ABSTRACT
Objective:
To evaluate the candidate genes PAX-8, NKX2-5, TSH-R and HES-1 in 63 confirmed cases of thyroid dysgenesis.
Subjects and methods:
Characterization of patients with congenital hypothyroidism into specific subtypes of thyroid dysgenesis with hormone levels (TT4 and TSH), thyroid ultrasound and scintigraphy. DNA was extracted from peripheral blood leukocytes and the genetic analysis was realized by investigating the presence of mutations in the transcription factor genes involved in thyroid development.
Results:
No mutations were detected in any of the candidate genes. In situ thyroid gland represented 71.1% of all cases of permanent primary congenital hypothyroidism, followed by hypoplasia (9.6%), ectopia (78%), hemiagenesis (6.0%) and agenesis (5.5%). The highest neonatal screening TSH levels were in the agenesis group (p < 0.001).
Conclusions:
Thyroid dysgenesis is possibly a polygenic disorder and epigenetic factors could to be implicated in these pathogeneses.
Keywords
Thyroid dysgenesis; congenital hypothyroidism; transcription factors
INTRODUCTION
Congenital hypothyroidism (CH) is the most common disorder of the endocrine system among newborns with a global incidence of about 1/3,000-4,000 (11. Fagman H, Nilsson M. Morphogenesis of the thyroid gland. Mol Cell Endocrinol. 2010;323(1):35-54.). Untreated CH can cause dwarfism and severe intellectual disability, with the delayed onset of thyroid replacement therapy by only a few weeks after birth being associated with reduced development of mental functions later on in life (22. Klein AH, Meltzer S, Kenny FM. Improved prognosis in congenital hypothyroidism treated before age three months. J Pediatr. 1972;81(5):912-5.). Therefore, neonatal screening programs have been implemented to identify these patients earlier to ensure proper somatic growth and development of the central nervous system in infants (33. Morreale de Escobar G. The role of thyroid hormone in fetal neurodevelopment. J Pediatr Endocrinol Metab. 2001;14 Suppl 6:1453-62.).
The causes of CH are broadly categorized into dyshormonogenesis accounting for 15% of the cases, and thyroid dysgenesis (TD) accounting for 85% of the cases (33. Morreale de Escobar G. The role of thyroid hormone in fetal neurodevelopment. J Pediatr Endocrinol Metab. 2001;14 Suppl 6:1453-62.
4. Lafranchi SH. Newborn screening strategies for congenital hypothyroidism: an update. J Inherit Metab Dis. 2010;33 (Suppl 2):S225-33.
5. Olney RS, Grosse SD, Vogt RF. Prevalence of congenital hypothyroidism--current trends and future directions: workshop summary. Pediatrics. 2010;125(Suppl):S31-6.-66. Szinnai G. Clinical genetics of congenital hypothyroidism. Endocr Dev. 2014;26:60-78.). Dyshormonogenesis is caused by autosomal recessive mutations of key molecules of thyroid hormone synthesis, in which thyroid hormone production fails in a structurally healthy thyroid gland (77. Rastogi MV, Lafranchi SH. Congenital hypothyroidism. Orphanet J Rare Dis. 2010;5:17.). TD is caused by a wide range of different structural malformations in the thyroid that result in a wide variety of different phenotypes of CH (66. Szinnai G. Clinical genetics of congenital hypothyroidism. Endocr Dev. 2014;26:60-78.,88. Szinnai G. Genetics of normal and abnormal thyroid development in humans. Best Pract Res Clin Endocrinol Metab. 2014;28(2):133-50.
9. De Felice M, Di Lauro R. Thyroid development and its disorders: genetics and molecular mechanisms. Endocr Rev. 2004;25(5): 722-46.-1010. Ramos HE, Nesi-França S, Maciel R. Novos aspectos da genética e dos mecanismos moleculares da morfogênese da tiróide para o entendimento da disgenesia tiroidiana: [revisão]. Arq Bras Endocrinol Metab. 2008;52(9):1403-15.). TD is subcategorized into: 1) thyroid agenesis - the most severe form, in which there is a complete lack of thyroid tissue (i.e. both lobes); 2) thyroid hemiagenesis - one of the thyroid lobes is completely missing; 3) thyroid hypoplasia - the gland is smaller but is still in a normal position; 4) thyroid ectopia - the gland is not positioned normally but rests along the migratory pathway of the primordium.
While TD appears sporadically in the vast majority of cases (88. Szinnai G. Genetics of normal and abnormal thyroid development in humans. Best Pract Res Clin Endocrinol Metab. 2014;28(2):133-50.), several findings have pointed to a genetic underpinning (1111. Carré A, Stoupa A, Kariyawasam D, Gueriouz M, Ramond C, Monus T, et al. Mutations in BOREALIN cause thyroid dysgenesis. Hum Mol Genet. 2017;26(3):599-610.
12. Hannoush ZC, Weiss RE. Defects of Thyroid Hormone Synthesis and Action. Endocrinol Metab Clin North Am. 2017;46(2):375-88.-1313. Abu-Khudir R, Larrivée-Vanier S, Wasserman JD, Deladoëy J. Disorders of thyroid morphogenesis. Best Pract Res Clin Endocrinol Metab. 2017;31(2):143-59.). Familial inheritance patterns have been observed in about 2% of cases (1414. Moreno JC, Vijlder JMJ, Vulsma T, Ris-Stalpers, C. Genetic basis of hypothyroidism: recent advances, gaps and strategies for future research. Trends Endocrinol Metab. 2003;14(7):318-26.) and a higher incidence has been found for girls (male to female ratio 1:2) and among the Hispanic and Caucasian ethno-racial groups in comparison with Afro-descendants (1515. Knobel M, Medeiros-Neto G. An outline of inherited disorders of the thyroid hormone generating system. Thyroid. 2003;13:771-801.). Animal models have shown that several genes have been implicated from animal models were putatively associated with TD, however results from genetic association studies in human have been controversial (66. Szinnai G. Clinical genetics of congenital hypothyroidism. Endocr Dev. 2014;26:60-78.).
The relevance of understanding the genetic underpinnings of TD has important implications not only for further understanding of the genotype/ phenotype relationship of the disorder, but also to assist in the correct management of patients and screening programs for CH.
The present study aimed investigate the etiology of the permanent CH diagnosed, with the purpose of identifying the cases due to TD, and evaluate the role of four different candidate genes that have been suggested as being involved in thyroid embryogenesis: (paired box gene 8 (PAX-8), thyroid stimulating hormone receptor (TSH-R), transcription factor related locus 5 (NKX2-5) and hairy/enhancer of split 1 (HES-1) and having an influence on these outcomes. We believe this study will contribute to the better knowledge of genetic basis of TD-caused CH.
SUBJECTS AND METHODS
Patients with primary CH over the age of 3 years were recruited between 2012 and 2015 from the Association for Parents and Friends of Disabled Individuals (APAE) - Salvador - Bahia.
Retrospective analysis of patient medical records was conducted to evaluate the clinical presentation including: 1) TSH; total T4 (TT4), and free T4 (fT4) at the time of neonatal screening, measured by immunofluorometric assays (Autodelfia®, Wallac Oy, Turku, Finland); and 2) congenital heart defects or other congenital malformations.
The patients were characterized for TD by: 1) measuring thyroglobulin (Tg) levels, per immunofluorometric assays (Autodelfia®, Wallac Oy, Turku, Finland); 2) thyroid ultrasound, performed by Portable Mindray DP-4900 – 7-10 Mhz focused on the thyroid gland and cervical region. Total thyroid volume was calculated as described by Ueda, 1999 (1616. Ueda D. Normal volume of the thyroid gland in children. J Clin Ultrasound. 1990;18(6):455-62.) and compared according to sex, age, height and body surface as described by Zimmermam and cols., 2004 (1717. Zimmermann MB, Hess SY, Molinari L, De Benoist B, Delange F, Braverman LE, et al. New reference values for thyroid volume by ultrasound in iodine-sufficient schoolchildren: a World Health Organization/Nutrition for Health and Development Iodine Deficiency Study Group Report. Am J Clin Nutr. 2004;79(2):231-7.); and 3) scintigraphy, performed by intravenous injection of 99Tc Pertechnetate.
This study was approved by both the Federal University of Bahia - Ethics Committee for Research Projects and the APAE-Salvador (N° 125/2011 and N° 22/2011, respectively). Participation was voluntary with written consent obtained from at least one parent or guardian.
Genetic testing
DNA was extracted from whole blood using PureLink® Genomic DNA Mini kit (Life Technology®, Carlsbad, California). The entire coding region and promoter region of the PAX-8 gene, including exon/intron boundaries, was amplified from genomic DNA by polymerase chain reaction (PCR) using standard techniques (1818. Ramos HE, Nesi-França S, Boldarine VT, Pereira RM, Chiamolera MI, Camacho CP, et al. Clinical and molecular analysis of thyroid hypoplasia: a population-based approach in southern Brazil. Thyroid. 2009;19(1):61-8.). All 10 individual TSH-R exons were sequenced, with exon 10 subdivided into 2 overlapping primers (1818. Ramos HE, Nesi-França S, Boldarine VT, Pereira RM, Chiamolera MI, Camacho CP, et al. Clinical and molecular analysis of thyroid hypoplasia: a population-based approach in southern Brazil. Thyroid. 2009;19(1):61-8.). The coding region of the NKX2-5 gene was studied and each of 2 exons were amplified by a total of 3 PCRs (1818. Ramos HE, Nesi-França S, Boldarine VT, Pereira RM, Chiamolera MI, Camacho CP, et al. Clinical and molecular analysis of thyroid hypoplasia: a population-based approach in southern Brazil. Thyroid. 2009;19(1):61-8.). HES-1 exons were sequenced entirely using 5 pairs of primers with exon 4 divided into two parts (1818. Ramos HE, Nesi-França S, Boldarine VT, Pereira RM, Chiamolera MI, Camacho CP, et al. Clinical and molecular analysis of thyroid hypoplasia: a population-based approach in southern Brazil. Thyroid. 2009;19(1):61-8.). PCR products were sequenced directly on a ABI Prism® 3100 Genetic Analyzer (Applied Biosystem®, Carlsbad, California) using the Big Dye TM® Terminator Sequencing Standard Kit (Applied Biosystems®, Carlsbad, California). The results were analyzed by comparison with the standard sequence (www.ncbi.nlm.nih.gov/pubmed) for each gene specifically (PAX-8; TSH-R; NKX2-5 and HES-1) using the bioinformatics program BioEdit Sequence Alignment Editor, Version 7.2.5.0 (Ibis Biosciences, Carlsbad, California). All primers and PCR conditions are available upon request.
Statistical analysis
The clinical parameters (TSH and fT4 serum levels) of the patients diagnosed with TD were compared with those of patients diagnosed with ISTG to assess the severity of conditions in patients with different phenotypes. Statistical analysis was conducted with SPSS 2.0 and Stata 12 software packages. Nonparametric Mann-Whitney U and Kuskall-Wallis tests were used whenever appropriate and a p-value of < 0.05 was considered significant. The Dunn test was used for multiple comparisons (1919. Dinno A. Nonparametric Pairwise Multiple Comparisons in Independent Groups Using Dunn's Test. Stata J. 2015;15:292-300.).
RESULTS
Of the 1,188 newborns diagnosed with CH up to the year 2016, abnormal TSH levels were confirmed in 773 newborns and they are being followed-up. Of these, 2.84% (N = 22) were found to be transitory primary congenital hypothyroidism, based upon spontaneous normalization of TSH between screening and diagnosis by the age of 3, thus resulting in 354 unrelated patients eligible for inclusion.
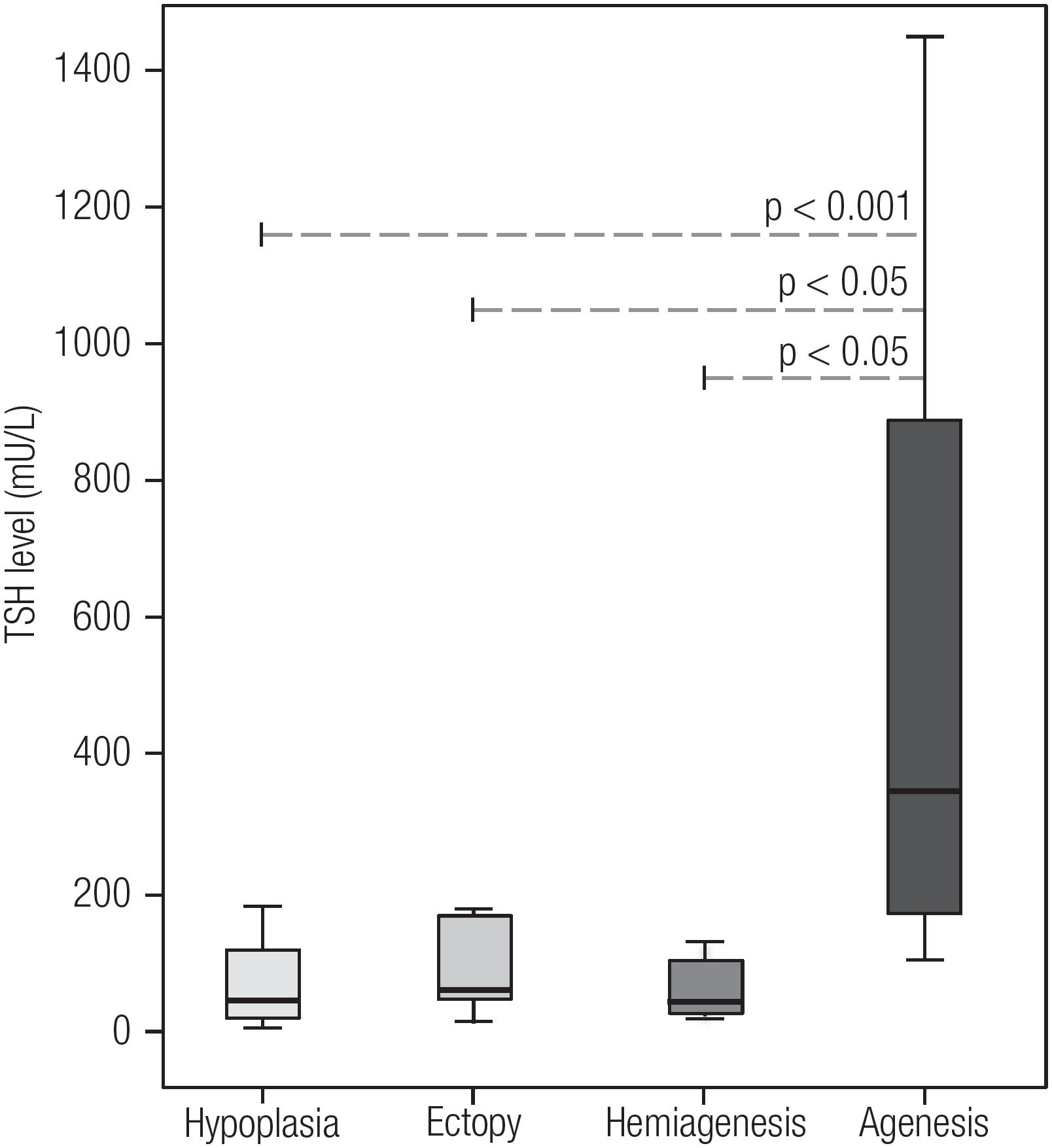
During the study period May 2012-June 2015, 218 patients underwent imaging tests for TD characterization. Of the 218, 155 (71.1%) had ISTG with the remaining 28.9% (N = 63) having some form of TD: ectopy (N = 17; 7.8%), agenesis (N = 12; 5.5%), hypoplasia (N = 21; 9.6%) and hemiagenesis (N = 13; 6.0%). The overall gender distribution was 118 females: 100 males. A summary of the clinical parameters evaluated in newborns is shown in Table 1. In a broader comparison, patients with TD compared with those with ISTG had significantly higher screening TSH levels (p < 0.001) and confirmatory serum TSH levels (p < 0.05) (Figure 1). This difference was probably due to the group of agenesis, as this group showed significantly higher levels than the other groups of TD (Figure 2). In addition, the agenesis subgroup had lower fT4 levels (0.4, IQR 0.3-1.2 μg/dL), followed by the ectopy group (0.8, IQR 0.3-1.1 μg/dL) when compared with the other groups.
Comparison of median TSH levels of patients with thyroid dysgenesis and those with in situ thyroid gland. A) Comparison of Neonatal screening TSH levels B) Initial serum TSH.
p-Value: Mann-Whitney U test.
Comparison of screening TSH levels among the different subtypes of thyroid dysgenesis.
P-Value: the Dunn test for multiple comparisons.
Genetics findings
Six known single nucleotide polymorphisms (SNP) were found in patients with TD. Two in the NKX2-5 gene: a) rs2277923 (g.173235021T>C) was found in 34/63 (54%) patients – 19 heterozygotes, 15 homozygotes; b) rs72554028 (g.173233001C>G) was found in one patient (1/63 – 2%). Four polymorphisms were found in the TSH-R gene: a) rs1991517 (g.81610583G>C) was found in 31/63 (49%) patients – 27 heterozygotes, 4 homozygotes; b) rs2234919 (g.81422178C>A) was found in 4/63 (6%) - all heterozygotes; c) rs752184247 (g.78929481G>A) was found in 14/63 (22%) - all heterozygotes and; d) rs200551849 (g.8589551C>T) was found in 2/63 (3%) patients - both compound heterozygotes with the rs1991517 polymorphism.
DISCUSSION
None of the 63 patients with TD (41 females and 22 males) had mutations in the studied candidate genes PAX-8, TSH-R, NKX2-5 and HES-1. The candidate genes PAX-8, NKX2-5, TSH-R and HES-1 proposed as those being the most likely cause of TD (88. Szinnai G. Genetics of normal and abnormal thyroid development in humans. Best Pract Res Clin Endocrinol Metab. 2014;28(2):133-50.,2020. Brust ES, Beltrao CB, Chammas MC, Watanabe T, Sapienza MT, Marui S. Absence of mutations in PAX-8, NKX2. 5, and TSH receptor genes in patients with thyroid dysgenesis. Arq Bras Endocrinol Metabol. 2012;56(3):173-7.
21. Mahjoubi F, Mohammadi MM, Montazeri M, Aminii M, Hashemipour M. Mutations in the gene encoding paired box domain (PAX-8) are not a frequent cause of congenital hypothyroidism (CH) in Iranian patients with thyroid dysgenesis. Arq Bras Endocrinol Metabol. 2010;54(6):555-9.
22. Beltrão CB, Juliano AG, Chammas MC, Watanabe T, Sapienza MT, Marui S. Etiology of congenital hypothyroidism using thyroglobulin and ultrasound combination. Endocr J. 2010;57(7):587-93.
23. Alves EA, Cruz CM, Pimentel CP, Ribeiro RC, Santos AK, Caldato MC, et al. High frequency of D727E polymorphisms in exon 10 of the TSHR gene in Brazilian patients with congenital hypothyroidism. J Pediatr Endocrinol Metab. 2010;23(12):1321-8.-2424. Carre A, Rachdi L, Tron E, Richard B, Castanet M, Schlumberger M, et al. HES-1 is required for appropriate morphogenesis and differentiation during mouse thyroid gland development. PLoS One. 2011;6(2):e16752.) were not responsible for the TD confirmed in our cohort. This finding implies that there may be other genes that are responsible for TD (66. Szinnai G. Clinical genetics of congenital hypothyroidism. Endocr Dev. 2014;26:60-78.
7. Rastogi MV, Lafranchi SH. Congenital hypothyroidism. Orphanet J Rare Dis. 2010;5:17.-88. Szinnai G. Genetics of normal and abnormal thyroid development in humans. Best Pract Res Clin Endocrinol Metab. 2014;28(2):133-50.). In the cases of the patients with agenesis and ectopia, there was a clear gender bias towards females that was not seen in the ISTG, hypoplasia or hemiagenesis groups, which had a virtually equal distribution between the two genders. Along with a greater gender discrepancy, those with ectopy (p ≤ 0.05) and agenesis (p ≤ 0.001) had medians of TSH that were notably higher at the initial newborn screening and confirmatory serum TSH testing compared with hypoplasia and hemiagenesis. To the best of our knowledge we are the first researchers in Bahia to perform this type of genetic analysis for candidate thyroid embryogenesis genes PAX-8, NKX2-5, TSH-R and HES-1 in confirmed cases of permanent CH and TD (2525. Cerqueira TL, Ramos Y, Strappa G, Martin DS, Jesus M, Gonzaga J, et al.The c.63A>G polymorphism in the NKX2.5 gene is associated with thyroid hypoplasia in children with thyroid dysgenesis. Arch Endocrinol Metab. 2015;59(6):562-7). It should be noted that we excluded NKX2-1 and FOXE-1 from our analysis, based upon the fact that the phenotype presenting in our cohort showed no signs of Bamforth-Lazarus syndrome or neurological findings (2626. Bamforth J, Hughes I, Lazarus J, Weaver C, Harper P. Congenital hypothyroidism, spiky hair, and cleft palate. J Med Genet. 1989;26(1):49-51.).
Overall, the incidence of CH found in the Brazilian neonatal screening is 1/2,595 to 1/4,795 (1010. Ramos HE, Nesi-França S, Maciel R. Novos aspectos da genética e dos mecanismos moleculares da morfogênese da tiróide para o entendimento da disgenesia tiroidiana: [revisão]. Arq Bras Endocrinol Metab. 2008;52(9):1403-15.,2727. Silvestrin SM, Leone C, Leone CR. Detecting congenital hypothyroidism with newborn screening: the relevance of thyroid-stimulating hormone cutoff values. J Pediatr (Rio J). 2017;93(3):274-280.) in accordance with the expected global incidence reported of 1/3,000-4,000 infants (33. Morreale de Escobar G. The role of thyroid hormone in fetal neurodevelopment. J Pediatr Endocrinol Metab. 2001;14 Suppl 6:1453-62.,66. Szinnai G. Clinical genetics of congenital hypothyroidism. Endocr Dev. 2014;26:60-78.,99. De Felice M, Di Lauro R. Thyroid development and its disorders: genetics and molecular mechanisms. Endocr Rev. 2004;25(5): 722-46.). ISTG was the most common etiology found in our cohort, with a rise in incidence from previously reported measures, primarily attributed to the diagnosis of milder cases of CH due to a lower TSH threshold used for screening (2828. Castanet M, Goischke A, Léger J, Thalassinos C, Rodrigue D, Cabrol S, et al.; on behalf of Fédération Parisienne pour le Dépistage et la Prévention des Handicaps de l'Enfant (FDPHE). Natural History and Management of Congenital Hypothyroidism with in situ Thyroid Gland. Horm Res Paediatr. 2015;83:102-10.).
The severity of hypothyroidism in children with TD has varied between studies. In our study, children with agenesis presented clinical parameters differing significantly from those of the other subtypes at both neonatal screening and confirmatory testing for hormone levels. This was consistent with the findings of other studies that have demonstrated that children with agenesis present worse neuropsychological development in comparison with children with hypoplasia, ectopy or dyshormonogenesis, and require higher doses of L-T4 (2929. Ruchała M, Szczepanek E, Sowiński J. Diagnostic value of radionuclide scanning and ultrasonography in thyroid developmental anomaly imaging. Nucl Med Rev Cent East Eur. 2011;14(1):21-8.
30. Cherella CE, Wassner AJ. Congenital hypothyroidism: insights into pathogenesis and treatment. Int J Pediatr Endocrinol. 2017;2017:11.-3131. Léger J, Olivieri A, Donaldson M, Torresani T, Krude H, van Vliet G, et al.; ESPE-PES-SLEP-JSPE-APEG-APPES-ISPAE; Congenital Hypothyroidism Consensus Conference Group. European Society for Pediatric Endocrinology consensus guidelines on screening, diagnosis, and management of congenital hypothyroidism. J Clin Endocrinol Metab. 2014;99(2):363-84.). Thus, it is possible that treatment and followup schedules for TD need to consider the unique hormonal patterns and different responses to therapy in each different etiological category (44. Lafranchi SH. Newborn screening strategies for congenital hypothyroidism: an update. J Inherit Metab Dis. 2010;33 (Suppl 2):S225-33.,77. Rastogi MV, Lafranchi SH. Congenital hypothyroidism. Orphanet J Rare Dis. 2010;5:17.,3030. Cherella CE, Wassner AJ. Congenital hypothyroidism: insights into pathogenesis and treatment. Int J Pediatr Endocrinol. 2017;2017:11.,3131. Léger J, Olivieri A, Donaldson M, Torresani T, Krude H, van Vliet G, et al.; ESPE-PES-SLEP-JSPE-APEG-APPES-ISPAE; Congenital Hypothyroidism Consensus Conference Group. European Society for Pediatric Endocrinology consensus guidelines on screening, diagnosis, and management of congenital hypothyroidism. J Clin Endocrinol Metab. 2014;99(2):363-84.).
Similar to our study, only a few mutations have previously been found in large panels of patients (1818. Ramos HE, Nesi-França S, Boldarine VT, Pereira RM, Chiamolera MI, Camacho CP, et al. Clinical and molecular analysis of thyroid hypoplasia: a population-based approach in southern Brazil. Thyroid. 2009;19(1):61-8.,2323. Alves EA, Cruz CM, Pimentel CP, Ribeiro RC, Santos AK, Caldato MC, et al. High frequency of D727E polymorphisms in exon 10 of the TSHR gene in Brazilian patients with congenital hypothyroidism. J Pediatr Endocrinol Metab. 2010;23(12):1321-8.). Mutations have previously been identified in familial groups with TD, however, in our population there are likely to be sporadic and unrelated cases, which could be an important factor to evaluate the impact of these genetic variants at population level (2828. Castanet M, Goischke A, Léger J, Thalassinos C, Rodrigue D, Cabrol S, et al.; on behalf of Fédération Parisienne pour le Dépistage et la Prévention des Handicaps de l'Enfant (FDPHE). Natural History and Management of Congenital Hypothyroidism with in situ Thyroid Gland. Horm Res Paediatr. 2015;83:102-10.). Of the polymorphisms found, rs1991517 in the TSH-R gene has frequently been reported in the Brazilian population (1818. Ramos HE, Nesi-França S, Boldarine VT, Pereira RM, Chiamolera MI, Camacho CP, et al. Clinical and molecular analysis of thyroid hypoplasia: a population-based approach in southern Brazil. Thyroid. 2009;19(1):61-8.,2020. Brust ES, Beltrao CB, Chammas MC, Watanabe T, Sapienza MT, Marui S. Absence of mutations in PAX-8, NKX2. 5, and TSH receptor genes in patients with thyroid dysgenesis. Arq Bras Endocrinol Metabol. 2012;56(3):173-7.,2222. Beltrão CB, Juliano AG, Chammas MC, Watanabe T, Sapienza MT, Marui S. Etiology of congenital hypothyroidism using thyroglobulin and ultrasound combination. Endocr J. 2010;57(7):587-93.) with a frequency as high as 10% (2323. Alves EA, Cruz CM, Pimentel CP, Ribeiro RC, Santos AK, Caldato MC, et al. High frequency of D727E polymorphisms in exon 10 of the TSHR gene in Brazilian patients with congenital hypothyroidism. J Pediatr Endocrinol Metab. 2010;23(12):1321-8.), representing the second highest reported alteration in patients with CH. Previous studies, have found PAX-8 mutations ranging from 0-3.4% (88. Szinnai G. Genetics of normal and abnormal thyroid development in humans. Best Pract Res Clin Endocrinol Metab. 2014;28(2):133-50.). Inactivating mutations on the human TSH-R gene can cause a resistance to TSH with a strongly variable type of disease. Several studies have found that TSH-R mutations are more frequent than expected in patients with TD with prevalence in cohorts between 3-6% and even as high as 16.5% (88. Szinnai G. Genetics of normal and abnormal thyroid development in humans. Best Pract Res Clin Endocrinol Metab. 2014;28(2):133-50.). HES-1 has yet to be found in patients with TD (2424. Carre A, Rachdi L, Tron E, Richard B, Castanet M, Schlumberger M, et al. HES-1 is required for appropriate morphogenesis and differentiation during mouse thyroid gland development. PLoS One. 2011;6(2):e16752.). NKX2-5, also associated with cardiac malformations, was found with 3 heterozygote mutations in a single study with a cohort of 241 patients (3232. Dentice M, Cordeddu V, Rosica A, Ferrara AM, Santarpia L, Salvatore D, et al. Missense mutation in the transcription factor NKX2-5: a novel molecular event in the pathogenesis of thyroid dysgenesis. J Clin Endocrinol Metab. 2006;91(4):1428-33.). In our study, seven patients (2 females and 5 males) presented congenital heart disease (CHD) associated with TD, previously described in electronic records (2525. Cerqueira TL, Ramos Y, Strappa G, Martin DS, Jesus M, Gonzaga J, et al.The c.63A>G polymorphism in the NKX2.5 gene is associated with thyroid hypoplasia in children with thyroid dysgenesis. Arch Endocrinol Metab. 2015;59(6):562-7).
Studies have hypothesized that the molecular mechanisms underway in the very early steps of thyroid organogenesis were underlying in the etiopathogenesis of TD (1111. Carré A, Stoupa A, Kariyawasam D, Gueriouz M, Ramond C, Monus T, et al. Mutations in BOREALIN cause thyroid dysgenesis. Hum Mol Genet. 2017;26(3):599-610.,3333. Amendola E, De Luca P, Macchia PE, Terracciano D, Rosica A, Chiappetta G, et al. A mouse model demonstrates a multigenic origin of congenital hypothyroidism. Endocrinology. 2005;146(12): 5038-47.,3434. Nilsson M, Fagman H. Development of the thyroid gland. Development. 2017;144(12):2123-40.). However, it continues to be difficult to draw many conclusions from the murine models, as many fundamental differences with human thyroid development have been reported (i.e. localization, mode of inheritance and penetrance) (3333. Amendola E, De Luca P, Macchia PE, Terracciano D, Rosica A, Chiappetta G, et al. A mouse model demonstrates a multigenic origin of congenital hypothyroidism. Endocrinology. 2005;146(12): 5038-47.,3535. Weller G. Development of the thyroid, parathyroid and the thymus glands in man. Contrib Embryol. 1933;24:93-140.
36. Pohlenz J, Dumitrescu A, Zundel D, Martiné U, Schönberger W, Koo E, et al. Partial deficiency of thyroid transcription factor 1 produces predominantly neurological defects in humans and mice. J Clin Invest. 2002;109(4):469-73.
37. Daladoey J, Vassart G, Van Vliet, G. Possible non-Mendelian mechanisms of thyroid dysgenesis. Endocr Dev. 2007;10:29-42.-3838. Fagman H, Nilsson M. Morphogenetics of early thyroid development. J Mol Endocrinol. 2011;46(1):R33-42.). For instance, while heterozygous PAX-8 mutations in murine models do not display a pathological phenotype, heterozygous PAX-8 mutations have been reported in sporadic and familial CH patients with thyroid hypoplasia or ectopy (3535. Weller G. Development of the thyroid, parathyroid and the thymus glands in man. Contrib Embryol. 1933;24:93-140.,3939. Macchia PE, Lapi P, Krude H, Pirro MT, Missero C, Chiovato L, et al. PAX-8 mutations associated with congenital hypothyroidism caused by thyroid dysgenesis. Nat Genet. 1998;19(1):83-6.
40. Hinton CF, Harris KB, Borgfeld L, Drummond-Borg M, Eaton R, Lorey F, et al.Trends in incidence rates of congenital hypothyroidism related to select demographic factors: data from the United States, California, Massachusetts, New York, and Texas. Pediatrics. 2010;125 Suppl 2:S37-47-4141. Olivieri A, Stazi MA, Mastroiacovo P, Fazzini C, Medda E, Spagnolo A, et al.; Study Group for Congenital Hypothyroidism. A population-based study on the frequency of additional congenital malformations in infants with congenital hypothyroidism: data from the Italian Registry for Congenital Hypothyroidism (1991-1998). J Clin Endocrinol Metab. 2002;87(2):557-62.). However, a heterogeneous biochemical and morphological phenotype has been observed among patients with the same PAX-8 mutations (4040. Hinton CF, Harris KB, Borgfeld L, Drummond-Borg M, Eaton R, Lorey F, et al.Trends in incidence rates of congenital hypothyroidism related to select demographic factors: data from the United States, California, Massachusetts, New York, and Texas. Pediatrics. 2010;125 Suppl 2:S37-47,4141. Olivieri A, Stazi MA, Mastroiacovo P, Fazzini C, Medda E, Spagnolo A, et al.; Study Group for Congenital Hypothyroidism. A population-based study on the frequency of additional congenital malformations in infants with congenital hypothyroidism: data from the Italian Registry for Congenital Hypothyroidism (1991-1998). J Clin Endocrinol Metab. 2002;87(2):557-62.). At present, findings have suggested that genetic background plays an important role in phenotypic presentation (1212. Hannoush ZC, Weiss RE. Defects of Thyroid Hormone Synthesis and Action. Endocrinol Metab Clin North Am. 2017;46(2):375-88.,3131. Léger J, Olivieri A, Donaldson M, Torresani T, Krude H, van Vliet G, et al.; ESPE-PES-SLEP-JSPE-APEG-APPES-ISPAE; Congenital Hypothyroidism Consensus Conference Group. European Society for Pediatric Endocrinology consensus guidelines on screening, diagnosis, and management of congenital hypothyroidism. J Clin Endocrinol Metab. 2014;99(2):363-84.,3333. Amendola E, De Luca P, Macchia PE, Terracciano D, Rosica A, Chiappetta G, et al. A mouse model demonstrates a multigenic origin of congenital hypothyroidism. Endocrinology. 2005;146(12): 5038-47.,3434. Nilsson M, Fagman H. Development of the thyroid gland. Development. 2017;144(12):2123-40.). TD is likely to be of polygenic origin (1212. Hannoush ZC, Weiss RE. Defects of Thyroid Hormone Synthesis and Action. Endocrinol Metab Clin North Am. 2017;46(2):375-88.,3131. Léger J, Olivieri A, Donaldson M, Torresani T, Krude H, van Vliet G, et al.; ESPE-PES-SLEP-JSPE-APEG-APPES-ISPAE; Congenital Hypothyroidism Consensus Conference Group. European Society for Pediatric Endocrinology consensus guidelines on screening, diagnosis, and management of congenital hypothyroidism. J Clin Endocrinol Metab. 2014;99(2):363-84.,3434. Nilsson M, Fagman H. Development of the thyroid gland. Development. 2017;144(12):2123-40.,3636. Pohlenz J, Dumitrescu A, Zundel D, Martiné U, Schönberger W, Koo E, et al. Partial deficiency of thyroid transcription factor 1 produces predominantly neurological defects in humans and mice. J Clin Invest. 2002;109(4):469-73.) and unlikely to occur from Mendelian transmission (1212. Hannoush ZC, Weiss RE. Defects of Thyroid Hormone Synthesis and Action. Endocrinol Metab Clin North Am. 2017;46(2):375-88.,3131. Léger J, Olivieri A, Donaldson M, Torresani T, Krude H, van Vliet G, et al.; ESPE-PES-SLEP-JSPE-APEG-APPES-ISPAE; Congenital Hypothyroidism Consensus Conference Group. European Society for Pediatric Endocrinology consensus guidelines on screening, diagnosis, and management of congenital hypothyroidism. J Clin Endocrinol Metab. 2014;99(2):363-84.,3434. Nilsson M, Fagman H. Development of the thyroid gland. Development. 2017;144(12):2123-40.,3737. Daladoey J, Vassart G, Van Vliet, G. Possible non-Mendelian mechanisms of thyroid dysgenesis. Endocr Dev. 2007;10:29-42.), with the existence of several modifier alleles contributing to a resulting phenotype (77. Rastogi MV, Lafranchi SH. Congenital hypothyroidism. Orphanet J Rare Dis. 2010;5:17.,2424. Carre A, Rachdi L, Tron E, Richard B, Castanet M, Schlumberger M, et al. HES-1 is required for appropriate morphogenesis and differentiation during mouse thyroid gland development. PLoS One. 2011;6(2):e16752.,3232. Dentice M, Cordeddu V, Rosica A, Ferrara AM, Santarpia L, Salvatore D, et al. Missense mutation in the transcription factor NKX2-5: a novel molecular event in the pathogenesis of thyroid dysgenesis. J Clin Endocrinol Metab. 2006;91(4):1428-33.,3939. Macchia PE, Lapi P, Krude H, Pirro MT, Missero C, Chiovato L, et al. PAX-8 mutations associated with congenital hypothyroidism caused by thyroid dysgenesis. Nat Genet. 1998;19(1):83-6.,3838. Fagman H, Nilsson M. Morphogenetics of early thyroid development. J Mol Endocrinol. 2011;46(1):R33-42.,4242. Vilain C, Rydlewski C, Duprez L, Heinrichs C, Abramowicz M, Malvaux P, et al. Autosomal Dominant Transmission of Congenital Thyroid Hypoplasia Due to Loss-of-Function Mutation of PAX-8 1. J Clin Endocrinol Metab. 2001;86(1):234-8.). This is consistent with the theory that suggests that the molecular mechanisms resulting in defective thyroid morphogenesis may be “modulated by the genetic constitution of the embryo and/or the hormonal milieu of the fetus” (4343. Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM, et al. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev. 2009;30(4):293-342.). While many genes have been identified as important contributors to survival, proliferation and migration of thyroid precursor cells in fact, act as an integrated and complex regulatory network (77. Rastogi MV, Lafranchi SH. Congenital hypothyroidism. Orphanet J Rare Dis. 2010;5:17.) and it will most probably require large samples of individuals to unravel the etiopathogenesis of TD.
One of the significant limitations of thyroid ultrasound is the poor sensitivity in visualizing the ectopic thyroid when compared with radionuclide scanning (2222. Beltrão CB, Juliano AG, Chammas MC, Watanabe T, Sapienza MT, Marui S. Etiology of congenital hypothyroidism using thyroglobulin and ultrasound combination. Endocr J. 2010;57(7):587-93.,4444. Perry RJ, Maroo S, Maclennan AC, Jones JH, Donaldson MD. Combined ultrasound and isotope scanning is more informative in the diagnosis of congenital hypothyroidism than single scanning. Arch Dis Child. 2006;91(12):972-6.). In attempts to reduce the chances of misdiagnosis of TD etiology we included only those patients with a complete description of TD. Our genetic analysis was performed following a standard protocol, however, we did not perform any analysis of other modifications found in the promoter (except for the PAX-8 gene) or complete intronic regions, which should also be considered.
In conclusion, we did not find any mutations in the proposed thyroid embryogenesis genes TSH-R, PAX-8, NKX2-5, and HES-1 in 63 confirmed cases of TD. We found that patients with agenesis had clinically distinctive hormone levels at the time of the neonatal screening compared with those with other types of CH. The few genetic polymorphisms identified in the TSH-R, PAX-8, NKX2-5, and HES-1 were not sufficient to elucidate the pathophysiology and the molecular mechanisms underlying defects in the cases of TD.
-
Financial support: this work was supported in part by grants from FAPESB (AMTL and HER) - Edital N°12/2012 and CNPq (HER) - Edital N° 14/2012. TLOC received financial support from CPqGM/Fiocruz-BA.
Acknowledgements:
the authors are grateful to Renata Arruti, Monica Lima and the entire team of APAE-Salvador. We appreciate the cooperation of all the patients and their families, and the support received from Fundação de Amparo à Pesquisa do Estado da Bahia (FAPESB) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq - National Counsel for Scientific Development and Technology).
REFERENCES
-
1Fagman H, Nilsson M. Morphogenesis of the thyroid gland. Mol Cell Endocrinol. 2010;323(1):35-54.
-
2Klein AH, Meltzer S, Kenny FM. Improved prognosis in congenital hypothyroidism treated before age three months. J Pediatr. 1972;81(5):912-5.
-
3Morreale de Escobar G. The role of thyroid hormone in fetal neurodevelopment. J Pediatr Endocrinol Metab. 2001;14 Suppl 6:1453-62.
-
4Lafranchi SH. Newborn screening strategies for congenital hypothyroidism: an update. J Inherit Metab Dis. 2010;33 (Suppl 2):S225-33.
-
5Olney RS, Grosse SD, Vogt RF. Prevalence of congenital hypothyroidism--current trends and future directions: workshop summary. Pediatrics. 2010;125(Suppl):S31-6.
-
6Szinnai G. Clinical genetics of congenital hypothyroidism. Endocr Dev. 2014;26:60-78.
-
7Rastogi MV, Lafranchi SH. Congenital hypothyroidism. Orphanet J Rare Dis. 2010;5:17.
-
8Szinnai G. Genetics of normal and abnormal thyroid development in humans. Best Pract Res Clin Endocrinol Metab. 2014;28(2):133-50.
-
9De Felice M, Di Lauro R. Thyroid development and its disorders: genetics and molecular mechanisms. Endocr Rev. 2004;25(5): 722-46.
-
10Ramos HE, Nesi-França S, Maciel R. Novos aspectos da genética e dos mecanismos moleculares da morfogênese da tiróide para o entendimento da disgenesia tiroidiana: [revisão]. Arq Bras Endocrinol Metab. 2008;52(9):1403-15.
-
11Carré A, Stoupa A, Kariyawasam D, Gueriouz M, Ramond C, Monus T, et al. Mutations in BOREALIN cause thyroid dysgenesis. Hum Mol Genet. 2017;26(3):599-610.
-
12Hannoush ZC, Weiss RE. Defects of Thyroid Hormone Synthesis and Action. Endocrinol Metab Clin North Am. 2017;46(2):375-88.
-
13Abu-Khudir R, Larrivée-Vanier S, Wasserman JD, Deladoëy J. Disorders of thyroid morphogenesis. Best Pract Res Clin Endocrinol Metab. 2017;31(2):143-59.
-
14Moreno JC, Vijlder JMJ, Vulsma T, Ris-Stalpers, C. Genetic basis of hypothyroidism: recent advances, gaps and strategies for future research. Trends Endocrinol Metab. 2003;14(7):318-26.
-
15Knobel M, Medeiros-Neto G. An outline of inherited disorders of the thyroid hormone generating system. Thyroid. 2003;13:771-801.
-
16Ueda D. Normal volume of the thyroid gland in children. J Clin Ultrasound. 1990;18(6):455-62.
-
17Zimmermann MB, Hess SY, Molinari L, De Benoist B, Delange F, Braverman LE, et al. New reference values for thyroid volume by ultrasound in iodine-sufficient schoolchildren: a World Health Organization/Nutrition for Health and Development Iodine Deficiency Study Group Report. Am J Clin Nutr. 2004;79(2):231-7.
-
18Ramos HE, Nesi-França S, Boldarine VT, Pereira RM, Chiamolera MI, Camacho CP, et al. Clinical and molecular analysis of thyroid hypoplasia: a population-based approach in southern Brazil. Thyroid. 2009;19(1):61-8.
-
19Dinno A. Nonparametric Pairwise Multiple Comparisons in Independent Groups Using Dunn's Test. Stata J. 2015;15:292-300.
-
20Brust ES, Beltrao CB, Chammas MC, Watanabe T, Sapienza MT, Marui S. Absence of mutations in PAX-8, NKX2. 5, and TSH receptor genes in patients with thyroid dysgenesis. Arq Bras Endocrinol Metabol. 2012;56(3):173-7.
-
21Mahjoubi F, Mohammadi MM, Montazeri M, Aminii M, Hashemipour M. Mutations in the gene encoding paired box domain (PAX-8) are not a frequent cause of congenital hypothyroidism (CH) in Iranian patients with thyroid dysgenesis. Arq Bras Endocrinol Metabol. 2010;54(6):555-9.
-
22Beltrão CB, Juliano AG, Chammas MC, Watanabe T, Sapienza MT, Marui S. Etiology of congenital hypothyroidism using thyroglobulin and ultrasound combination. Endocr J. 2010;57(7):587-93.
-
23Alves EA, Cruz CM, Pimentel CP, Ribeiro RC, Santos AK, Caldato MC, et al. High frequency of D727E polymorphisms in exon 10 of the TSHR gene in Brazilian patients with congenital hypothyroidism. J Pediatr Endocrinol Metab. 2010;23(12):1321-8.
-
24Carre A, Rachdi L, Tron E, Richard B, Castanet M, Schlumberger M, et al. HES-1 is required for appropriate morphogenesis and differentiation during mouse thyroid gland development. PLoS One. 2011;6(2):e16752.
-
25Cerqueira TL, Ramos Y, Strappa G, Martin DS, Jesus M, Gonzaga J, et al.The c.63A>G polymorphism in the NKX2.5 gene is associated with thyroid hypoplasia in children with thyroid dysgenesis. Arch Endocrinol Metab. 2015;59(6):562-7
-
26Bamforth J, Hughes I, Lazarus J, Weaver C, Harper P. Congenital hypothyroidism, spiky hair, and cleft palate. J Med Genet. 1989;26(1):49-51.
-
27Silvestrin SM, Leone C, Leone CR. Detecting congenital hypothyroidism with newborn screening: the relevance of thyroid-stimulating hormone cutoff values. J Pediatr (Rio J). 2017;93(3):274-280.
-
28Castanet M, Goischke A, Léger J, Thalassinos C, Rodrigue D, Cabrol S, et al.; on behalf of Fédération Parisienne pour le Dépistage et la Prévention des Handicaps de l'Enfant (FDPHE). Natural History and Management of Congenital Hypothyroidism with in situ Thyroid Gland. Horm Res Paediatr. 2015;83:102-10.
-
29Ruchała M, Szczepanek E, Sowiński J. Diagnostic value of radionuclide scanning and ultrasonography in thyroid developmental anomaly imaging. Nucl Med Rev Cent East Eur. 2011;14(1):21-8.
-
30Cherella CE, Wassner AJ. Congenital hypothyroidism: insights into pathogenesis and treatment. Int J Pediatr Endocrinol. 2017;2017:11.
-
31Léger J, Olivieri A, Donaldson M, Torresani T, Krude H, van Vliet G, et al.; ESPE-PES-SLEP-JSPE-APEG-APPES-ISPAE; Congenital Hypothyroidism Consensus Conference Group. European Society for Pediatric Endocrinology consensus guidelines on screening, diagnosis, and management of congenital hypothyroidism. J Clin Endocrinol Metab. 2014;99(2):363-84.
-
32Dentice M, Cordeddu V, Rosica A, Ferrara AM, Santarpia L, Salvatore D, et al. Missense mutation in the transcription factor NKX2-5: a novel molecular event in the pathogenesis of thyroid dysgenesis. J Clin Endocrinol Metab. 2006;91(4):1428-33.
-
33Amendola E, De Luca P, Macchia PE, Terracciano D, Rosica A, Chiappetta G, et al. A mouse model demonstrates a multigenic origin of congenital hypothyroidism. Endocrinology. 2005;146(12): 5038-47.
-
34Nilsson M, Fagman H. Development of the thyroid gland. Development. 2017;144(12):2123-40.
-
35Weller G. Development of the thyroid, parathyroid and the thymus glands in man. Contrib Embryol. 1933;24:93-140.
-
36Pohlenz J, Dumitrescu A, Zundel D, Martiné U, Schönberger W, Koo E, et al. Partial deficiency of thyroid transcription factor 1 produces predominantly neurological defects in humans and mice. J Clin Invest. 2002;109(4):469-73.
-
37Daladoey J, Vassart G, Van Vliet, G. Possible non-Mendelian mechanisms of thyroid dysgenesis. Endocr Dev. 2007;10:29-42.
-
38Fagman H, Nilsson M. Morphogenetics of early thyroid development. J Mol Endocrinol. 2011;46(1):R33-42.
-
39Macchia PE, Lapi P, Krude H, Pirro MT, Missero C, Chiovato L, et al. PAX-8 mutations associated with congenital hypothyroidism caused by thyroid dysgenesis. Nat Genet. 1998;19(1):83-6.
-
40Hinton CF, Harris KB, Borgfeld L, Drummond-Borg M, Eaton R, Lorey F, et al.Trends in incidence rates of congenital hypothyroidism related to select demographic factors: data from the United States, California, Massachusetts, New York, and Texas. Pediatrics. 2010;125 Suppl 2:S37-47
-
41Olivieri A, Stazi MA, Mastroiacovo P, Fazzini C, Medda E, Spagnolo A, et al.; Study Group for Congenital Hypothyroidism. A population-based study on the frequency of additional congenital malformations in infants with congenital hypothyroidism: data from the Italian Registry for Congenital Hypothyroidism (1991-1998). J Clin Endocrinol Metab. 2002;87(2):557-62.
-
42Vilain C, Rydlewski C, Duprez L, Heinrichs C, Abramowicz M, Malvaux P, et al. Autosomal Dominant Transmission of Congenital Thyroid Hypoplasia Due to Loss-of-Function Mutation of PAX-8 1. J Clin Endocrinol Metab. 2001;86(1):234-8.
-
43Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM, et al. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev. 2009;30(4):293-342.
-
44Perry RJ, Maroo S, Maclennan AC, Jones JH, Donaldson MD. Combined ultrasound and isotope scanning is more informative in the diagnosis of congenital hypothyroidism than single scanning. Arch Dis Child. 2006;91(12):972-6.
Publication Dates
-
Publication in this collection
Aug 2018
History
-
Received
03 Dec 2016 -
Accepted
09 May 2018