Abstract
Obtaining rare earth elements (REE) is a complicated task, due mainly to the difficulty of separating and purifying them. Solvent extraction is the most widely used technique for separating REE, and the most common extractants used are organophosphorus acids. The low selectivity of this technique leads to the need for a high number of extraction cells. To increase the selectivity and separation and avoid the practice of saponification of the extractant, the use of complexing agents has been studied, such as low-molecular-weight and biodegradable weak organic acids. The objective of this research was to study the separation of the rare earth elements Gd and Eu by the solvent extraction technique using the organophosphonic extractant 2-ethylhexyl phosphonic acid mono-2-ethylhexyl ester (P507). Assays were performed using non-saponified and saponified P507 extractant and adding lactic acid to the aqueous phase prior to extraction. Experimental factorial planning was used to study the effect on the extraction and separation of the Eu and Gd of the variables extractant concentration, initial feed pH, saponification degree, and lactic acid concentration. The greatest Gd extractions were obtained when lactic acid was added to the feed solution. Also, saponification of the extractant and lactic acid addition improved the Gd/Eu separation. The number of stages required to extract Gd using McCabe-Thiele diagrams was estimated, whether or not the solution was conditioned with lactic acid. The largest extraction percentage was obtained by adding lactic acid in a continuous counter-current extraction assay, achieving 94% Gd extraction.
Keywords:
rare earth elements; solvent extraction; 2-ethylhexylphosphonic acid mono-2-ethylexyl ester; saponification; lactic acid
1. Introduction
The rare earth elements (REE) constitute the group of lanthanides (atomic number between 51 and 71) in addition to the elements Y and Sc, and can be found widely distributed throughout the earth's crust (Gupta, 2004GUPTA, C.K.; KRISHNAMURTHY, N. Extractive metallurgy of Rare Earths. 1st ed. Boca Raton, USA: CRC Press Taylor&Francis Group, 2004. cap. 2, p. 57-131.). These elements have great economic importance due to the large number of applications in high-technology industries (Lapido-Loureiro, 2011LAPIDO-LOUREIRO, F. E. Terras-Raras: Tipos de Depósitos, Recursos Identificados e Alvos Prospectivos no Brasil. In: SEMINÁRIO BRASILEIRO DE TERRAS-RARAS, 1., 2011, Rio de Janeiro. Bases para o desenvolvimento de Terras raras no Brasil. Rio de Janeiro: Cetem - Centro de Tecnologia Mineral, 2011.). But to obtain oxides of these individual elements, it is necessary to determine the best means to achieve their separation.
Solvent extraction (SX) or liquid-liquid extraction using liquid organic extractants is currently one of the main techniques for industrial-scale separation, purification and concentration of metals, including REE. Because of the similarity between the chemical properties of these elements, the separation and purification of their mixtures is a complicated task. There are well-known separation routes for REE through this technique (Ritcey, 2006RITCEY, G. M. Solvent extraction: principles and application to process metallurgy. 2nd ed. Ottawa, Canada: G.M. Ritcey and Associates Incorporates, 2006. v. 2., p. 423-452.). However, new research is needed to find other separation routes by solvent extraction that are more efficient and have less impact on the environment. The extraction reaction of a metal with an organophosphorus acid extractant is denoted by the general Equation 1:
In order to increase the selectivity of the separation of these elements, modifications in the purification processes by SX have been studied. Among the modifications are saponification of the extractant, such as 2-ethylhexyl phosphonic acid 2-ethylhexyl ester (P507), with commercial bases such as sodium hydroxide, ammonium hydroxide and ammonium carbonate, as well as alternative basic compounds like Mg(HCO3)2, a solution produced from dolomite and magnesium chloride (Lee et al., 2005LEE M.S.; LEE J.Y.; KIMB J.S.; LEE G.S. Solvent extraction of neodymium ions from hydrochloric acid solution using PC88A and saponified PC88A. Separation and Purification Technology, v. 46, p. 72-78, 2005.; Feng et al., 2012FENG, Z.; HUANG, X.; LIU, H.; WANG, M.; LONG, Z.; YU, Y. Study on preparation and application of novel saponification agent for organic phase of rare earths extraction. Journal of Rare Earths, v. 30, n. 9, p. 903-908, 2012.).
The saponification of the organic acid extractant causes the acidity of the spent aqueous liquor (raffinate) to decrease less dramatically during extraction of the metals from the aqueous phase, which not only increases the total extraction, but also increases selectivity. However, the use of saponified extracts generates an aqueous effluent with a high content of ammonium, sodium, calcium or magnesium (depending on the agent used for saponification). These effluents must be treated before being disposed of in the environment (Al-Marzooqi et al., 2014AL-MARZOOQI, F. A.; AL GHAFERI, A. A.; SAADAT, I.; HILAL, N. Application of Capacitive deionization in water desalination: a review. Desalination, v. 342, p. 3-15, 2014.). As an alternative to the saponification process, the conditioning of the feed with complexing agents is considered a promising option because it is environmentally friendly and these agents improve the separation of REE just as saponification does (Sun et al., 2006SUN, X.; WANG, Y.; LI, D. Selective separation of yttrium by CA-100 in the presence of a complexing agent. Journal of Alloys and Compounds, v. 408-412, p. 999-1002, 2006.). The improvement of the separation, when complexants such as lactic acid (HLa) are used, is due to the buffering effect exerted by the acid in the aqueous phase, which avoids the decrease of pH in the aqueous phase and favors the extraction of REE (Yin et al., 2010YIN, S.; WU, W.; ZHANG, B.; ZHANG, F.; LUO, Y.; LI, S.; BIN, X. Study on separation technology of Pr and Nd in D2EHPA-HCl-LA coordination extraction system. Journal of Rare Earths, v. 28, p. 111-115, 2010.). The effect of lactic acid on the separation efficiency of light rare earth elements, praseodymium and lanthanum, was also observed by Gomes et al. (2017)GOMES R. C.; SERUFF L. A.; SCAL M. L. W.; VERA Y. M. The influence of lactic acid concentration on the separation of light rare earth elements by continuous liquid-liquid extraction with 2-ethylhexyl phosphonic acid mono-2-ethylhexyl ester. Journal Metallurgical and Materials Transactions B, v. 49, n. 1, p. 460-465, 2018..
The objective of this study was to evaluate the efficiency of gadolinium and europium separation by the solvent extraction technique when two modifications were made to the extraction system. The first modification was saponification of the organophosphorus acid P507 extractant and the second was conditioning of the feed with lactic acid. The two modifications were compared with the separation process not including extractant saponification or conditioning of the lactic acid feed. In addition, the McCabe-Thiele method was used to plot isotherms, to guide the extraction plant operation by providing important parameters such as number of stages and A/O ratio. Solvent extraction circuits were run with and without lactic acid.
2. Material and methods
2.1 Solvent extraction condition assays
Experimental factorial planning was applied with three levels and two variables; the total number of experiments being equal to 9 (32) plus 3 experiments at the central point, for a total of 12 extraction tests.
In the tests without modification, the two variables evaluated were the extractant concentration and pH of the feed. The organic extractant used was 2-ethylhexyl phosphonic acid 2-ethylhexyl ester, at concentrations of 0.28 mol L-1 (10% v/v), 0.43 mol L-1 (15% v/v) and 0.57 mol L-1 (20% v/v), in all cases diluted with isoparaffin.
The pH values evaluated were 2.5, 3.0 and 3.5. In the extraction assays with the saponified extractant, the two variables evaluated were the degree of extractant saponification and the feed pH. The values of the extractor saponification grades evaluated were 10%, 20% and 30% (with extractant concentration of approximately 0.43 mol L-1).
In the extraction tests with addition of lactic acid, the two variables evaluated were the lactic acid concentration and feed pH. The lactic acid concentrations investigated were 0.10, 0.15 and 0.20 mol L-1 (with extractant at 0.43 mol L-1). The response variables considered were the gadolinium extraction and the Gd/Eu separation factor.
For the extraction tests, a synthetic solution containing Gd and Eu was used with concentrations simulating these elements in real liquor from monazite leaching. The REE liquor was prepared from the digestion of the oxides of these elements with concentrated HCl (12 mol L-1). The concentrations of the two REE in the feed liquor expressed as oxide were 18.12 g L-1 Gd2O3 and 4.86 g L-1 Eu2O3.
Saponification of the extracts was performed by adding a suitable amount of a 10 mol L-1 NaOH solution to neutralize the desired amount of the extractant.
For extraction, equal volumes of the aqueous and organic phase (0.020 L) were placed in a capped flask and stirred for 30 min. Then the mixture was allowed to settle for 30 minutes. All the experiments were conducted at room temperature.
2.2 Extraction isotherms for extraction of gadolinium
To prepare the isotherms, extraction was done in the same way as in solvent extraction condition assays, but varying the ratio between the volumes of aqueous and organic phases (A/O) with and without lactic acid. The A/O ratios used were 0.083, 0.100, 0.125, 0.25, 0.50, 1, 2, 4, 8, 10 and 12. The extractant concentration was 0.43 mol L-1 (15% v/v) and pH of the feed solution was 3.0. All the experiments were conducted at room temperature.
2.3 Continuous counter-current extraction tests
Continuous extraction experiments using mixer-settler extractors were conducted. The volumes of the mixing and settling chambers were 242 and 372 mL, respectively.
Extraction without lactic acid had 15 stages, while with lactic acid (0.2 mol L-1) there were 10 stages. These conditions were defined in previous batch tests.
The organic and aqueous phases were the same used in the extraction isotherm experiments. All the experiments were conducted at room temperature and with feed solution at pH of 3.0. The aqueous and organic volumetric flow rates were respectively of 15 and 30 mL min-1. The residence time in each stage at A/O 1:2 was 10.2 minutes.
2.4 Rare earths elements' chemical analysis
The chemical analysis of europium in the aqueous solutions before and after extraction was performed by UV-Vis spectrometry and the absorbance was read at 394 nm. The total concentration of the REE was determined by complexometric titration using ethylenediamine tetraacetic acid (EDTA) as titrant and xylenol orange as indicator (Kinnunen and Wennerstrand, 1957KINNUNEN, J.; WENNERSTRAND, B. Some further applications of xylenol orange as an indicator in the EDTA titration. Chemist Analyst, v. 46, p. 92-93, 1957.). The gadolinium concentration was determined from the difference between the total concentration of REE and the concentration of europium.
3. Results and discussion
The effects (as indicated by Pareto diagrams with 95% confidence) and the response surfaces for Gd extraction and the Gd/Eu separation factor in the non-saponified P507 extraction tests, without addition of lactic acid, as a function of the extractant concentration and the feed pH, are depicted respectively in the Figures 1, 2, 3 and 4.
Pareto diagram of the effects of extractant concentration and feed pH on the extraction of Gd.
Pareto diagram of the effects of extractant concentration and feed pH on the Gd/Eu pair separation factor.
Response surface for the Gd/Eu pair separation factor as a function of extractant concentration and feed pH.
From the analysis, it can be stated that the concentration of the extractant and the feed pH had the greatest effects on the extraction of the heavier element, Gd (Figure 1). The positive influence of pH and concentration on the gadolinium extraction was confirmed by the surface response curve because it showed that the highest extractions of gadolinium were obtained with the highest pH value and extractant concentration (Figure 3). Regarding the separation factor, only the pH had a statistically significant effect, which one was positive (Figure 2). This fact is confirmed by the response surface curve because it shows that the largest separation factor was obtained at the highest pH value (Figure 4).
The increase in the pH and concentration of the extractant favored the extraction reaction, since by decreasing the concentration of H+ ions in the aqueous phase and increasing the concentration of extractant, the reaction moved towards the formation of a compound between the element and the extractant (see Equation 1). The increase in P507 concentration did not result in higher selectivity because no preferential extraction occurred. As such, only pH had a significant effect on selectivity.
The effects (indicated by Pareto diagrams with 95% confidence) and the response surfaces related to the extraction of Gd and the separation factor of the Gd/Eu in the extraction tests with saponified P507, as a function of the degree of saponification and pH of the feed, are shown respectively in the Figures 5, 6, 7 and 8.
Pareto diagram of the effects of the saponification degree and feed pH on the Gd/Eu pair separation factor.
Response surface for the extraction of Gd as a function of the saponification degree and feed pH.
Response surface for the Gd/Eu pair separation factor as a function of the saponification degree and feed pH.
It can be seen that the saponification of the extractant had the strongest positive influence on the extraction of Gd (Figure 5). This is also observed in the response surface curve, which shows that at the highest saponification (30%), the extraction values were high regardless of the initial pH value of the solution (Figure 7). In the case of the separation factor, the variable that had the greatest (positive) influence was pH. The saponification degree negatively influenced the Gd/Eu separation (Figure 6). The response surface curve confirms these results, since the best separations were obtained at higher pH and the increase in the saponification degree caused a decrease in selectivity (Figure 8).
The partial neutralization of the extractant and the increase of the initial pH cause a lesser reduction of the final pH, which increases the extraction of rare earth elements.
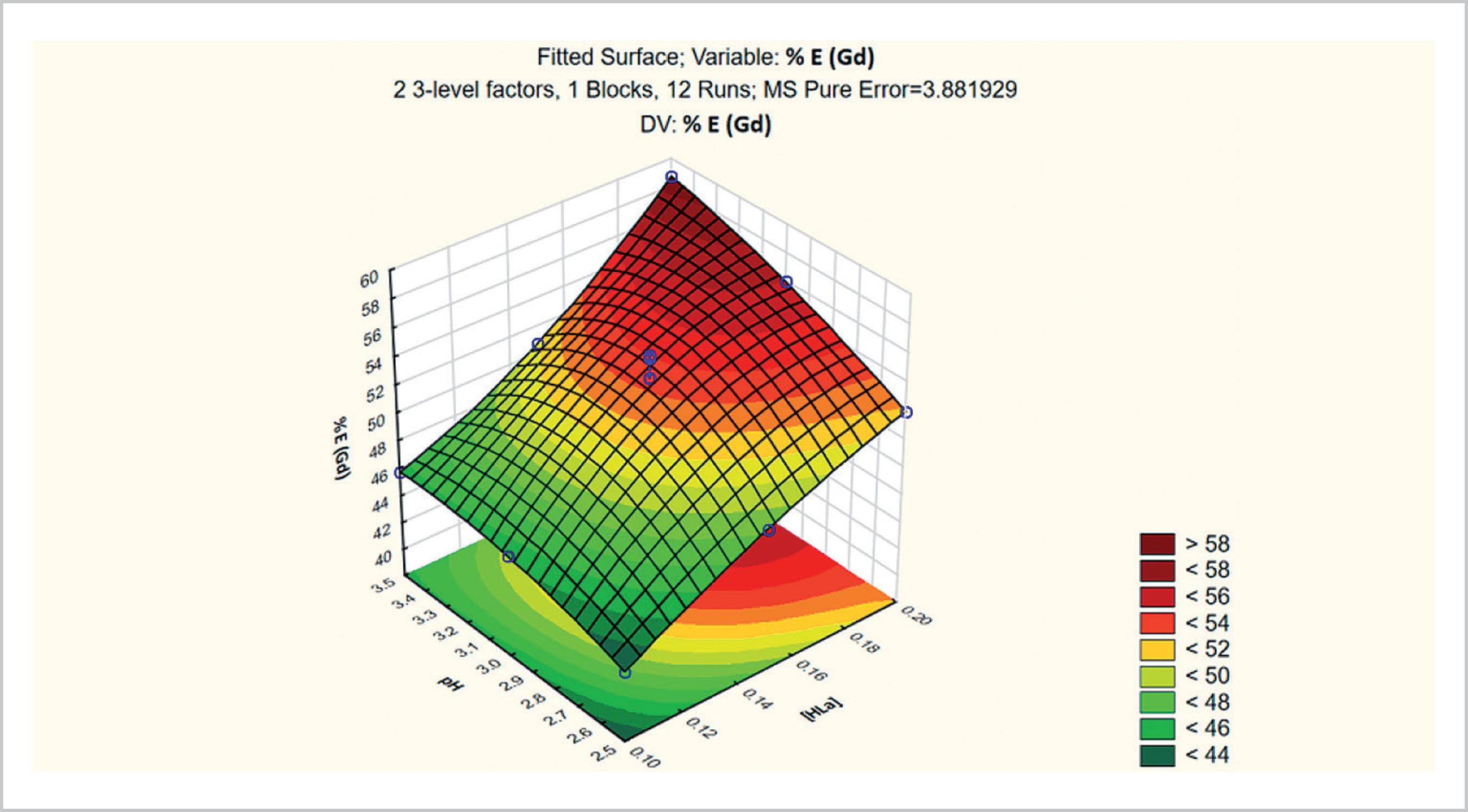
The effects (indicated by Pareto diagrams with 95% confidence) and the response surfaces for Gd extraction and the Gd/Eu separation factor in the P507 extraction tests with the addition of lactic acid to the aqueous phase, as a function of the lactic acid concentration and feed pH, are shown in respectively in the Figures 9, 10, 11 and 12.
Pareto diagram of the effects of lactic acid concentration and feed pH on the Gd/Eu pair separation factor.
Response surface for Gd extraction as a function of lactic acid concentration and feed pH.
Response surface for the Gd/Eu pair separation factor as a function of lactic acid concentration and feed pH.
As can be seen, the concentration of lactic acid had the strongest effects on the extraction of Gd (Figure 9). This fact is also shown in the response surface curve, which indicates that the highest extractions were obtained at the highest concentration of lactic acid (0.2 mol L-1) and at the highest pH (3.5) (Figure 11). The concentration of lactic acid was the variable that most positively influenced the Gd/Eu separation factor (Figure 10), which is in agreement with the response surface (Figure 12).
The lactic acid in the extraction system exerts a pH buffering role during extraction, reducing the pH drop and shifting the equilibrium to the right [1].
The test of normality of the residuals was carried out and in all the cases, the residuals had normal distribution.
Figures 13 and 14 show the comparison between the three tests in the working pH range. In these tests, the concentration of the extractant P507 was always the same, 0.43 mol L-1.
Both the saponification (30% saponification) and the addition of lactic acid (0.2 mol L-1), together with the increase in pH, improved the extraction of the heavier element (Gd) and also the separation of the Gd/Eu pair when compared to the extractant without saponification and without adding lactic acid. The best results were obtained when lactic acid was added, but at some points the results were statistically the same as those obtained in the tests where the saponified extractant was used. That is, the magnitudes of the effects obtained with saponification and with the addition of lactic acid were similar. The buffering effect of lactic acid in the aqueous phase, which avoids a drastic decrease in pH during extraction and partial neutralization of the saponified extractant, favored the separation of the elements in a similar manner at the levels analyzed.
Despite the better Eu/Gd separation at pH 3.5 (Figure 14), extraction isotherms were obtained at pH 3.0 due to precipitation of Gd and Eu lactate complexes at pH 3.5, which precipitated in very few hours, making it difficult to operate a continuous solvent extraction.
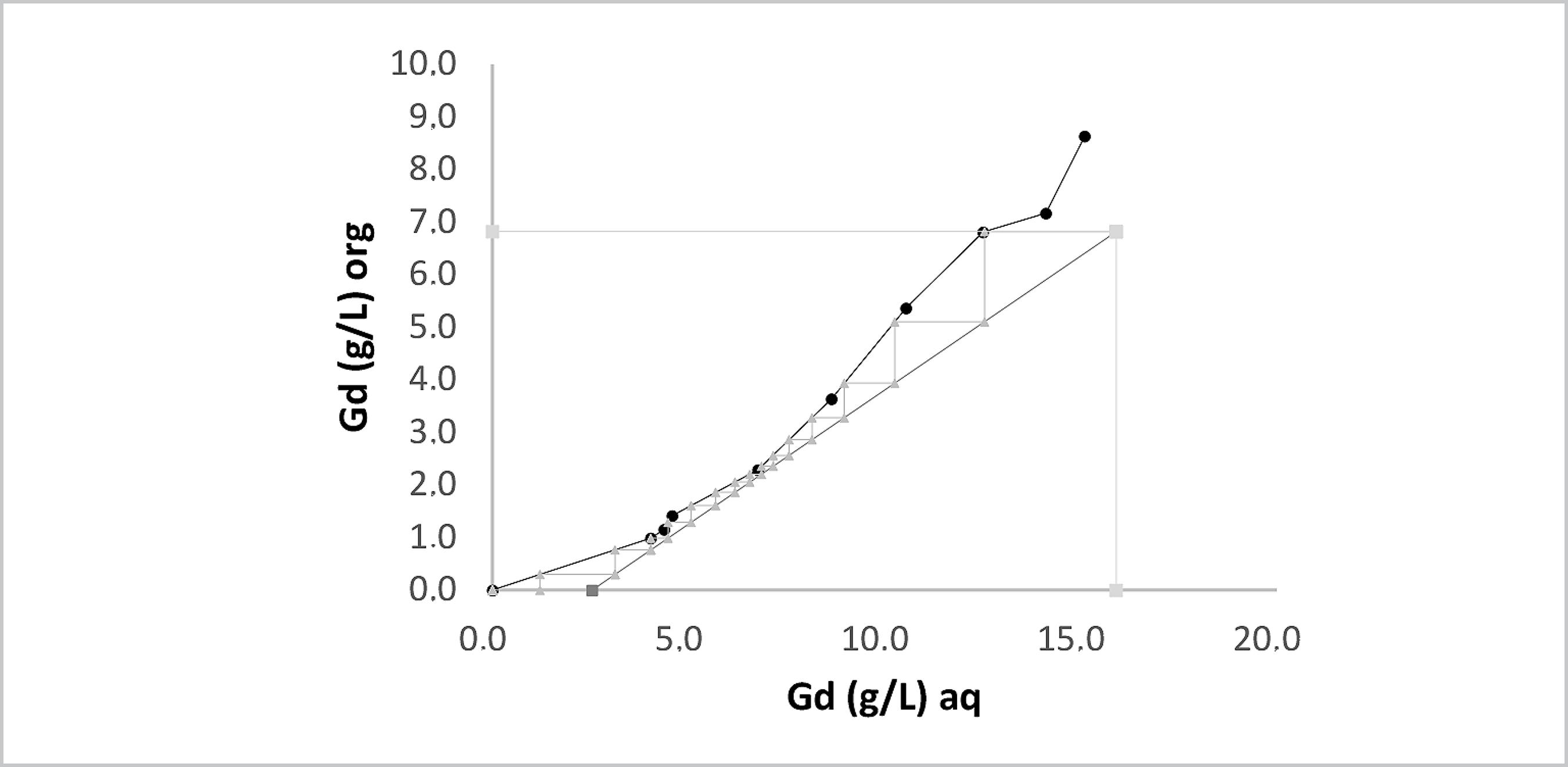
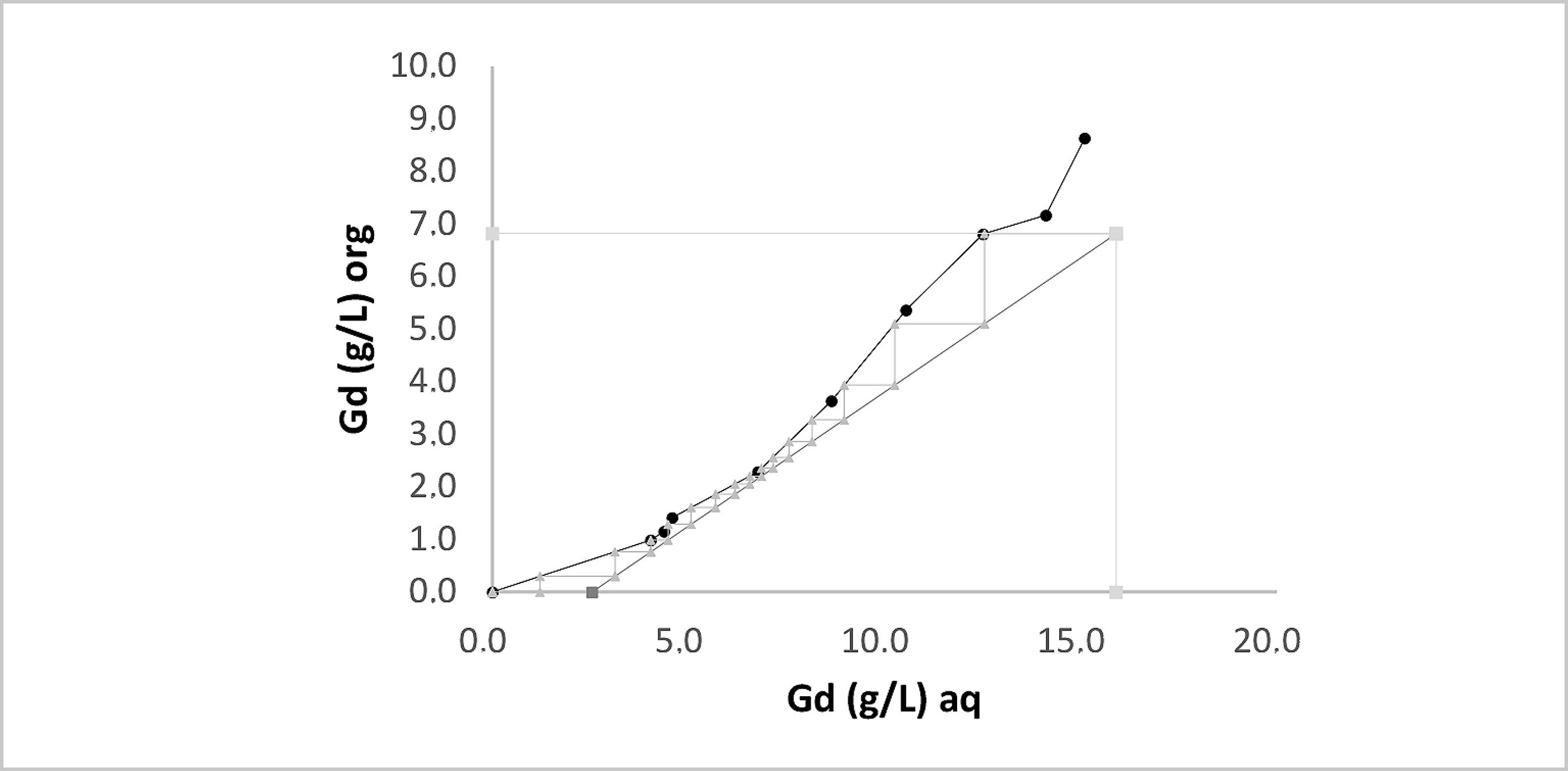
The extraction isotherms plotted using the McCabe-Thiele method are shown in Figures 15 and 16 for the heaviest element Gd. The difference between them is the use of lactic acid. The total molar concentration of rare earth elements in the feed solution was 0.13 mol L-1, the pH was 3.0, and the A/O ratio was 0.5 for both isotherms.
McCabe-Thiele plot for Gd extraction with P507 at 0.43 mol L-1 from Eu and Gd solution containing lactic acid 0.2 mol L-1 at pH 3.0.
McCabe-Thiele plot for Gd extraction with P507 at 0.43 mol L-1 from Eu and Gd solution at pH 3.0.
The McCabe-Thiele diagram for the counter-current circuit using 0.2 mol L-1 of lactic acid at pH 3.0 and A/O ratio of 1:2 from an aqueous solution of REE shows that ten stages are required to obtain raffinate with 0.16 g L-1 of Gd and 99% extraction percentage.
The McCabe-Thiele diagram for the counter-current circuit without lactic acid at pH 3.0 and A/O ratio of 1:2 from an aqueous solution of REE led to a 15-stage circuit, which provided raffinate with 2.55 g L-1 of Gd and extraction percentage of 84%. This demonstrates that the presence of lactic acid improved the extraction.
Figure 17 shows the extraction results for Gd and Eu in continuous circuits with and without lactic acid, using conditions defined before in McCabe-Thiele diagrams (Figures 15 and 16).
Accumulated extraction values of Gd and Eu per cell in the tests performed in the semi-pilot circuit with lactic acid (on the left) and without lactic acid (on the right).
It can be observed that with the use of lactic acid, higher extraction values were obtained for both gadolinium and europium than when lactic acid was not used. Europium and gadolinium extraction rates were 83.8 and 94.1%, respectively, with a 1:2 aqueous/organic volume flow rate and containing 0.2 mol L-1 of lactic acid. Moreover, raffinate was obtained containing 0.66 g L-1 of Eu and 0.96 g L-1 of Gd, and the organic load compositions were 1.8 g L-1 of Eu and 8.2 g L-1 of Gd. The gadolinium purity reached in the organic load was 81.7% and europium purity reached in the raffinate was 40.7%.
Furthermore, europium and gadolinium extraction rates were 46.1 and 73.0%, respectively, with 1:2 aqueous/organic volume flow rate and without lactic acid. The raffinate compositions were 2.2 g L-1 of Eu, and 4.4 g L-1 of Gd and organic load was obtained containing 1.0 g L-1 of Eu and 6.4 g L-1 of Gd. The gadolinium purity reached in the organic load was 86.3% and europium purity reached in the raffinate was 33.4%.
The extraction reaction without the addition of a pH buffering agent (lactic acid) did not produce extraction values as high as those obtained with lactic acid, even when operating with more cells (15 extraction cells).
4. Conclusion
It can be concluded that both saponifying the extractant and adding lactic acid to the aqueous feed not only increase the Gd extraction but also improve the separation of the Gd/Eu pair when using P507 as extractant.
Based on the McCabe-Thiele diagrams, a counter-current simulation study was defined to determine the number of theoretical stages for europium and gadolinium separation in the presence and absence of lactic acid. The results of this study indicated that lactic acid added in the aqueous feed at pH 3.0 greatly increased the extraction of Gd and reduced the number of stages.
The lactic acid addition in continuous liquid-liquid extraction provided greater extraction of Gd, with fewer stages. Lactic acid acted as a pH buffer in the aqueous solution, so the extraction reaction was favored, leading to high extraction percentage values. Despite higher Gd extraction when lactic acid was present in the system, the loaded organic phase obtained was impure. Future studies will be focused on the loaded organic scrubbing step in order to remove europium.
Acknowledgements
The authors thank the National Council for Scientific and Technological Development (CNPq) for the research grant.
References
- AL-MARZOOQI, F. A.; AL GHAFERI, A. A.; SAADAT, I.; HILAL, N. Application of Capacitive deionization in water desalination: a review. Desalination, v. 342, p. 3-15, 2014.
- FENG, Z.; HUANG, X.; LIU, H.; WANG, M.; LONG, Z.; YU, Y. Study on preparation and application of novel saponification agent for organic phase of rare earths extraction. Journal of Rare Earths, v. 30, n. 9, p. 903-908, 2012.
- GOMES R. C.; SERUFF L. A.; SCAL M. L. W.; VERA Y. M. The influence of lactic acid concentration on the separation of light rare earth elements by continuous liquid-liquid extraction with 2-ethylhexyl phosphonic acid mono-2-ethylhexyl ester. Journal Metallurgical and Materials Transactions B, v. 49, n. 1, p. 460-465, 2018.
- GUPTA, C.K.; KRISHNAMURTHY, N. Extractive metallurgy of Rare Earths 1st ed. Boca Raton, USA: CRC Press Taylor&Francis Group, 2004. cap. 2, p. 57-131.
- KINNUNEN, J.; WENNERSTRAND, B. Some further applications of xylenol orange as an indicator in the EDTA titration. Chemist Analyst, v. 46, p. 92-93, 1957.
- LAPIDO-LOUREIRO, F. E. Terras-Raras: Tipos de Depósitos, Recursos Identificados e Alvos Prospectivos no Brasil. In: SEMINÁRIO BRASILEIRO DE TERRAS-RARAS, 1., 2011, Rio de Janeiro. Bases para o desenvolvimento de Terras raras no Brasil. Rio de Janeiro: Cetem - Centro de Tecnologia Mineral, 2011.
- LEE M.S.; LEE J.Y.; KIMB J.S.; LEE G.S. Solvent extraction of neodymium ions from hydrochloric acid solution using PC88A and saponified PC88A. Separation and Purification Technology, v. 46, p. 72-78, 2005.
- RITCEY, G. M. Solvent extraction: principles and application to process metallurgy. 2nd ed. Ottawa, Canada: G.M. Ritcey and Associates Incorporates, 2006. v. 2., p. 423-452.
- SUN, X.; WANG, Y.; LI, D. Selective separation of yttrium by CA-100 in the presence of a complexing agent. Journal of Alloys and Compounds, v. 408-412, p. 999-1002, 2006.
- YIN, S.; WU, W.; ZHANG, B.; ZHANG, F.; LUO, Y.; LI, S.; BIN, X. Study on separation technology of Pr and Nd in D2EHPA-HCl-LA coordination extraction system. Journal of Rare Earths, v. 28, p. 111-115, 2010.
Publication Dates
-
Publication in this collection
20 Dec 2019 -
Date of issue
Jan-Mar 2020
History
-
Received
17 Apr 2019 -
Accepted
12 Aug 2019