metabol. 2010; 4:477-81.
-
34Chambers EF, Turski PA, LaMasters D, Newton TH. Regions of low density in the contrast-enhanced pituitary gland: normal and pathologic processes. Radiology. 1982;144:109-13.
-
35Hall WA, Luciano MG, Doppman JL, Patronas NJ, Oldfield EH. Pituitary magnetic resonance imaging in normal human volunteers: occult adenomas in the general population. Ann Intern Med. 1994;120:817-20.
-
36Clemmons DR. Consensus statement on the standardization and evaluation of growth hormone and insulin-like growth factor assays. Clin Chem. 2011;57:555-9.
-
a
Patients seen by general practitioners at primary health care units, ranging in age from 18 to 70 years, without known pituitary disease and excluding pregnant women.
-
b
Diabetes mellitus, thyroid dysfunction, kidney or liver disease, anorexia nervosa, weight loss > 5% in the last 3 months and BMI < 18.5 kg/m2, and oral estrogens.
Publication Dates
-
Publication in this collection
Feb 2015
History
-
Received
29 May 2014 -
Accepted
24 Oct 2014