ABSTRACT
BACKGROUND AND OBJECTIVES:
Inflammation is a defense response of the body to a cellular damage caused by physical, chemical or biological agents, which triggers, among other factors, pain. Although inflammation plays an important role in the protection and regeneration of tissue injury, inflammatory pain results in decreased quality of life. In view of this, the development of safe and less invasive forms for the treatment of inflammatory pain is of great importance. The objective of this study was to evaluate the antihyperalgesic potential of the culture supernatant of keratinocytes and human fibroblasts in an experimental model of inflammatory hyperalgesia.
METHODS:
Evaluation of carrageenan induced inflammatory hyperalgesia through the use of electronic von Frey in animal models treated with culture supernatant of keratinocytes and fibroblasts.
RESULTS:
Local administration of naloxone, a nonselective opioid antagonist, in peripheral tissue, has been observed to inhibit the antihyperalgesic effect of the keratinocyte culture supernatant. Fibroblast culture supernatant on days 1 and 3 reverses for 2 hours the carrageenan induced inflammatory hyperalgesia, which is mediated by µ opioid agonist.
CONCLUSION:
This study indicates that culture supernatant of fibroblasts and keratinocytes is capable of inducing antinociception in inflammatory hyperalgesia, mediated by the release of endogenous opioids. In addition, it has been observed that the analgesic effect of the fibroblast culture supernatant is mediated specifically by the µ opioid agonist, having a duration of 2 hours.
Keywords:
Analgesia; Fibroblasts; Keratinocytes; Peripheral nervous system; Skin
RESUMO
JUSTIFICATIVA E OBJETIVOS:
A inflamação é uma resposta de defesa do organismo a uma lesão celular causada por agentes físicos, químicos ou biológicos, a qual desencadeia, entre outros fatores, a dor. Apesar da inflamação possuir um importante papel na proteção e regeneração da lesão tecidual, a dor inflamatória culmina na diminuição da qualidade de vida. Diante disso, é de grande importância o desenvolvimento de formas seguras e menos invasivas para o tratamento da dor inflamatória. O objetivo deste estudo foi avaliar o potencial anti-hiperalgésico do sobrenadante de cultura de queratinócitos e fibroblastos humanos em modelo experimental de hiperalgesia inflamatória.
MÉTODOS:
Avaliação da hiperalgesia inflamatória induzida por carragenina através do uso de von Frey eletrônico em modelos animais tratados com sobrenadante de cultura de queratinócitos e fibroblastos.
RESULTADOS:
Observou-se que a administração local de naloxona, antagonista opioide não seletivo, em tecido periférico inibiu o efeito anti-hiperalgésico do sobrenadante da cultura de queratinócitos. Sobrenadante de cultura de fibroblastos dos dias 1 e 3 reverte por 2h a hiperalgesia inflamatória induzida por carragenina, sendo esta mediada por agonista µ opioide.
CONCLUSÃO:
Este estudo indicou que sobrenadante de cultura de fibroblastos e queratinócitos foi capaz de induzir antinocicepção em hiperalgesia inflamatória, mediada pela liberação de opioides endógenos. Além disso, foi observado que o efeito analgésico do sobrenadante de cultura de fibroblastos é mediado especificamente por agonista µ opioide, tendo uma duração de 2 horas.
Descritores:
Analgesia; Fibroblastos; Pele; Queratinócitos; Sistema nervoso periférico
INTRODUCTION
Inflammation is a defense response to cellular damage caused by physical, chemical or biological agents(11 Abbas AK, Janeway CA Jr. Immunology: improving on nature in the twenty-first century. Cell. 2000;100(1):129-38.). Inflammatory process is characterized by a series of interrelated events that seek to recover tissue integrity, among which are: increased blood flow to the affected region, increased vascular permeability, fluid leakage, migration and accumulation of defense cells(22 Dawes JM, Anderson DA, Bennett DL, Bevan S, McMahon SB. Inflammatory mediators and modulators of pain. Wall and Melzack's Textbook of Pain. 2013;6:48-67.). The resulting characteristic signs of this process, the cardinal signs, are: pain, tumor, redness, loss of function and heat(11 Abbas AK, Janeway CA Jr. Immunology: improving on nature in the twenty-first century. Cell. 2000;100(1):129-38.
2 Dawes JM, Anderson DA, Bennett DL, Bevan S, McMahon SB. Inflammatory mediators and modulators of pain. Wall and Melzack's Textbook of Pain. 2013;6:48-67.-33 Levine JD, Taiwo Y. Inflammatory Pain. In: Wall PD, Melzack R, Bonica JJ. Textbook of Pain. 3rd ed. Edinburgh, Scothand, Churchill Livingstone; 1994. 45-56p.).
Although it’s an important mechanism for warning and protection of a possible tissue injury, pain creates suffering, which leads to a decreased quality of life(44 Carvalho MMMJ. O Sofrimento da dor em câncer. In: Carvalho MMMJ. Introdução à psiconcologia.1ª ed. São Paulo; 2003. 103-18p.). It’s known that perception of pain is triggered by the activation of specialized cells, called nociceptors(55 Messlinger K. What is a nociceptor? Anaesthesist. 1997;46(2):142-53.). The nerve endings that detect this type of stimulus are the Aδ and C fibers. While the Aδ fibers are myelinized, with rapid transmission of the painful stimulus and therefore responsible for the acute phase of pain, the non-myelinized, slow-conducting C fibers have a greater role in inflammatory and chronic pain(66 Besson JM. The complexity of physiopharmacologic aspects of pain. Drugs. 1997;53(Suppl.2):1-9.,77 Webster KE. Somaesthetic pathways. Br Med Bull. 1977;33(2):113-20.).
During the inflammatory process, algogenic substances are released, which are able to sensitize the nociceptors, reducing the excitability threshold. Among them, the following can be mentioned: acetylcholine, bradycinine, histamine, serotonin, leukotriene, substance P, among others(88 Woolf CJ. Recent advances in the pathophysiology of acute pain. Br J Anaesth. 1989;63(2):139-46.). The persistence of inflammation leads to changes in the peripheral nervous system, where there will be an exacerbation of the response to painful stimuli, known as hyperalgesia(99 Stein C, Pflüger M, Yassouridis A, Hoelzl J, Lehrberger K, Welte C, et al. No tolerance to peripheral morphine analgesia in presence of opioid expression in inflamed synovia. J Clin Invest. 1996;98(3):793-9.).
Furthermore, endogenous mechanisms that counterbalance the changes caused by inflammation triggered by the initial lesion are released in order to regulate it. A characteristic example is the release of endogenous opioids and peptides derived from protein precursors synthesized by synovial cells, mast cells, lymphocytes, neutrophils, monocytes and skin cells, such as keratinocytes and fibroblasts that migrated to the lesion sites(99 Stein C, Pflüger M, Yassouridis A, Hoelzl J, Lehrberger K, Welte C, et al. No tolerance to peripheral morphine analgesia in presence of opioid expression in inflamed synovia. J Clin Invest. 1996;98(3):793-9.
10 Stein C, Gramsch C, Herz A. Intrinsic mechanisms of antinociception in inflammation: local opioid receptors and beta-endorphin. J Neurosci. 1990;10(4):1292-8.
11 Garcia JB, Cardoso MG, Dos-Santos MC. Opioids and the immune system: clinical relevance. Rev Bras Anestesiol. 2012;62(5):709-18.-1212 Slominski AT, Zmijewski MA, Skobowiat C, Zbytek B, Slominski RM, Steketee JD. Sensing the environment: regulation of local and global homeostasis by the skin's neuroendocrine system. Adv Anat Embryol Cell Biol. 2012;212:v, vii, 1-155.). Another example is the increased expression of receptors in peripheral sensory neurons and the rupture of the perineural barrier, facilitating the interaction of opioids and their receptors(1313 Vetter I, Kapitzke D, Hermanussen S, Monteith GR, Cabot PJ. The effects of pH on beta-endorphin and morphine inhibition of calcium transients in dorsal root ganglion neurons. J Pain. 2006;7(7):488-99.).
There are three families of endogenous opioids described in the literature: endorphins (derived from proopiomelanocortin - POMC), enkephalins (derived from proenkephalin - PENK) and dynorphins (derived from prodynorphin)(1414 Hollt V. Opioid peptide processing and receptor selectivity. Annu Rev Pharmacol Toxicol. 1986;26(1):59-77.). Each one presents specificity with different opioid receptors: µ (endorphin and enkephalin), δ (enkephalin and endorphin) and κ (dynorphin)(1212 Slominski AT, Zmijewski MA, Skobowiat C, Zbytek B, Slominski RM, Steketee JD. Sensing the environment: regulation of local and global homeostasis by the skin's neuroendocrine system. Adv Anat Embryol Cell Biol. 2012;212:v, vii, 1-155.,1515 Nakanishi S, Inoue A, Kita T, Nakamura M, Chang AC, Cohen SN, et al. Nucleotide sequence of cloned cDNA for bovine corticotropin-beta-lipotropin precursor. Nature. 1979;278(5703):423-7.).
Production of endogenous opioids is known to be performed by cells of the immune system(1111 Garcia JB, Cardoso MG, Dos-Santos MC. Opioids and the immune system: clinical relevance. Rev Bras Anestesiol. 2012;62(5):709-18.,1616 Sibinga NE, Goldstein A. Opioids peptides and opioid receptors in cell of the immune system. Annu Rev Immunol. 1988;6:219-49.). However, studies demonstrate their production also in keratinocytes and fibroblasts(1717 Wintzen M, Yaar M, Avila E, Vermeer BJ, Gilchrest BA. Keratinocytes produce b-endorphin and b-lipotropic hormone after stimulation by UV, IL-1a or phorbol esters. J Invest Dermatol. 1995;104:641.https://www.scopus.com/record/display.uri?eid=2-s2.0-0000723419&origin=inward&txGid=7b951b39c5d52e89782d718c1d65b2be.
https://www.scopus.com/record/display.ur...
18 Schauer E, Trautinger F, Köck A, Schwarz A, Bhardwaj R, Simon M, et al. Proopiomelanocortin-derived peptides are synthesized and released by human keratinocytes. J Clin Invest. 1994;93(5):2258-62.
19 Bigliardi PL, Bigliardi-Qi M, Buechner S, Rufli T. Expression of mµ-opiate receptor in human epidermis and keratinocytes. J Invest Dermatol. 1998;111(2):297-301.
20 Lo HH, Tseng LF, Wei E, Li CH. Endorphin is a potent analgesic agent. Proc Natl Acad Sci. USA. 1976;7(8):2895-8.
21 Slominski AT, Zmijewski MA, Zbytek B, Brozyna AA, Granese J, Pisarchik A, et al. Regulated proenkephalin expression in human skin and cultured skin cells. J Invest Dermatol. 2011;131(3):613-22.-2222 Bigliardi-Qi M, Sumanovski LT, Büchner S, Rufli T, Bigliardi PL. Mu-opiate receptor and beta-endorphin expression in nerve endings and keratinocytes in human skin. Dermatology. 2004;209(3):183-9.). Authors have observed that fibroblasts and keratinocytes express functional proenkephalin messenger (PENK) RNA, being able to synthesize and secrete PENK-derived peptides, such as enkephalins(2121 Slominski AT, Zmijewski MA, Zbytek B, Brozyna AA, Granese J, Pisarchik A, et al. Regulated proenkephalin expression in human skin and cultured skin cells. J Invest Dermatol. 2011;131(3):613-22.). The release of inflammatory factors, especially interleukin 1β (IL-1β), induces the expression and release of endogenous opioids, which, once attached to the receptors of peripheral nerve fibers, trigger an increase in potassium currents and a decrease in calcium currents in the bodies of sensory neurons, inhibiting neuronal triggering and transmitter release(2323 Schaible HG. Pathophysiology of pain. Orthopade. 2006;36(1):8-16.,2424 Schaible HG. Peripheral and central mechanisms of pain generation. Hand Exp Pharmacol. 2007;(177):3-28.).
Considering that inflammatory hyperalgesia triggers negative emotional and physical responses compromising quality of life(33 Levine JD, Taiwo Y. Inflammatory Pain. In: Wall PD, Melzack R, Bonica JJ. Textbook of Pain. 3rd ed. Edinburgh, Scothand, Churchill Livingstone; 1994. 45-56p.), the development of safe forms of treatment is very important. Exogenous opioids are still the most used options in the treatment of different algetic stimuli(2525 Siderov J, Zalcberg JR. Prescribing opioids--a painful experience. Med J Aust. 1994;161(9):515-6.). However, important side effects associated with these pharmacological options remain a major disadvantage to their use(2626 Benyamin R, Trescot AM, Datta S, Buenaventura R, Adlaka R, Sehgal N, et al. Opioid complications and side effects. Pain Physician. 2008;11(2 Suppl):S105-20.
27 Chou R, Turner JA, Devine EB, Hansen RN, Sullivan SD, Blazina I, et al. The effectiveness and risks of long-term opioid therapy for chronic pain: a systematic review for a National Institutes of Health Pathways to Prevention Workshop. Ann Intern Med. 2015;162(14):276-86.-2828 Eriksen J, Sjogren P, Bruera E, Ekholm O, Rasmussen NK. Critical issues on opioids in chronic non-cancer pain: an epidemiological study. Pain. 2006;125(1-2):172-9.). The direct relationship between the peripheral nervous system, keratinocytes and fibroblasts is highlighted as a promising fact in the search for different safe forms of analgesia, especially because it’s a local action. Thus, the present study evaluated the antihyperalgesic potential of human keratinocyte and fibroblast culture supernatant in an experimental model of inflammatory hyperalgesia.
METHODS
72 male Wistar rats from 6 to 8 weeks of age (200-250g) from the Multidisciplinary Center for Biological Research in the Field of Laboratory Animal Science - CEMIB were used. The animals were put in appropriate cages containing 4 animals/box, remaining in ventilated shelves with controlled temperature and humidity 22ºC and 55%, respectively, with a 12h light/dark cycle. The animals received water and feed Ad libitum throughout the research. The animals were randomly divided into groups. After the study, the animals were anesthetized and then euthanized by decapitation. The size of group (n) for each experimental group is presented below:
Processing of biological material (skin)
The fragments of human skin from blepharoplasties of healthy individuals performed by the team of Ophthalmologic Plastics of the University Hospital were sent to the Skin Cell Culture Laboratory under protocol number 16013-2/2016 and processed in a sterile area, clean room ISO 7 class, the fat tissue was discarded. The fragments were placed on a Petri dish and divided into two portions, one of which was frozen in a -80ºC freezer. The other portion was sectioned into 2 to 3 mm fragments using a surgical instrument under laminar flow in order to keep the entire procedure sterile. The smaller skin fragments were submitted to enzymatic treatment with 10 mL of 0.25% trypsin solution and 1 mM of ethylenediaminetetraacetic acid (with the epidermis always facing up) and incubated at 37ºC with a 5% tension of CO2 for four hours, resulting in separation of the epidermis from the dermis.
Isolation and culture of fibroblasts
After incubation of the fragments, trypsin was inactivated with the same volume of culture medium and the dermis fragments were placed in a culture vial with M199 fibroblast medium supplemented with L-glutamine 2mM, penicillin 100UI/mL, streptomycin 0.1mg/mL and 10% bovine fetal serum (BFS). The cells were nourished by changing the culture medium every three days. When these cells obtained a 90% confluence, approximately 7 days after the culture started, the cellular replication was performed with the help of a 0.25% trypsin solution and 1mM ethylenediaminetetraacetic acid. The cells were used between 2-3th passages. At the time, the obtained fibroblasts were washed three times with Hank’s solution.
Isolation and culture of keratinocytes
After the epidermis was separated, trypsin was neutralized using the same volume of specific culture medium for keratinocytes. The cell suspension obtained was filtered through a 40µm nylon filter and centrifuged at 1200rpm and 4ºC for 10 minutes. The cell pool obtained, consisting of keratinocytes and melanocytes, was counted and plated in culture flasks, with 1x105 cells per cm(22 Dawes JM, Anderson DA, Bennett DL, Bevan S, McMahon SB. Inflammatory mediators and modulators of pain. Wall and Melzack's Textbook of Pain. 2013;6:48-67.), incubated at 37ºC, with 5% tension of CO2, in keratinocyte specific culture medium, complemented with L-glutamine 2mM/mL, penicillin 100UI/mL and streptomycin 0.1mg/mL. The primary keratinocytes culture was obtained from the adhesion of the cells to the culture flasks which occurred in approximately 48h. The cells were nourished by changing the culture medium every three days. The cellular replication was performed in approximately 7 days when the cells reached 90% of confluence and were used in the second passage.
Drugs
Carrageenan at 100µg/50µL/paw (Sigma) was used for induction of inflammatory hyperalgesia. Administration of carrageenan (100µg/50uL) in the subcutaneous tissue of the rear leg induces inflammatory hyperalgesia for 6 hours, whose pain peak occurs in the third hour(3131 Henriques MG, Silva PM, Martins MA, Flores CA, Cunha FQ, Assreuy-Filho J, et al. Mouse paw edema. A new model for inflammation? Braz J Med Biol Res. 1987;20(2):243-9.).
The selective opioid receptors antagonists used were: CTOP (Sigma Aldrich/ P5296, subunit µ antagonist), Nor- BNI (Norbinaltorphimine/Sigma Aldrich; subunit kappa antagonist) and N115 (Naltrindole/ HCL - Sigma Aldrich; subunit delta antagonist). The non-selective opioid receptor antagonist used was naloxone (nalo, Sigma yperal).
Intraplantar injection of drugs
The administration of drugs through the intraplantar route (i.p.l.) was performed using a hypodermic BD Ultra-Fine® needle (29G) which was inserted after antisepsis into the subcutaneous tissue of the plantar surface of the right posterior limb.
Behavioral test (von Frey electronic test)
The evaluation of mechanical hyperalgesia in the paw of rats was performed through electronic von Frey in basal conditions and after the stimulus in the paw. In this method the electronic anesthesiometer was used, consisting of a pressure transducer connected to a cable and to a digital force detector in which the force exerted was expressed in grams. At the end of the transducer there is a tip through which a force in a straight angle was applied in the central region of the animal’s rear leg with gradually increasing pressure. The stimulus was interrupted after the feature observation of paw removal (flinches).
For the experiment, 6 acrylic boxes measuring 12x20x17cm were used, the flooring was composed of an iron mesh net, in which there was, 25cm below the experimental boxes, an inclined mirror used for visualization of the lower part of the paw, facilitating the application of the tip. Before the experiment, the animals were kept for 15 minutes inside the boxes for adaptation. Three measurements per animal were performed, the final value was the average of the measurements. The intensity of mechanical hypernociception was measured as the D reaction pressure variation in grams, obtained by subtracting the value observed before the experimental procedure (basal) from the reaction value after the administration of the inflammatory stimulus. The test sessions were performed during the clear phase, between 9:00 a.m. and 5:00 p.m., in a quiet room, with room temperature maintained at 23ºC.
Evaluation of the hyperalgesic effect of the keratinocyte culture supernatant
In a first phase, carrageenan (100µg/50uL/paw) was administered via i.p.l., which induced inflammatory hyperalgesia for the 6 hours of evaluation. After two hours of administration of the hyperalgesic agent, a non-selective opioid antagonist (naloxone) was administered at the same site. After 2h30min, 50µL of keratinocyte culture supernatant (treated groups, n=6) of 3 days or 50µL of keratinocyte culture medium (control group, n=6) were administered at the same site. After 30 minutes of administration of the supernatant, mechanical hyperalgesia was evaluated using the von Frey test (Results item 1).
Evaluation of the time-response curve of the different days (1 or 3 days) of keratinocytes or fibroblasts supernatant culture against inflammatory yperalgesia
The electronic test of mechanical hyperalgesia (von Frey) was performed prior to the study. After 1h, carrageenan (100µg/50µL/paw) was administered via i.p.l. of the right posterior paw. After 2h of the administration of the hyperalgesic agent, in the same place, 50µL of supernatant of the keratinocyte culture (treated groups, n=6) of different days (1 and 3 days) or 50µL of culture medium for keratinocytes (control group, n=6) was administered in the periods already described. To establish the time-response curve, after 0.5, 1, 2, 4 and 6 hours of administration of the supernatant, mechanical hyperalgesia was evaluated using the von Frey test (Results item 2).
Participation of opioid receptors and their subunits in the antihyperalgesic effect of the supernatant from keratinocyte culture against inflammatory yperalgesia
The electronic test of mechanical hyperalgesia (von Frey) was performed prior to the study. After 1h, carrageenan (100µg/50µL/paw) was administered via i.p.l. of the right posterior paw. After 2h of the administration of the hyperalgesic agent, selective antagonists from the mµ (CTOP, 20µg/50µL/paw), kappa (Nor-BNI, 10µg/50 20µg/paw) and delta (N115, 3µg/50µL/paw) opioid sub-units were administered via the intraplantar route of the right posterior paw. After 2.5 hours of carrageenan administration, the supernatant of the keratinocyte culture (treated group) was administered (n=6/group). After 30 minutes of administration of the supernatant, mechanical hyperalgesia was evaluated using the von Frey test (Results item 3).
The experiments followed the guidelines of the Ethics Committee for Animal Research of the University, under protocol number 4654-1/2017, and the standards established by the International Association for the Study of Pain (IASP).
Statistical analysis
The results were expressed as mean±standard mean error (s.m.e.). The data analysis was performed by the ANOVA One-way or Two-way Variance analysis test, followed by the Bonferroni test for multiple comparisons. The significance level was from p<0.05.
RESULTS
1. Local administration of naloxone to the peripheral tissue inhibits the antihyperalgesic effect of the supernatant from keratinocyte culture
The administration of carrageenan (100µg/50µL/paw) i.p.l. induced inflammatory hyperalgesia for the 6 hours of evaluation. After two hours of administration of the hyperalgesic agent, a non-selective opioid antagonist (naloxone) was administered at the same site. After 2.50 hours, 50µL of keratinocyte culture supernatant (treated groups, n=6) of 3 days or 50µL of keratinocyte culture medium (C.M., 50µL) (control group, n=6) were administered at the same site.
As shown in figure 1, supernatant of keratinocyte culture (C.M., 50µL) reversed carrageenan induced mechanical hyperalgesia. The analgesic effect of C.M. was reversed by local pre-treatment with naloxone (Nalo, 10µg), a non-selective opioid receptor antagonist. Administration of C.M. (50µL) in the contralateral paw did not alter the hyperalgesic threshold of the carrageenan, showing that the effect of C.M. has a local and non-systemic effect. Administration of pure culture medium (P.M.) does not alter the hyperalgesic threshold of carrageenan.
3-day keratinocyte culture medium reverses carrageenan induced mechanical hyperalgesia
3-day keratinocyte culture supernatant. C.M = medium for keratinocyte culture; P.M. = pure medium for keratinocyte culture; NaCl = 0.9% sodium chloride; Nalo = naloxone; Cg = carrageenan.
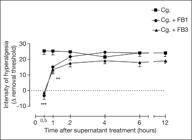
2. Supernatant day 1 and 3 fibroblast culture temporarily reverses carrageenan induced inflammatory hiperalgesia
Since the culture of keratinocytes for clinical use has a 3:1 ratio (keratinocytes: fibroblast), the possibility of the culture alone having the same analgesic effect was analyzed. The supernatant of isolated fibroblast culture (FB1 and FB3, 50µL) on days 1 and 3 reversed carrageenan induced hyperalgesia (Cg.) at a time of 0.5 and 1h, as shown in figure 2. However, this effect was temporary, with a resumption of hyperalgesia after 2h. The administration of FB1 and FB3 (50µL) in the contralateral paw did not change the hyperalgesic threshold of carrageenan, showing that the effect of FB1 and FB2 is local and non-systemic. Administration of pure culture medium (M199) does not change the hyperalgesic threshold of carrageenan.
Local administration of fibroblast culture supernatant in peripheral tissue temporarily reduces carrageenan induced mechanical hyperalgesia
Cg = carrageenan; FB1 = 1-day fibroblast culture supernatant; FB3 = 3-day fibroblast culture supernatant.
Comparing the time-response curve of BF1 and BF3 for 12h, there is no difference in time and duration of hyperalgesia reduction (Cg. + BF1 and Cg. + BF3) between 1 or 3 day supernatant of isolated culture of fibroblasts.
3. The antihyperalgesic effect of the supernatant from the fibroblast culture is mediated by the µ opioid receptor
Since the analgesic effect of the supernatant from the fibroblast culture is measured by opioid receptors, which opioid receptor is specifically involved in this analgesic effect was analyzed. Selective antagonists from the µ opioid (CTOP, 20µg/50µL), kappa (Nor-BNI, 10µg/50µL) and delta (N115, 3µg/50µL) sub-units were administered through an intraplantar way 30 minutes before the administration of the fibroblast culture supernatant. As shown in Figure 3, carrageenan induced hyperalgesia, which was reversed by FB1 (Two-way ANOVA, Bonferroni test, ***p<0.001). CTOP, but not Nor-BNI or N115, inhibited the antihyperalgesic effect of fibroblast culture supernatant on carrageenan induced hyperalgesia (Two-way ANOVA, Bonferroni test, p>0.05).
Opioid µ subunit antagonist inhibits the supernatant analgesic effect of fibroblast culture
Cg = carrageenan; FB1 = 1-day fibroblast culture supernatant; CTOP = µ subunit antagonist; Nor-BNI = kappa subunit antagonist; N115 = delta subunit antagonist.
DISCUSSION
Studies have shown that besides being synthesized by immune cells, other cells such as keratinocytes and fibroblasts also play an important role in endogenous peripheral antinociception, since such cells express functional PENK mRNA, being able to synthesize and secrete PENK-derived peptides such as enkephalins. The release of inflammatory factors, notably IL-1b, induces the expression and release of opioids by these cells, which, once attached to the receptors of peripheral nerve fibers, inhibit neuronal triggering and transmitter release(1212 Slominski AT, Zmijewski MA, Skobowiat C, Zbytek B, Slominski RM, Steketee JD. Sensing the environment: regulation of local and global homeostasis by the skin's neuroendocrine system. Adv Anat Embryol Cell Biol. 2012;212:v, vii, 1-155.
13 Vetter I, Kapitzke D, Hermanussen S, Monteith GR, Cabot PJ. The effects of pH on beta-endorphin and morphine inhibition of calcium transients in dorsal root ganglion neurons. J Pain. 2006;7(7):488-99.
14 Hollt V. Opioid peptide processing and receptor selectivity. Annu Rev Pharmacol Toxicol. 1986;26(1):59-77.
15 Nakanishi S, Inoue A, Kita T, Nakamura M, Chang AC, Cohen SN, et al. Nucleotide sequence of cloned cDNA for bovine corticotropin-beta-lipotropin precursor. Nature. 1979;278(5703):423-7.-1616 Sibinga NE, Goldstein A. Opioids peptides and opioid receptors in cell of the immune system. Annu Rev Immunol. 1988;6:219-49.).
The present study demonstrated that supernatant from keratinocyte and fibroblast culture promotes analgesia during inflammatory pain induced by carrageenan in a model of mechanical hyperalgesia, corroborating previous studies(1616 Sibinga NE, Goldstein A. Opioids peptides and opioid receptors in cell of the immune system. Annu Rev Immunol. 1988;6:219-49.
17 Wintzen M, Yaar M, Avila E, Vermeer BJ, Gilchrest BA. Keratinocytes produce b-endorphin and b-lipotropic hormone after stimulation by UV, IL-1a or phorbol esters. J Invest Dermatol. 1995;104:641.https://www.scopus.com/record/display.uri?eid=2-s2.0-0000723419&origin=inward&txGid=7b951b39c5d52e89782d718c1d65b2be.
https://www.scopus.com/record/display.ur...
-1818 Schauer E, Trautinger F, Köck A, Schwarz A, Bhardwaj R, Simon M, et al. Proopiomelanocortin-derived peptides are synthesized and released by human keratinocytes. J Clin Invest. 1994;93(5):2258-62.,3232 Bodnar RJ. Endogenous opiates and behavior: 2017. Peptides. 2020;124:170223.
33 Leong C, Neumann C, Ramasamy S, Rout B, Wee LY, Bigliardi-Qi M, et al. Investigating endogenous µ-opioid receptors in human keratinocytes as pharmacological targets using novel fluorescent ligand. PloS one. 2017;12(12):e0188607.-3434 Li X, Zhu J, Tao Y, Tao K. Elevated endogenous opioids in obstructive jaundice: the possible skin mechanisms. Med Hypotheses. 2018;116:119-21.). The data obtained suggest that the opioid receptor seems to be involved in the analgesic effect of keratinocyte culture. The administration of a non-selective opioid antagonist (naloxone) inhibited the antihyperalgesic effect of the supernatant from the 3-day keratinocyte culture in a model of inflammatory hyperalgesia, demonstrating that the effect of the supernatant from the keratinocyte culture is mediated by the release of opioids (Figure 1).
Additionally, the results demonstrate that the inflammatory hyperalgesia induced by carrageenan was totally reversed when applied, 30 minutes before its peak of action, supernatant of 1 and 3-day fibroblasts culture. The total reversion occurred 30 minutes after the application of the fibroblast culture, and its reducing effect of hyperalgesia was maintained until one hour later, when it was re-established. After 2h of fibroblast culture application, no significant difference was observed between the groups treated with fibroblast culture and the control group in the reversion of carrageenan induced hyperalgesia.
In order to verify the participation of opioid receptors and their sub-units in the analgesia verified by the fibroblast culture supernatant, selective antagonists of the mu opioid sub-units (CTOP, 20ug/50uL/paw), kappa (Nor-BNI, 10ug/50uL/paw) and delta (N115.3ug/50uL/paw) were administered intraplantarly. The present results demonstrated a significant difference between the groups that received selective antagonists from the kappa and delta sub-units before treatment with supernatant from the fibroblast culture and the group treated only with carrageenan. However, there was no significant difference between the group treated with selective antagonist of the mu subunit and the control group treated with carrageenan. This suggests that only the selective antagonist of the mu subunit (CTOP, 20ug/50uL/paw) was able to inhibit the analgesic effect of the supernatant from the fibroblast culture, demonstrating that the kappa and delta receptors do not participate in the analgesic mediation induced by the fibroblasts. Thus, its was possibly to notice that the opioid group involved in the antinociception induced by fibroblast culture seems to be µ agonist.
Therefore, a direct relationship between the peripheral nervous system, keratinocytes, fibroblasts, and analgesia could be observed. Such correlation is a potential and safe target for the development of new antinociceptive methods that seek the reduction of known adverse effects with the wide use of exogenous opioids.
CONCLUSION
The present study indicated that fibroblasts and keratinocytes supernatant culture was able to induce antinociception in carrageenan induced inflammatory hyperalgesia, which is mediated by the release of endogenous opioids. In addition, it was observed that the reduction of hyperalgesia by fibroblast culture supernatant is specifically mediated by the µ opioid agonist.
-
Sponsoring sources: CNPq under number 138343/2017-9.
REFERENCES
-
1Abbas AK, Janeway CA Jr. Immunology: improving on nature in the twenty-first century. Cell. 2000;100(1):129-38.
-
2Dawes JM, Anderson DA, Bennett DL, Bevan S, McMahon SB. Inflammatory mediators and modulators of pain. Wall and Melzack's Textbook of Pain. 2013;6:48-67.
-
3Levine JD, Taiwo Y. Inflammatory Pain. In: Wall PD, Melzack R, Bonica JJ. Textbook of Pain. 3rd ed. Edinburgh, Scothand, Churchill Livingstone; 1994. 45-56p.
-
4Carvalho MMMJ. O Sofrimento da dor em câncer. In: Carvalho MMMJ. Introdução à psiconcologia.1ª ed. São Paulo; 2003. 103-18p.
-
5Messlinger K. What is a nociceptor? Anaesthesist. 1997;46(2):142-53.
-
6Besson JM. The complexity of physiopharmacologic aspects of pain. Drugs. 1997;53(Suppl.2):1-9.
-
7Webster KE. Somaesthetic pathways. Br Med Bull. 1977;33(2):113-20.
-
8Woolf CJ. Recent advances in the pathophysiology of acute pain. Br J Anaesth. 1989;63(2):139-46.
-
9Stein C, Pflüger M, Yassouridis A, Hoelzl J, Lehrberger K, Welte C, et al. No tolerance to peripheral morphine analgesia in presence of opioid expression in inflamed synovia. J Clin Invest. 1996;98(3):793-9.
-
10Stein C, Gramsch C, Herz A. Intrinsic mechanisms of antinociception in inflammation: local opioid receptors and beta-endorphin. J Neurosci. 1990;10(4):1292-8.
-
11Garcia JB, Cardoso MG, Dos-Santos MC. Opioids and the immune system: clinical relevance. Rev Bras Anestesiol. 2012;62(5):709-18.
-
12Slominski AT, Zmijewski MA, Skobowiat C, Zbytek B, Slominski RM, Steketee JD. Sensing the environment: regulation of local and global homeostasis by the skin's neuroendocrine system. Adv Anat Embryol Cell Biol. 2012;212:v, vii, 1-155.
-
13Vetter I, Kapitzke D, Hermanussen S, Monteith GR, Cabot PJ. The effects of pH on beta-endorphin and morphine inhibition of calcium transients in dorsal root ganglion neurons. J Pain. 2006;7(7):488-99.
-
14Hollt V. Opioid peptide processing and receptor selectivity. Annu Rev Pharmacol Toxicol. 1986;26(1):59-77.
-
15Nakanishi S, Inoue A, Kita T, Nakamura M, Chang AC, Cohen SN, et al. Nucleotide sequence of cloned cDNA for bovine corticotropin-beta-lipotropin precursor. Nature. 1979;278(5703):423-7.
-
16Sibinga NE, Goldstein A. Opioids peptides and opioid receptors in cell of the immune system. Annu Rev Immunol. 1988;6:219-49.
-
17Wintzen M, Yaar M, Avila E, Vermeer BJ, Gilchrest BA. Keratinocytes produce b-endorphin and b-lipotropic hormone after stimulation by UV, IL-1a or phorbol esters. J Invest Dermatol. 1995;104:641.https://www.scopus.com/record/display.uri?eid=2-s2.0-0000723419&origin=inward&txGid=7b951b39c5d52e89782d718c1d65b2be
» https://www.scopus.com/record/display.uri?eid=2-s2.0-0000723419&origin=inward&txGid=7b951b39c5d52e89782d718c1d65b2be -
18Schauer E, Trautinger F, Köck A, Schwarz A, Bhardwaj R, Simon M, et al. Proopiomelanocortin-derived peptides are synthesized and released by human keratinocytes. J Clin Invest. 1994;93(5):2258-62.
-
19Bigliardi PL, Bigliardi-Qi M, Buechner S, Rufli T. Expression of mµ-opiate receptor in human epidermis and keratinocytes. J Invest Dermatol. 1998;111(2):297-301.
-
20Lo HH, Tseng LF, Wei E, Li CH. Endorphin is a potent analgesic agent. Proc Natl Acad Sci. USA. 1976;7(8):2895-8.
-
21Slominski AT, Zmijewski MA, Zbytek B, Brozyna AA, Granese J, Pisarchik A, et al. Regulated proenkephalin expression in human skin and cultured skin cells. J Invest Dermatol. 2011;131(3):613-22.
-
22Bigliardi-Qi M, Sumanovski LT, Büchner S, Rufli T, Bigliardi PL. Mu-opiate receptor and beta-endorphin expression in nerve endings and keratinocytes in human skin. Dermatology. 2004;209(3):183-9.
-
23Schaible HG. Pathophysiology of pain. Orthopade. 2006;36(1):8-16.
-
24Schaible HG. Peripheral and central mechanisms of pain generation. Hand Exp Pharmacol. 2007;(177):3-28.
-
25Siderov J, Zalcberg JR. Prescribing opioids--a painful experience. Med J Aust. 1994;161(9):515-6.
-
26Benyamin R, Trescot AM, Datta S, Buenaventura R, Adlaka R, Sehgal N, et al. Opioid complications and side effects. Pain Physician. 2008;11(2 Suppl):S105-20.
-
27Chou R, Turner JA, Devine EB, Hansen RN, Sullivan SD, Blazina I, et al. The effectiveness and risks of long-term opioid therapy for chronic pain: a systematic review for a National Institutes of Health Pathways to Prevention Workshop. Ann Intern Med. 2015;162(14):276-86.
-
28Eriksen J, Sjogren P, Bruera E, Ekholm O, Rasmussen NK. Critical issues on opioids in chronic non-cancer pain: an epidemiological study. Pain. 2006;125(1-2):172-9.
-
29Vivancos GG, Verri WA Jr, Cunha TM, Schivo IR, Parada CA, Cunha FQ, et al. An electronic pressure-meter nociception paw test for rats. Braz J Med Biol Res. 2004;37(3):391-9.
-
30Rosland JH. The formalin test in mice: the influence of ambient temperature. Pain. 1991;45(2):211- 6.
-
31Henriques MG, Silva PM, Martins MA, Flores CA, Cunha FQ, Assreuy-Filho J, et al. Mouse paw edema. A new model for inflammation? Braz J Med Biol Res. 1987;20(2):243-9.
-
32Bodnar RJ. Endogenous opiates and behavior: 2017. Peptides. 2020;124:170223.
-
33Leong C, Neumann C, Ramasamy S, Rout B, Wee LY, Bigliardi-Qi M, et al. Investigating endogenous µ-opioid receptors in human keratinocytes as pharmacological targets using novel fluorescent ligand. PloS one. 2017;12(12):e0188607.
-
34Li X, Zhu J, Tao Y, Tao K. Elevated endogenous opioids in obstructive jaundice: the possible skin mechanisms. Med Hypotheses. 2018;116:119-21.
Publication Dates
-
Publication in this collection
19 June 2020 -
Date of issue
Jul-Sep 2020
History
-
Received
20 Feb 2020 -
Accepted
10 May 2020