Abstract
Objective
There is a growing body of data supporting the association between diabetes and microcirculatory disfunction. We aimed to study e-selectin levels, and their associations with serum markers of inflammation and arterial stiffness in prediabetes and newly diagnosed diabetes patients in this study.
Subjects and methods
Sixty patients (25 females) with a newly established elevated fasting serum glucose [20 impaired fasting glucose (IFG), 20 impaired glucose tolerance (IGT), 20 newly diagnosed diabetes (T2DM)] and 17 healthy controls (13 females) were included in the study. Serum e-selectin and hs-CRP levels, and arterial stiffness parameters of the patients were studied.
Results
Fasting serum glucose was the most important predictor of serum e-selectin levels. Pulse wave velocity and central aortic pressures were significantly higher in IFG, IGT and T2DM groups, compared to controls (p = 0.001, < 0.001, 0.013 and 0.015, 0.002, 0.009, respectively). The mean arterial pressure did not show any significant association with serum e-selectin and hs-CRP levels (β coefficient: 0.092, p = 0.358; and β coefficient: 0.189, p = 0.362, respectively).
Conclusion
Prediabetes patients have increasing e-selectin levels through the diagnosis of T2DM. E-selectin is associated with serum glucose levels. Prediabetic and newly diagnosed diabetics have higher arterial stiffness measurements. Serum e-selectin may be a good marker of endothelial inflammation and dysfunction increasing in parallel with serum glucose levels, predicting future cardiovascular events.
E-selectin; arterial stiffness; inflammation; cardiovascular risk; hypertension
INTRODUCTION
Diabetes mellitus is characterized with increased serum glucose levels deriving as a result of disturbances in insulin production or sensitivity. The prevalence of type 2 diabetes mellitus (T2DM) is rising rapidly and its key feature, insulin resistance, results mainly from low physical activity and obesity. In fact, the disease do not show an abrupt onset like type 1 diabetes and patients acquire T2DM after a particular time. At first, minimal high fasting blood glucose levels indicate impaired fasting glucose (IFG). Then, rising glucose levels show increasing insulin resistance and failure to lower postmeal blood glucose indicating impaired glucose tolerance (IGT). The patient is accepted to have T2DM after a particular serum glucose level. This disease course represents a spectrum, so drawing clear lines between these stages may be inaccurate, and probably the endothelial inflammation and microcirculatory disfunction starts with the first rises in blood glucose levels (11 Haffner SM, Stern MP, Hazuda HP, Mitchell BD, Patterson JK. Cardiovascular risk factors in confirmed prediabetic individuals. Does the clock for coronary heart disease start ticking before the onset of clinical diabetes? JAMA. 1990;263(21):2893-8.,22 Song MK, Davies NM, Roufogalis BD, Huang TH-W. Management of cardiorenal metabolic syndrome in diabetes mellitus: a phytotherapeutic perspective. J Diabetes Res. 2014;2014.).
T2DM is associated to bad cardiovascular outcomes in time (33 Kannel WB, McGee DL. Diabetes and cardiovascular risk factors: the Framingham study. Circulation. 1979;59(1):8-13.). Insomuch that, diabetes has been accepted as a cardiovascular disease equivalent, meaning that these patients should be accepted as having atherosclerotic disease at the time of diagnosis. The blamed underlying factors of these risks are the mentioned endothelial inflammation and microcirculatory dysfunction (44 Muris DM, Houben AJ, Schram MT, Stehouwer CD. Microvascular dysfunction is associated with a higher incidence of type 2 diabetes mellitus: a systematic review and meta-analysis. Arterioscler Thromb Vasc Biol. 2012;32(12):3082-94.). These patients will benefit much from any intervention in the diagnosis, follow-up, and therapy. Since about a decade, a growing number of studies have reported the association between various estimates of microvascular dysfunction in T2DM and IFG (55 De Vriese A, Verbeuren TJ, Van de Voorde J, Lameire NH, Vanhoutte PM. Endothelial dysfunction in diabetes. Br J Pharmacol. 2000;130(5):963-74.).
Selectin family are the molecules that generate the first adhesive stage contributing to the rolling of leukocytes into the vascular endothelium for the distribution of immune cells to the tissue of inflammation (66 Noble KE, Panayiotidis P, Collins PW, Hoffbrand AV, Yong KL. Monocytes induce E-selectin gene expression in endothelial cells: role of CD11/CD18 and extracellular matrix proteins. Eur J Immunol. 1996;26(12):2944-51.). Soluble isoforms of e-selectin is found in the supernatant of in vitro cultured endothelial cells after 24 hours of cytokine activation and are composed through a largely caspase-dependent shedding process (77 Newman W, Beall LD, Carson CW, Hunder GG, Graben N, Randhawa ZI, et al. Soluble E-selectin is found in supernatants of activated endothelial cells and is elevated in the serum of patients with septic shock. J Immunol. 1993;150(2):644-54.,88 Cummings CJ, Sessler CN, Beall LD, Fisher BJ, Best AM, Fowler AA 3rd. Soluble E-selectin levels in sepsis and critical illness. Correlation with infection and hemodynamic dysfunction. Am J Respir Crit Care Med. 1997;156(2 Pt 1):431-7.). In healthy subjects, low levels of soluble e-selectin may be found in serum, but the levels increase in patients with an inflammatory condition. Higher concentrations of this marker is derived mainly from microcirculatory endothelium, so increased e-selectin levels reflect further microvascular endothelial dysfunction and is associated with cardiovascular disease (99 de Jager J, Dekker JM, Kooy A, Kostense PJ, Nijpels G, Heine RJ, et al. Endothelial dysfunction and low-grade inflammation explain much of the excess cardiovascular mortality in individuals with type 2 diabetes: the Hoorn Study. Arterioscler Thromb Vasc Biol. 2006;26(5):1086-93.,1010 To SS, Newman PM, Hyland VJ, Robinson BG, Schrieber L. Regulation of adhesion molecule expression by human synovial microvascular endothelial cells in vitro. Arthritis Rheum. 1996;39(3):467-77.).
Arterial stiffness measurement has high cardiovascular predictive value, reproducibility, availability and cost effectiveness in determining the future cardiovascular risk (1111 Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Böhm M, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2013;34(28):2159-219.). Increased arterial stiffness is a common indicator for the atherosclerotic involvement of the vascular system and is known to occur as a result of atherosclerotic risk factors, such as diabetes mellitus, smoking, hypertension, hypercholesterolemia, and aging (1212 Kostis JB, Lawrence-Nelson J, Ranjan R, Wilson AC, Kostis WJ, Lacy CR. Association of increased pulse pressure with the development of heart failure in SHEP. Systolic Hypertension in the Elderly (SHEP) Cooperative Research Group. Am J Hypertens. 2001;14(8 Pt 1):798-803.). Increased arterial stiffness is also associated with coronary artery disease, cerebrovascular disease and peripheral arterial disease (1313 Heitzer T, Schlinzig T, Krohn K, Meinertz T, Münzel T, Munzel T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation. 2001;104(22):2673-8.).
In this study, we aimed to study e-selectin levels, serum markers of inflammation and their associations with arterial stiffness in prediabetes and newly diagnosed T2DM patients.
SUBJECTS AND METHODS
Patients
The study was conducted in a tertiary referral center. A total of 77 subjects from internal medicine and cardiology outpatient clinics were consecutively included in the study. Seventeen subjects were controls and they were entirely from healthy subjects coming to outpatient clinic for control reasons. Sixty were patients with a newly detected elevated fasting serum blood glucose level above the normal range, 100 mg/dL. Patients were stratified to have IFG, IGT or T2DM according to the American Diabetes Association (ADA) guidelines, as 100 mg/dL < [fasting serum glucose] < 126 mg/dL, 140 mg/l < [second hour serum glucose of a 75 g glucose loading test] < 200 mg/dL, and [two times fasting serum glucose > 126 mg/dL] or [serum glucose of a 2 hour 75 g glucose loading test > 200 mg/dL], respectively (1414 Position statement. Standards of medical care in diabetes – 2012. Diabetes Care. 2012;35(Supp 1):S12.). Patients with evidence of any target organ damage from high blood pressure (BP), thyroid dysfunction, liver disease, renal failure, malignancy and other chronic diseases, inflammatory conditions, and those who were receiving any medications were excluded. The patients were asked to sit down for 10 minutes before the BP was recorded using a manual mercury sphygmomanometer. The mean of two BP measurements was considered. The mean arterial blood pressure was calculated according to the following formula: MAP = DBP + ((SBP-DBP)/3). A complete blood count, renal and hepatic function tests, electrolytes, serum lipid levels, and thyroid hormones of the patient and control groups were measured. The demographic parameters, antropometric measurements, blood pressures, medications and laboratory results of the participants were directly recorded in a Microsoft Excel 2010 file. All participants gave written informed consent. The study protocol was approved by The Research and Ethics Committees of Gulhane Military Medical Academy. A part of data was published elsewhere.
Anthropometric measurements
Weight (in kg) and height (in cm) were measured and BMI was calculated as body weight/height2 (kg/m2).
Measurement of arterial stiffness
TensioMed measurement of arterial stiffness (TensoMed Ltd. Budapest-Hungary) device was used. Systolic and diastolic pressures, pulse wave velocity (PWV), augmentation index (AIX) and central aortic pressure (CAP) measurements were recorded.
PWV, AIX and CAP analysis
TensioMed arteriography measurement device automatically reported the measurements. Measurements were made after 5 minutes rest of the patients, non smoking-or taking caffeinated beverages for at least the last 30 minutes, in the sitting position in a reserved quiet room away from external stimuli. The distance between the jugular notch and the symphysis pubis of the patients were measured and the data was recorded. During the measurement, brachial artery occlusion was made (only 8-20 seconds) and the blood flow was stopped as a part of the process. Then, the systolic pressure, diastolic pressure, mean arterial blood pressure, pulse rate, pulse pressure, PWV, AIX and CAP were taken from the device. The pressure waves were recorded along with the AIX, PWV and CAP values.
Blood sample collection and analysis of parameters
All blood samples were drawn in the morning after at least 10 h of fasting. Routine laboratory tests and biochemistry including a full blood count, serum glucose, urea, creatinine, sodium, potassium, liver enzymes were performed during the outpatient visit. Serum samples for e-selectin were run in the same assay. These samples were promptly centrifuged, the plasma and serum were separated and stored at −80° C. Fasting plasma glucose, total cholesterol (TC), triglyceride and high-density lipoprotein cholesterol (HDL-C) levels were measured by an enzymatic colorimetric method with an Olympus AU2700 auto analyzer using reagents from Olympus Diagnostics (GmbH, Hamburg, Germany). Low-density lipoprotein cholesterol (LDL-C) was calculated by Friedewald’s Formula (1515 Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499-502.). ELISA kits were used according to the manufacturer’s instructions (Uscnlife Science & Technology Co., Ltd, Wuhan, China) to determine e-selectin levels. We measured all samples in duplicates and used the mean values. hs-CRP was measured in serum by high sensitive C-reactive protein (hs-CRP) ELISA Kit (Cell Biolabs, Inc, San Diego, USA).
Statistical analysis
Statistical analysis was carried out using IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp. Independent samples t test, Chi-square test, Mann-Whitney U statistical analysis, Pearson-rho correlation, univariate analysis and linear regression analysis tests were used. Quantitative variables were expressed as mean ± standard deviation. The Kolmogorov-Smirnov and Shapiro Wilkins tests were used to determine the distribution characteristics of variables and Levene’s test was used to determine the equality of variance. Differences between groups were studied for significance by independent samples t-test as appropriate. Categorical variables were compared by Chi-square test. Pearson and Spearman correlation analysis were used to evaluate the relationship between variables. Univariate analysis was used to adjust the effects of covariates on variables. Results of the analysis were expressed as percent for qualitative variables and mean ± standard deviation for continuous variables. A two-sided p < 0.05 was considered significant.
RESULTS
General characteristics of the patients
A total of 60 patients with a newly established elevated fasting serum glucose (25 females) and 17 healthy controls (13 females) were included in the study. The mean ages were 50.4 ± 12.1 and 40.2 ± 15.1 years, respectively, for patient and control groups (p = 0.007). Of patients, the mean age was 49.5 ± 16.1 years in males (n = 35) and 51.6 ± 13.7 years in females (n = 25) (p = 0.592). The mean BMI’s of the male and female patients were 27.2 ± 4.7 and 28.9 ± 4.5, respectively (p = 0.158). There was no significant difference in terms of age and BMI between male and female patients. The patient group were divided into three groups according to ADA criteria, considering the fasting serum glucose measurements, as IFG, IGT and newly diagnosed T2DM groups (Table 1). The IFG and IGT groups were not age and BMI-matched to the control group, but T2DM group was the same as control group in terms of these parameters. There were differences in terms of blood pressure measurements between groups (p < 0.001). The T2DM group did not match in terms of sex to the control group (p = 0.001).
Arterial stiffness measurements
PWV and CAP measurements were significantly higher in IFG, IGT and T2DM groups compared to controls (Table 1). Aix measurements were similar in all groups. There was only a weak negative correlation between e-selectin levels and Aix in the patient group (Pearson, r = -0.304, p = 0.021.
Laboratory findings
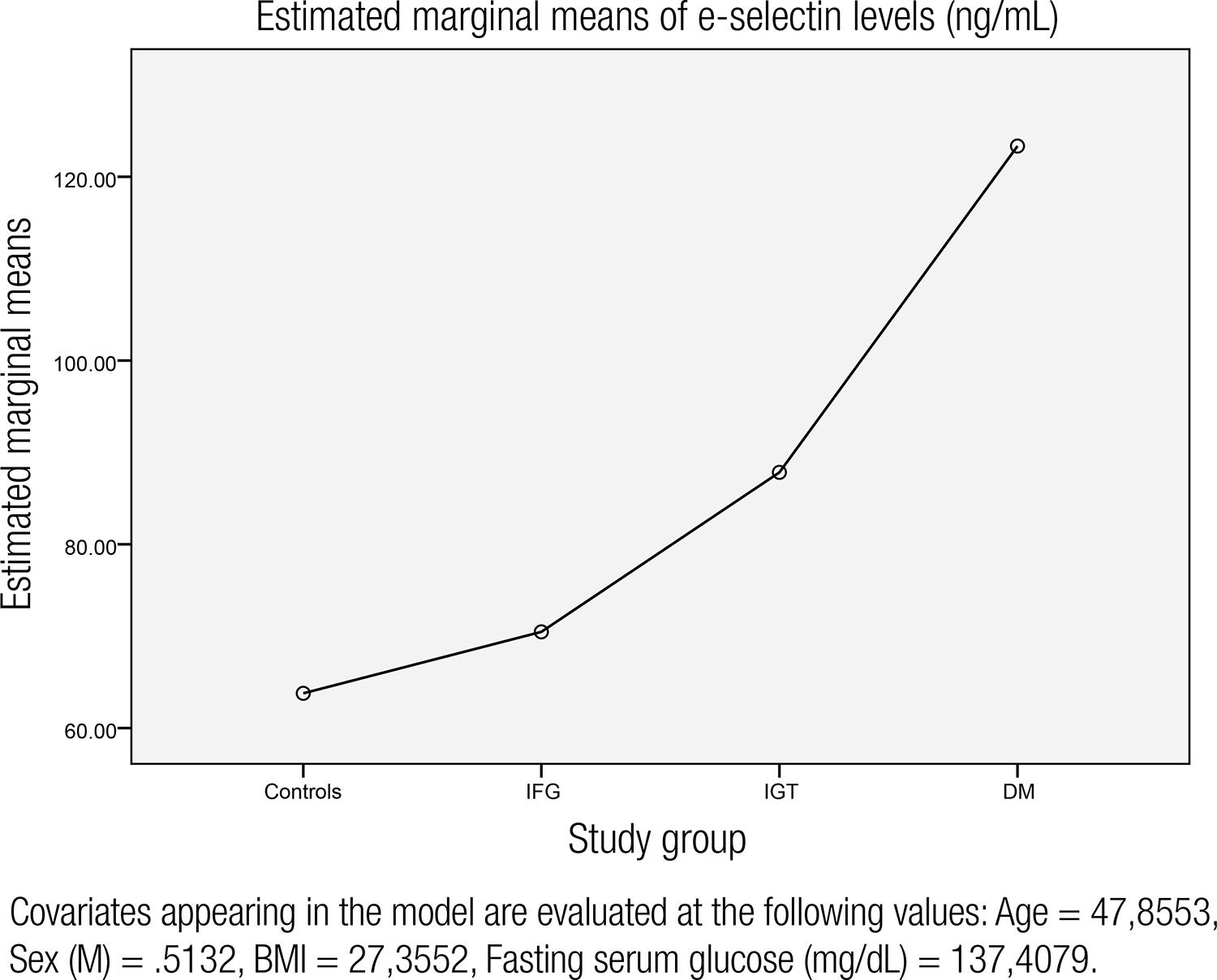
The laboratory findings are shown in table 1. There was no statistically significant difference in terms of LDL, total cholesterol, LDL, creatinine levels between groups. Fasting serum glucose levels were 88.8, 112.1, 115.8, and 223.8 mg/dL, respectively, for controls, IFG, IGT and T2DM groups (p < 0.001, for each groups). The mean e-selectin levels were significantly higher in IGT, IFG and T2DM groups compared to controls. In regression analysis, e-selectin levels were significanly associated with fasting serum glucose, hs-CRP levels, sex, BMI and age (Table 2). Fasting serum glucose was the most important predictor of e-selectin levels. The mean arterial pressure did not show any significant association with serum e-selectin levels and was excluded by the regression analysis (β coefficient: 0.092, p = 0.358). Also, the mean arterial pressure did not have any influence on hs-CRP levels (β coefficient: 0.189, p = 0.362). So, the intergroup blood pressure differences were disregarded. Charts showing the association between e-selectin levels, fasting serum glucose and hs-CRP levels in the entire group are given in figures 1 and 2. After adjustment for age, BMI, sex, and fasting blood glucose, e-selectin levels were increasing through the initiation of T2DM and are shown in figure 3.
Chart showing the association between fasting serum glucose and e-selectin levels in the entire group
Chart showing the association between serum e-selectin levels and hs-CRP in the entire group.
The chart representing mean e-selectin levels in controls, impaired fasting glucose (IFG), impaired glucose tolerance (IGT) and newly diagnosed T2DM groups after adjustment for serum glucose, BMI, age and sex.
DISCUSSION
In this study, it has been found that e-selectin levels increase in prediabetes patients, consistently with rising glucose levels, through the diagnosis of T2DM. The fasting glucose levels are the most powerful predictor of e-selectin levels. Also, a significant association exists with serum hs-CRP levels. These data provide an evidence that diabetes candidates have an increasing endothelial dysfunction (ED) and inflammation with rising blood glucose levels, through the diagnosis of T2DM. ED is a mostly recognized part of macrovascular and microvascular disease complications in diabetes (1616 Tooke JE. Microvascular function in human diabetes. A physiological perspective. Diabetes. 1995;44(7):721-6.). ED may also precede diabetes onset and may promote insulin resistance and glucose dysregulation leading to diabetes, but there is still controversy about the exact role of ED in patients with prediabetes and diabetes (1717 Rossi R, Cioni E, Nuzzo A, Origliani G, Modena MG. Endothelial-dependent vasodilation and incidence of type 2 diabetes in a population of healthy postmenopausal women. Diabetes Care. 2005;28(3):702-7.,1818 Meigs JB, O’donnell CJ, Tofler GH, Benjamin EJ, Fox CS, Lipinska I, et al. Hemostatic markers of endothelial dysfunction and risk of incident type 2 diabetes: the Framingham Offspring Study. Diabetes. 2006;55(2):530-7.).
ED and atherosclerosis play an important role in the pathogenesis of end organ damage. The endothelial layer has many functions, such as homeostasis, vascular permeability, vasoregulation, angiogenesis and inflammatory response (1919 Kharbanda RK, Deanfield JE. Functions of the healthy endothelium. Coron Artery Dis. 2001;12(6):485-91.). It is widely accepted that ED is the atherosclerosis precursor. Previously, flow-mediated dilatation has been investigated as a gold standard indicator of endothelial damage and dysfunction (2020 Yilmaz MI, Sonmez A, Saglam M, Gulec M, Kilic S, Eyileten T, et al. Hemoglobin is inversely related to flow-mediated dilatation in chronic kidney disease. Kidney Int. 2009;75(12):1316-21.). Lately, investigators focused on non-invasive methods in defining ED and atherosclerosis, such as arterial stiffness measurements and biomarkers. Arterial stiffness has high cardiovascular predictive value, reproducibility, cost effectiveness and availability in many healthcare facilities and it is suggested as an additional test for the evaluation of hypertensive patients according to the current guidelines (1111 Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Böhm M, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2013;34(28):2159-219.).
E-selectin is also known as CD62E or endothelial leukocyte adhesion molecule-1 (ELAM1). It is a type 1 membrane protein belonging to the surface molecules of the selectin family (CD62P and CD62L). Selectins are C type cell surface lectins playing a role in leukocyte adhesion to the endothelium of the vessel wall. CD62E (E-selectin) is an endothelial cell specific selectin expressed on cytokine induced endothelial cells, but only after a proinflammatory cytokine led activation (88 Cummings CJ, Sessler CN, Beall LD, Fisher BJ, Best AM, Fowler AA 3rd. Soluble E-selectin levels in sepsis and critical illness. Correlation with infection and hemodynamic dysfunction. Am J Respir Crit Care Med. 1997;156(2 Pt 1):431-7.,1010 To SS, Newman PM, Hyland VJ, Robinson BG, Schrieber L. Regulation of adhesion molecule expression by human synovial microvascular endothelial cells in vitro. Arthritis Rheum. 1996;39(3):467-77.). TNFalpha, IL1, and some bacterial components like lipopolysaccharides trigger the transcription of CD62E in an NFkB dependent signal cascade. CD62E is associated with endothelium of the blood vessel in different inflammatory situations. Previously published data have demonstrated that e-selectin is a proatherogenic and proinflammatory cytokine associated with insulin resistance, obesity, and cardiovascular disease (2121 Adamska A, Karczewska-Kupczewska M, Nikołajuk A, Otziomek E, Górska M, Kowalska I, et al. Relationships of serum soluble E-selectin concentration with insulin sensitivity and metabolic flexibility in lean and obese women. Endocrine. 2014;45(3):422-9.). In a previous study, it has been suggested that circulating e-selectin concentration may be a biomarker for indicating subsequent development of metabolic diseases and in particular cardiovascular diseases from a healthy state (2222 Mochizuki K, Inoue S, Miyauchi R, Misaki Y, Shimada M, Kasezawa N, et al. Plasma sE-selectin level is positively correlated with neutrophil count and diastolic blood pressure in Japanese men. J Nutr Sci Vitaminol (Tokyo). 2013;59(5):447-53.). Higher levels of endothelial inflammation may also be associated to the chronic complications of diabetes mellitus. In a recent study by Sasongko and cols. it has been mentioned that higher CRP levels, but not markers of endothelial function, may be related to more severe diabetic retinopathy suggesting that inflammatory processes are involved in severe diabetic retinopathy (2323 Sasongko MB, Wong TY, Jenkins AJ, Nguyen TT, Shaw JE, Wang JJ. Circulating markers of inflammation and endothelial function, and their relationship to diabetic retinopathy. Diabet Med. 2015;32(5):686-91.). Our findings indicate that prediabetes patients have higher e-selectin levels as their serum glucose rise, and it looks as they get highest with the diabetes diagnosis. This is compatible with a hypothesis of rising endothelial inflammation with progression to diabetes mellitus.
In fact, hypertension is a condition with endothelial dysfunction and inflammation and this has been documented in previous studies (2424 Schulz E, Gori T, Münzel T. Oxidative stress and endothelial dysfunction in hypertension. Hypertens Res. 2011;34(6):665-73.,2525 Davignon J, Ganz P. Role of endothelial dysfunction in atherosclerosis. Circulation. 2004;109(23 Suppl 1):III27-32.). It may be expected that the rising blood pressure levels in our prediabetes and diabetes groups are responsible from the increase in e-selectin levels. But the regression analysis did not reveal a significant association between e-selectin levels; arterial blood pressure was excluded in terms of any significant effect on e-selectin levels by regression analysis; so we could not show any association between blood pressure and e-selectin levels, which is a good marker to show endothelial inflammation. This may perhaps be due to any “covering effect” of diabetes, leading to higher levels of endothelial inflammation than hypertension, so the effect of increase in blood pressure on e-selectin levels is lower than the increase in blood glucose in this patient group. Also, there may be factors influencing e-selectin levels in diabetics rather than endothelial inflammation and this may be another mechanism to make these results.
Our study did not find any significant association between e-selectin levels and arterial stiffness parameters except Aix measurements. In fact, higher Aix measurements represent higher cardiovascular risk of these patients, and we may expect a positive correlation between these two parameters. This still carries a controversy. However, in a study by Llauradó and cols., impaired endothelial function was not associated with arterial stiffness in adults with type 1 diabetes, and they suggested that endothelial dysfunction occurs earlier than arterial stiffness in type 1 diabetes (2626 Llauradó G, Ceperuelo-Mallafré V, Vilardell C, Simó R, Albert L, Berlanga E, et al. Impaired endothelial function is not associated with arterial stiffness in adults with type 1 diabetes. Diabetes Metab. 2013;39(4):355-62.). This may also be due to our previously called thesis, the covering effect, than blood glucose levels. Further studies are needed to reveal these associations.
Our study has some limitations. First, our study sample is relatively small. Secondly, we had difficulties in matching groups in terms of demographic and some laboratory parameters, so had difficulties in discussing the results. Thirdly, the differences in blood pressure measurements may be a challenge, but we neglected the effect of blood pressure on regression analysis. Fourth, about the newly diagnosed diabetic term, the patients have come to our outpatient clinics for control reasons or examination for other disease or conditions. We do not exactly know how long they are diabetic, or have IFG or IGT. This may have caused some disturbances in our observations.
CONCLUSION
Our results show that prediabetes patients have increasing e-selectin levels, which is a marker showing endothelial inflammation, through the diagnosis of T2DM. e-selectin levels are significantly correlated with fasting serum glucose levels. Prediabetic and newly diagnosed diabetics have higher arterial stiffness measurements. E-selectin and hs-CRP levels are not influenced by blood pressure and arterial stiffness parameters. These results suggest that e-selectin may be a good marker of endothelial inflammation and dysfunction increasing in parallel with serum glucose levels in prediabetes and newly diagnosed diabetes patients.
REFERENCES
-
1Haffner SM, Stern MP, Hazuda HP, Mitchell BD, Patterson JK. Cardiovascular risk factors in confirmed prediabetic individuals. Does the clock for coronary heart disease start ticking before the onset of clinical diabetes? JAMA. 1990;263(21):2893-8.
-
2Song MK, Davies NM, Roufogalis BD, Huang TH-W. Management of cardiorenal metabolic syndrome in diabetes mellitus: a phytotherapeutic perspective. J Diabetes Res. 2014;2014.
-
3Kannel WB, McGee DL. Diabetes and cardiovascular risk factors: the Framingham study. Circulation. 1979;59(1):8-13.
-
4Muris DM, Houben AJ, Schram MT, Stehouwer CD. Microvascular dysfunction is associated with a higher incidence of type 2 diabetes mellitus: a systematic review and meta-analysis. Arterioscler Thromb Vasc Biol. 2012;32(12):3082-94.
-
5De Vriese A, Verbeuren TJ, Van de Voorde J, Lameire NH, Vanhoutte PM. Endothelial dysfunction in diabetes. Br J Pharmacol. 2000;130(5):963-74.
-
6Noble KE, Panayiotidis P, Collins PW, Hoffbrand AV, Yong KL. Monocytes induce E-selectin gene expression in endothelial cells: role of CD11/CD18 and extracellular matrix proteins. Eur J Immunol. 1996;26(12):2944-51.
-
7Newman W, Beall LD, Carson CW, Hunder GG, Graben N, Randhawa ZI, et al. Soluble E-selectin is found in supernatants of activated endothelial cells and is elevated in the serum of patients with septic shock. J Immunol. 1993;150(2):644-54.
-
8Cummings CJ, Sessler CN, Beall LD, Fisher BJ, Best AM, Fowler AA 3rd. Soluble E-selectin levels in sepsis and critical illness. Correlation with infection and hemodynamic dysfunction. Am J Respir Crit Care Med. 1997;156(2 Pt 1):431-7.
-
9de Jager J, Dekker JM, Kooy A, Kostense PJ, Nijpels G, Heine RJ, et al. Endothelial dysfunction and low-grade inflammation explain much of the excess cardiovascular mortality in individuals with type 2 diabetes: the Hoorn Study. Arterioscler Thromb Vasc Biol. 2006;26(5):1086-93.
-
10To SS, Newman PM, Hyland VJ, Robinson BG, Schrieber L. Regulation of adhesion molecule expression by human synovial microvascular endothelial cells in vitro. Arthritis Rheum. 1996;39(3):467-77.
-
11Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Böhm M, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2013;34(28):2159-219.
-
12Kostis JB, Lawrence-Nelson J, Ranjan R, Wilson AC, Kostis WJ, Lacy CR. Association of increased pulse pressure with the development of heart failure in SHEP. Systolic Hypertension in the Elderly (SHEP) Cooperative Research Group. Am J Hypertens. 2001;14(8 Pt 1):798-803.
-
13Heitzer T, Schlinzig T, Krohn K, Meinertz T, Münzel T, Munzel T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation. 2001;104(22):2673-8.
-
14Position statement. Standards of medical care in diabetes – 2012. Diabetes Care. 2012;35(Supp 1):S12.
-
15Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499-502.
-
16Tooke JE. Microvascular function in human diabetes. A physiological perspective. Diabetes. 1995;44(7):721-6.
-
17Rossi R, Cioni E, Nuzzo A, Origliani G, Modena MG. Endothelial-dependent vasodilation and incidence of type 2 diabetes in a population of healthy postmenopausal women. Diabetes Care. 2005;28(3):702-7.
-
18Meigs JB, O’donnell CJ, Tofler GH, Benjamin EJ, Fox CS, Lipinska I, et al. Hemostatic markers of endothelial dysfunction and risk of incident type 2 diabetes: the Framingham Offspring Study. Diabetes. 2006;55(2):530-7.
-
19Kharbanda RK, Deanfield JE. Functions of the healthy endothelium. Coron Artery Dis. 2001;12(6):485-91.
-
20Yilmaz MI, Sonmez A, Saglam M, Gulec M, Kilic S, Eyileten T, et al. Hemoglobin is inversely related to flow-mediated dilatation in chronic kidney disease. Kidney Int. 2009;75(12):1316-21.
-
21Adamska A, Karczewska-Kupczewska M, Nikołajuk A, Otziomek E, Górska M, Kowalska I, et al. Relationships of serum soluble E-selectin concentration with insulin sensitivity and metabolic flexibility in lean and obese women. Endocrine. 2014;45(3):422-9.
-
22Mochizuki K, Inoue S, Miyauchi R, Misaki Y, Shimada M, Kasezawa N, et al. Plasma sE-selectin level is positively correlated with neutrophil count and diastolic blood pressure in Japanese men. J Nutr Sci Vitaminol (Tokyo). 2013;59(5):447-53.
-
23Sasongko MB, Wong TY, Jenkins AJ, Nguyen TT, Shaw JE, Wang JJ. Circulating markers of inflammation and endothelial function, and their relationship to diabetic retinopathy. Diabet Med. 2015;32(5):686-91.
-
24Schulz E, Gori T, Münzel T. Oxidative stress and endothelial dysfunction in hypertension. Hypertens Res. 2011;34(6):665-73.
-
25Davignon J, Ganz P. Role of endothelial dysfunction in atherosclerosis. Circulation. 2004;109(23 Suppl 1):III27-32.
-
26Llauradó G, Ceperuelo-Mallafré V, Vilardell C, Simó R, Albert L, Berlanga E, et al. Impaired endothelial function is not associated with arterial stiffness in adults with type 1 diabetes. Diabetes Metab. 2013;39(4):355-62.
-
Fundings: this research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Publication Dates
-
Publication in this collection
21 July 2015 -
Date of issue
Oct 2015
History
-
Received
11 Feb 2015 -
Accepted
08 Mar 2015