Abstract
When propagated in vitro, explants receive all the nutrients needed for their growth, including carbohydrates, from the culture medium. However, it is not well understood how the type and concentration of carbohydrates can affect the functioning of the photosynthetic apparatus (particularly photosystem II) of these plants. The aim was to assess the morphophysiological responses of Billbergia zebrina plants in function of sources and concentrations of carbohydrates during in vitro culture. Side shoots of plants previously established in vitro were individualized and transferred to a culture medium containing fructose, glucose or sucrose in four concentrations (0, 15, 30 or 45 g L−1). After growth for 55 days, the chlorophyll a fluorescence transient, leaf anatomy and growth were analyzed. The concentration and type of carbohydrate employed during in vitro culture did not decrease the photosynthetic apparatus performance. However, concentrations above 30 g L−1 led to anatomical modifications, revealing some degree of stress suffered by the plants. When grown in concentrations of 15 and 30 g L−1, irrespective of the carbohydrate used, the plants presented greater stomatal density. The supplementation of the culture medium with monosaccharides caused alterations in the development of the xylem vessels, such as increased number and diameter, allowing adjustment to the microenvironmental conditions. The in vitro conditions influenced the photosynthetic and anatomical responses of plants. The concentration interval from 15 to 30 g L−1 sucrose had a better effect by not causing large changes in the performance of the photosynthetic apparatus and anatomy of plants.
Keywords:
bromeliad; chlorophyll a fluorescence; plant anatomy; plant physiology; plant tissue culture
Resumo
Quando propagados in vitro, os explantes recebem todos os nutrientes necessários para o seu crescimento, incluindo carboidratos do meio de cultura. No entanto, ainda não se sabe como o tipo e a concentração de carboidratos pode afetar o funcionamento do aparato fotossintético (particularmente o fotossistema II) dessas plantas. O objetivo foi avaliar as respostas morfofisiológicas de plantas de Billbergia zebrina em função das fontes e concentrações de carboidratos durante o cultivo in vitro. Brotos laterais das plantas previamente estabelecidos in vitro foram individualizados e transferidos para um meio de cultura contendo frutose, glicose ou sacarose em quatro concentrações (0, 15, 30 ou 45 g L−1). Após 55 dias de cultivo foram analisadas a fluorescência transiente da clorofila a, anatomia foliar e crescimento. A concentração e tipo de carboidrato empregados durante a cultivo in vitro não diminuíram o desempenho do aparelho fotossintético. Entretanto, concentrações acima de 30 g L−1 levaram a modificações anatômicas, revelando um grau de estresse sofrido pelas plantas. Quando cultivadas nas concentrações de 15 e 30 g L−1, independentemente do carboidrato utilizado, as plantas apresentaram maior densidade estomática. A suplementação da cultura média com monossacarídeos causou alterações no desenvolvimento dos vasos xilemáticos, como aumento do número e diâmetro, permitindo o ajuste das condições microambientais. As condições in vitro influenciaram as respostas fotossintéticas e anatômicas das plantas. O intervalo de concentração de 15 a 30 g L−1 de sacarose teve melhor efeito por não causar grandes alterações no desempenho do aparato fotossintético e na anatomia das plantas.
Palavras-chave:
bromélia; fluorescência da clorofila a; anatomia vegetal; fisiologia vegetal; cultura de tecidos vegetais
Introduction
Micropropagation is the most common method used for large-scale cloning ornamental horticulture plant species (Kumari et al., 2017KUMARI, A.; BASKARAN, P.; STADEN, J.V. In vitro regeneration of Begonia homonyma - A threatened plant. South African Journal of Botany, v.109, p.174-177, 2017. DOI: https://doi.org/10.1016/j.sajb.2016.12.027
https://doi.org/10.1016/j.sajb.2016.12.0...
; Naidoo et al., 2017NAIDOO, D.; AREMU, A.O.; STADEN, J.V.; FINNIE J.F. In vitro plant regeneration and alleviation of physiological disorders in Scadoxus puniceus. South African Journal of Botany, v.109, p.316-322, 2017. DOI: https://doi.org/10.1016/j.sajb.2017.01.010
https://doi.org/10.1016/j.sajb.2017.01.0...
; Bezerra et al., 2019BEZERRA, G.A.; GABRIEL, A.V.M.D.; MARIANO, E.D.; CARDOSO, J.C. In vitro culture and greenhouse acclimatization of Oncidium varicosum (Orchidaceae) with microorganisms isolated from its roots. Ornamental Horticulture, v.25, n.4, p.407-416, 2019. DOI: https://doi.org/10.1590/2447-536X.v25i4.2046
https://doi.org/10.1590/2447-536X.v25i4....
). Among the ornamental species frequently micropropagated are bromeliads. Billbergia zebrina is a bromeliad species native to the Atlantic Rainforest of Brazil, which presents commercial value as an ornamental due to the quality of its leaves and inflorescence (Vesco et al., 2011VESCO, L.L.D.; STEFENON, V.M.; WELTER, L.J.; SCHERER, R.F.; GUERRA, M.P. Induction and scale-up of Billbergia zebrina nodule cluster cultures: implications for mass propagation, improvement and conservation. Scientia Horticulturae, v.28, n.4, p.515-522, 2011. DOI: https://doi.org/10.1016/j.scienta.2011.02.018
https://doi.org/10.1016/j.scienta.2011.0...
).
Conventional in vitro propagation methods require the use of sealed environments. Therefore, the exchange of CO2 as happens in natural conditions is prevented, the relative humidity within the flasks is high and the luminosity is low (Eckstein et al., 2012ECKSTEIN, A.; ZIEBA, P.; GABRYS, H. Sugar and light effects on the condition of the photosynthetic apparatus of Arabidopsis thaliana cultured in vitro. Journal of Plant Growth Regulation, v.31, n.1, p.90-101, 2012. DOI: https://doi.org/10.1007/s00344-011-9222-z
https://doi.org/10.1007/s00344-011-9222-...
). These characteristics generate low photosynthetic activity, one of the main factors limiting the efficiency of micropropagation (Ševčíková et al., 2019ŠEVČÍKOVÁ, H.; LHOTÁKOVÁ, Z.; HAMET, J.; LIPAVSKÁ, H. Mixotrophic in vitro cultivations: the way to go astray in plant physiology. Physiologia plantarum, v.167, n.4, p.365-377, 2019. DOI: https://doi.org/10.1111/ppl.12893
https://doi.org/10.1111/ppl.12893...
). During in vitro culture, plants partially lose their autotrophism, i.e., they do not totally produce their own energy source, so they need an exogenous source of carbohydrates (Martins et al., 2019MARTINS, J.P.R.; RODRIGUES, L.C.A.; CONDE, L.T.; GONTIJO A.B.P.L.; FALQUETO, A.R. Anatomical and physiological changes of in vitro-propagated Vriesea imperialis (Bromeliaceae) in the function of sucrose and ventilated containers. Plant Biosystems, 2019. DOI: https://doi.org/10.1080/11263504.2019.1635223
https://doi.org/10.1080/11263504.2019.16...
).
The carbohydrates added to the culture medium play an important role in regulating the in vitro growth and development of plants and can affect the quality of micropropagated plants as well as their physiological state. Carbohydrates are essential for the intermediate and respiratory metabolism, besides being a substrate for synthesis of complex carbon hydrates such as starch and cellulose (Chen et al., 2017CHEN, Y.; WANG, H.; HU, W.; WANG, S.; SNIDER, J. L.; ZHOU, Z. Co-occurring elevated temperature and waterlogging stresses disrupt cellulose synthesis by altering the expression and activity of carbohydrate balance-associated enzymes during fiber development in cotton. Environmental and Experimental Botany, v.135, p.106-117, 2017. DOI: https://doi.org/10.1016/j.envexpbot.2016.12.012
https://doi.org/10.1016/j.envexpbot.2016...
). Each carbohydrate has a distinct function, and can have a different influence depending on the species of plant studied.
Martins et al. (2016b)MARTINS, J.P.R.; VERDOODT, V.; PASQUAL, P.; DE PROFT, M. Physiological responses by Billbergia zebrina (Bromeliaceae) when grown under controlled microenvironmental conditions. African Journal of Biotechnology, v.15, n.36, p.1952-1961, 2016b. DOI: https://doi.org/10.5897/AJB2016.15584
https://doi.org/10.5897/AJB2016.15584...
observed that the ornamental bromeliad B. zebrina, when grown in vitro, could release the enzyme invertase in the culture medium to cleave sucrose into fructose and glucose, and that this cleavage reduces the hydric potential of the culture medium. In this study, the quantification of carbohydrates showed that these monosaccharides were accumulated in the roots and aerial part. These authors suggested that the accumulation of a soluble carbohydrate probably facilitated the flow of water from the culture medium to the plants. They also analyzed the contents of chlorophyll and malic acid in the leaves, which declined with increasing concentration of sucrose in the medium. Therefore, that study brought some questions related to how each carbohydrate (fructose, glucose and sucrose) can influence the photosynthetic performance and anatomy of plants of that species.
Carbohydrates in general are known to suppress the expression of genes involved in photosynthesis and accumulation of chlorophyll (Rolland et al., 2006ROLLAND, F.; BAENA-GONZALEZ, E.; SHEEN, J. Sugar sensing and signaling in plants: conserved and novel mechanisms. Annual Review of Plant Biology, v.57, p.675-709, 2006. DOI: https://doi.org/10.1146/annurev.arplant.57.032905.105441
https://doi.org/10.1146/annurev.arplant....
). Sucrose may have negative feedback effects on photosynthesis, reducing the quantity and activity of Rubisco (Badr et al., 2015BADR, A.; ANGERS, P.; DESJARDINS, Y. Comprehensive analysis of in vitro to ex vitro transition of tissue cultured potato plantlets grown with or without sucrose using metabolic profiling technique. Plant Cell, Tissue and Organ Culture, v.122, n.2, p.491-508, 2015. DOI: https://doi.org/10.1007/s11240-015-0786-3
https://doi.org/10.1007/s11240-015-0786-...
). Nevertheless, some studies have shown that exogenous carbohydrate improved the photosynthetic performance of plants grown in vitro (Eckstein et al., 2012ECKSTEIN, A.; ZIEBA, P.; GABRYS, H. Sugar and light effects on the condition of the photosynthetic apparatus of Arabidopsis thaliana cultured in vitro. Journal of Plant Growth Regulation, v.31, n.1, p.90-101, 2012. DOI: https://doi.org/10.1007/s00344-011-9222-z
https://doi.org/10.1007/s00344-011-9222-...
; Sáez et al., 2016SÁEZ, P.L.; BRAVO, L.A.; SÁNCHEZ-OLATE, M.; BRAVO, P.B.; RÍOS, D.G. Effect of photon flux density and exogenous sucrose on the photosynthetic performance during in vitro culture of Castanea sativa. American Journal of Plant Sciences, v.7, n.4, p.2087-2105, 2016. DOI: https://doi.org/10.4236/ajps.2016.714187
https://doi.org/10.4236/ajps.2016.714187...
).
The supply of exogenous carbohydrate, besides improving the in vitro photosynthetic performance of some plants, also may enhance the ex vitro acclimatization process (Badr et al., 2015BADR, A.; ANGERS, P.; DESJARDINS, Y. Comprehensive analysis of in vitro to ex vitro transition of tissue cultured potato plantlets grown with or without sucrose using metabolic profiling technique. Plant Cell, Tissue and Organ Culture, v.122, n.2, p.491-508, 2015. DOI: https://doi.org/10.1007/s11240-015-0786-3
https://doi.org/10.1007/s11240-015-0786-...
; Lembrechts et al., 2017LEMBRECHTS, R.; CEUSTERS, N.; DE PROFT, M.; CEUSTERS, J. Sugar and starch dynamics in the medium-root-leaf system indicate possibilities to optimize plant tissue culture. Scientia Horticulturae, v.224, p.226–231, 2017. DOI: https://doi.org/10.1016/j.scienta.2017.06.015
https://doi.org/10.1016/j.scienta.2017.0...
). This is caused by the increase in the stock of endogenous carbohydrates such as starch, sucrose, fructose and glucose, which are used as energy sources by micropropagated plants (Badr et al., 2015BADR, A.; ANGERS, P.; DESJARDINS, Y. Comprehensive analysis of in vitro to ex vitro transition of tissue cultured potato plantlets grown with or without sucrose using metabolic profiling technique. Plant Cell, Tissue and Organ Culture, v.122, n.2, p.491-508, 2015. DOI: https://doi.org/10.1007/s11240-015-0786-3
https://doi.org/10.1007/s11240-015-0786-...
; Martins et al., 2016bMARTINS, J.P.R.; VERDOODT, V.; PASQUAL, P.; DE PROFT, M. Physiological responses by Billbergia zebrina (Bromeliaceae) when grown under controlled microenvironmental conditions. African Journal of Biotechnology, v.15, n.36, p.1952-1961, 2016b. DOI: https://doi.org/10.5897/AJB2016.15584
https://doi.org/10.5897/AJB2016.15584...
).
The carbohydrates used during in vitro culture may affect both the morphology/anatomy and physiology of bromeliads (Martins et al., 2015cMARTINS, J.P.R.; VERDOODT, V.; PASQUAL, P.; DE PROFT, M. Impacts of photoautotrophic and photomixotrophic conditions on in vitro propagated Billbergia zebrina (Bromeliaceae). Plant Cell, Tissue and Organ Culture, v.123, n.1, p.121-132, 2015c. DOI: https://doi.org/10.1007/s11240-015-0820-5
https://doi.org/10.1007/s11240-015-0820-...
; Martins et al., 2016aMARTINS, J.P.R.; MARTINS, A.D.; PIRES, M.F.; BRAGA, R.A.; JR.; REIS, R.O.; DIAS, G.M.G.; PASQUAL, M. Anatomical and physiological responses of Billbergia zebrina (Bromeliaceae) to copper excess in a controlled microenvironment. Plant Cell, Tissue and Organ Culture, v.126, n.1, p.43-57, 2016a. DOI: https://doi.org/10.1007/s11240-016-0975-8
https://doi.org/10.1007/s11240-016-0975-...
, 2016b). Anatomical analysis of micropropagated plants is an excellent tool to ascertain how the in vitro conditions can affect the success of all the micropropagation stages, including transfer to ex vitro conditions (Martins et al., 2015cMARTINS, J.P.R.; VERDOODT, V.; PASQUAL, P.; DE PROFT, M. Impacts of photoautotrophic and photomixotrophic conditions on in vitro propagated Billbergia zebrina (Bromeliaceae). Plant Cell, Tissue and Organ Culture, v.123, n.1, p.121-132, 2015c. DOI: https://doi.org/10.1007/s11240-015-0820-5
https://doi.org/10.1007/s11240-015-0820-...
; Eburneo et al., 2017EBURNEO, L.; RIBEIRO-JÚNIOR, N.G.; KARSBURG, I.V.; ROSSI, A.A.B.; SILVA, I.V. Anatomy and micromorphometric analysis of leaf Catasetum x apolloi Benelli & Grill with addition of potassium silicate under different light sources. Brazilian Journal of Biology, v.77, n.1, p.140-149, 2017. DOI: http://dx.doi.org/10.1590/1519-6984.12015
http://dx.doi.org/10.1590/1519-6984.1201...
). Studies have also measured the chlorophyll a fluorescence to determine the performance of the photosynthetic apparatus of plants grown in vitro (Dobránszki and Drienyovszki, 2014DOBRÁNSZKI, J.; MENDLER-DRIENYOVSZKI, N.M. Cytokinin-induced changes in the chlorophyll content and fluorescence of in vitro apple leaves. Journal of Plant Physiology, v.171, n.16, p.1472-1478, 2014. DOI: https://doi.org/10.1016/j.jplph.2014.06.015
https://doi.org/10.1016/j.jplph.2014.06....
; Matysiak and Gabryszewska, 2016MATYSIAK, B.; GABRYSZEWSKA, E. The effect of in vitro culture conditions on the pattern of maximum photochemical efficiency of photosystem II during acclimatisation of Helleborus niger plantlets to ex vitro conditions. Plant Cell, Tissue and Organ Culture, v.125, n.3, p.585–593, 2016. DOI: https://doi.org/10.1007/s11240-016-0972-y
https://doi.org/10.1007/s11240-016-0972-...
; Rosa et al., 2018ROSA, W.S.; MARTINS, J.P.R.; RODRIGUES, E.S.; RODRIGUES, L.C.A.; GONTIJO, A.B.P.L.; FALQUETO, A.R. Photosynthetic apparatus performance in function of the cytokinins used during the in vitro multiplication of Aechmea blanchetiana (Bromeliaceae). Plant Cell, Tissue and Organ Culture, v.133, n.3, p.339-350, 2018. DOI: https://doi.org/10.1007/s11240-018-1385-x
https://doi.org/10.1007/s11240-018-1385-...
). Fluorescence measurement has become an important tool for studies of plant physiology, because it is nondestructive, sensitive, fast and can be applied in both field and laboratory conditions.
So far it is not clear how the carbohydrates employed in the culture medium can influence the anatomy and performance of the photosynthetic apparatus, particularly photosystem II (PSII) of plants propagated in vitro. We hypothesized that: (1) the performance of the photosynthetic apparatus will decline in function of higher concentrations of carbohydrates added to the culture medium; and (2) the employment of only monosaccharides as carbohydrate source can induce physiological disturbances in plants due to the osmotic potential of the culture medium. Therefore, the aim of this study was to assess the morphophysiological responses of B. zebrina in function of the sources and concentrations of carbohydrates during conventional in vitro culture.
Material and Methods
Plant material, in vitro establishment and multiplication
B. zebrina plantlets were previously established in vitro using seeds and the procedures were according to Martins et al. (2015a)MARTINS, J.P.R.; PASQUAL, M.; MARTINS, A.D.; RIBEIRA, S.F. Effects of salts and sucrose concentrations on in vitro propagation of Billbergia zebrina (Herbert) Lindley (Bromeliaceae). Australian Journal of Crop Science, v.9, n.1, p.85-91, 2015a.. B. zebrina plants previously established in vitro were used to obtain the explants. The plants were multiplied in stationary liquid MS culture medium (Murashige and Skoog, 1962MURASHIGE, T.; SKOOG, F.A revised medium for rapid growth and biossays with tabacco tissue culture. Physiologia Plantarum, v.15, n.3, p.473-497, 1962. DOI: https://doi.org/10.1111/j.1399-3054.1962.tb08052.x
https://doi.org/10.1111/j.1399-3054.1962...
) supplemented with 13 µM 6-benzylaminopurine (BAP) and 30 g L−1 sucrose. After growth for 45 days, the explants with induced side shoots were subcultured for 45 days in 268 mL glass flasks containing 50 mL of stationary liquid MS culture medium, without plant growth regulators, supplemented with 30 g L−1 sucrose (Martins et al., 2015aMARTINS, J.P.R.; PASQUAL, M.; MARTINS, A.D.; RIBEIRA, S.F. Effects of salts and sucrose concentrations on in vitro propagation of Billbergia zebrina (Herbert) Lindley (Bromeliaceae). Australian Journal of Crop Science, v.9, n.1, p.85-91, 2015a.). The culture media were adjusted to pH 5.8 before autoclaving at 120 °C during 20 minutes. After inoculation of the explants in a laminar flow chamber, the material was kept in a growth room at 26±2°C and 16 h photoperiod (8:00 a.m.- midnight), under fluorescent lamps (Empalux® FT8 HO, 36W/6400K) emitting 90 μmol m−2 s−1 of photosynthetically active radiation (PAR).
In vitro culture conditions
The side shoots obtained (2 cm in length, in average) after the subculture step were individualized with a scalpel and transferred to 268 mL glass flasks containing 50 mL of MS medium solidified with 6.5 g L−1 agar (Vetec®), supplemented with one of three carbohydrate types (fructose, glucose or sucrose) at four concentrations (0, 15, 30 and 45 g L−1). Five shoots were placed in each flask. The pH of the media was adjusted to 5.9 before autoclaving at 120 °C for 20 minutes. Then the explants were inoculated in a laminar flow chamber and the material was kept in a growth room for 55 days at 26±2 °C and 16 h photoperiod (8:00 a.m.- midnight), under fluorescent lamps (Empalux® FT8 HO, 36W/6400K) emitting 90 μmol m−2 s−1 of PAR.
Chlorophyll a fluorescence analysis
The chlorophyll a fluorescence was evaluated after culturing for 55 days of growth in 15 plants of each treatment. The data were collected with a Handy PEA portable fluorimeter (Hansatech, King's Lynn, Norfolk, United Kingdom) between 7:30-8:30 a.m. The measurements were made on leaves (third completely expanded leaf in the central rosette) of in vitro cultured plants after being dark-adapted for 30 min using a leaf clip (Hansatech®). The device's saturating light is 3000 μmol (photons) m−2 s−1. The JIP-test data (Table 1) were evaluated from the OJIP transients based on the theory of energy fluxes in biomembranes. The OJIP transient is defined by O, J, I, and P steps, corresponding to the redox states of photosystem (PS) II and PS I and to the efficiencies of electron transfer through the intersystem chain to the end electron acceptors at the PSI acceptor side (Strasser et al., 2004STRASSER, R.J.; TSIMILLI-MICHAEL, M.; SRIVASTAVA, A. Analysis of the chlorophyll a fluorescence transient. In: PAPAGEORGIOU, G.C.; GOVINDJEE. (ed) Chlorophyll fluorescence: a signature of photosynthesis. Kluwer Academic Publishers Press, Dordrecht, 2004. p.321-362. DOI: https://doi.org/10.1007/978-1-4020-3218-9_12
https://doi.org/10.1007/978-1-4020-3218-...
).
Abbreviations of the parameters, formulas and description of the data derived from the transient fluorescence of chlorophyll a
Leaf anatomy analysis
The stomatal density and characteristics of the xylem vessels of the B. zebrina plants were determined in four plants of each treatment. The samples were collected randomly and fixed in FAA (formaldehyde, acetic acid and 50% ethanol, 0.5:/0.5:/9, v/v) for 72 hours, followed by storage in 50% ethanol (Johansen, 1940Johansen, D.A. Plant microtechnique, 2ed. Mc Graw-Hill, New York, 1940. 523p.). Cross sections were obtained with a double edge razor in the middle region of the first fully expanded leaf from the rosette. The stomatal density was measured on the abaxial face of the second fully expanded leaf. The sections were clarified with 2.5% sodium hypochlorite (v/v) and then stained with safrablau solution. For paradermal characterization, the leaf sections were clarified with 15% sodium hypochlorite (v/v) and stained with 1% safranin. The sections were mounted on slides with 50% glycerin. To quantify the stomatal density, photomicrographs were taken from three leaf regions (base, middle and tip). The sections were visualized, and the images captured with a Leica DM 1000 light microscope coupled to a Leica ICC50 HD digital camera (Wetzlar, Germany). Photomicrographs of the paradermal sections were taken from four different leaves and in four distinct fields of each leaf region. Photographs were also taken of two cross sections each from four leaves to measure the diameter and number of xylem vessels. The UTHSCSA-Imagetool® software was used to measure the anatomical characteristics as revealed by the photomicrographs.
Analysis of growth
The growth of the plants was evaluated in 20 plants from each treatment, which were collected and divided into four parcels. Each parcel was weighed on a precision scale to determine the fresh weight in milligrams (mg). Then the average of each parcel was divided by 5 to obtain the average weight per plant.
Statistical analysis
The experimental design was completely randomized in a factorial scheme, consisting of four concentrations (0, 15, 30 or 45 g L−1) and three carbohydrates (fructose, glucose and sucrose). The resulting data were submitted to analysis of variance (ANOVA) and the means were compared by Scott-Knott test at 5% probability. The Sisvar® program was used for all the statistical analysis.
Results
Chlorophyll a fluorescence transients
The analysis of chlorophyll a fluorescence transients revealed physiological differences in the B. zebrina plants cultured with different types and concentrations of carbohydrates. Figures 1A-C show the typical OJIP curves for the plants cultured under the three carbohydrates. It was also possible to observe in the plants grown in culture medium not supplemented with carbohydrate a reduction of the maximum fluorescence (FM) and increase of initial fluorescence (F0). This difference between FM and F0 caused a reduction of the variable fluorescence (FV). When the plants were grown with sucrose the values of FV were higher than for the other two carbohydrate types. The carbohydrate type and concentration significantly influenced the FM and FV, while only the concentration influenced F0 (Figures 1A, 1B and 1C).
Chlorophyll a fluorescence transients and variable fluorescence (FV) of Billbergia zebrina during in vitro cultivation in function of types and concentrations of carbohydrates (A-C). Relative variable fluorescence between F0 and FM (VOP) showing the variable relative fluorescence at J-step (VJ) and at I-step (VI) as a function of the in vitro treatments (D-F). Means (± SD) followed by the same letter, in each carbohydrate type, are not significantly different according to a Scott–Knott's test, at 5 %. For each concentration (g L−1) analyzed in each relative variable fluorescence phase, means (± SD) followed by an asterisk are significantly different according to a Scott–Knott's test, at 5 %
The relative fluorescence curves showed a substantial increase of VJ (∼0.65) and reduction of I-step (VI = ∼0.831) in the plants cultured without supplementation of carbohydrates. On the other hand, for the plants grown in media supplemented with carbohydrates, the values of VJ (∼0.51) and VI (∼0.87) were lower and higher, respectively, in relation to the control plants (Figures 1D, 1E and 1F).
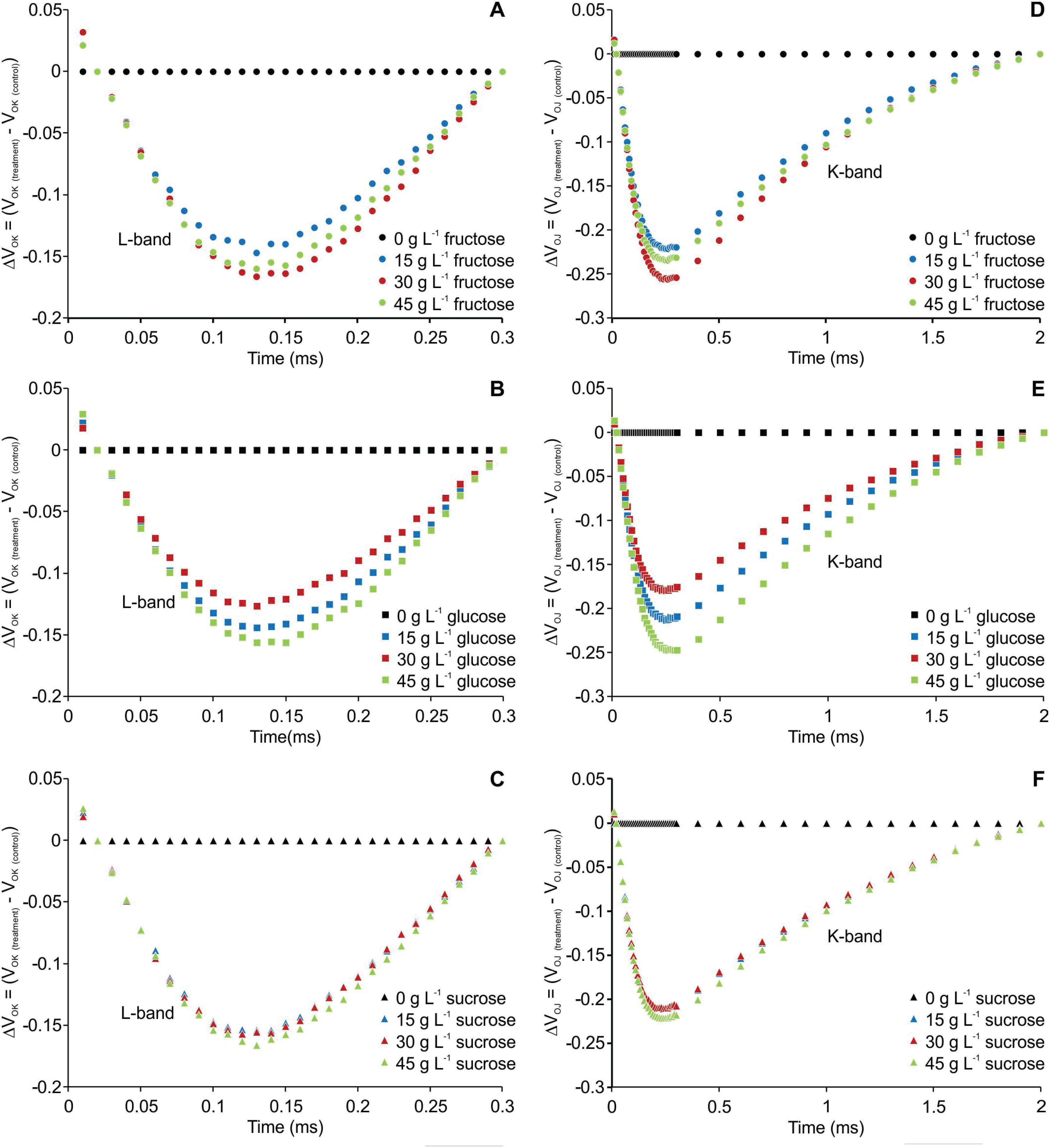
The transient fluorescence of the OJIP curve between points O (20 μs) and K (300 μs) and between points O (20 μs) and J (2 ms) were normalized and are presented as kinetic differences [ΔVOK = VOK treatment - VOK control] and [ΔVOJ = VOJ treatment - VOJ control], respectively. Negative deviations were observed for L- and K-bands in all the treatments compared to the plants cultured without carbohydrate (Figure 2).
Kinetic differences of relative fluorescence of Billbergia zebrina during steps O to K = ΔVOK = VOK(treatment) − VOK(control) (A-C) and O to J = ΔVOJ = VOJ(treatment) − VOJ(control) (D-F), showing the L- and K-bands, respectively.
The phenomenological parameters of JIP-test per cross section (CS) varied significantly in function of the concentrations and types of carbohydrate, except for dissipation (DI0/CSM), for which only concentration had an influence. When restricting the carbohydrate source, the absorption (ABS/CSM), trapping (TR0/CSM), electron transport per cross section (ET0/CSM) and number of active reaction centers (CS/ABS) were lower and statistically different from the other treatments. Inversely, the dissipation (DI0/CSM) presented higher values for plants without carbohydrate supplementation. The plants grown with sucrose at concentration of 30 g L−1 had higher values of the parameters identified above compared to those with cultured with fructose and glucose (Figure 3).
Models of phenomenological energy fluxes per excited cross section approximated by FM (CSM = P-step) in Billbergia zebrina leaves at 55 days as a function of types and concentrations of carbohydrates. CSM/ABS - active reaction centers (RCs) are indicated by black circles. In each parameter, means followed by the same letter (upper case for the carbohydrate type in each concentration and lower case for concentration in each carbohydrate type) are not significantly different according to a Scott–Knott's test, at 5 %
The biophysical parameters of the JIP-test were influenced only by the concentrations (Table 2). The values of the quantum yield of the primary photochemistry (φP0) and electron transport (φE0) of the plants supplemented with exogenous carbohydrate were higher than for the plants that did not receive carbohydrates. On the other hand, the quantum yield of energy dissipation (φD0) and net rate of PSII closure (M0) were lower independently of the carbohydrate utilized in the culture medium.
Net rate of photosystem II (PS II) closure (M0) and quantum yields based on fluorescence emission kinetics as a function of types and concentrations of carbohydrates
Anatomical analysis of leaves
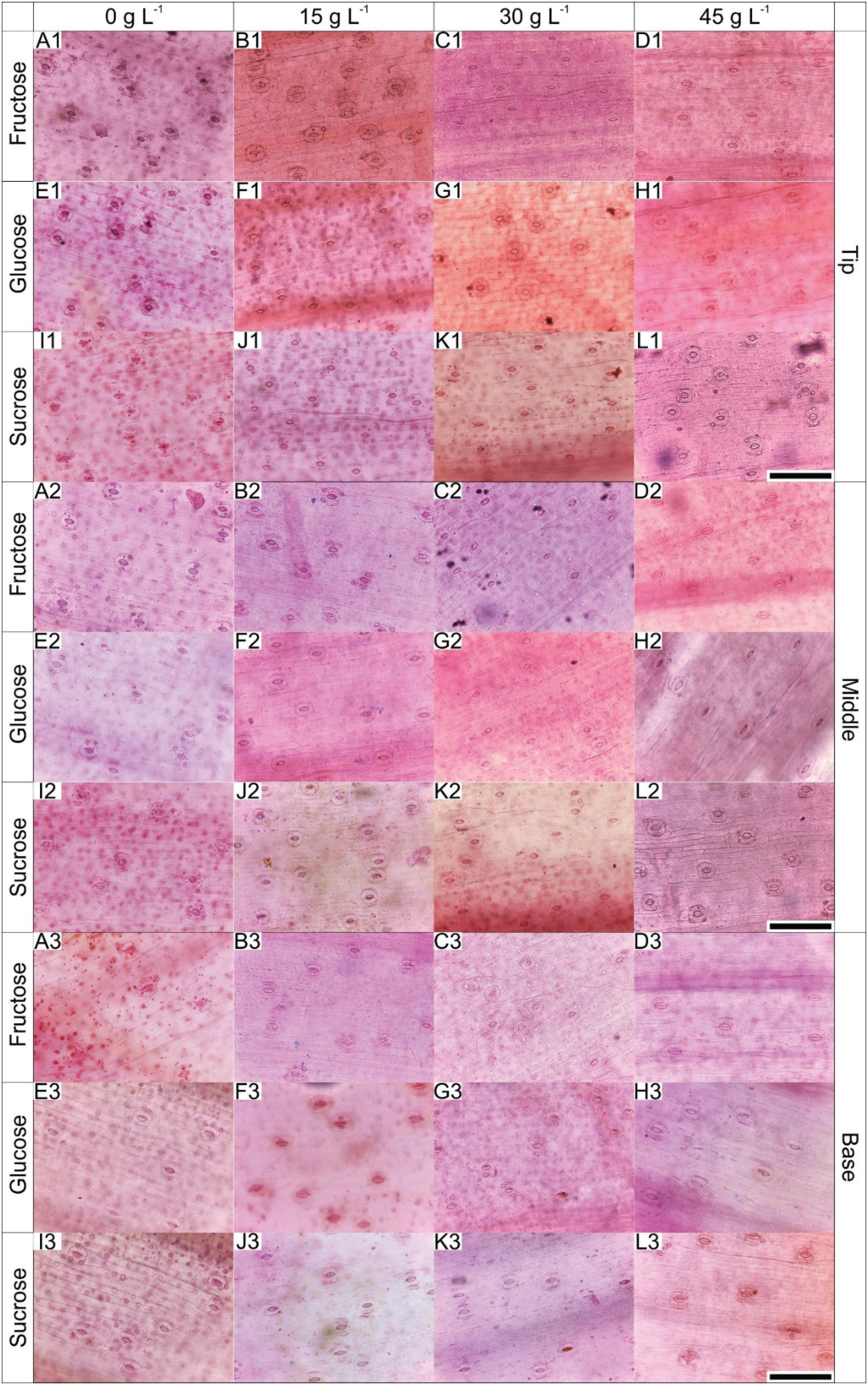
The leaves of B. zebrina plants grown in vitro presented progressively rising stomatal density from the base to the tip, irrespective of the carbohydrate type (Figure 4).
Paradermal sections of the different leaf regions of B. zebrina at 55 days of in vitro culture as a function of concentrations (0, 15, 30 or 45 g L−1) of fructose, glucose or sucrose. Bar = 100 μm.
The comparison of the treatments revealed that only the concentrations influenced the stomatal density of the three leaf regions. The highest density was observed in the plants cultured with 15 and 30 g L−1 of exogenous carbohydrate supplemented in the medium (Figure 4, Table 3).
Stomatal density (0.1 mm2) on B. zebrina’s leaves as a function of carbohydrate concentrations (g L−1) supplemented in the culture medium
The diameter and number of xylem vessels were influenced both by the concentration and types of carbohydrates. Xylem vessels of plants cultured with glucose were larger, particularly at the concentrations of 15 and 30 g L−1 (Figures 5, 6). However, the largest number of vessels was observed in the plants that received fructose at concentrations of 15 and 30 g L−1 (Figures 5, 6).
Cross sections of B. zebrina leaves at 55 days of in vitro culture as a function of concentrations (0, 15, 30 or 45 g L−1) of fructose, glucose or sucrose. Bar = 50 μm.
Diameter and number of xylem vessels as a function of types and concentrations of carbohydrates. Means (± SD) followed by the same letter, upper case at each concentration level and lower case to the type of carbohydrate, do not differ according to the Scott-Knott test at 5%.
Analysis of growth
After culturing for 55 days, rooting was observed in all treatments. However, the plants supplemented with glucose had a larger number of roots compared to those cultured with the same concentration of sucrose and fructose (data not shown). This had a direct influence on fresh weight.
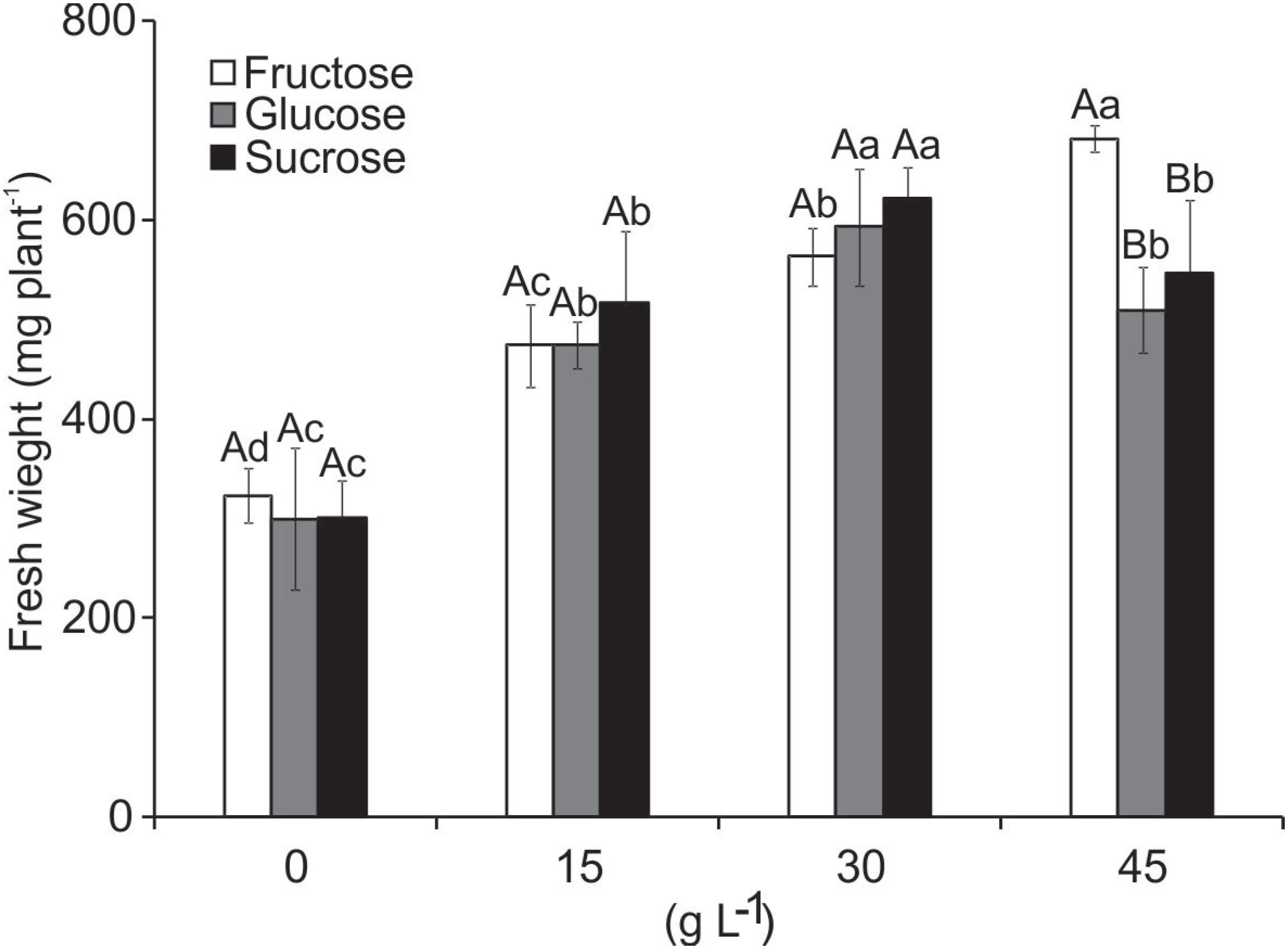
The fresh weight of the plants presented significant interaction with both variation factors. The fresh weight increased with higher concentration of carbohydrate added to the medium from 15 to 30 L−1, but declined when 45 g L−1 was added (glucose and sucrose). Although the weight was higher in the treatment with 45 g L−1 fructose, this was due to the formation of multiple shoots at the base of the explants (Figure 7).
Fresh weight (mg plant−1) of B. zebrina plants grown in vitro as a function of types and concentrations of carbohydrates. Means (± SD) followed by the same letter, upper case at each concentration level and lower case to the type of carbohydrate, do not differ according to the Scott-Knott test at 5%.
Discussion
All B. zebrina plants presented OJIP curves with characteristic polyphase increase, indicating they were photosynthetically active (Kalaji et al., 2018KALAJI, H.M.; RASTOGI, A.; ŽIVČÁK, M.; BRESTIC, M.; DASZKOWSKA-GOLEC, A.; SITKO, K.; ALSHARAFA, K.Y.; LOTFI, R.; STYPIŃSKI, P.; SAMBORSKA, A.I.; CETNER, M.D. Prompt chlorophyll fluorescence as a tool for crop phenotyping: an example of barley landraces exposed to various abiotic stress factors. Photosynthetica, v.56, n.3, p.953-961, 2018. DOI: https://doi.org/10.1007/s11099-018-0766-z
https://doi.org/10.1007/s11099-018-0766-...
). The increase of F0 values of the plants cultured without supplementation of exogenous carbohydrate is related to partial inhibition of the reaction center of PSII, with reduction of the flow of electrons from quinone A (QA) to quinone B (QB) (Goltsev et al., 2016GOLTSEV, V.N.; KALAJI, H. M.; PAUNOV, M.; BĄBA, W.; HORACZEK, T.; MOJSKI, J.; KOCIEL, H.; ALLAKHVERDIEV, S.I. Variable Chlorophyll Fluorescence and its use for Assessing Physiological Condition of Plant Photosynthetic Apparatus. Russian Journal of Plant Physiology, v.63, n.6, p.881-907, 2016. DOI: https://doi.org/10.1134/S1021443716050058
https://doi.org/10.1134/S102144371605005...
). This reduction in the transfer of electrons in PSII means an accumulation of QA−, resulting in an increase of VJ due to a partial inhibition of the donor side of PSII, a common situation in plants that are under stress (Chen et al., 2015CHEN, S.; KANG, Y.; ZHANG, M.; WANG, X.; STRASSER, R.J.; ZHOU, B.; QIANG, S. Differential sensitivity to the potential bioherbicide tenuazonic acid probed by the JIP-test based on fast chlorophyll fluorescence kinetics. Environmental and Experimental Botany, v.112, p.1-15, 2015. DOI: https://doi.org/10.1016/j.envexpbot.2014.11.009
https://doi.org/10.1016/j.envexpbot.2014...
; Cai et al., 2016CAI, W.; GAO, X.; HU, J.; CHEN, L.; LI, X.; LIU, Y.; WANG, G. UV-B radiation inhibits the photosynthetic electron transport chain in Chlamydomonas reinhardtii. Pakistan Journal of Botany, v.48, n.6, p.2587-2593, 2016.). Inversely, the lower values of F0 and J-step independently of the carbohydrate added to the culture medium indicates that the energy absorbed followed the pathway for reduction of QA to QB−, closing the reaction centers, causing a lower fluorescence emission rate.
The increase in the values of FM and FV in the plants cultured with supplementation of exogenous carbohydrate can indicate greater efficiency in reducing plastoquinone, with lower loss of energy by non-photochemical dissipation (Kalaji et al., 2014KALAJI, H.M.; SCHANSKER, G.; LADLE, R.J. Frequently asked questions about in vivo chlorophyll fluorescence: pratical issue. Photosynthesis Research, v.122, n.2, p.121-158, 2014. DOI: https://doi.org/10.1007/s11120-014-0024-6
https://doi.org/10.1007/s11120-014-0024-...
). The higher values of FM and FV verified in the plants cultured with exogenous sucrose may be related to the fundamental role of sucrose in protecting PSII against photoinhibition and oxidative stress, because it acts as a source of energy for synthesis of the protein that acts in the chloroplasts when under some type of stress (Downs et al., 1999DOWNS, C.A.; RYAN, S.L.; HECKATHORN, S.A. The chloroplast small heat-shock protein: evidence for a general role in protecting photosystem II against oxidative stress and photoinhibiton. Journal of Plant Physiology, v.155, n.4-5, p.488-496, 1999. DOI: https://doi.org/10.1016/S0176-1617(99)80043-1
https://doi.org/10.1016/S0176-1617(99)80...
).
Plants that do not receive carbohydrate in the culture medium may present a limited photosynthesis rate, only allowing them to survive for a short period due to consumption of the endogenous carbohydrate reserves. After this period, the plants undergo a senescence process due to the imbalance in the consumption/production of hydrates of carbon (Martins et al., 2016bMARTINS, J.P.R.; VERDOODT, V.; PASQUAL, P.; DE PROFT, M. Physiological responses by Billbergia zebrina (Bromeliaceae) when grown under controlled microenvironmental conditions. African Journal of Biotechnology, v.15, n.36, p.1952-1961, 2016b. DOI: https://doi.org/10.5897/AJB2016.15584
https://doi.org/10.5897/AJB2016.15584...
). This was demonstrated by the decrease of VI and CS/ABS. When the leaf senescence processes start, the number of active reaction centers declines along with the values of VI. This causes alterations in the capacity to transfer electrons from the donor to the receptor side of the PSII reaction center due to these processes inherent to leaf senescence (Wang et al., 2016WANG, Q.Y.; ZHANG, J.; ZHAO, B.Z.; XIN, X.L.; DENG, X.H.; ZHANG, H. Influence of long term fertilization on selenium accumulation in soil and uptake by crops. Pedosphere, v.26, n.1, p.120-129, 2016. DOI: https://doi.org/10.1016/S1002-0160(15)60028-5
https://doi.org/10.1016/S1002-0160(15)60...
).
In this study, the analysis of the transient fluorescence kinetics allowed identifying L- and K-bands. According to Kalaji et al. (2016)KALAJI, H.M.; JAJOO, A.; OUKARROUM, A.; BRESTIC, M.; ZIVCAK, M.; SAMBORSKA, I.A.; CETNER, M.D.; ŁUKASIK, I.; GOLTSEV, V.; LADLE, R.J. Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions. Acta Physiologiae Plantarum, v.38, n.4, p.102, 2016. DOI: https://doi.org/10.1007/s11738-016-2113-y
https://doi.org/10.1007/s11738-016-2113-...
, it is possible to use these bands to characterize physiological disturbances in plants. The L-band is an indication of the functionality of PSII and is related to the stacking and unstacking of thylakoids (Strasser and Stirbet, 1998STRASSER, R.J.; STIRBET, A.D. Heterogeneity of photosystem II probed by the numerically simulated chlorophyll a fluorescence rise (O–J–I–P). Mathematics and Computers in Simulation, v.48, n.1, p.3-9, 1998. DOI: https://doi.org/10.1016/S0378-4754(98)00150-5
https://doi.org/10.1016/S0378-4754(98)00...
). In turn, the K-band is associated with the donation of electrons from the oxygen evolution complex (OEC) to PSII (Falqueto et al., 2017FALQUETO, A.R.; SILVA JÚNIOR, R.A.; GOMES, M.T.G.; MARTINS, J.P.R.; SILVA, D.M.; PARTELLI, F.L. Effects of drought stress on chlorophyll a fluorescence in two rubber tree clones. Scientia Horticulturae, v.224, p.238-243, 2017. DOI: https://doi.org/10.1016/j.scienta.2017.06.019
https://doi.org/10.1016/j.scienta.2017.0...
; Rosa et al., 2018ROSA, W.S.; MARTINS, J.P.R.; RODRIGUES, E.S.; RODRIGUES, L.C.A.; GONTIJO, A.B.P.L.; FALQUETO, A.R. Photosynthetic apparatus performance in function of the cytokinins used during the in vitro multiplication of Aechmea blanchetiana (Bromeliaceae). Plant Cell, Tissue and Organ Culture, v.133, n.3, p.339-350, 2018. DOI: https://doi.org/10.1007/s11240-018-1385-x
https://doi.org/10.1007/s11240-018-1385-...
). Thus, the appearance of negative bands in the plants supplemented with carbohydrates suggests that in conventional in vitro culture conditions, the plants can use any exogenous source of carbohydrate to maintain their vital functions.
The in vitro culture conditions influenced the efficiency of the flow of energy through the leaf cross section. The results showed high absorption (ABS/CSM), trapping (TR0/CSM) and transport (ET0/CSM) and low dissipation (DI0/CSM) when the plants were cultured in the medium containing sucrose at concentration of 30 g L−1. This can indicate greater efficiency of the photosynthetic apparatus (Sun et al., 2016SUN, Z.W.; REN, L.K.; FAN, J.W.; LI, Q.; WANG, K.J.; GUO, M.M.; WANG, L.; LI, J.; ZHANG, G.X.; YANG, Z.Y.; CHEN, F.; LI, X.N. Salt response of photosynthetic electron transport system in wheat cultivars with contrasting tolerance. Plant, Soil and Environment, v. 62, n.11, p.515-521, 2016. DOI: https://doi.org/10.17221/529/2016-PSE
https://doi.org/10.17221/529/2016-PSE...
), because the excitation of the chlorophyll molecules was absorbed and captured, impelling electrons to reduce pheophytin, QA and the other receptors of the electron transport chain. That condition allowed an increase of FM and consequently an increase of FV and lower energy dissipation (φD0).
The reduction of the active reaction centers (CS/ABS) in plants not receiving exogenous carbohydrate can induce greater energy dissipation and susceptibility to photoinhibition (Chen et al., 2014CHEN, S.G.; STRASSER, R.J.; QIANG, S. In vivo assessment of effect of phytotoxin tenuazonic acid on PSII reaction centers. Plant Physiology and Biochemistry, v.84, p.10-21, 2014. DOI: https://doi.org/10.1016/j.plaphy.2014.09.004
https://doi.org/10.1016/j.plaphy.2014.09...
; Zushi and Matsuzoe, 2017ZUSHI, K.; MATSUZOE, N. Using of chlorophyll a fluorescence OJIP transients for sensing salt stress in the leaves and fruits of tomato. Scientia Horticulturae, v.219, p.216-221, 2017. DOI: https://doi.org/10.1016/j.scienta.2017.03.016
https://doi.org/10.1016/j.scienta.2017.0...
). This reduction of CS/ABS can indicate senescence of plants (Wang et al., 2016WANG, Q.Y.; ZHANG, J.; ZHAO, B.Z.; XIN, X.L.; DENG, X.H.; ZHANG, H. Influence of long term fertilization on selenium accumulation in soil and uptake by crops. Pedosphere, v.26, n.1, p.120-129, 2016. DOI: https://doi.org/10.1016/S1002-0160(15)60028-5
https://doi.org/10.1016/S1002-0160(15)60...
), as well as induce increased energy dissipation (φD0 and DI0/CSM). This suggests the existence of a negative regulation mechanism of photosynthesis for dissipation of the excess energy absorbed and thus protect against possible photodamages (Holland et al., 2014HOLLAND, V.; KOLLER, S.; BRÜGGEMANN, W. Insight into the photosynthetic apparatus in evergreen and deciduous European oaks during autumn senescence using OJIP fluorescence transient analysis. Plant Biology, v.16, n.4, p.801-808, 2014. DOI: https://doi.org/10.1111/plb.12105
https://doi.org/10.1111/plb.12105...
; Kalaji et al., 2014KALAJI, H.M.; SCHANSKER, G.; LADLE, R.J. Frequently asked questions about in vivo chlorophyll fluorescence: pratical issue. Photosynthesis Research, v.122, n.2, p.121-158, 2014. DOI: https://doi.org/10.1007/s11120-014-0024-6
https://doi.org/10.1007/s11120-014-0024-...
; Wang et al., 2016WANG, Q.Y.; ZHANG, J.; ZHAO, B.Z.; XIN, X.L.; DENG, X.H.; ZHANG, H. Influence of long term fertilization on selenium accumulation in soil and uptake by crops. Pedosphere, v.26, n.1, p.120-129, 2016. DOI: https://doi.org/10.1016/S1002-0160(15)60028-5
https://doi.org/10.1016/S1002-0160(15)60...
). These results emphasize the importance of supplementing the culture medium with an exogenous source of carbohydrate during conventional in vitro culture. Carbohydrates supply energy for plants’ metabolism and act as regulators to control the physiology, metabolism, development and expression of genes that can act in photosynthesis (Pessoni et al., 2015PESSONI, R.A.; TERSAROTTO, C.C.; MATEUS, C.A.; ZERLIN, J.K.; SIMÕES, K.; DE CÁSSIA, L.; FIGUEIREDO-RIBEIRO, R.; BRAGA, M.R. Fructose Affecting Morphology and Inducing β-Fructofuranosidases in Penicillium janczewskii. SpringerPlus, v.4, p.487, 2015. DOI: https://doi.org/10.1186/s40064-015-1298-7
https://doi.org/10.1186/s40064-015-1298-...
), besides acting as osmotic regulators (Martins et al., 2016bMARTINS, J.P.R.; VERDOODT, V.; PASQUAL, P.; DE PROFT, M. Physiological responses by Billbergia zebrina (Bromeliaceae) when grown under controlled microenvironmental conditions. African Journal of Biotechnology, v.15, n.36, p.1952-1961, 2016b. DOI: https://doi.org/10.5897/AJB2016.15584
https://doi.org/10.5897/AJB2016.15584...
).
The increase in the values of M0 for the plants cultured without added carbohydrate suggests a reduction in the closure of the PSII reaction centers (Wang et al., 2016WANG, Q.Y.; ZHANG, J.; ZHAO, B.Z.; XIN, X.L.; DENG, X.H.; ZHANG, H. Influence of long term fertilization on selenium accumulation in soil and uptake by crops. Pedosphere, v.26, n.1, p.120-129, 2016. DOI: https://doi.org/10.1016/S1002-0160(15)60028-5
https://doi.org/10.1016/S1002-0160(15)60...
; Goltsev et al., 2016GOLTSEV, V.N.; KALAJI, H. M.; PAUNOV, M.; BĄBA, W.; HORACZEK, T.; MOJSKI, J.; KOCIEL, H.; ALLAKHVERDIEV, S.I. Variable Chlorophyll Fluorescence and its use for Assessing Physiological Condition of Plant Photosynthetic Apparatus. Russian Journal of Plant Physiology, v.63, n.6, p.881-907, 2016. DOI: https://doi.org/10.1134/S1021443716050058
https://doi.org/10.1134/S102144371605005...
), indicating delay of the flow of electrons from QA to QB. On the other hand, the values of φP0 and φE0 in the plants cultured with exogenous carbohydrate, regardless of type, can indicate that the absorbed energy was captured and used to reduce QA with lower loss of energy by dissipation (φD0) (Kalaji et al., 2016KALAJI, H.M.; JAJOO, A.; OUKARROUM, A.; BRESTIC, M.; ZIVCAK, M.; SAMBORSKA, I.A.; CETNER, M.D.; ŁUKASIK, I.; GOLTSEV, V.; LADLE, R.J. Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions. Acta Physiologiae Plantarum, v.38, n.4, p.102, 2016. DOI: https://doi.org/10.1007/s11738-016-2113-y
https://doi.org/10.1007/s11738-016-2113-...
). The results show that the use of any type of carbohydrate (mono or disaccharide) in the in vitro culture medium is important to maintain the PSII quantum yield, since there was a decrease of φP0 and φE0 or increase of φD0, even when using concentrations considered to be high, like 45 g L−1. It should be borne in mind that the species studied accumulates large quantities of fructose and glucose in the aerial part (Martins et al., 2016bMARTINS, J.P.R.; VERDOODT, V.; PASQUAL, P.; DE PROFT, M. Physiological responses by Billbergia zebrina (Bromeliaceae) when grown under controlled microenvironmental conditions. African Journal of Biotechnology, v.15, n.36, p.1952-1961, 2016b. DOI: https://doi.org/10.5897/AJB2016.15584
https://doi.org/10.5897/AJB2016.15584...
). The excess of these monosaccharides can suppress genes related to the photosynthetic apparatus, by affecting the signaling system of carbohydrates (Sheen, 1990SHEEN, J. Metabolic repression of transcription in higher plants. Plant Cell, v.2, p.1027-1038, 1990. DOI: https://doi.org/10.1105/tpc.2.10.1027
https://doi.org/10.1105/tpc.2.10.1027...
; Sheen, 1994SHEEN, J. Feedback-control of gene-expression. Photosynthesis Research, v.39, p.427-438, 1994. DOI: https://doi.org/10.1007/BF00014596
https://doi.org/10.1007/BF00014596...
). On the other hand, monosaccharides are important sources of molecules for structural constituents and energy, necessary to incite biochemical processes. The low content of monosaccharides might have been caused by the degradation of photosynthetic pigments, contributing to a reduction of photosynthesis (Piotrowska et al., 2010PIOTROWSKA, A.; BAJGUZ, A.; GODLEWSKA-ŻYŁKIEWICZ, B.; ZAMBRZYCKA, E. Changes in growth, biochemical components, and antioxidant activity in aquatic plant Wolffia arrhiza (Lemnaceae) exposed to cadmium and lead. Archives of Environmental Contamination and Toxicology, v.58, n.3, p.594-604, 2010. DOI: https://doi.org/10.1007/s00244-009-9408-6
https://doi.org/10.1007/s00244-009-9408-...
).
The plants grown in culture medium without carbohydrate supplementation presented low φP0 (0.55), much lower than 0.75, which suggests that the plants were under stress, with probable damages to PSII and negative regulation of photosynthesis (Martins et al., 2015bMARTINS, J.P.R.; SCHIMILDT, E.R.; ALEXANDRE, R.S.; FALQUETO, A.R.; OTONI, W.C. Chlorophyll a fluorescence and growth of Neoregelia concentrica (Bromeliaceae) during acclimatization in response to light levels In Vitro Cellular & Developmental Biology - Plant, v.51, n.4, p.471–481, 2015b. DOI: https://doi.org/10.1007/s11627-015-9711-z
https://doi.org/10.1007/s11627-015-9711-...
; Matysiak and Gabryszewska, 2016MATYSIAK, B.; GABRYSZEWSKA, E. The effect of in vitro culture conditions on the pattern of maximum photochemical efficiency of photosystem II during acclimatisation of Helleborus niger plantlets to ex vitro conditions. Plant Cell, Tissue and Organ Culture, v.125, n.3, p.585–593, 2016. DOI: https://doi.org/10.1007/s11240-016-0972-y
https://doi.org/10.1007/s11240-016-0972-...
). This stress response was evidenced by the energy flow parameters, where ABS/CSM, TR0/CSM, ET0/CSM and CS/ABS were lower and DI0/CSM was higher, showing that the lack of carbohydrate in the culture medium can cause debilities in the plants, including reduced photosynthetic efficiency (Tang et al., 2015TANG, G.L.; LI, X.Y.; LIN, L.S.; ZENG, F.J.; GU, Z.Y. Girdling-induced Alhagi sparsifolia senescence and chlorophyll fluorescence changes. Photosynthetica, v.53, n.4, p.585, 2015. DOI: https://doi.org/10.1007/s11099-015-0148-8
https://doi.org/10.1007/s11099-015-0148-...
).
It is known from the literature that carbohydrates may have a negative effect on photosynthesis during in vitro culture (Badr et al., 2015BADR, A.; ANGERS, P.; DESJARDINS, Y. Comprehensive analysis of in vitro to ex vitro transition of tissue cultured potato plantlets grown with or without sucrose using metabolic profiling technique. Plant Cell, Tissue and Organ Culture, v.122, n.2, p.491-508, 2015. DOI: https://doi.org/10.1007/s11240-015-0786-3
https://doi.org/10.1007/s11240-015-0786-...
; Ribeiro et al., 2017RIBEIRO, R.V.; MACHADO, E.C.; MAGALHÃES, J.R.F.; LOBO, A.K.M.; MARTINS, M.O.; SILVEIRA, J.A.G.; YIN, X.; STRUIK, P.C. Increased sink strength offsets the inhibitory effect of sucrose on sugarcane photosynthesis. Journal of Plant Physiology, v.20, p.61-69, 2017. DOI: https://doi.org/10.1016/j.jplph.2016.11.005
https://doi.org/10.1016/j.jplph.2016.11....
). However, the results of chlorophyll a fluorescence observed in this study suggest that during conventional culture of B. zebrina the employment of carbohydrates is essential to maintain the activities of the photosynthetic apparatus and does not cause damages in the exogenous concentration interval utilized (even at the highest concentration). Other authors have also reported that the supply of exogenous carbohydrate maintains the photosynthetic performance of plants in vitro (Eckstein et al., 2012ECKSTEIN, A.; ZIEBA, P.; GABRYS, H. Sugar and light effects on the condition of the photosynthetic apparatus of Arabidopsis thaliana cultured in vitro. Journal of Plant Growth Regulation, v.31, n.1, p.90-101, 2012. DOI: https://doi.org/10.1007/s00344-011-9222-z
https://doi.org/10.1007/s00344-011-9222-...
; Sáez et al., 2016SÁEZ, P.L.; BRAVO, L.A.; SÁNCHEZ-OLATE, M.; BRAVO, P.B.; RÍOS, D.G. Effect of photon flux density and exogenous sucrose on the photosynthetic performance during in vitro culture of Castanea sativa. American Journal of Plant Sciences, v.7, n.4, p.2087-2105, 2016. DOI: https://doi.org/10.4236/ajps.2016.714187
https://doi.org/10.4236/ajps.2016.714187...
).
The treatments also influenced the anatomical structures of the leaves. B. zebrina plants have stomata on the abaxial face of the leaves, as has been described for other bromeliads (Martins et al., 2014MARTINS, J.P.R.; SCHIMILDT, E.R.; ALEXANDRE, R.S.; CASTRO, E.M.; NANI, T.F.; PIRES, M.F.; PASQUAL, M. Direct organogenesis and leaf-anatomy modifications in vitro of Neoregelia concentrica (Vellozo) L.B. Smith (Bromeliaceae). Pakistan Journal of Botany, v.46, n.6, p.2179-2187, 2014.; Martins et al., 2019MARTINS, J.P.R.; RODRIGUES, L.C.A.; CONDE, L.T.; GONTIJO A.B.P.L.; FALQUETO, A.R. Anatomical and physiological changes of in vitro-propagated Vriesea imperialis (Bromeliaceae) in the function of sucrose and ventilated containers. Plant Biosystems, 2019. DOI: https://doi.org/10.1080/11263504.2019.1635223
https://doi.org/10.1080/11263504.2019.16...
) as well as for this species (Martins et al., 2015cMARTINS, J.P.R.; VERDOODT, V.; PASQUAL, P.; DE PROFT, M. Impacts of photoautotrophic and photomixotrophic conditions on in vitro propagated Billbergia zebrina (Bromeliaceae). Plant Cell, Tissue and Organ Culture, v.123, n.1, p.121-132, 2015c. DOI: https://doi.org/10.1007/s11240-015-0820-5
https://doi.org/10.1007/s11240-015-0820-...
; Martins et al., 2016aMARTINS, J.P.R.; MARTINS, A.D.; PIRES, M.F.; BRAGA, R.A.; JR.; REIS, R.O.; DIAS, G.M.G.; PASQUAL, M. Anatomical and physiological responses of Billbergia zebrina (Bromeliaceae) to copper excess in a controlled microenvironment. Plant Cell, Tissue and Organ Culture, v.126, n.1, p.43-57, 2016a. DOI: https://doi.org/10.1007/s11240-016-0975-8
https://doi.org/10.1007/s11240-016-0975-...
). The progressive increase of the stomatal density from the base to the tip of the leaves can be explained by the fact that in monocots, leaves grow in one direction, where the new cells are produced in the meristem region near the base and growth moves toward the tip as the cells divide and elongate (Skinner and Nelson, 1994SKINNER, R.H.; NELSON, C.J. Epidermal cell division and the coordination of leaf and tiller development. Annals of Botany, v.74, n.1, p.9-16, 1994. DOI: https://doi.org/10.1006/anbo.1994.1088
https://doi.org/10.1006/anbo.1994.1088...
).
On the leaf surface, the stomatal density is one of the most important factors to detect stress. It is a very important ecophysiological parameter that can affect photosynthesis and gas exchange (Kaluthota et al., 2015KALUTHOTA, S.; PEARCE, D.W.; EVANS, L.M.; LETTS, M.G.; WHITHAM, T.G.; ROOD, S.B. Higher photosynthetic capacity from higher latitude: foliar characteristics and gas exchange of southern, central and northern populations of Populus angustifolia. Tree Physiology, v.35, n.9, p.936-948, 2015. DOI: https://doi.org/10.1093/treephys/tpv069
https://doi.org/10.1093/treephys/tpv069...
). We found that the stomatal density of the B. zebrina leaves behaved similarly in the three regions analyzed. The number of stomata increased progressively with concentrations of 15 and 30 g L−1 in comparison with no supplementation, and then declined at the concentration of 45 g L−1. The addition of carbohydrates in the medium leads to alteration of the osmotic potential, thus affecting the flow of water from the culture medium to the plant (Martins et al., 2016bMARTINS, J.P.R.; VERDOODT, V.; PASQUAL, P.; DE PROFT, M. Physiological responses by Billbergia zebrina (Bromeliaceae) when grown under controlled microenvironmental conditions. African Journal of Biotechnology, v.15, n.36, p.1952-1961, 2016b. DOI: https://doi.org/10.5897/AJB2016.15584
https://doi.org/10.5897/AJB2016.15584...
). Moderate water deficits may have a positive effect on the number of stomata in monocots, but more severe deficits cause reduction, a response described by a parabolic curve (Xu and Zhou, 2008XU, Z.; ZHOU, G. Responses of leaf stomatal density to water status and its relationship with photosynthesis in a grass. Journal of Experimental Botany, v.59, n.12, p.3317-3325, 2008. DOI: https://doi.org/10.1093/jxb/ern185
https://doi.org/10.1093/jxb/ern185...
).
The plants cultured with monosaccharides presented alterations in the number or diameter of xylem vessels. This may be related to the metabolic processes that occur naturally in plants, since free fructose and glucose must be phosphorylated by fructokinase (FRK) or hexokinase (HXK) before being metabolized (Granot, 2007GRANOT, D. Role of tomato hexose kinases. Functional Plant Biology, v.34, n.6, p.564-570, 2007. DOI: https://doi.org/10.1071/FP06207
https://doi.org/10.1071/FP06207...
). Some genes that encode FRKs are essential to the development of the xylem and phloem (Stein et al., 2016STEIN, O.; DAMARI WEISSLER, H.; SECCHI, F.; RACHAMILEVITCH, S.; GERMAN, M.A.; YESELSON, Y.; AMIR, R.; SCHAFFER, A.; HOLBROOK, N.M.; ALONI, R.; ZWIENIECKI, M.A.; GRANOT, D. The tomato plastidic fructokinase SlFRK3 plays a role in xylem development. New Physiologist, v.209, n.4, p.1484-1495, 2016. DOI: https://doi.org/10.1111/nph.13705
https://doi.org/10.1111/nph.13705...
). Fructose in the form of non-phosphorylated hexose can be found more abundantly in the vascular tissue, which points to the importance of FRKs in vascular development (Stein et al., 2016STEIN, O.; DAMARI WEISSLER, H.; SECCHI, F.; RACHAMILEVITCH, S.; GERMAN, M.A.; YESELSON, Y.; AMIR, R.; SCHAFFER, A.; HOLBROOK, N.M.; ALONI, R.; ZWIENIECKI, M.A.; GRANOT, D. The tomato plastidic fructokinase SlFRK3 plays a role in xylem development. New Physiologist, v.209, n.4, p.1484-1495, 2016. DOI: https://doi.org/10.1111/nph.13705
https://doi.org/10.1111/nph.13705...
). Likewise, phosphorylated glucose turns into UDP-glucose, which in following the metabolic pathway affects the biosynthesis of hemicellulose through the action of uridine diphosphoglucose dehydrogenase (UGDH). This enzyme is also involved in the development of the xylem (Li et al., 2017LI, N.N.; CHEN, L.; LI, X.H.; LI, Q.; ZHANG, W.B.; TAKECHI, K.; TAKANO, H.; LIN, X.F. Overexpression of UDP-glucose dehydrogenase from Larix gmelinii enhances growth and cold tolerance in transgenic Arabidopsis thaliana. Biologia Plantarum, v.61, n.1, p.95-105, 2017. DOI: https://doi.org/10.1007/s10535-016-0657-8
https://doi.org/10.1007/s10535-016-0657-...
). Our hypothesis is that the high concentration of these monosaccharides transported via the xylem from the culture medium induced greater expression of the genes that encode FRKs and UGDHs, consequently influencing the development of the xylem of the B. zebrina plants.
The alterations in the xylem observed in the plants cultured with exogenous monosaccharides enhanced the efficiency of the water transport. The greater density of the vessels increases the water conduction area (Evans and Ortega, 2019EVANS, L.S.; ORTEGA, H. Xylem conductivities in grasses. Flora, v.257, p.151420, 2019. DOI: https://doi.org/10.1016/j.flora.2019.151420
https://doi.org/10.1016/j.flora.2019.151...
). Likewise, larger diameter also increases the potential to carry more water in less time by reducing the resistance to water flow (Martins et al., 2015cMARTINS, J.P.R.; VERDOODT, V.; PASQUAL, P.; DE PROFT, M. Impacts of photoautotrophic and photomixotrophic conditions on in vitro propagated Billbergia zebrina (Bromeliaceae). Plant Cell, Tissue and Organ Culture, v.123, n.1, p.121-132, 2015c. DOI: https://doi.org/10.1007/s11240-015-0820-5
https://doi.org/10.1007/s11240-015-0820-...
). This increase in transport might have been due to the accumulation of soluble carbohydrates in the leaves (Martins et al., 2016bMARTINS, J.P.R.; VERDOODT, V.; PASQUAL, P.; DE PROFT, M. Physiological responses by Billbergia zebrina (Bromeliaceae) when grown under controlled microenvironmental conditions. African Journal of Biotechnology, v.15, n.36, p.1952-1961, 2016b. DOI: https://doi.org/10.5897/AJB2016.15584
https://doi.org/10.5897/AJB2016.15584...
). This accumulation of carbohydrates increases the osmotic pressure in the sap of the xylem, providing additional force for absorption of water by the roots (Boyer, 1985BOYER, J.S. Water transport. Annual Review of Plant Biology, v.36, p.473-516, 1985. DOI: https://doi.org/10.1146/annurev.pp.36.060185.002353
https://doi.org/10.1146/annurev.pp.36.06...
). The osmotic adjustment of the plants analyzed in this study might have favored maintenance of the cell turgor and volume, allowing similar growth of the plants supplemented with the three carbohydrates.
Besides this, it appears that monosaccharides are preferable to this species, as found by Martins et al. (2016b)MARTINS, J.P.R.; VERDOODT, V.; PASQUAL, P.; DE PROFT, M. Physiological responses by Billbergia zebrina (Bromeliaceae) when grown under controlled microenvironmental conditions. African Journal of Biotechnology, v.15, n.36, p.1952-1961, 2016b. DOI: https://doi.org/10.5897/AJB2016.15584
https://doi.org/10.5897/AJB2016.15584...
. Good evidence exists indicating that sucrose is perceived as a distinct sugar, which cannot be substituted by glucose or fructose for control of various processes of plant development, as well in the storage of complex carbohydrates in the organs (Li and Sheen, 2016LI, L.; SHEEN, J. Dynamic and diverse sugar signalling. Current Opinion in Plant Biology, v.33, p.116-125, 2016. DOI: https://doi.org/10.1016/j.pbi.2016.06.018
https://doi.org/10.1016/j.pbi.2016.06.01...
). It has been proposed that FRKs play a fundamental role in the synthesis of starch, and also increase the activity of two key enzymes involved in the biosynthesis of starch, ADP-glucose pyrophosphorylase and amido-synthase (Schaffer and Petreikov, 1997SCHAFFER, A.A.; PETREIKOV, M. Sucrose to starch metabolism in tomato fruit undergoing transient starch accumulation. Plant Physiology, v.113, p.739-746, 1997. DOI: https://doi.org/10.1104/pp.113.3.739
https://doi.org/10.1104/pp.113.3.739...
).
The concentrations and types of carbohydrates had an impact on the root development of B. zebrina. This process demands large amounts of energy, and the larger number of roots observed in the plants cultured with glucose was likely due to its rapid and effective metabolization, which according to other researchers is more effective in inducing mitotic divisions (Corrêa et al., 2005CORRÊA, L.R.; PAIM, D.C.; SCHWAMBACH, J.; FETT-NETO, A.G. Carbohydrates as regulatory factors on the rooting of Eucalyptus saligna Smith and Eucalyptus globulus Labill. Plant Growth Regulation, v.45, n.1, p.63-73, 2005. DOI: https://doi.org/10.1007/s10725-004-6125-z
https://doi.org/10.1007/s10725-004-6125-...
; Rolland et al., 2006ROLLAND, F.; BAENA-GONZALEZ, E.; SHEEN, J. Sugar sensing and signaling in plants: conserved and novel mechanisms. Annual Review of Plant Biology, v.57, p.675-709, 2006. DOI: https://doi.org/10.1146/annurev.arplant.57.032905.105441
https://doi.org/10.1146/annurev.arplant....
). Carbohydrates such as glucose have been reported to regulate the expression of hormonal components. Furthermore, this carbohydrate may have activities similar to those of hormones in the growth of roots, cell proliferation and leaf expansion (Yanaglsawa et al., 2003YANAGLSAWA, S.; YOO, S.D.; SHEEN, J. Differential regulation of EIN3 stability by glucose and ethylene signalling in plants. Nature, v.425, p.521-525, 2003. DOI: https://doi.org/10.1038/nature01984
https://doi.org/10.1038/nature01984...
).
The higher fresh weight of the plants obtained in this study with carbohydrate concentration of 30 g L−1, except for fructose, can be explained by the greater availability of carbohydrates to supply energy and biomass to the molecular networks that drive cell division and elongation (Lastdrager et al., 2014LASTDRAGER, J.; HANSON, J.; SMEEKENS, S. Sugar signals and the control of plant growth and development. Journal of Experimental Botany, v.65, n.3, p.799–807, 2014. DOI: https://doi.org/10.1093/jxb/ert474
https://doi.org/10.1093/jxb/ert474...
). Besides this, carbohydrates can modulate the metabolism and transport of auxins in plants (Rolland, 2006ROLLAND, F.; BAENA-GONZALEZ, E.; SHEEN, J. Sugar sensing and signaling in plants: conserved and novel mechanisms. Annual Review of Plant Biology, v.57, p.675-709, 2006. DOI: https://doi.org/10.1146/annurev.arplant.57.032905.105441
https://doi.org/10.1146/annurev.arplant....
; Lastdrager et al., 2014LASTDRAGER, J.; HANSON, J.; SMEEKENS, S. Sugar signals and the control of plant growth and development. Journal of Experimental Botany, v.65, n.3, p.799–807, 2014. DOI: https://doi.org/10.1093/jxb/ert474
https://doi.org/10.1093/jxb/ert474...
). Auxins are essential for plants’ growth and development, by promoting cell expansion. However, carbohydrate concentrations higher than 30 g L−1 in the culture medium can induce osmotic stress in plants grown in vitro (Martins et al., 2016bMARTINS, J.P.R.; VERDOODT, V.; PASQUAL, P.; DE PROFT, M. Physiological responses by Billbergia zebrina (Bromeliaceae) when grown under controlled microenvironmental conditions. African Journal of Biotechnology, v.15, n.36, p.1952-1961, 2016b. DOI: https://doi.org/10.5897/AJB2016.15584
https://doi.org/10.5897/AJB2016.15584...
). When bromeliads are faced with a stressful condition, the apical dominance may be broken, inducing the proliferation to side shoots (Martins et al. 2019MARTINS, J.P.R.; RODRIGUES, L.C.A.; CONDE, L.T.; GONTIJO A.B.P.L.; FALQUETO, A.R. Anatomical and physiological changes of in vitro-propagated Vriesea imperialis (Bromeliaceae) in the function of sucrose and ventilated containers. Plant Biosystems, 2019. DOI: https://doi.org/10.1080/11263504.2019.1635223
https://doi.org/10.1080/11263504.2019.16...
). This can explain the results obtained with the plants cultured with 45 g L−1 fructose. These plants were small and had many side shoots, indicating a higher level of stress than in the plants cultured with the same concentration of sucrose or glucose. That effect culminated in greater accumulation of fresh weight in the plants of this treatment.
Conclusions
The in vitro conditions influenced the photosynthetic performance and anatomical traits of Billbergia zebrina plants. During conventional in vitro culture, the supply of exogenous carbohydrates as a carbon source is essential for the plants to maintain their vital functions. However, our first hypothesis was refuted, because the performance of the photosynthetic apparatus did not decline in function of the concentration or type of carbohydrate. The employment of carbohydrate concentrations greater than 30 g L−1 induced morphological or anatomical modifications, indicating stress on the plants. Alterations in the development of xylem vessels were important for adjustment to the microenvironmental conditions when using monosaccharides in the medium (lower osmotic potential). The use of sucrose can have a better effect in the concentration interval between 15 and 30 g L−1 by not causing large changes in the performance of the photosynthetic apparatus and anatomy of the plants.
Acknowledgements
The authors would like to acknowledge the scholarship awarded by Capes (Coordination for the Improvement of Higher Education Personnel)
References
- BADR, A.; ANGERS, P.; DESJARDINS, Y. Comprehensive analysis of in vitro to ex vitro transition of tissue cultured potato plantlets grown with or without sucrose using metabolic profiling technique. Plant Cell, Tissue and Organ Culture, v.122, n.2, p.491-508, 2015. DOI: https://doi.org/10.1007/s11240-015-0786-3
» https://doi.org/10.1007/s11240-015-0786-3 - BEZERRA, G.A.; GABRIEL, A.V.M.D.; MARIANO, E.D.; CARDOSO, J.C. In vitro culture and greenhouse acclimatization of Oncidium varicosum (Orchidaceae) with microorganisms isolated from its roots. Ornamental Horticulture, v.25, n.4, p.407-416, 2019. DOI: https://doi.org/10.1590/2447-536X.v25i4.2046
» https://doi.org/10.1590/2447-536X.v25i4.2046 - BOYER, J.S. Water transport. Annual Review of Plant Biology, v.36, p.473-516, 1985. DOI: https://doi.org/10.1146/annurev.pp.36.060185.002353
» https://doi.org/10.1146/annurev.pp.36.060185.002353 - CAI, W.; GAO, X.; HU, J.; CHEN, L.; LI, X.; LIU, Y.; WANG, G. UV-B radiation inhibits the photosynthetic electron transport chain in Chlamydomonas reinhardtii Pakistan Journal of Botany, v.48, n.6, p.2587-2593, 2016.
- CHEN, S.G.; STRASSER, R.J.; QIANG, S. In vivo assessment of effect of phytotoxin tenuazonic acid on PSII reaction centers. Plant Physiology and Biochemistry, v.84, p.10-21, 2014. DOI: https://doi.org/10.1016/j.plaphy.2014.09.004
» https://doi.org/10.1016/j.plaphy.2014.09.004 - CHEN, S.; KANG, Y.; ZHANG, M.; WANG, X.; STRASSER, R.J.; ZHOU, B.; QIANG, S. Differential sensitivity to the potential bioherbicide tenuazonic acid probed by the JIP-test based on fast chlorophyll fluorescence kinetics. Environmental and Experimental Botany, v.112, p.1-15, 2015. DOI: https://doi.org/10.1016/j.envexpbot.2014.11.009
» https://doi.org/10.1016/j.envexpbot.2014.11.009 - CHEN, Y.; WANG, H.; HU, W.; WANG, S.; SNIDER, J. L.; ZHOU, Z. Co-occurring elevated temperature and waterlogging stresses disrupt cellulose synthesis by altering the expression and activity of carbohydrate balance-associated enzymes during fiber development in cotton. Environmental and Experimental Botany, v.135, p.106-117, 2017. DOI: https://doi.org/10.1016/j.envexpbot.2016.12.012
» https://doi.org/10.1016/j.envexpbot.2016.12.012 - CORRÊA, L.R.; PAIM, D.C.; SCHWAMBACH, J.; FETT-NETO, A.G. Carbohydrates as regulatory factors on the rooting of Eucalyptus saligna Smith and Eucalyptus globulus Labill. Plant Growth Regulation, v.45, n.1, p.63-73, 2005. DOI: https://doi.org/10.1007/s10725-004-6125-z
» https://doi.org/10.1007/s10725-004-6125-z - DOBRÁNSZKI, J.; MENDLER-DRIENYOVSZKI, N.M. Cytokinin-induced changes in the chlorophyll content and fluorescence of in vitro apple leaves. Journal of Plant Physiology, v.171, n.16, p.1472-1478, 2014. DOI: https://doi.org/10.1016/j.jplph.2014.06.015
» https://doi.org/10.1016/j.jplph.2014.06.015 - DOWNS, C.A.; RYAN, S.L.; HECKATHORN, S.A. The chloroplast small heat-shock protein: evidence for a general role in protecting photosystem II against oxidative stress and photoinhibiton. Journal of Plant Physiology, v.155, n.4-5, p.488-496, 1999. DOI: https://doi.org/10.1016/S0176-1617(99)80043-1
» https://doi.org/10.1016/S0176-1617(99)80043-1 - EBURNEO, L.; RIBEIRO-JÚNIOR, N.G.; KARSBURG, I.V.; ROSSI, A.A.B.; SILVA, I.V. Anatomy and micromorphometric analysis of leaf Catasetum x apolloi Benelli & Grill with addition of potassium silicate under different light sources. Brazilian Journal of Biology, v.77, n.1, p.140-149, 2017. DOI: http://dx.doi.org/10.1590/1519-6984.12015
» http://dx.doi.org/10.1590/1519-6984.12015 - ECKSTEIN, A.; ZIEBA, P.; GABRYS, H. Sugar and light effects on the condition of the photosynthetic apparatus of Arabidopsis thaliana cultured in vitro Journal of Plant Growth Regulation, v.31, n.1, p.90-101, 2012. DOI: https://doi.org/10.1007/s00344-011-9222-z
» https://doi.org/10.1007/s00344-011-9222-z - EVANS, L.S.; ORTEGA, H. Xylem conductivities in grasses. Flora, v.257, p.151420, 2019. DOI: https://doi.org/10.1016/j.flora.2019.151420
» https://doi.org/10.1016/j.flora.2019.151420 - FALQUETO, A.R.; SILVA JÚNIOR, R.A.; GOMES, M.T.G.; MARTINS, J.P.R.; SILVA, D.M.; PARTELLI, F.L. Effects of drought stress on chlorophyll a fluorescence in two rubber tree clones. Scientia Horticulturae, v.224, p.238-243, 2017. DOI: https://doi.org/10.1016/j.scienta.2017.06.019
» https://doi.org/10.1016/j.scienta.2017.06.019 - GOLTSEV, V.N.; KALAJI, H. M.; PAUNOV, M.; BĄBA, W.; HORACZEK, T.; MOJSKI, J.; KOCIEL, H.; ALLAKHVERDIEV, S.I. Variable Chlorophyll Fluorescence and its use for Assessing Physiological Condition of Plant Photosynthetic Apparatus. Russian Journal of Plant Physiology, v.63, n.6, p.881-907, 2016. DOI: https://doi.org/10.1134/S1021443716050058
» https://doi.org/10.1134/S1021443716050058 - GRANOT, D. Role of tomato hexose kinases. Functional Plant Biology, v.34, n.6, p.564-570, 2007. DOI: https://doi.org/10.1071/FP06207
» https://doi.org/10.1071/FP06207 - HOLLAND, V.; KOLLER, S.; BRÜGGEMANN, W. Insight into the photosynthetic apparatus in evergreen and deciduous European oaks during autumn senescence using OJIP fluorescence transient analysis. Plant Biology, v.16, n.4, p.801-808, 2014. DOI: https://doi.org/10.1111/plb.12105
» https://doi.org/10.1111/plb.12105 - Johansen, D.A. Plant microtechnique, 2ed. Mc Graw-Hill, New York, 1940. 523p.
- KALAJI, H.M.; JAJOO, A.; OUKARROUM, A.; BRESTIC, M.; ZIVCAK, M.; SAMBORSKA, I.A.; CETNER, M.D.; ŁUKASIK, I.; GOLTSEV, V.; LADLE, R.J. Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions. Acta Physiologiae Plantarum, v.38, n.4, p.102, 2016. DOI: https://doi.org/10.1007/s11738-016-2113-y
» https://doi.org/10.1007/s11738-016-2113-y - KALAJI, H.M.; RASTOGI, A.; ŽIVČÁK, M.; BRESTIC, M.; DASZKOWSKA-GOLEC, A.; SITKO, K.; ALSHARAFA, K.Y.; LOTFI, R.; STYPIŃSKI, P.; SAMBORSKA, A.I.; CETNER, M.D. Prompt chlorophyll fluorescence as a tool for crop phenotyping: an example of barley landraces exposed to various abiotic stress factors. Photosynthetica, v.56, n.3, p.953-961, 2018. DOI: https://doi.org/10.1007/s11099-018-0766-z
» https://doi.org/10.1007/s11099-018-0766-z - KALAJI, H.M.; SCHANSKER, G.; LADLE, R.J. Frequently asked questions about in vivo chlorophyll fluorescence: pratical issue. Photosynthesis Research, v.122, n.2, p.121-158, 2014. DOI: https://doi.org/10.1007/s11120-014-0024-6
» https://doi.org/10.1007/s11120-014-0024-6 - KALUTHOTA, S.; PEARCE, D.W.; EVANS, L.M.; LETTS, M.G.; WHITHAM, T.G.; ROOD, S.B. Higher photosynthetic capacity from higher latitude: foliar characteristics and gas exchange of southern, central and northern populations of Populus angustifolia Tree Physiology, v.35, n.9, p.936-948, 2015. DOI: https://doi.org/10.1093/treephys/tpv069
» https://doi.org/10.1093/treephys/tpv069 - KUMARI, A.; BASKARAN, P.; STADEN, J.V. In vitro regeneration of Begonia homonyma - A threatened plant. South African Journal of Botany, v.109, p.174-177, 2017. DOI: https://doi.org/10.1016/j.sajb.2016.12.027
» https://doi.org/10.1016/j.sajb.2016.12.027 - LASTDRAGER, J.; HANSON, J.; SMEEKENS, S. Sugar signals and the control of plant growth and development. Journal of Experimental Botany, v.65, n.3, p.799–807, 2014. DOI: https://doi.org/10.1093/jxb/ert474
» https://doi.org/10.1093/jxb/ert474 - LEMBRECHTS, R.; CEUSTERS, N.; DE PROFT, M.; CEUSTERS, J. Sugar and starch dynamics in the medium-root-leaf system indicate possibilities to optimize plant tissue culture. Scientia Horticulturae, v.224, p.226–231, 2017. DOI: https://doi.org/10.1016/j.scienta.2017.06.015
» https://doi.org/10.1016/j.scienta.2017.06.015 - LI, N.N.; CHEN, L.; LI, X.H.; LI, Q.; ZHANG, W.B.; TAKECHI, K.; TAKANO, H.; LIN, X.F. Overexpression of UDP-glucose dehydrogenase from Larix gmelinii enhances growth and cold tolerance in transgenic Arabidopsis thaliana Biologia Plantarum, v.61, n.1, p.95-105, 2017. DOI: https://doi.org/10.1007/s10535-016-0657-8
» https://doi.org/10.1007/s10535-016-0657-8 - LI, L.; SHEEN, J. Dynamic and diverse sugar signalling. Current Opinion in Plant Biology, v.33, p.116-125, 2016. DOI: https://doi.org/10.1016/j.pbi.2016.06.018
» https://doi.org/10.1016/j.pbi.2016.06.018 - MARTINS, J.P.R.; MARTINS, A.D.; PIRES, M.F.; BRAGA, R.A.; JR.; REIS, R.O.; DIAS, G.M.G.; PASQUAL, M. Anatomical and physiological responses of Billbergia zebrina (Bromeliaceae) to copper excess in a controlled microenvironment. Plant Cell, Tissue and Organ Culture, v.126, n.1, p.43-57, 2016a. DOI: https://doi.org/10.1007/s11240-016-0975-8
» https://doi.org/10.1007/s11240-016-0975-8 - MARTINS, J.P.R.; PASQUAL, M.; MARTINS, A.D.; RIBEIRA, S.F. Effects of salts and sucrose concentrations on in vitro propagation of Billbergia zebrina (Herbert) Lindley (Bromeliaceae). Australian Journal of Crop Science, v.9, n.1, p.85-91, 2015a.
- MARTINS, J.P.R.; RODRIGUES, L.C.A.; CONDE, L.T.; GONTIJO A.B.P.L.; FALQUETO, A.R. Anatomical and physiological changes of in vitro-propagated Vriesea imperialis (Bromeliaceae) in the function of sucrose and ventilated containers. Plant Biosystems, 2019. DOI: https://doi.org/10.1080/11263504.2019.1635223
» https://doi.org/10.1080/11263504.2019.1635223 - MARTINS, J.P.R.; SCHIMILDT, E.R.; ALEXANDRE, R.S.; CASTRO, E.M.; NANI, T.F.; PIRES, M.F.; PASQUAL, M. Direct organogenesis and leaf-anatomy modifications in vitro of Neoregelia concentrica (Vellozo) L.B. Smith (Bromeliaceae). Pakistan Journal of Botany, v.46, n.6, p.2179-2187, 2014.
- MARTINS, J.P.R.; SCHIMILDT, E.R.; ALEXANDRE, R.S.; FALQUETO, A.R.; OTONI, W.C. Chlorophyll a fluorescence and growth of Neoregelia concentrica (Bromeliaceae) during acclimatization in response to light levels In Vitro Cellular & Developmental Biology - Plant, v.51, n.4, p.471–481, 2015b. DOI: https://doi.org/10.1007/s11627-015-9711-z
» https://doi.org/10.1007/s11627-015-9711-z - MARTINS, J.P.R.; VERDOODT, V.; PASQUAL, P.; DE PROFT, M. Impacts of photoautotrophic and photomixotrophic conditions on in vitro propagated Billbergia zebrina (Bromeliaceae). Plant Cell, Tissue and Organ Culture, v.123, n.1, p.121-132, 2015c. DOI: https://doi.org/10.1007/s11240-015-0820-5
» https://doi.org/10.1007/s11240-015-0820-5 - MARTINS, J.P.R.; VERDOODT, V.; PASQUAL, P.; DE PROFT, M. Physiological responses by Billbergia zebrina (Bromeliaceae) when grown under controlled microenvironmental conditions. African Journal of Biotechnology, v.15, n.36, p.1952-1961, 2016b. DOI: https://doi.org/10.5897/AJB2016.15584
» https://doi.org/10.5897/AJB2016.15584 - MATYSIAK, B.; GABRYSZEWSKA, E. The effect of in vitro culture conditions on the pattern of maximum photochemical efficiency of photosystem II during acclimatisation of Helleborus niger plantlets to ex vitro conditions. Plant Cell, Tissue and Organ Culture, v.125, n.3, p.585–593, 2016. DOI: https://doi.org/10.1007/s11240-016-0972-y
» https://doi.org/10.1007/s11240-016-0972-y - MURASHIGE, T.; SKOOG, F.A revised medium for rapid growth and biossays with tabacco tissue culture. Physiologia Plantarum, v.15, n.3, p.473-497, 1962. DOI: https://doi.org/10.1111/j.1399-3054.1962.tb08052.x
» https://doi.org/10.1111/j.1399-3054.1962.tb08052.x - NAIDOO, D.; AREMU, A.O.; STADEN, J.V.; FINNIE J.F. In vitro plant regeneration and alleviation of physiological disorders in Scadoxus puniceus South African Journal of Botany, v.109, p.316-322, 2017. DOI: https://doi.org/10.1016/j.sajb.2017.01.010
» https://doi.org/10.1016/j.sajb.2017.01.010 - PESSONI, R.A.; TERSAROTTO, C.C.; MATEUS, C.A.; ZERLIN, J.K.; SIMÕES, K.; DE CÁSSIA, L.; FIGUEIREDO-RIBEIRO, R.; BRAGA, M.R. Fructose Affecting Morphology and Inducing β-Fructofuranosidases in Penicillium janczewskii SpringerPlus, v.4, p.487, 2015. DOI: https://doi.org/10.1186/s40064-015-1298-7
» https://doi.org/10.1186/s40064-015-1298-7 - PIOTROWSKA, A.; BAJGUZ, A.; GODLEWSKA-ŻYŁKIEWICZ, B.; ZAMBRZYCKA, E. Changes in growth, biochemical components, and antioxidant activity in aquatic plant Wolffia arrhiza (Lemnaceae) exposed to cadmium and lead. Archives of Environmental Contamination and Toxicology, v.58, n.3, p.594-604, 2010. DOI: https://doi.org/10.1007/s00244-009-9408-6
» https://doi.org/10.1007/s00244-009-9408-6 - RIBEIRO, R.V.; MACHADO, E.C.; MAGALHÃES, J.R.F.; LOBO, A.K.M.; MARTINS, M.O.; SILVEIRA, J.A.G.; YIN, X.; STRUIK, P.C. Increased sink strength offsets the inhibitory effect of sucrose on sugarcane photosynthesis. Journal of Plant Physiology, v.20, p.61-69, 2017. DOI: https://doi.org/10.1016/j.jplph.2016.11.005
» https://doi.org/10.1016/j.jplph.2016.11.005 - ROLLAND, F.; BAENA-GONZALEZ, E.; SHEEN, J. Sugar sensing and signaling in plants: conserved and novel mechanisms. Annual Review of Plant Biology, v.57, p.675-709, 2006. DOI: https://doi.org/10.1146/annurev.arplant.57.032905.105441
» https://doi.org/10.1146/annurev.arplant.57.032905.105441 - ROSA, W.S.; MARTINS, J.P.R.; RODRIGUES, E.S.; RODRIGUES, L.C.A.; GONTIJO, A.B.P.L.; FALQUETO, A.R. Photosynthetic apparatus performance in function of the cytokinins used during the in vitro multiplication of Aechmea blanchetiana (Bromeliaceae). Plant Cell, Tissue and Organ Culture, v.133, n.3, p.339-350, 2018. DOI: https://doi.org/10.1007/s11240-018-1385-x
» https://doi.org/10.1007/s11240-018-1385-x - SÁEZ, P.L.; BRAVO, L.A.; SÁNCHEZ-OLATE, M.; BRAVO, P.B.; RÍOS, D.G. Effect of photon flux density and exogenous sucrose on the photosynthetic performance during in vitro culture of Castanea sativa American Journal of Plant Sciences, v.7, n.4, p.2087-2105, 2016. DOI: https://doi.org/10.4236/ajps.2016.714187
» https://doi.org/10.4236/ajps.2016.714187 - ŠEVČÍKOVÁ, H.; LHOTÁKOVÁ, Z.; HAMET, J.; LIPAVSKÁ, H. Mixotrophic in vitro cultivations: the way to go astray in plant physiology. Physiologia plantarum, v.167, n.4, p.365-377, 2019. DOI: https://doi.org/10.1111/ppl.12893
» https://doi.org/10.1111/ppl.12893 - SCHAFFER, A.A.; PETREIKOV, M. Sucrose to starch metabolism in tomato fruit undergoing transient starch accumulation. Plant Physiology, v.113, p.739-746, 1997. DOI: https://doi.org/10.1104/pp.113.3.739
» https://doi.org/10.1104/pp.113.3.739 - SHEEN, J. Metabolic repression of transcription in higher plants. Plant Cell, v.2, p.1027-1038, 1990. DOI: https://doi.org/10.1105/tpc.2.10.1027
» https://doi.org/10.1105/tpc.2.10.1027 - SHEEN, J. Feedback-control of gene-expression. Photosynthesis Research, v.39, p.427-438, 1994. DOI: https://doi.org/10.1007/BF00014596
» https://doi.org/10.1007/BF00014596 - SKINNER, R.H.; NELSON, C.J. Epidermal cell division and the coordination of leaf and tiller development. Annals of Botany, v.74, n.1, p.9-16, 1994. DOI: https://doi.org/10.1006/anbo.1994.1088
» https://doi.org/10.1006/anbo.1994.1088 - STEIN, O.; DAMARI WEISSLER, H.; SECCHI, F.; RACHAMILEVITCH, S.; GERMAN, M.A.; YESELSON, Y.; AMIR, R.; SCHAFFER, A.; HOLBROOK, N.M.; ALONI, R.; ZWIENIECKI, M.A.; GRANOT, D. The tomato plastidic fructokinase SlFRK3 plays a role in xylem development. New Physiologist, v.209, n.4, p.1484-1495, 2016. DOI: https://doi.org/10.1111/nph.13705
» https://doi.org/10.1111/nph.13705 - STRASSER, R.J.; STIRBET, A.D. Heterogeneity of photosystem II probed by the numerically simulated chlorophyll a fluorescence rise (O–J–I–P). Mathematics and Computers in Simulation, v.48, n.1, p.3-9, 1998. DOI: https://doi.org/10.1016/S0378-4754(98)00150-5
» https://doi.org/10.1016/S0378-4754(98)00150-5 - STRASSER, R.J.; TSIMILLI-MICHAEL, M.; SRIVASTAVA, A. Analysis of the chlorophyll a fluorescence transient. In: PAPAGEORGIOU, G.C.; GOVINDJEE. (ed) Chlorophyll fluorescence: a signature of photosynthesis Kluwer Academic Publishers Press, Dordrecht, 2004. p.321-362. DOI: https://doi.org/10.1007/978-1-4020-3218-9_12
» https://doi.org/10.1007/978-1-4020-3218-9_12 - SUN, Z.W.; REN, L.K.; FAN, J.W.; LI, Q.; WANG, K.J.; GUO, M.M.; WANG, L.; LI, J.; ZHANG, G.X.; YANG, Z.Y.; CHEN, F.; LI, X.N. Salt response of photosynthetic electron transport system in wheat cultivars with contrasting tolerance. Plant, Soil and Environment, v. 62, n.11, p.515-521, 2016. DOI: https://doi.org/10.17221/529/2016-PSE
» https://doi.org/10.17221/529/2016-PSE - TANG, G.L.; LI, X.Y.; LIN, L.S.; ZENG, F.J.; GU, Z.Y. Girdling-induced Alhagi sparsifolia senescence and chlorophyll fluorescence changes. Photosynthetica, v.53, n.4, p.585, 2015. DOI: https://doi.org/10.1007/s11099-015-0148-8
» https://doi.org/10.1007/s11099-015-0148-8 - VESCO, L.L.D.; STEFENON, V.M.; WELTER, L.J.; SCHERER, R.F.; GUERRA, M.P. Induction and scale-up of Billbergia zebrina nodule cluster cultures: implications for mass propagation, improvement and conservation. Scientia Horticulturae, v.28, n.4, p.515-522, 2011. DOI: https://doi.org/10.1016/j.scienta.2011.02.018
» https://doi.org/10.1016/j.scienta.2011.02.018 - WANG, Q.Y.; ZHANG, J.; ZHAO, B.Z.; XIN, X.L.; DENG, X.H.; ZHANG, H. Influence of long term fertilization on selenium accumulation in soil and uptake by crops. Pedosphere, v.26, n.1, p.120-129, 2016. DOI: https://doi.org/10.1016/S1002-0160(15)60028-5
» https://doi.org/10.1016/S1002-0160(15)60028-5 - XU, Z.; ZHOU, G. Responses of leaf stomatal density to water status and its relationship with photosynthesis in a grass. Journal of Experimental Botany, v.59, n.12, p.3317-3325, 2008. DOI: https://doi.org/10.1093/jxb/ern185
» https://doi.org/10.1093/jxb/ern185 - YANAGLSAWA, S.; YOO, S.D.; SHEEN, J. Differential regulation of EIN3 stability by glucose and ethylene signalling in plants. Nature, v.425, p.521-525, 2003. DOI: https://doi.org/10.1038/nature01984
» https://doi.org/10.1038/nature01984 - ZUSHI, K.; MATSUZOE, N. Using of chlorophyll a fluorescence OJIP transients for sensing salt stress in the leaves and fruits of tomato. Scientia Horticulturae, v.219, p.216-221, 2017. DOI: https://doi.org/10.1016/j.scienta.2017.03.016
» https://doi.org/10.1016/j.scienta.2017.03.016
Publication Dates
-
Publication in this collection
11 May 2020 -
Date of issue
Jan-Mar 2020
History
-
Received
18 Sept 2019 -
Accepted
05 Dec 2019