Abstract
Objective
Our aim in the present study was to elucidate how type 1 diabetes mellitus (T1DM) and sleep parameters interact, which was rarely evaluated up to the moment.
Materials and methods
Eighteen T1DM subjects without chronic complications, and 9 control subjects, matched for age and BMI, were studied. The following instruments used to evaluate sleep: the Epworth Sleepiness Scale, sleep diaries, actimeters, and polysomnography in a Sleep Lab. Glycemic control in T1DM individuals was evaluated through: A1C, home fingertip glucometer for 10 days (concomitant with the sleep diary and actimeter), and CGM or concomitant with continuous glucose monitoring (during the polysomnography night).
Results
Comparing with the control group, individuals with diabetes presented more pronounced sleep extension from weekdays to weekends than control subjects (p = 0.0303). Among T1DM, glycemic variability (SD) was positively correlated with sleep latency (r = 0.6525, p = 0.0033); full awakening index and arousal index were positively correlated with A1C (r = 0.6544, p = 0.0081; and r = 0.5680, p = 0.0272, respectively); and mean glycemia values were negatively correlated with sleep quality in T1DM individuals with better glycemic control (mean glycemia < 154 mg/dL).
Conclusion
Our results support the hypothesis of an interaction between sleep parameters and T1DM, where the glycemic control plays an important role. More studies are needed to unveil the mechanisms behind this interaction, which may allow, in the future, clinicians and educators to consider sleep in the effort of regulating glycemic control. Arch Endocrinol Metab. 2015;59(1):71-8
Type 1 diabetes mellitus; sleep; blood glucose; sleep-wake cycle disorders
INTRODUCTION
There is growing evidence that sleep disorders and sleep loss affect glucose
metabolism and insulin resistance (11 Leproult R, Balbo M, Spiegel K. Role of sleep duration in the
regulation of glucose metabolism and appetite. Best Pract Res Clin Endocrinol
Metab. 2010;24:687-702.
2 Tasali E, Leproult R, Ehrmann DA, Van Cauter E. Slow-wave sleep and
the risk of type 2 diabetes in humans. Proc Natl Acad Sci U S A.
2008;105:1044-9.
3 Meisinger C, Heier M, Loewel H, Study MKAC. Sleep disturbance as a
predictor of type 2 diabetes mellitus in men and women from the general
population. Diabetologia. 2005;48:235-41.
4 Mallon L, Broman JE, Hetta J. High incidence of diabetes in men with
sleep complaints or short sleep duration: a 12-year follow-up study of a
middle-aged population. Diabetes Care. 2005;28:2762-7.-55 Gonzalez-Ortiz M, Martinez-Abundis E, Balcazar-Munoz BR,
Pascoe-Gonzalez S. Effect of sleep deprivation on insulin sensitivity and
cortisol concentration in healthy subjects. Diabetes Nutr. Metab.
2000;13:80-3.). At the same time, altered glucose metabolism
may impact sleep quality (66 Spiegel K, Tasali E, Leproult R, Van Cauter E. Effects of poor and
short sleep on glucose metabolism and obesity risk. Nat Rev Endocrinol.
2009;5:253-61.,77 Hayashino Y, Yamazaki S, Nakayama T, Sokejima S, Fukuhara S.
Relationship between diabetes mellitus and excessive sleepiness during driving.
Exp Clin Endocrinol Diabetes. 2008;116:1-5.). Relationships between type 2
diabetes mellitus (T2DM) and sleep disordered breathing have
been extensively studied. Patients with T2DM present an extremely high prevalence of
obstructive sleep apnea, which in turn contributes to poor glycemic control (66 Spiegel K, Tasali E, Leproult R, Van Cauter E. Effects of poor and
short sleep on glucose metabolism and obesity risk. Nat Rev Endocrinol.
2009;5:253-61.,88 Pamidi S, Aronsohn RS, Tasali E. Obstructive sleep apnea: role in
the risk and severity of diabetes. Best Pract Res Clin Endocrinol Metab.
2010;24:703-15.). In
contrast, the relationship between type 1 diabetes mellitus (T1DM)
and sleep has not been frequently investigated.
In the present study we evaluate a possible interaction between T1DM and sleep, as proposed before (99 Barone MTU, Menna-Barreto L. Diabetes and sleep: a complex cause-and-effect relationship. Diabetes Res Clin Pract. 2011;91:129-37.). We performed this study in order to broaden the understanding of this vicious circle. Differences between sleep variables in people with T1DM and their peers have already been shown (1010 Yeshayahu Y, Mahmud FH. Altered sleep patterns in adolescents with type 1 diabetes: implications for insulin regimen. Diabetes Care. 2010;33:e14.). Furthermore, the glycemic control was shown to affect sleep in T1DM, and apnea was positively associated with glycated hemoglobin (A1C) and with the disease duration (1111 Villa MP, Multari G, Montesano M, Pagani J, Cervoni M, Midulla F, et al. Sleep apnoea in children with diabetes mellitus: effect of glycaemic control. Diabetologia. 2000;43:696-702.,1212 Pillar G, Schuscheim G, Weiss R, Malhotra A, McCowen KC, Shlitner A, et al. Interactions between hypoglycemia and sleep architecture in children with type 1 diabetes mellitus. J Pediatr. 2003;142:163-8.). Moreover, nocturnal hypoglycemia was associated with sleep deepening, whereas glycemic variation has been considered to cause awakenings among T1DM children (1212 Pillar G, Schuscheim G, Weiss R, Malhotra A, McCowen KC, Shlitner A, et al. Interactions between hypoglycemia and sleep architecture in children with type 1 diabetes mellitus. J Pediatr. 2003;142:163-8.). In addition, authors comparing T1DM adults with controls reported that T1DM subjects presented changes in sleep architecture, with increased proportion of stage 2, besides higher levels of epinephrine and growth hormone during the entire night, and higher ACTH and cortisol during part of the first half of the night (1313 Jauch-Chara K, Schmid SM, Hallschmid M, Born J, Schultes B. Altered neuroendocrine sleep architecture in patients with type 1 diabetes. Diabetes Care. 2008;31:1183-8.). As a consequence, T1DM subjects report less restorative sleep (1313 Jauch-Chara K, Schmid SM, Hallschmid M, Born J, Schultes B. Altered neuroendocrine sleep architecture in patients with type 1 diabetes. Diabetes Care. 2008;31:1183-8.), and present higher risk for obstructive sleep apnea and poorer sleep quality than control subjects (1414 van Dijk M, Dinga E, van Dijk JG, Lammers GJ, van Kralingen K, Dekkers OM, et al. Disturbed subjective sleep characteristics in adult patients with long-standing type 1 diabetes mellitus. Diabetologia. 2011;54:1967-76.).
Therefore, considering the evidence that sleep quality and duration may affect glucose metabolism (66 Spiegel K, Tasali E, Leproult R, Van Cauter E. Effects of poor and short sleep on glucose metabolism and obesity risk. Nat Rev Endocrinol. 2009;5:253-61.,1515 Knutson KL, Ryden AM, Mander BA, Van Cauter E. Role of sleep duration and quality in the risk and severity of type 2 diabetes mellitus. Arch Intern Med. 2006;166:1768-74.), and with the evidence presented above that sleep quality and duration may be altered in T1DM, we evaluated several sleep and sleep related parameters to broaden the understanding associations of glycemic control and sleep parameters in T1DM patients. Our hypothesis is that poor glycemic control may impact several sleep parameters, including: architecture, quality, and duration. At the same time, sleep impairments may hinder glycemic control in T1DM patients.
MATERIALS AND METHODS
Subjects
Eighteen young adults with T1DM, free of diabetes chronic complications, and not taking drugs that could affect sleep, were recruited. They were no night or shift workers and had no previous diagnosis of sleep disorders. In order to select individuals without chronic complications, all T1DM volunteers were subjected to retinal inspection by ophthalmologists, measurement of 24 h microalbuminuria and creatinin levels, 10 g monofilament sensation, vibration perception with a 128-Hz tuning fork, resting heart rate, and pressure adaptation when standing up. Individuals presenting impairment in any of these tests, interpreted according to ADA’s Standards of Medical Care in Clinical in Diabetes (1616 American Diabetes Association. Standards of medical care in diabetes--2011. Diabetes Care. 2011;34:S11-61.), were not included in the study.
Nine control subjects, matched for age and BMI, free of drugs with effect on sleep, no night or shift workers, and without previous diagnosis of sleep disorders, were recruited. Even though these individuals were not obese and had no diabetes symptoms, all of them did a blood glucose test after fasting of 8 hours at a reference laboratory.
Instruments
Data from all individuals were collected with sleep diaries and actimeters (Tempatilumi, wrist accelerometer produced by CEBrasil) simultaneously during 10 consecutive days, between the end of February and beginning of March. The actimeter was continuously worn on the non-dominant wrist, removed only for shower or any water activity. All volunteers were instructed to press the actimeter event button immediately before removing it, in order to identify unworn periods. During these 10 days, T1DM individuals performed 6-10 home blood glucose tests a day, with a Performa® glucometer (Roche Diagnostics, Mannheim, Germany). The sleep diary was adapted from the Pittsburgh Sleep Diary Wake Time (1717 Monk TH, Reynolds CF, Kupfer DJ, Buysse DJ, Coble PA, Hayes AJ, et al. The Pittsburgh sleep diary. J Sleep Res. 1994;3:111-20.), excluding mood and alertness items. Sleep quality was reported by individuals upon awakening also using a Visual Analogue Scale – VAS (1818 Aitken RCB. Measurement of feelings using visual analogue scales. Proc R Soc Med. 1969;62:989-93.). For the individuals with diabetes a question about hypoglycemia and hyperglycemia during the night and the glycemic value, if measured, was included in the diary. In addition, all volunteers were subjected to: 1 night of polysomnography, and the Epworth Sleepiness Scale (ESS). T1DM subjects wore a Continuous Glucose Monitoring System (CGMS®, Medtronic) during the polysomnography (PSG); and had their A1C measured after the collection of all other data. The polysomnography equipment used was the EMBLA (Flagra hf. Medical Devices, Reykjavik, Iceland), performed in a blinded manner, at the InCor-HCFMUSP Sleep Lab. Monitoring included 4 channels of electroencephalogram, 2 channels of electrooculogram, besides channels for: electrocardiogram, chin and anterior tibial electromyogram, pulse oxymetry, airflow (oronasal thermistor and cannula pressure), and chest and abdominal straps.
Analysis
In order to perform some of the analyses, the T1DM group was divided two groups according to the individual mean glycemia (MG), considering all the measurements performed during the 10 consecutive days. The 9 individuals with mean glycemia below 154 mg/dL (which corresponds to an A1C of 7.0% (53 mmol/mol) (1919 Nathan DM, Kuenen J, Borg R, Zheng H, Schoenfeld D, Heine RJ; for the A1c-Derived Average Glucose (ADAG) Study Group. Translating the A1C assay into estimated average glucose values. Diabetes Care. 2008;31:1-6.) composed the low mean glycemia group (LMG), whereas the other 9 (mean glycemia above 154 mg/dL) were considered the high mean glycemia group (HMG). This division was made considering the American Diabetes Association A1C goal (< 7.0%, 53 mmol/mol) to most adults with diabetes (1616 American Diabetes Association. Standards of medical care in diabetes--2011. Diabetes Care. 2011;34:S11-61.).
In addition, the two other variables considered to assess glycemic control were: A1C and glycemic variability (GV), calculated here as the standard deviation (SD) of the 10 consecutive days of measured glycemias.
Polysomnography tests were scored blindly and analyzed by an experienced physician, according to the American Academy of Sleep Medicine manual (2020 American Academy of Sleep Medicine. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. American Academy of Sleep Medicine, Westchester, 2007.).
Data from the actimeters were processed with El Temps software (Díez-Noguera, 1, 209+, Barcelona), in order to identify period, amplitude, potency, and acrophase of each subjects’ rest/activity rhythm.
All collected data were analyzed using the following tests: Shapiro-Wilk’s W test for normality (data from all variables presented p value greater than 0.05); Pearson correlation coefficient, for comparison between variables; T-test for independent samples, when comparing two groups; ANOVA one-way, when comparing data from more than two groups. All results with p value under 0.05 were considered significant. All algorithms used are part of the Statistic software (StatSoft, Inc., 2004, version 6).
Ethics
The study was approved by the Ethics Committee for Research in Human Beings of Instituto de Ciências Biomédicas, University of São Paulo, number 873/CEP. It was also approved by all other partner institutions, Associação de Diabetes Juvenil, Hospital das Clínicas e Instituto do Coração da Faculdade de Medicina da Universidade de São Paulo. Informed consent has been obtained from all participants.
RESULTS
T1DM subjects’ age was between 20 and 38 years (26.3 y ± 5.1 y). They were non-obese (BMI: 23 ± 2.9 kg/m2), 8 were male, and 10 female, and their disease duration was 12.9 y ± 7.2 y. The nine control subjects’ age was between 23 and 38 years (28.8 y ± 5.3 y), BMI: 22 ± 2.7 kg/m2, and 4 of them were male. Other information concerning T1DM individuals’ glycemic control, and sleep parameters from both groups, T1DM and control, are found on table 1.
Sleep diary
No difference between the sleep duration of T1DM and control individuals was observed (p = 0.3146). Sleep duration was neither correlated with mean glycemia, nor with glycemic variability (r = 0.3043, p = 0.2196; r = 0.1075, p = 0.6710; respectively).
Among our volunteers, 78% (14 from the T1DM group, and 7 from the control group) presented longer sleep duration during the weekends (Friday to Saturday and Saturday to Sunday) compared to weekdays (Monday to Tuesday, Tuesday to Wednesday, Wednesday to Thursday, and Thursday to Friday). When comparing this sleep duration difference between both groups, the 14 T1DM individuals difference was of 2 h 01 min 33 s ± 1 h 07 min 19 s, while in the 9 control individuals it was of 57 min 17 s ± 36 min 24 s (p = 0.0303). The high duration of sleep time on weekend nights or during holidays compared to weekday nights is suggestive of behaviorally induced insufficient sleep, according to Hublin and Sallinen (2121 Hublin C, Sallinen M. Behaviorally induced insufficient sleep. Sleep Med Clin. 2012;7:313-23.).
Sleep quality did not correlate with the mean glycemia, nor with the glycemic variability of T1DM individuals (p = 0.4381, p = 0.4046, respectively). When the T1DM individuals were separated by the mean glycemia values, only the Low Mean Glycemia group presented significant negative correlation of mean glycemia with sleep quality (r = -0.8508, p = 0.0036). No difference between the sleep latency of T1DM and control individuals was observed (p = 0.6347). The average of the sleep latency, reported by the individuals during the 10 days, was positively correlated with the glycemic variability (r = 0.6525, p = 0.0033, Figure 1).
Actimeter
The rest/activity rhythm was analyzed in terms of phases duration, period, amplitude, potency, acrophase, and difference between the observed period and 24 hours. No significant difference was found between the T1DM and control groups in any of these variables.
Night rest duration did not present a significant correlation with mean glycemia (r = 0.5404, p = 0.0697), whereas it presents a tendency of positive correlation with glycemic variability (r = 0.5706, p = 0.0527). Interestingly, when considering just the Low Mean Glycemia group, the correlation of the mean glycemia with the night rest duration was negative (r = -0.8987, p = 0.0381).
Polysomnography
None of our volunteers (T1DM and control) presented sleep disorders. The only sleep parameters correlated with the mean glycemia were the full awakenings index (r = 0.5684, p = 0.0271, Figure 2A) and the apnea-hypopnea index. Surprisingly the correlation with apnea-hypopnea index was negative (r = -0.5573, p = 0.0309), as well as a tendency of correlation between apnea-hypopnea index and glycemic variability (r = -0.4928, p = 0.0620). It is possible to observe, though, that this result reflects especially the highest apnea-hypopnea index of the 5 individuals with lowest mean glycemia (p = 0.0011). Similarly to the mean glycemia, A1C was positively correlated with awakenings index (r = 0.6544, p = 0.0081, Figure 2B) and arousal index (r = 0.5680, p = 0.0272, Figure 2C). The 25% highest glycemic variability T1DM individuals had a significantly higher awakenings index (p = 0.0092, Figure 2D).
(A-B) Correlation of full awakening index with mean glycemia (r = 0.5684, p = 0.0271), and with A1C (r = 0.6544, p = 0.0081), respectively; (C) correlation between arousal index and A1C (r = 0.5680, p = 0.0272); (D) full awakening index of the 25% highest glycemic variability (group 2), comparing to the others (75%, groups 1) (p = 0.0092).
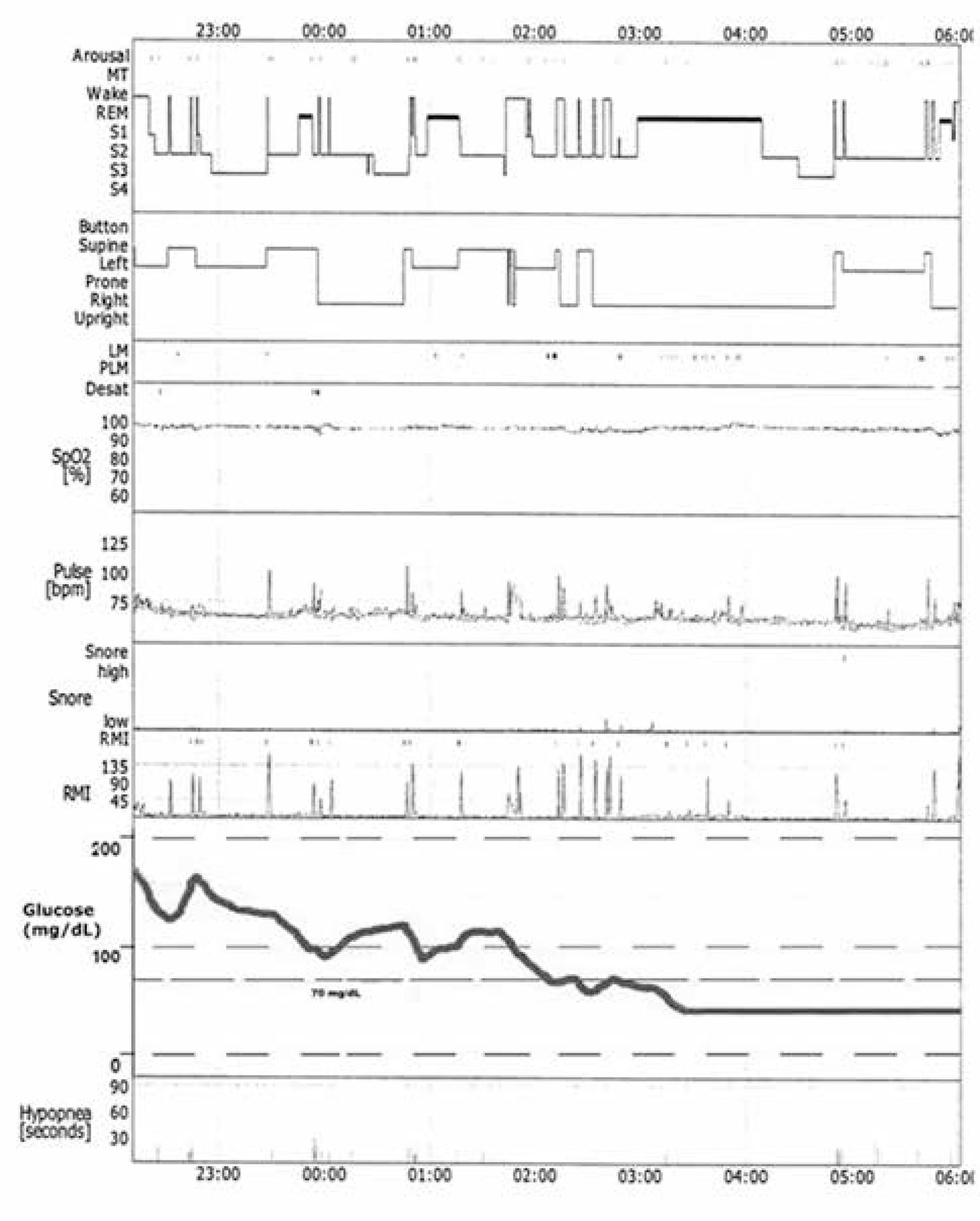
Only one individual presented low glucose levels (< 70 mg/dL) during polysomnography. His glucose stayed low for more than 3 hours. During this time, which was 42.4% of the total sleep duration, this individual did not wake up, and presented less hypopneas (33.3%) and awakenings (31.6%) than during the rest of the night. Additionally, REM sleep was prevalent during this period as seen on figure 3.
Polysomnography register of the T1DM individual who presented hypoglycemia (< 70 mg/dL) for more than 3 hours during this night.
Epworth Sleepiness Scale
There was no difference between the control and the T1DM group, nor between High Mean Glycemia and Low Mean Glycemia groups (p = 0.1065, p = 0.4791, respectively). On the other hand the percentage of individuals with a score higher than 10 in the T1DM group was much higher than in the control group (39%, and 11%, respectively).
DISCUSSION
The correlations observed between sleep aspects and glycemic control are suggestive of the hypothesized associations. Although our sample size was limited, it was similar to those of previously published studies with T1DM. However, in contrast, we have used a wide range of validated measurement instruments to access sleep parameters, besides more extensive data collection through sleep diaries and actimeters.
In the case of mean sleep latency, a positive correlation with glycemic variability was observed. In other terms, this may be understood as higher difficulty in falling asleep in case of higher glycemic variability. Although this was shown for the first time in our study, it coincides with informal observations both from clinicians and especially from individuals with diabetes, who sometimes report trouble to sleep when experiencing glycemic variations.
The absence of correlation between sleep duration and glycemic control was not surprising, since other groups have reported similar results before (1010 Yeshayahu Y, Mahmud FH. Altered sleep patterns in adolescents with type 1 diabetes: implications for insulin regimen. Diabetes Care. 2010;33:e14.,1414 van Dijk M, Dinga E, van Dijk JG, Lammers GJ, van Kralingen K, Dekkers OM, et al. Disturbed subjective sleep characteristics in adult patients with long-standing type 1 diabetes mellitus. Diabetologia. 2011;54:1967-76.). In our study, differently from findings of other groups that studied T1DM children and adolescents (1010 Yeshayahu Y, Mahmud FH. Altered sleep patterns in adolescents with type 1 diabetes: implications for insulin regimen. Diabetes Care. 2010;33:e14.,1212 Pillar G, Schuscheim G, Weiss R, Malhotra A, McCowen KC, Shlitner A, et al. Interactions between hypoglycemia and sleep architecture in children with type 1 diabetes mellitus. J Pediatr. 2003;142:163-8.), and in accordance with a study with T1DM adults (1414 van Dijk M, Dinga E, van Dijk JG, Lammers GJ, van Kralingen K, Dekkers OM, et al. Disturbed subjective sleep characteristics in adult patients with long-standing type 1 diabetes mellitus. Diabetologia. 2011;54:1967-76.), sleep duration was not different between T1DM and control subjects. While Pillar and cols. reported in 2003 decreased sleep duration in children with T1DM (1212 Pillar G, Schuscheim G, Weiss R, Malhotra A, McCowen KC, Shlitner A, et al. Interactions between hypoglycemia and sleep architecture in children with type 1 diabetes mellitus. J Pediatr. 2003;142:163-8.), Yeshayahu and Mahmud reported in 2010 longer sleep durations in adolescents with T1DM (1010 Yeshayahu Y, Mahmud FH. Altered sleep patterns in adolescents with type 1 diabetes: implications for insulin regimen. Diabetes Care. 2010;33:e14.). The methods used in those two studies were different, whereas the first gauged sleep duration during the polysomnography night, the second considered reported sleep duration. Moreover, there is an age difference that should be considered.
Even though sleep and rest duration were not different between T1DM and control, the duration of sleep extension during weekends was more than twice as long in T1DM patients as it was for control subjects. Taking into account these observations, we speculate that in an environment free of social demands, most of the T1DM individuals would present longer sleep duration than controls, supporting Yeshayahu and Mahmud findings (1010 Yeshayahu Y, Mahmud FH. Altered sleep patterns in adolescents with type 1 diabetes: implications for insulin regimen. Diabetes Care. 2010;33:e14.).
Moreover, knowing that the sleep extension presented is suggestive of behaviorally
induced insufficient sleep (2121 Hublin C, Sallinen M. Behaviorally induced insufficient sleep. Sleep
Med Clin. 2012;7:313-23.), which may
result of daily sleep deprivation, we understand that the glycemic control in those
individuals may be affected. Several authors have reported the negative impact of
sleep deprivation, as well as of behaviorally induced insufficient sleep, on insulin
resistance and glucose tolerance, as well as learning, memory, attention, immune
response, cardiovascular function, and neuro-hormonal regulation (2121 Hublin C, Sallinen M. Behaviorally induced insufficient sleep. Sleep
Med Clin. 2012;7:313-23.
22 Walker MP. Cognitive consequences of sleep and sleep loss. Sleep
Med. 2008;9:S29-34.
23 Van Cauter E, Spiegel K, Tasali E, Leproult R. Metabolic
consequences of sleep and sleep loss. Sleep Med. 2008;9:S23-8.
24 Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on
metabolic and endocrine function. Lancet. 1999;354:1435-9.
25 Spiegel K, Knutson K, Leproult R, Tasali E, Van Cauter E. Sleep
loss: a novel risk factor for insulin resistance and Type 2 diabetes. J Appl
Physiol. 2005;99:2008-19.-2626 Schmid SM, Hallschmid M, Jauch-Chara K, Wilms B, Lehnert H, Born J,
et al. Disturbed glucoregulatory response to food intake after moderate sleep
restriction. Sleep. 2011;34:371-7.).
Concerning specifically T1DM, a study revealed that a single partially deprived
night is enough to produce insulin resistance (2727 Donga E, van Dijk M, van Dijk JG, Biermasz NR, Lammers GJ, van
Kralingen K, et al. Partial sleep restriction decreases insulin sensitivity in
type 1 diabetes. Diabetes Care. 2010;33:1573-7.). In this study individuals were allowed to sleep for 4 hours at
night, and in the following day their insulin sensitivity was reduced in 14-21%.
We consider sleep quality an important parameter, since it was shown, in T2DM, to be negatively correlated with A1C (1515 Knutson KL, Ryden AM, Mander BA, Van Cauter E. Role of sleep duration and quality in the risk and severity of type 2 diabetes mellitus. Arch Intern Med. 2006;166:1768-74.). In addition, worse sleep quality was previously reported in individuals with T1DM (1313 Jauch-Chara K, Schmid SM, Hallschmid M, Born J, Schultes B. Altered neuroendocrine sleep architecture in patients with type 1 diabetes. Diabetes Care. 2008;31:1183-8.,1414 van Dijk M, Dinga E, van Dijk JG, Lammers GJ, van Kralingen K, Dekkers OM, et al. Disturbed subjective sleep characteristics in adult patients with long-standing type 1 diabetes mellitus. Diabetologia. 2011;54:1967-76.), which we did not observe in the present study. In Jauch-Chara and cols.’s study a questionnaire was applied after a polysomnography night (1313 Jauch-Chara K, Schmid SM, Hallschmid M, Born J, Schultes B. Altered neuroendocrine sleep architecture in patients with type 1 diabetes. Diabetes Care. 2008;31:1183-8.), whereas our individuals reported their sleep quality on a visual analogue scale every morning. When taking into account ESS score, our results points towards the same direction of Jauch-Chara and cols.’s. (1313 Jauch-Chara K, Schmid SM, Hallschmid M, Born J, Schultes B. Altered neuroendocrine sleep architecture in patients with type 1 diabetes. Diabetes Care. 2008;31:1183-8.). While in the control group only one individual (11% of the total) had an ESS score higher than 10, in the T1DM group, 7 individuals (39% of this group) presented this score level. This finding may be relevant, since a score of 10 points or higher in the ESS is suggestive of low sleep quality and sleep disorders (2828 Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540-5.). At the same time, although we found no association of mean glycemia or glycemic variability with reported sleep quality, when groups were divided, at least the glycemic parameters of the individuals with a better metabolic control (Low Mean Glycemia group) seemed to be correlated with sleep quality. These results are difficult to interpret, but we suspect that from a certain mean glycemia, sleep quality may not change much, whereas below that level, sleep quality varies in association with glycemic control.
Concerning polysomnography data, differently from other groups (1111 Villa MP, Multari G, Montesano M, Pagani J, Cervoni M, Midulla F, et al. Sleep apnoea in children with diabetes mellitus: effect of glycaemic control. Diabetologia. 2000;43:696-702.,1212 Pillar G, Schuscheim G, Weiss R, Malhotra A, McCowen KC, Shlitner A, et al. Interactions between hypoglycemia and sleep architecture in children with type 1 diabetes mellitus. J Pediatr. 2003;142:163-8.), we did not find sleep disorders in T1DM group. This result is probably due to our strict selection of participants without chronic complications. Mondini and Guilleminault (2929 Mondini S, Guilleminault C. Abnormal breathing patterns during sleep in diabetes. Ann Neurol. 1985;17:391-5.), for example, found sleep-disordered breathing in 41.7% of T1DM individuals. They also reported that this disorder was associated with the presence of neuropathy. Nevertheless, in our study, arousal index and awakenings index were positively correlated with the A1C. This may be understood as an association between these two sleep aspects and glycemic control evident only in a broader angle, which is provided by the 2-3 months of glycemic mean reflected by the A1C value. Therefore, we understand that arousal and awakenings indexes may result of a longer term poor glycemic control. At the same time, we found that the A1C is strongly associated with glycemic variability (r = 0.6228, p = 0.0058), which, as discussed earlier, may promote sleep changes. These results reveal a significant correlation between the stability or fragmentation of sleep and glycemic control. Moreover, we show that the rate of full awakenings was correlated with the 10 days mean glycemia. Therefore, we conclude that individuals with poor glycemic control have more fragmented sleep, with more arousals and awakenings. At the same time, sleep fragmentation has been associated with increases in insulin resistance and adrenocortical and sympathetic nervous system activity, which, in turn, may hinder the maintenance of good glycemic control (3030 Stamatakis KA, Punjabi NM. Effects of sleep fragmentation on glucose metabolism in normal subjects. Chest. 2010;137:95-101.,3131 Clarenbach CF, West SD, Kohler M. Is obstructive sleep apnea a risk factor for diabetes? Discov Med. 2011;12:17-24.).
The results of higher apnea-hypopnea index observed in individuals with low mean glycemia was surprising. Our results, in this case are just the opposite of another group’s (1111 Villa MP, Multari G, Montesano M, Pagani J, Cervoni M, Midulla F, et al. Sleep apnoea in children with diabetes mellitus: effect of glycaemic control. Diabetologia. 2000;43:696-702.), who found, in children with T1DM, that the poorer the glycemic control (A1C values) or the greater the disease duration, the greater the apnea index. For this reason, we decided to divide the group, and found that 25% of the individuals (5 individuals) with the lowest GV values (below 59 mg/dL) were the ones with the highest apnea-hypopnea index (4.3 ± 2.39 events per hour, comparing to 2.9 ± 1.64). Surprisingly, excluding from this 25% lowest GV sample the 2 individuals with the highest apnea-hypopnea index, the remaining 3 individuals present a mean apnea-hypopnea index very similar to the remaining group (2.7 ± 1.72). And more than that, with this exclusion, the correlation becomes non-significant (r = -0.2747, p = 0.3637). Exactly the same happened with the negative correlation between mean glycemia and apnea-hypopnea index, it became non-significant when the two extreme values (AHI > 6) are excluded from the sample (r = -0.3975, p = 0.1786). For this reason, although the first results favored a conclusion of higher apnea-hypopnea index in individuals with better metabolic control, it seems that it is just an artifact resulting from 2 individuals’ extreme data. It is also worth mentioning that according to the American Academy of Sleep Medicine (3232 American Academy of Sleep Medicine. The international classification of sleep disorders, 2nd ed. American Academy of Sleep Medicine, Westchester, 2005.), none of our individuals would be considered to have sleep-disordered breathing, which is diagnosed in cases of at least 15 events per hour, or more than 5 events per hour with clear symptoms.
The difference between stages duration observed by Jauch-Chara and cols. (1313 Jauch-Chara K, Schmid SM, Hallschmid M, Born J, Schultes B. Altered neuroendocrine sleep architecture in patients with type 1 diabetes. Diabetes Care. 2008;31:1183-8.), when comparing T1DM with control individuals, was not observed in our study, not even between the different T1DM groups (control vs. T1DM - % of REM sleep: 21.5% ± 5.3% vs. 19.9% ± 7.7%, p = 0.6216; % stage 3, 21.7% ± 8.4% vs. 20.6% ± 8.3%: p = 0.7668).
The only case of hypoglycemia during the polysomnography night presented interesting consequences on the sleep parameters. During the hypoglycemia several parameters seemed to become more stable, with more REM sleep, and less hypopneas and awakenings than the rest of the night. Different authors have also found results indicating that the sleep inhibits counter-regulatory responses, and that the hypoglycemia deepens sleep (1212 Pillar G, Schuscheim G, Weiss R, Malhotra A, McCowen KC, Shlitner A, et al. Interactions between hypoglycemia and sleep architecture in children with type 1 diabetes mellitus. J Pediatr. 2003;142:163-8.,3333 Banarer S, Cryer PE. Sleep-related hypoglycemia-associated autonomic failure in type 1 diabetes: reduced awakening from sleep during hypoglycemia. Diabetes. 2003;52:1195-203.). These results reinforce the importance of keeping track of glycemia during the night, avoiding hypoglycemia, since if the individual does not wake up, and the glycemia decreases even more, it would put the individual’s life in risk (3434 Schultes B, Jauch-Chara K, Gais S, Hallschimd M, Reiprich E, Kern W, et al. Defective awakening response to nocturnal hypoglycemia in patients with type 1 diabetes mellitus. PLoS Med. 2007;4:e69.,3535 Gill GV, Woodward A, Casson IF, Weston PJ. Cardiac arrhythmia and nocturnal hypoglycaemia in type 1 diabetes--the ‘dead in bed’ syndrome revisited. Diabetologia. 2009;52:42-5.). In addition, we recommend monitoring glucose continuously when performing polysomnography in DM individuals, since, as observed, the occurrence of hypoglycemia may blunt its results.
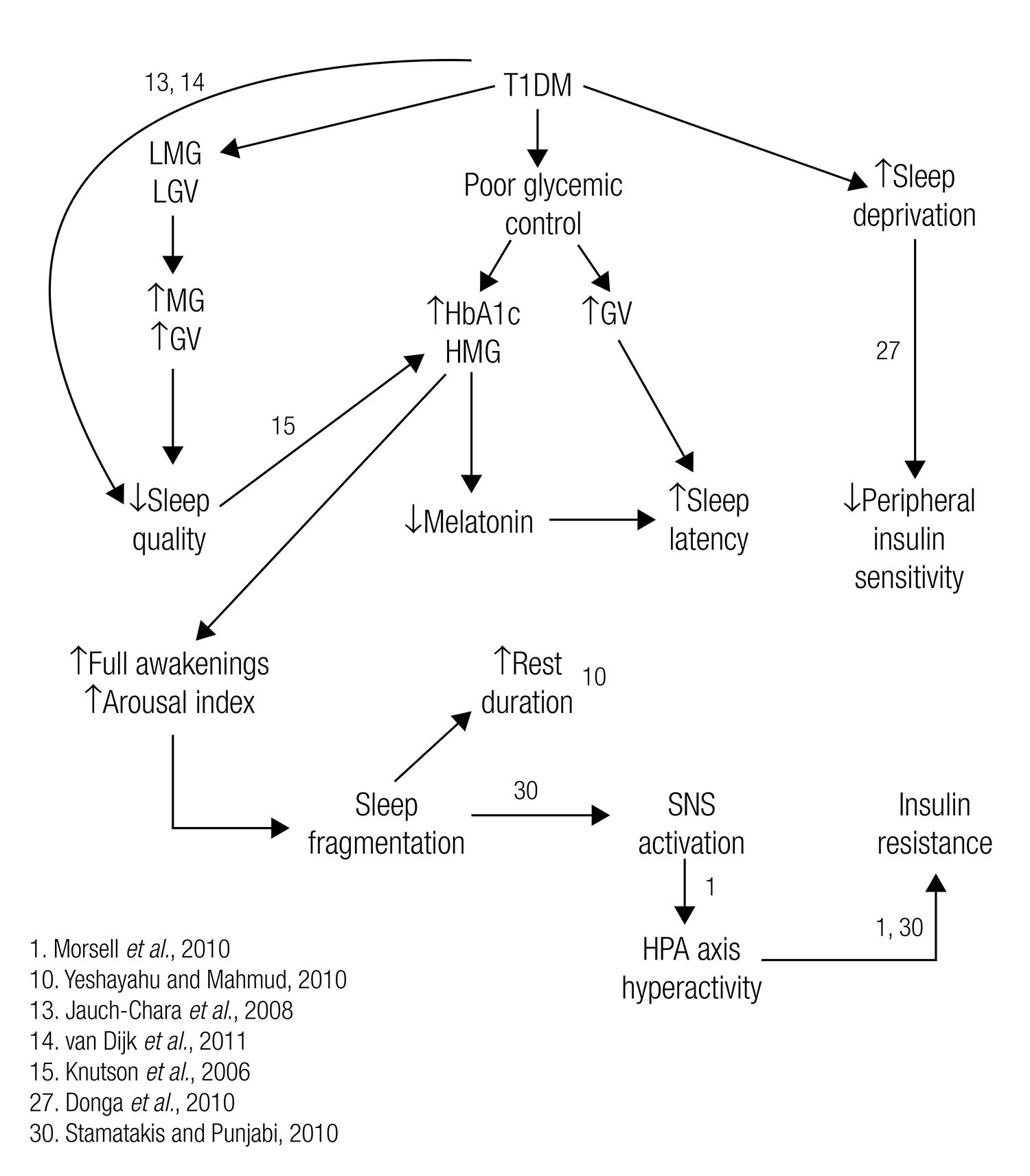
The results found in this study support at least partially our hypothesis, and are in accordance with results from other groups pointing towards the existence of a vicious circle involving T1DM and sleep (Figure 4). In this vicious circle, poor glycemic control in T1DM affects sleep parameters, which in turn compromises the glycemic control. It is similar to the vicious circle that associates sleep with T2DM. Similarly to Knutson and cols.’s proposal for individuals with T2DM (1515 Knutson KL, Ryden AM, Mander BA, Van Cauter E. Role of sleep duration and quality in the risk and severity of type 2 diabetes mellitus. Arch Intern Med. 2006;166:1768-74.), we suggest sleep duration and quality to be considered part of T1DM’s therapy. Although in the present study we did not isolate variables with the intention to find cause-and-effect relationships, we understand that our results in association to other groups’ results make we believe that improving glycemic control may lead to improvements on sleep quality. At the same time, a good night’s sleep, with adequate duration and high quality may help to regulate metabolism and sympathoadrenal activity, improving the glycemic control.
We understand that more studies are needed to confirm our findings. Some of the tendencies observed may become significant when analyzing data from more subjects, and unexpected results may be clarified. In addition, more studies are needed to unveil the links connecting glucose metabolism and diabetes with sleep.
Acknowledgment
We acknowledge support from André Gonçalves, Fernando Fonseca and their team from Faculdade de Medicina do ABC, for their generosity. We thank Lucy Aihara and Cinthya Ugliara for their assistance, and all volunteers for participating in the study and the technicians for their dedicated work.
REFERENCES
-
1Leproult R, Balbo M, Spiegel K. Role of sleep duration in the regulation of glucose metabolism and appetite. Best Pract Res Clin Endocrinol Metab. 2010;24:687-702.
-
2Tasali E, Leproult R, Ehrmann DA, Van Cauter E. Slow-wave sleep and the risk of type 2 diabetes in humans. Proc Natl Acad Sci U S A. 2008;105:1044-9.
-
3Meisinger C, Heier M, Loewel H, Study MKAC. Sleep disturbance as a predictor of type 2 diabetes mellitus in men and women from the general population. Diabetologia. 2005;48:235-41.
-
4Mallon L, Broman JE, Hetta J. High incidence of diabetes in men with sleep complaints or short sleep duration: a 12-year follow-up study of a middle-aged population. Diabetes Care. 2005;28:2762-7.
-
5Gonzalez-Ortiz M, Martinez-Abundis E, Balcazar-Munoz BR, Pascoe-Gonzalez S. Effect of sleep deprivation on insulin sensitivity and cortisol concentration in healthy subjects. Diabetes Nutr. Metab. 2000;13:80-3.
-
6Spiegel K, Tasali E, Leproult R, Van Cauter E. Effects of poor and short sleep on glucose metabolism and obesity risk. Nat Rev Endocrinol. 2009;5:253-61.
-
7Hayashino Y, Yamazaki S, Nakayama T, Sokejima S, Fukuhara S. Relationship between diabetes mellitus and excessive sleepiness during driving. Exp Clin Endocrinol Diabetes. 2008;116:1-5.
-
8Pamidi S, Aronsohn RS, Tasali E. Obstructive sleep apnea: role in the risk and severity of diabetes. Best Pract Res Clin Endocrinol Metab. 2010;24:703-15.
-
9Barone MTU, Menna-Barreto L. Diabetes and sleep: a complex cause-and-effect relationship. Diabetes Res Clin Pract. 2011;91:129-37.
-
10Yeshayahu Y, Mahmud FH. Altered sleep patterns in adolescents with type 1 diabetes: implications for insulin regimen. Diabetes Care. 2010;33:e14.
-
11Villa MP, Multari G, Montesano M, Pagani J, Cervoni M, Midulla F, et al. Sleep apnoea in children with diabetes mellitus: effect of glycaemic control. Diabetologia. 2000;43:696-702.
-
12Pillar G, Schuscheim G, Weiss R, Malhotra A, McCowen KC, Shlitner A, et al. Interactions between hypoglycemia and sleep architecture in children with type 1 diabetes mellitus. J Pediatr. 2003;142:163-8.
-
13Jauch-Chara K, Schmid SM, Hallschmid M, Born J, Schultes B. Altered neuroendocrine sleep architecture in patients with type 1 diabetes. Diabetes Care. 2008;31:1183-8.
-
14van Dijk M, Dinga E, van Dijk JG, Lammers GJ, van Kralingen K, Dekkers OM, et al. Disturbed subjective sleep characteristics in adult patients with long-standing type 1 diabetes mellitus. Diabetologia. 2011;54:1967-76.
-
15Knutson KL, Ryden AM, Mander BA, Van Cauter E. Role of sleep duration and quality in the risk and severity of type 2 diabetes mellitus. Arch Intern Med. 2006;166:1768-74.
-
16American Diabetes Association. Standards of medical care in diabetes--2011. Diabetes Care. 2011;34:S11-61.
-
17Monk TH, Reynolds CF, Kupfer DJ, Buysse DJ, Coble PA, Hayes AJ, et al. The Pittsburgh sleep diary. J Sleep Res. 1994;3:111-20.
-
18Aitken RCB. Measurement of feelings using visual analogue scales. Proc R Soc Med. 1969;62:989-93.
-
19Nathan DM, Kuenen J, Borg R, Zheng H, Schoenfeld D, Heine RJ; for the A1c-Derived Average Glucose (ADAG) Study Group. Translating the A1C assay into estimated average glucose values. Diabetes Care. 2008;31:1-6.
-
20American Academy of Sleep Medicine. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. American Academy of Sleep Medicine, Westchester, 2007.
-
21Hublin C, Sallinen M. Behaviorally induced insufficient sleep. Sleep Med Clin. 2012;7:313-23.
-
22Walker MP. Cognitive consequences of sleep and sleep loss. Sleep Med. 2008;9:S29-34.
-
23Van Cauter E, Spiegel K, Tasali E, Leproult R. Metabolic consequences of sleep and sleep loss. Sleep Med. 2008;9:S23-8.
-
24Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435-9.
-
25Spiegel K, Knutson K, Leproult R, Tasali E, Van Cauter E. Sleep loss: a novel risk factor for insulin resistance and Type 2 diabetes. J Appl Physiol. 2005;99:2008-19.
-
26Schmid SM, Hallschmid M, Jauch-Chara K, Wilms B, Lehnert H, Born J, et al. Disturbed glucoregulatory response to food intake after moderate sleep restriction. Sleep. 2011;34:371-7.
-
27Donga E, van Dijk M, van Dijk JG, Biermasz NR, Lammers GJ, van Kralingen K, et al. Partial sleep restriction decreases insulin sensitivity in type 1 diabetes. Diabetes Care. 2010;33:1573-7.
-
28Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540-5.
-
29Mondini S, Guilleminault C. Abnormal breathing patterns during sleep in diabetes. Ann Neurol. 1985;17:391-5.
-
30Stamatakis KA, Punjabi NM. Effects of sleep fragmentation on glucose metabolism in normal subjects. Chest. 2010;137:95-101.
-
31Clarenbach CF, West SD, Kohler M. Is obstructive sleep apnea a risk factor for diabetes? Discov Med. 2011;12:17-24.
-
32American Academy of Sleep Medicine. The international classification of sleep disorders, 2nd ed. American Academy of Sleep Medicine, Westchester, 2005.
-
33Banarer S, Cryer PE. Sleep-related hypoglycemia-associated autonomic failure in type 1 diabetes: reduced awakening from sleep during hypoglycemia. Diabetes. 2003;52:1195-203.
-
34Schultes B, Jauch-Chara K, Gais S, Hallschimd M, Reiprich E, Kern W, et al. Defective awakening response to nocturnal hypoglycemia in patients with type 1 diabetes mellitus. PLoS Med. 2007;4:e69.
-
35Gill GV, Woodward A, Casson IF, Weston PJ. Cardiac arrhythmia and nocturnal hypoglycaemia in type 1 diabetes--the ‘dead in bed’ syndrome revisited. Diabetologia. 2009;52:42-5.
-
Funding: Fundação de Amparo à Pesquisa do Estado de São Paulo (Fapesp) (Grant: 2008/11026-2).
-
ErratumArch Endocrinol Metab. 2015;59(1):71-8Where you read:
Figure 2.(A-B) Correlation of full awakening index with mean glycemia (r = 0.5684, p = 0.0271), and with A1C (r = 0.6544, p = 0.0081), respectively;(C) correlation between arousal index and A1C (r = 0.5680, p = 0.0272);(D) full awakening index of the 25% highest glycemic variability (group 2), comparing to the others (75%, groups 1) (p = 0.0092).
Should read:
Figure 2.(A-B) Correlation of full awakening index with mean glycemia (r = 0.5684, p = 0.0271), and with A1C (r = 0.6544, p = 0.0081), respectively;(C) correlation between arousal index and A1C (r = 0.5680, p = 0.0272);(D) full awakening index of the 25% highest glycemic variability (group 2), comparing to the others (75%, groups 1) (p = 0.0092).
Where you read:Should read:
Publication Dates
-
Publication in this collection
Feb 2015
History
-
Received
11 June 2014 -
Accepted
24 Oct 2014