Abstract
In the Conceição - MG mine, there are significant reserves of dolomitic itabirite, which is considered overburden material. This study aimed to develop a flotation route for the concentration of this dolomitic BIF according to the requirements of the steel industry. The characterization of samples, comminution, liberation size, microflotation of the pure dolomite and hematite minerals were performed to establish the conditions for selective separation in bench flotation tests. The microflotation tests showed that the soybean oil at pH 6 preferably floats the dolomite in relation to the hematite. The corn starch depressant at a pH higher than10 preferably depresses the hematite relative to the dolomite. The bench flotation tests revealed interactions between the factors pH, percentage of solids in the pulp, soybean oil and starch dosage. The optimal results for bench flotation tests were achieved under the following conditions: pH at 10.5, percentage of solids of 27wt%, dosages of soybean oil and corn starch of 400g/ton and 351g/ton, respectively.
keywords:
dolomitic BIF; iron concentration; flotation
1. Introduction
The high demand for iron ore products and the exhaustion of reserves with a high iron grade in the "Quadrilátero Ferrífero" of Minas Gerais in Brazil have led to the development of beneficiation of low grade "banded iron formations" (BIF) with the removal of overburden materials. They are composed of minerals without economic value, which are deposited in piles or replaced after mining. The overburden material represents costs in the operation of a mine, as well as issues related to safety and the environment (Aragão and Oliveira Filho, 2011ARAGÃO, G. A. S., OLIVEIRA FILHO, W. L de. Classificação de pilhas de estéril na mineração de ferro. Rem - Revista Escola de Minas, v. 64, n. 2, p. 193-198, 2011.). When there is a process route for its concentration, this material can be considered as ore. The dolomitic BIF is the main overburden material of iron ore exploitation at the Conceição mine (Itabira, Minas Gerais, Brazil). It has a similar texture of the siliceous BIF, with alternating bands of carbonate and iron, composed of dolomite, hematite and smaller quantities of quartz, and may also contain varying amounts of calcite, talc, chlorite and amphiboles (Rosière et al., 2008ROSIÈRE, C. A. et. al. The itabirites of the Quadrilátero Ferrífero and related high-grade iron ore deposits: an overview. Reviews in Economic Geology, v. 15, p. 223-254. 2008.).
The concentration by flotation is the most used process in mining, both in quantity of processed ores and in diversity of applications (Peres et al., 2007PERES, A. E. C., et. al. Non-sulfide minerals plant practice. In: FUERSTENAU, M. C., JAMESON, G., YOON, R. H. Froth flotation: a century of innovation. Colorado: SME, 2007. p. 845-868.). It is based on the principle of selectively inducing hydrophobicity to the surface of the mineral to be floated by chemical agents, separating it from other hydrophilic minerals. Saponified vegetable oils are surfactants widely used in flotation as a collector. They are composed of mixtures of carboxylic acids (RCOOH) of long hydrocarbon chain, which presents a hydrophobic character, and the carboxyl polar group, which is hydrophilic (Leja, 1982LEJA, J. Flotation surfactants. In: Surface Chemistry of Froth Flotation. New York: Plenum Press, 1982. p. 205-333.; Pearse, 2005PEARSE, M. J. An overview of the use of chemical reagents in mineral. processing. Minerals Engineering, v. 18, n. 2, p.139-149, 2005.). Soaps or salts of these oils, obtained by saponification, are used in the anionic flotation of slightly soluble and oxide minerals (Kulkarni and Somasundaran, 1980KULKARNI, R. D., SOMASUNDARAN, P. Flotation chemistry of hematite/oleate system. Colloids and Surfaces, v. 1, n. 3-4, p.387-405, 1980.; Guimarães et al., 2005GUIMARÃES, R. C., ARAUJO, A. C de, PERES, A. E. C. Reagents in igneous phosphate ores flotation. Minerals Engineering, v. 18, n. 2, p.199-204, 2005.). Starches of different origins are used as depressants in several flotation systems (Leja, 1982LEJA, J. Flotation surfactants. In: Surface Chemistry of Froth Flotation. New York: Plenum Press, 1982. p. 205-333.; Hanna and Somasundaran, 1976HANNA, H. S., SOMASUNDARAN, P. Flotation of salt-type minerals. In: FUERSTENAU, M. C. Flotation. New York: American Institute of Mining, Metallurgical and Petroleum Engineers, 1976. p. 48-196.). Nunes and Peres (2011)NUNES, A. P. L., PERES, A. E. C. Reagentes depressores de carbonatos: uma revisão. Rio de Janeiro: CETEM, 2011. (Serie Tecnologia Mineral, 89). point them out as being excellent carbonate depressants. In Brazil, starches are widely used in flotation of siliceous BIF as a depressant of iron minerals (Monte and Peres, 2004MONTE, M. B. M de, PERES, A. E. C. Química de superfície na flotação. In: LUZ, A. B da, SAMPAIO, J. A., ALMEIDA, S. L. M. de. Tratamento de Minérios. (4. ed.). Rio de Janeiro: CETEM/MCT, 2004. p. 339-402.).
The objective of this study was to obtain a concentrate of iron ore by means of flotation from the dolomitic BIF.
2. Materials and methods
The sample of dolomitic BIF was obtained in blocks at the Conceição mine (Figure 1). The sample was analyzed by X-ray diffraction (XRD), X-ray fluorescence (FRX), scanning electron microscopy (SEM), conversion electron Mössbauer spectroscopy (CEMS), loss on ignition, thermogravimetry and density by helium pycnometry. The liberation size was determined according to Gaudin (1939)GAUDIN, A. M. Principles of mineral dressing. New Delhi: Tata McGraw-Hill, 1939.. The ore blocks underwent primary crushing on a jaw crusher, whereby the material from this stage was classified by a 1/4" sieve, whereupon the retained fraction fed the secondary crushing in a roller crusher to obtain fractions below 1/4", feeding the rod mill grinder. According to the liberation size, the grinding was carried out long enough for 5% of the material to be retained in the 74µm sieve. The ground material underwent wet sieving, using the 37µm sieve. The +37µm fraction was dry classified in an electromagnetic stirrer using the 100, 74, 53, 44 and 37µm sieve series. The -37µm fraction was classified in a cyclosizer at the sizes 24.7, 18.4, 12.6, 9.0 and 7.2µm.
The dolomite and hematite for the microflotation tests were obtained from the ore itself (Figure 1). The ore blocks were comminuted in a jaw crusher, followed by manual pick up of fractions rich in dolomite and hematite. Each of these fractions were ground in a disc mill and classified in the 149µm +74µm range. The purification of these fractions was done in a dry magnetic separator of high intensity field. The microflotation tests were performed on a modified Hallimond tube using distilled water, 1.0 g of mineral, 4 minutes of conditioning for soybean oil and 2 minutes for corn starch, 1 minute of flotation and nitrogen flushing of 70 cm3/min. The concentrations of soybean oil were 2, 4, 8, 16 and 32 mg/L, the concentrations of corn starch were 1.25, 2.5, 10, 20 and 40 mg/L, and the pH were 4, 6, 8, 10 and 12.
The reverse anionic flotation tests were conducted in a bench flotation cell using 1kg of ore, conditioning time of 4 minutes for soybean oil and 2 minutes for corn starch, 6 minutes of flotation, 1200 rpm of the rotor. A 24 factorial design (Table 1) was performed to evaluate the effects of the factors pH, concentration of solids in the pulp (CS), dosage of soybean oil (SO) and dosage of corn starch (ST) on grade of iron (Fe), CO3 2- (carbonate), and SiO2 (silica) in the concentrate and in the tailings. The results were analyzed with the aid of Minitab 17 software.
The soybean oil was supplied by Pirapora and saponified with sodium hydroxide at 70ºC. The corn starch (corn meal, Flotamil 75), supplied by Caramuru Alimentos SA, was gelatinized with sodium hydroxide. Sodium hydroxide and hydrochloric acid were used for pH adjustments.
3. Results and discussion
3.1 Characterization
The mineralogical analysis by X-ray diffraction showed that the sample consists of dolomite (majoritarian) and hematite (intermediate) and small amounts of chlorite and talc. In addition to the hematite, in the Fe3+ oxidation state, analysis by conversion electron Mössbauer spectroscopy, identified another iron phase, ferrihydrite, with the hematite being the predominant phase (98.00%).
The results of the X-ray fluorescence are shown in Table 2. The thermogravimetry showed that at 691ºC the sample lost 11wt% due to the release of CO2 from the carbonated phase, which is the explanation for the high value of the loss on ignition (LOI).
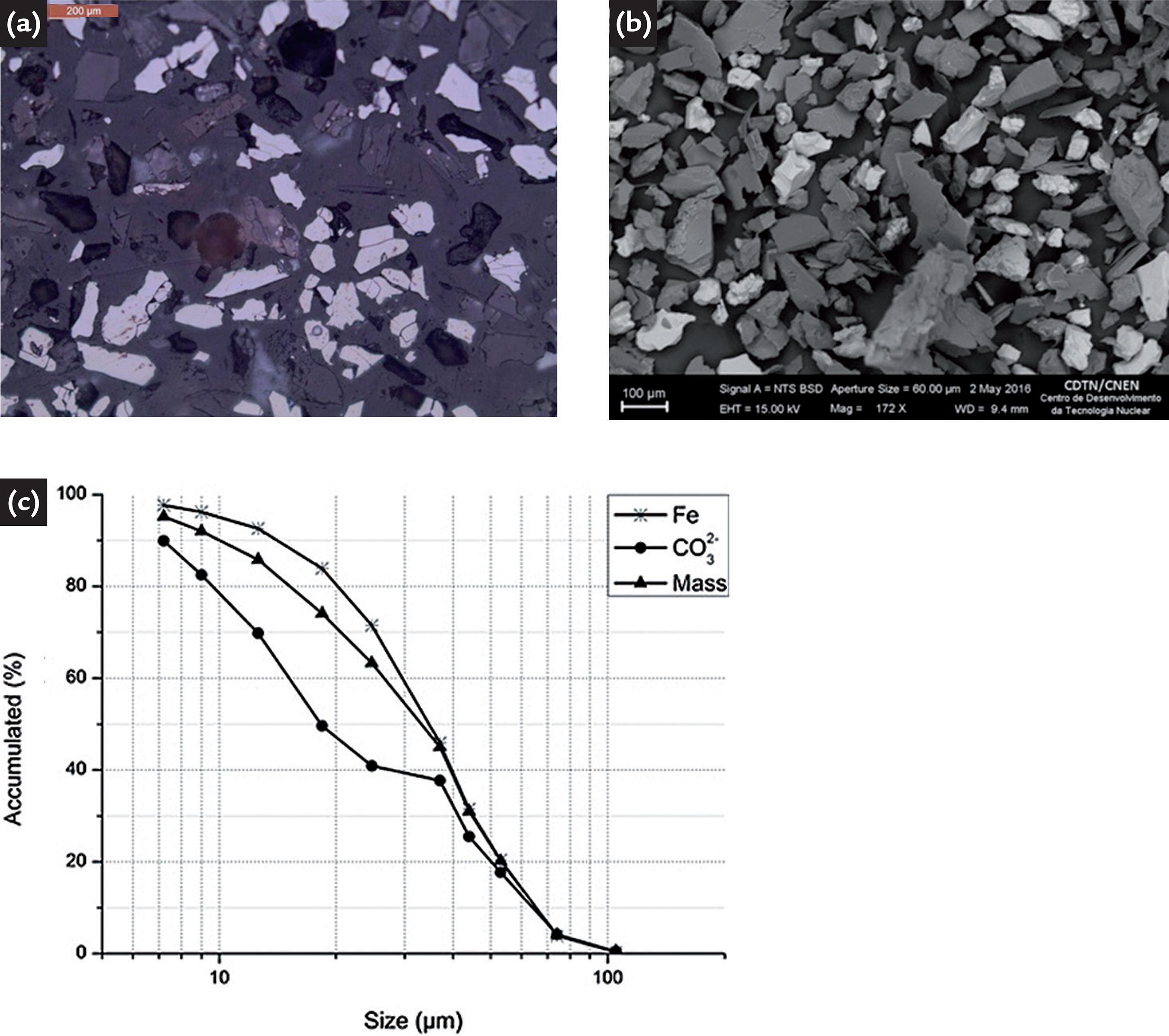
In the determination of the liberation size of the minerals, the hematite/gangue ratio was considered, where the gangue constitutes all the other minerals of the sample. The liberation degree of 88.56% is when the particles are practically all liberated (Figure 2a).
(a) Optical microscopic image of the fraction -74 + 53µm, showing free particles of hematite (light particles);(b) BSD/MEV image of the dolomitic BIF: milling time 12.5 min, P95 at 74µm, showing free particles of hematite (light particles); (c) mass, Fe and CO32- distribution as a function of particle size
According to the liberation size, the results of the grinding tests showed that for 12.5 min, P95 is 74µm. Figure 2b shows the backscattered electron image (BSD) generated in scanning electron microscopy of the ground sample for 12.5 min. In this size fraction, the particles are practically all liberated, being possible to observe the existence of individualized fractions of the hematite (white fractions).The size analysis (Figure 2c) showed that the CO3 2- distribution is concentrated in the finer fractions and the Fe fraction in the coarser fractions, with 45.9% Fe being distributed in the fractions >37µm and 37.7% in CO3 2- in the fractions <37µm. The density of the sample by helium picnometry was 4.13g/cm3.
3.2 Microflotation
Figure 3 shows the results of the microflotation tests. The flotability of hematite as a function of pH and soybean oil concentration (Figure 3a) is more complex. It decreases with the increasing of the pH, showing a maximum around 10 mg/L, and increases steadily with the increasing of soybean oil concentrations higher than 15 mg/L. The dolomite flotability (Figure 3b) is low for high pH and low concentrations of soybean oil and increases with the increasing of the soybean oil concentration. It shows a maximum around pH 6 for low concentrations of soybean oil. The hematite flotability decreases very fast with the increasing the starch concentration for an addition of 32 mg/L of soybean (Figure 3c); the dolomite flotability decreases with the increasing of the starch concentration for an addition of 32 mg/L of soybean oil. According to Chen and Tao (2004)CHEN, G., TAO, D. Effect of solution chemistry on flotability of magnesite and dolomite. International Journal of Mineral Processing, v.74, n.1-4, p.343-357, 2004., for dolomite the Mg2+ and Ca2+ species predominate at pH 6, which favor the adsorption on the collector and lead to the high flotability of this mineral.
Surface plots for the microflotability. The dots are the experimental points and the surfaces were interpolated with the software Minitab 17 by the distance method. Flotability of hematite (a) and of dolomite (b) as a function pH and soybean oil concentration; Flotability of hematite (c) and dolomite (d) as a function of pH and starch with the addition of 32 mg/L of soybean oil
The flotability of both minerals decreased under the alkaline conditions (Figures 3a and 3b), especially for hematite. This behavior was also observed by Lopes and Lima (2009)LOPES, G. M., LIMA, R. M. F. Flotação direta de minério de ferro com oleato de sódio. Rem - Revista Escola de Minas, v. 62, n. 3, p. 323-329, 2009. and Kulkarni and Somasundaran (1980)KULKARNI, R. D., SOMASUNDARAN, P. Flotation chemistry of hematite/oleate system. Colloids and Surfaces, v. 1, n. 3-4, p.387-405, 1980., who obtained low hematite flotabilities for pH> 10 using a fatty acid as the collector. Figures 3c and 3d show that the corn starch preferentially depresses the hematite, as also reported by Brandão (2005)BRANDÃO, P. R. G. A seletividade na flotação reversa de minério de ferro: adsorção dos reagentes. In: ENCONTRO NACIONAL DE TRATAMENTO DE MINÉRIOS E METALURGIA EXTRATIVA, 21. 2005. Natal. Anais... Natal, v. 2, p. 22-33, 2005..
3.3 Bench flotation tests
Table 3 shows the results of the grade of Fe, CO3 2- and SiO2 in the concentrate and in the tailings. Tables 4 and 5 show the results of the calculations of the main effects and the interactions carried out with Minitab 17 for the concentrate and the tailings by means of a stepwise analysis with input alpha of 0.10. Note the complexity of the system due to the interactions between the factors, suggesting the existence of physical and chemical interactions between the reagents. Only the effects of factors and interactions with p≤0.100 are considered statistically significant.
Grades of Fe, CO32- and SiO2 (%) in the concentrate and in the tailings obtained in the bench flotation tests (the settings of each test is shown in Table 1).
Calculation of the main factors effects and interactions for the concentrate by means of stepwise analysis (α=0.100).
Calculation of the main factors effects and interactions for the tailings by means of stepwise analysis (α=0.100).
The optimal conditions were calculated with the Response Optimizer of Minitab 17 to obtain in the concentrate the maximum grade of Fe and the minimum grade of CO3 2- and SiO2 , as well as in the tailings the minimum grade of Fe and maximum grades of CO3 2- and SiO2. The optimal settings, the expected grades and the results obtained in confirmatory tests are shown in Tables 6 and 7.
Result for the response optimizer calculated with Minitab 17 to achieve in the concentrate: the maximum grade of Fe and the minimum the grades of CO32- and SiO2; in the tailings: the minimum grade of Fe and the maximum grades of CO32- and SiO2.
Figure 4 shows the mass and metallurgical balance of the flotation test performed under optimized conditions (T18 of Table 7) and with roughing, cleaning and scavenging stages. These stages were carried out to reduce the CO3 2- and Fe grades in the concentrate and in the tailings, respectively. In the cleaning stage, 50g/ton of soybean oil was added to float carbonates to clean the final concentrate. In the scavenging stage, the addition of reagents was not necessary, since the material of this stage comes from the flotation roughing. At all stages, the pH was adjusted to 10.5. The final (cleaner) concentrate had grades of Fe, CO3 2- and SiO2 of 62.94, 4.13 and 0.82%, respectively. It should be noted that the tailings of the cleaner as well as the scavenger concentrate still contain high iron grade, close to that of the feed, so they can be returned to the roughing stage. Tables 8 shows the results of the complete chemical analysis of the final concentrate.
Mass and metallurgical balance of the flotation test performed under optimized conditions (T18 of Table 7) and with rougher, cleaner and scavenger stages
4. Conclusion
The dolomitic BIF of the Conceição mine is composed of dolomite and hematite, being the dolomite the majoritarian mineral. At -74µm the dolomite and hematite are practically liberated. Microflotation tests showed that at alkaline conditions, the corn starch preferably depresses the hematite and the soybean oil preferably floats the dolomite. The results of the studies of characterization, liberation size, milling and microflotation allowed the concentration of this ore by flotation. According to the optimization of bench flotation tests, good performance is achieved under the following conditions: pH 10.5, 27wt% of solids, 351 g/ton of corn starch and 400 g/ton of soybean oil. The cleaner stage improved the levels of Fe, CO32- and SiO2 in the concentrate to 62.94, 4.1 and 0.82%, respectively.
Acknowledgments
The authors thank the Mineral Technology Department of CDTN, CAPES and Vale S.A.
References
- ARAGÃO, G. A. S., OLIVEIRA FILHO, W. L de. Classificação de pilhas de estéril na mineração de ferro. Rem - Revista Escola de Minas, v. 64, n. 2, p. 193-198, 2011.
- BRANDÃO, P. R. G. A seletividade na flotação reversa de minério de ferro: adsorção dos reagentes. In: ENCONTRO NACIONAL DE TRATAMENTO DE MINÉRIOS E METALURGIA EXTRATIVA, 21. 2005. Natal. Anais... Natal, v. 2, p. 22-33, 2005.
- CHEN, G., TAO, D. Effect of solution chemistry on flotability of magnesite and dolomite. International Journal of Mineral Processing, v.74, n.1-4, p.343-357, 2004.
- GAUDIN, A. M. Principles of mineral dressing New Delhi: Tata McGraw-Hill, 1939.
- GUIMARÃES, R. C., ARAUJO, A. C de, PERES, A. E. C. Reagents in igneous phosphate ores flotation. Minerals Engineering, v. 18, n. 2, p.199-204, 2005.
- HANNA, H. S., SOMASUNDARAN, P. Flotation of salt-type minerals. In: FUERSTENAU, M. C. Flotation New York: American Institute of Mining, Metallurgical and Petroleum Engineers, 1976. p. 48-196.
- KULKARNI, R. D., SOMASUNDARAN, P. Flotation chemistry of hematite/oleate system. Colloids and Surfaces, v. 1, n. 3-4, p.387-405, 1980.
- LEJA, J. Flotation surfactants. In: Surface Chemistry of Froth Flotation New York: Plenum Press, 1982. p. 205-333.
- LOPES, G. M., LIMA, R. M. F. Flotação direta de minério de ferro com oleato de sódio. Rem - Revista Escola de Minas, v. 62, n. 3, p. 323-329, 2009.
- MONTE, M. B. M de, PERES, A. E. C. Química de superfície na flotação. In: LUZ, A. B da, SAMPAIO, J. A., ALMEIDA, S. L. M. de. Tratamento de Minérios (4. ed.). Rio de Janeiro: CETEM/MCT, 2004. p. 339-402.
- NUNES, A. P. L., PERES, A. E. C. Reagentes depressores de carbonatos: uma revisão Rio de Janeiro: CETEM, 2011. (Serie Tecnologia Mineral, 89).
- PEARSE, M. J. An overview of the use of chemical reagents in mineral. processing. Minerals Engineering, v. 18, n. 2, p.139-149, 2005.
- PERES, A. E. C., et. al. Non-sulfide minerals plant practice. In: FUERSTENAU, M. C., JAMESON, G., YOON, R. H. Froth flotation: a century of innovation Colorado: SME, 2007. p. 845-868.
- ROSIÈRE, C. A. et. al. The itabirites of the Quadrilátero Ferrífero and related high-grade iron ore deposits: an overview. Reviews in Economic Geology, v. 15, p. 223-254. 2008.
Publication Dates
-
Publication in this collection
Apr-Jun 2019
History
-
Received
09 May 2018 -
Accepted
29 Oct 2018