ABSTRACT
Capirona decorticans (Rubiaceae) is popularly used to treat warts, wounds, mycoses and scabies, and is also a component of the Ayahuasca tea. Despite its popular use, the phytochemical and pharmacological research on this species is limited. Therefore, this work quantified phenolic compounds in the ethanolic extract (EE) and hydromethanolic fraction (FM) (406, 293 mgEAG g-1, respectively) from leaves of C. decorticans. We identified flavonoids by LC-MS/MS-MMR-ESI (apigenin, rutin, luteolin, miricetin, quercetin, quercetin-3-β-D-glucoside, quercetrin), and evaluated oxidative stress and mutagenic/antimutagenic effect of EE and FM through an in vivo experiment using Swiss mice and cyclophosphamide (CP) as an inducer of DNA damage and oxidative stress. Mice were pretreated for 15 consecutive days with EE or FM (250 mg kg-1) and then intraperitoneally injected with CP (25 mg kg-1). Carbonylated proteins, ascorbic acid, catalase and thiobarbituric acid-reactive substances were measured in hepatic and renal tissues. The mutagenic/antimutagenic effect was evaluated through the Micronucleus Test. Protein carbonylation in the liver of animals exposed to CP was reduced by FM. There was no significant effect on other markers of oxidative stress. The groups treated with the extracts showed a significant percentage reduction (EE = 96% and FM = 71%) in the frequency of micronucleated polychromatic erythrocytes induced by CP. EE showed mutagenicity when used alone. The EE and FM of C. decorticans leaves showed antioxidant potential equivalent to that observed in other species, did not cause oxidative stress, nor toxicity, and had a protective and antimutagenic effect, although the EE showed signs of mutagenicity.
Keywords:
oxidative stress; cyclophosphamide; genotoxicity; phenolic compounds
RESUMO
Capirona decorticans (Rubiaceae) é popularmente usada para tratar verrugas, feridas, micoses e sarna, e como um componente do chá de Ayahuasca. Apesar do uso popular, são limitadas as pesquisas fitoquímicas e farmacológicas sobre a espécie. Portanto, este estudo quantificou compostos fenólicos no extrato etanólico (EE) e na fração hidrometanólica (FM) (406 e 293 mgEAG g-1, respectivamente) de folhas de C. decorticans. Identificamos flavonoides por LC-MS / MS-MMR-ESI (apigenina, rutina, luteolina, miricetina, quercetina, quercetina-3-β-D-glicosídeo, quercetrina), e avaliamos o estresse oxidativo e o efeito mutagênico/antimutagênico de EE e FM em um experimento in vivo utilizando camundongos Swiss e ciclofosfamida (CP) como um indutor de danos no DNA e estresse oxidativo. Os camundongos foram pré-tratados por 15 dias consecutivos com EE ou FM (250 mg kg-1) e injetados intraperitonealmente com CP (25 mg kg-1). Proteínas carboniladas, ácido ascórbico, catalase e substâncias reativas ao ácido tiobarbitúrico foram dosadas em tecidos hepáticos e renais. O efeito mutagênico/antimutagênico foi avaliado através do Teste de Micronúcleo. Houve carbonilação protéica no fígado de animais expostos à CP, que foi reduzida pela FM. Não houve efeito significativo sobre outros marcadores de estresse oxidativo. Os grupos tratados com os extratos apresentaram uma redução percentual significativa (EE = 96% e FM = 71%) na frequência de eritrócitos policromáticos micronucleados induzidos pela PC. O EE também apresentou mutagenicidade quando utilizado isoladamente. O EE e FM das folhas de C. decorticans apresentaram potencial antioxidante equivalente ao observado em outras espécies, não causaram estresse oxidativo, nem toxicidade, e tiveram efeito protetor e antimutagênico, embora a EE tenha apresentado mutagenicidade.
Palavras-chave:
estresse oxidativo; ciclofosfamida; genotoxicidade; compostos fenólicos
INTRODUCTION
Capirona decorticans Spruce (Rubiaceae) (C. decorticans) is popularly known in Brazil as perna de moça or mulateiro. This tree occurs in the Amazon forest (including Colombia, Venezuela, Bolivia, Peru, Ecuador, Guianas and Brazil) (Taylor et al. 2007Taylor, A.; Campos, M.; Zappi, D. 2007. Flora da Reserva Ducke, Amazonas, Brasil: Rubiaceae. Rodriguesia, 58: 549-616.). Plants of the Rubiaceae family are widely used in folk medicine, with numerous uses described (antimicrobial, anti-malarial, hepatoprotective, antioxidant, anticancer and psychoactive activities), being the source of several bioactive compounds including alkaloids, flavonoids, terpenes, anthraquinones and coumarins (Barrabé et al. 2014Barrabé, L.; Maggia, L.; Pillon, Y., Rigault, F.F.; Mouly, A.; Davis, A.P.; Buerki, S. 2014. New Caledonian lineages of Psychotria (Rubiaceae) reveal different evolutionary histories and the largest documented plant radiation for the archipelago. Molecular Phylogenetics Evolution, 71: 15-35.; Martins and Nunes 2015Martins, D.; Nunez, C.V. 2015. Secondary metabolites from Rubiaceae species.Molecules, 20: 13422-95.). Castillo et al. (2007Castillo, D.; Arevalo, J.; Herrera, F.; Ruiz, C.; Rojas, R.; Rengifo, E.; et al. 2007. Spirolactoneiridoids might be responsible for the antileishmanial activity of a Peruvian traditional remedy made with Himatanthus sucuuba (Apocynaceae). Journal of Ethnopharmacology, 112: 410-414.) reported the popular use of C. decorticans for the treatment of warts, wounds, mycoses and scabies.
Since the 1980s, medicinal plants and their active ingredients have received increasing attention (Kren and Walterova 2005Kren, V.; Walterova, D. 2005. Silybin and silymarin - new effects and applications. Biomedical Papers, 149: 29-41.). Medicinal plants serve as therapeutic alternatives, being safer and effective treatment options, and an increasing number of these plants and their extracts have been shown to produce beneficial therapeutic effects, including antioxidant, anti-inflammatory, anticancer, antimicrobial and immune modulatory effects (Arafa 2009Arafa, H.M. 2009. Uroprotective effects of curcumin in cyclophosphamide-induced haemorrhagic cystitis paradigm. Basic Clinical Pharmacology and Toxicology, 104: 393-399.; Garcia-Nino and Pedraza-Chaverri 2014Garcia-Nino, W.R.; Pedraza-Chaverri, J. 2014. Protective effect of curcumin against heavy metals-induced liver damage. Food and Chemistry Toxicology, 69: 182-201.). The potential antioxidant activity of medicinal plants has been evaluated in many studies (Ramkissoon et al. 2013Ramkissoon, J.S.; Mahomoodally, M.F.; Ahmed, N.; Subratty, A.H. 2013. Antioxidant and anti-glycation activities correlates with phenolic composition of tropical medicinal herbs. Asian Pacific Journal of Tropical Medicine, 6: 561-569.; Terpinc et al. 2016Terpinc, P.; Cigic, B.; Polak, T.; Hribar, J.; Porl, T. 2016. LC-MS analysis of phenolic compounds and antioxidant activity of buckwheat at different stages of malting. Food Chemistry, 210: 9-17.) and may be an important source of protection of the endogenous system from free radicals (Cândido et al. 2015Cândido, T.L.N.; Silva, M.R.; Agostini-Costa, T.S. 2015. Bioactive compounds and antioxidant capacity of buriti (Mauritia flexuosa L.f.) from the Cerrado and Amazon biomes. Food Chemistry, 177: 313-319.). Natural products have been increasingly targeted as a source of antioxidants to counter the harmful effects of oxidative stress to body functions, specially polyphenols, that are present in many plant species (Coulibaly et al. 2014Coulibaly, A.Y.; Hashim, R.; Sulaiman, S.F.; Sulaiman, O.; Ang, L.Z.P.; Ooi, K.L. 2014. Bioprospecting medical plants for antioxidant components. Asian Pacific Journal of Tropical Medicine, 7: S553-S559.). In addition, phenolic compounds may have positive, inert or deleterious effects on mutagenesis, which makes them a frequent object of bioprospecting studies (Bourgaud et al. 2001Bourgaud, F.; Gravot, A.; Milesi, S.; Gontier, E. 2001. Production of plant secondary metabolites: A historical perspective. Plant Science, 161: 839-851.). These phenolic compounds have the ability to suppress lipid peroxidation, prevent DNA oxidative damage, and scavenge free radicals, which cause the depletion of the immune system, changes in gene expression, and induction of abnormal proteins, resulting in degenerative diseases and aging (Cao and Cao 1999Cao, Y.; Cao, R. 1999. Angiogenesis inhibited by drinking tea. Nature, 398: 381.).
Cyclophosphamide (CP) is an alkylating agent widely used in the treatment of cancer and is classified as a human carcinogen based on the extensive evidence found not only in animal experimentation, but also in studies with humans (IARC 2012). The metabolites generated by CP induce oxidative stress and cause damage to DNA and toxicity to various target organs (Korkmaz et al. 2007Korkmaz, A.; Topal, T.; Obter, S. 2007. Pathophysiological aspects of cyclophosphamide and ifosfamide induced hemorrhagic cystitis; implication of reactive oxygen and nitrogen species as well as PARP activation. Cell Biology and Toxicology, 23: 303-12.).
Considering the above, the present work aimed to evaluate the phytochemical profile of the ethanolic extract of C. decorticans leaves and to determine the antioxidant action and mutagenic/antimutagenic effect of the ethanolic extract and the hydromethanolic fraction. We evaluated the effects of C. decorticans extracts on the oxidative damage induced in vivo in mice exposed to cyclophosphamide.
MATERIAL AND METHODS
Collection and botanical identification
Leaves of C. decorticans were obtained in March 2015, during the rainy season, from an adult tree in a forest fragment in the city of Alta Floresta (Mato Grosso state, Brazil) (9°52’44.11”S, 56°6’7.00”W), at an altitude of 299 meters. The material was labeled and a sample was deposited in the Centro Norte Mato Grossense (CNMT) herbarium of Universidade Federal de Mato Grosso (UFMT) - Campus Sinop (deposit number 6557).
Preparation and fractionation of the extract
A sample (6800 g) of the leaves harvested was dried in an oven with forced air circulation at a mean temperature of 40 °C until completely dried. The dried material (1650 g) was ground and subjected to exhaustive extraction by maceration in ethanol at room temperature. The ethanolic extract (EE) was obtained by removing the ethanol with rotavaporation, with a pressure of -700 mmHg at 40 °C. Chlorophyll was removed from the crude extract by partitioning into methanol/water (1:1) (Morais et al. 2015Morais, M.G.; da Costa., G.A.F.; Aleixo, A.A.; de Oliveira, G.T.; Alves, L.F.; Duarte-Almeida, J.M.; Ferreira, J.M.S.; dos Santos Lima, L.A.R. 2015. Antioxidant, antibacterial and cytotoxic potential of the ripe fruits ofSolanum lycocarpumA. St. Hil. (Solanaceae). Natural Product Research, 29: 480-483), followed by filtration and evaporation under reduced pressure, resulting in 30 g EE.
The fractionation was carried out with 10 g of EE, which was dissolved in a methanol/water solution (9:1) and subjected to the liquid-liquid partitioning process by polarity gradient with hexane, dichloromethane and ethyl acetate. After this procedure, the hydromethanolic fraction (FM) (4 g) was obtained. Chemical and biological tests were conducted with EE and FM at the concentration of 250 mg kg-1. The procedure followed Castillo et al. (2007Castillo, D.; Arevalo, J.; Herrera, F.; Ruiz, C.; Rojas, R.; Rengifo, E.; et al. 2007. Spirolactoneiridoids might be responsible for the antileishmanial activity of a Peruvian traditional remedy made with Himatanthus sucuuba (Apocynaceae). Journal of Ethnopharmacology, 112: 410-414.), with adaptations.
Phenols, flavonoids and antiradical activity
To quantify the total phenols, the Folin-Ciocalteau method was used, as described by Costa et al. (2010Costa, D.A.; Chaves, M.H.; Silva, W.C.S.; Costa, C.L.S. 2010. Chemical constituents, total phenolics and antioxidant activity of Sterculia striata St. Hil. et Naud. Acta Amazonica, 40: 207-212.). The phenol content was determined by linear regression, with a standard curve of gallic acid, with concentrations between 10 and 350 μg mL-1. The analyses were performed in triplicate and the results were expressed in milligram gallic acid equivalent per gram of extract (mgEAG g-1).
The total flavonoids were dosed in EE and FM by the colorimetric method with aluminum chloride (AlCl3), according to Costa et al. (2010Costa, D.A.; Chaves, M.H.; Silva, W.C.S.; Costa, C.L.S. 2010. Chemical constituents, total phenolics and antioxidant activity of Sterculia striata St. Hil. et Naud. Acta Amazonica, 40: 207-212.), with adaptations. The flavonoid contents were obtained by linear regression, with a calibration curve made with quercetin, with concentrations of 5, 10, 15, 20, 25 and 30 μg mL-1. The results were expressed in milligram quercetin equivalent per gram of extract (mgEQ g-1).
The anti-radical potential of the samples was determined by the free radical sequestration method, using 2,2-diphenyl-1-picrylhydrazyl (DPPH), according to Costa et al. (2010Costa, D.A.; Chaves, M.H.; Silva, W.C.S.; Costa, C.L.S. 2010. Chemical constituents, total phenolics and antioxidant activity of Sterculia striata St. Hil. et Naud. Acta Amazonica, 40: 207-212.). The CE50 of DPPH consumption of the EE and FM samples was calculated using rutin and ascorbic acid as standards in the antiradical activity.
Flavonoid analysis by LC-MS/MS
The presence of flavonoids in EE and FM was confirmed by sequential mass spectrometry (LC-MS/MS), in the Multiple Reaction Monitoring (MRM) mode of acquisition and transition ions previously established by flavonoid pattern analysis, using a UHPLC 1290 Infinnity equipped with 6460 Triple Quad LC/MS, both from Agilent Technologies. The procedure followed Ares et al. (2016Ares, A.M.; Valverde, S.; Nozal, M.J.; Bernal, J.L.; Bernal, J. 2016. Development and validation of a specific method to quantify intact glucosinolates in honey by LC-MS/MS. Journal Food Composition Analysis, 46: 114-122. ) and Terpinc et al. (2016Terpinc, P.; Cigic, B.; Polak, T.; Hribar, J.; Porl, T. 2016. LC-MS analysis of phenolic compounds and antioxidant activity of buckwheat at different stages of malting. Food Chemistry, 210: 9-17.). The following parameters were used in the analysis: injected sample volume of 20 μL, mobile phase flow at 0.5 mL min-1 with solvent A compound of water and formic acid 0.1%, solvent B acetonitrile and formic acid 0.1%, gradient 5% B 0-30 min, 100% B 30-32 min, 5% B 32-33 min; Column Zorbax Eclipse AAA, C-18, (4.6 x 150 mm, 3.5 μm), maintained at 25 °C; negative ionization mode by electrospray. In the mass spectrometer, the capillary voltage was adjusted to 3.5 kv, source at 300 °C and desolvation at 250 °C. Nitrogen was used for nebulization and as drying gas. The results were compared to the standards and to literature references.
In vivo experiment
Animals- Male Swiss mice aged 6-7 weeks (weighting 30-35 g), were obtained from the breeding colonies of the experimental animal facilities of Universidade Federal de Mato Grosso - UFMT, Campus Cuiabá, Mato Grosso, Brazil. The experimental procedures were authorized by the Ethics Committee for Animal Research of UFMT (CEUA/UFMT) (protocol number 23108.717375/2016-85). The animals were kept in plastic cages in an experimental room under controlled conditions of temperature (22 ± 2 ºC), relative humidity (55 ± 10%) and light cycle (12 h light, 12 h dark), and were fed standardized commercial feed and filtered water ad libitum.
Experimental design- The animals were divided into 6 groups (treatments) containing 8 animals each. Treatments are defined in Table 1. The EE or FM dosage administered was established according to the Malone Hippocratic test (Malone 1983Malone, M.H. 1983. The pharmacological evaluation of natural products - general and specific approachs to screening ethnopharmaceuticals. Journal of Ethnopharmacology, 8: 127-147.). The animals were treated orally by gavage (0.3 mL by day by animal of EE or FM) dissolved in filtered water, at a concentration of 250 mg kg-1 for 15 consecutive days. Cyclophosphamide (CP) at a dosage of 25 mg kg-1 was used as positive control, administered intraperitoneally (single dose) by injection on the 15th day of treatment (Delmanto et al. 2001Delmanto, R.D.; de Lima, P.L.A.; Sugui, M.M.; da Eira, A.F.; Salvadori, D.M.F.; Speit, G.; Ribeiro, L.R. 2001. Antimutagenic effect of Agaricus blazei Murrill mushroom on the by cyclophosphamide. Mutation Research, 496: 15-21.). Twenty-four hours after intraperitoneal injection, the mice were anesthetized with Ketamine 50 mg kg-1, Xylazine 20 mg kg-1 and Acepromazine 20 mg kg-1, followed by cervical dislocation and removal of femur, liver and kidneys. The liver and kidneys were washed with 0.9% NaCl and immediately frozen.
Specification of the treatments used in the in vivo experiment to evaluate the mutagenic effect of the ethanolic extract (EE) and hydromethanolic fraction (FM) of Capirona decorticans leaves. CP = cyclophosphamide.
Oxidative stress parameters
Carbonylated proteins- The oxidative damage to proteins in the liver and kidneys was assessed by determination of carbonyl groups, according to Yan et al. (1995Yan, L.J.; Traber, M.G.; Packer, L. 1995. Spectrophotometric method for determination of carbonyls in oxidatively modified apolipoprotein B of human low-density lipoproteins. Analytical Biochemistry, 228: 349-351.). Each tissue was diluted in 10 mM Tris/HCl buffer (pH 7.4), in proportion 1:80 (w/v), in a blank and triplicate test. The amount of carbonylated proteins (PC) of samples was read at 370 nm in quartz cuvette and the results were expressed as nmol of carbonyl mg protein-1.
Thiobarbituric acid reactive species (TBARS)- Oxidative damage to lipids in the liver was assessed by determining the levels of thiobarbituric acid reactive substances (TBARS) spectrophotometrically, according to Buege and Aust (1978Buege, J.A.; Aust, S.D. 1978. Microsomal lipid peroxidation. Methods in Enzymology, 52: 302-309.) with some modifications. The liver was homogenized 1:8 (w/v) in 20 mM TFK pH 7.5 and centrifuged at 4000 rpm for 15 min. The reaction mixture (500 μL of the supernatant were collected, 250 μL of 10% TCA and 1000 μL thiobarbituric acid (TBA) 0.67%) was then incubated in a water bath at 100 ºC for 30 minutes, and cooled and centrifuged at 3600 rpm for 10 minutes. The supernatant was read at 535 nm and compared to a calibration curve made with malonyldialdehyde (MDA). The amount of lipid peroxidation was expressed as nmol MDA mg-1 protein-1.
Ascorbic acid- The endogenous non-enzymatic antioxidant dosed for liver and kidneys was ascorbic acid, following Roe (1954Roe, J.H. 1954. Chemical determination of ascorbic, dehydroascorbic, and diketogulonic acids. In: Glick, D. (Ed.). Methods of Biochemical Analysis, v.1. Interscience, New York, p.115.). The tissue was diluted 1:15 (w/v) in 10 mMTris/HCl, and centrifuged at 2000 rpm for 10 minutes at 4 °C. The solution was read at 520 nm and the results were compared to a standard calibration curve of ascorbic acid. The result was expressed in μmol ASA g-1of tissue.
Catalase- The enzymatic antioxidant activity of catalase (CAT) followed Nelson and Kiesow (1972Nelson, D.P.; Kiesow, L.A. 1972. Enthalphy of decomposition of hydrogen peroxide by catalase at 25 °C (with molar extinction coefficients of H2O2 solution in the UV). Analytical Biochemistry, 49: 474-478.). The principle is based on decomposition of H2O2 and measured spectrophotometrically at 240 nm. Tissues were homogenized in the following proportions: liver 1:30 and kidney 1:20 (w/v) in 20 mM potassium phosphate buffer (TFK) containing Triton® X-100, NaCl, pH 7.4, then centrifuged at 10000 rpm for 15 minutes at 4 °C. The reaction mixture contained 25 μL of the supernatant, 1000 μL of 50 mM TFK pH 7.0 and 25 μL H2O2 added to a quartz cuvette and the absorbances were read every 15 seconds for 1 minute. The result was expressed in μmol H2O2 consumed min-1 mg protein-1.
Protein content of tissues- The protein content of the samples (except ASA) was determined with the Bradford method (1976Bradford, M.M. 1976. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72: 248-254.) at 595 nm using bovine serum albumin as standard for calibration curve construction.
Micronucleus test- Micronuclei (MN) are small nuclei that arise from chromosome fragmentation and that are deposited in young erythrocytes of the bone marrow. The test allows the assessment of the clastogenic effects that damage the chromosome and aneugenic effects that induce aneuploidy or abnormal chromosome segregation. The test consists of assessing the frequency of micronucleated polychromatic erythrocytes (MNPCE) after exposure to the chemical agent under investigation (MacGregor et al. 1987MacGregor, J.T.; Heddle, J.A.; Hite, M.; Marcolin, B.H.; Ramel, C.; Salamone, M.F.; Tice, R.R.; Wild, D. 1987. Guidelines for the conduct of micronucleus assays in mammalian bone marrow erythrocytes. Mutation Research, 189: 103-112.). The procedures followed Ribeiro et al. (2003Ribeiro, L.R.; Salvadori, D.M.F.; Marques, E.K. 2003. Mutagênese ambiental. Editora da ULBRA, Canoas-RS, 356p.). The number of micronucleated cells was counted in 1000 polychromatic erythrocytes (PCE) per animal. The slides were analyzed in a blind test, using a light microscope with a 100x immersion objective for PCE.
The percentage reduction in the frequency of MNPCE was determined according to Manoharan and Banerjee (1985Manoharan, K.; Banerjee, M. 1985. β-Carotene reduces sister chromatid exchange induce chemical carcinogens in mouse mammary cells in organ culture. Cell Biology International Reports, London, 9: 783-789.) and Waters et al. (1990Waters, M.D.; Brady, A.L.; Stack, H.F.; Brockman, H.E. 1990. Antimutagenicity profiles for some model compounds. Mutation Research: Reviews in Genetic Toxicology, 238: 57-85.), by the following formula:
where: A is the group treated with CP (positive control); B is the group treated with extracts of C. decorticans plus CP; and C is the group treated with 0.9% NaCl (negative control).
Statistical analysis- Results are presented as mean ± SD (standard deviation) and analyzed by one-way Anova followed by the post-hoc Tukey test. In all cases, a level of significance was accepted at p < 0.05. The frequency of micronucleated bone marrow cells was compared among treatments using a Chi-square test (Pereira 1991Pereira, C.A.B. 1991. Teste estatístico para comparar proporções em problemas de citogenética. In: Rabello-Gay, M.N.; Rodrigues, M.A.; Montelleone-Neto, L.A.R. (Ed.) Mutagênese, teratogênese e carcinogênese: métodos e critérios de avaliação. Ed. FCA, São Paulo, p.113-21. ).
RESULTS
The results of chemical analysis showed high concentration of phenolic compounds, low concentration of total flavonoids and high antiradical potential (Table 2). Seven flavonoids were identified in the EE and FM samples: rutin, quercetin-3-β-D-glucoside, quercitrin, myricetin, quercetin, luteolin and apigenin (Table 3, Figure 1).
Total phenol content, total flavonoid content, and antiradical activity (IC50) of the ethanolic extract (EE) and hydromethanolic fraction (FM) of Capirona decorticans leaves.
Characterization of the flavonoids identified in the ethanolic extract (EE) and hydromethanolic fraction (FM) of Capirona decorticans leaves, and the results of the LC-MS / MS analysis, MRM mode, with the main ions presented in the molecular breakdown of each compound.
Flavonoid compounds 1 to 7 identified by LC-MS/MS in MRM mode in ethanolic extract (EE) samples of Capirona decorticans, showing the retention time, mass of molecular ions, and main fragment of the identified flavonoids.
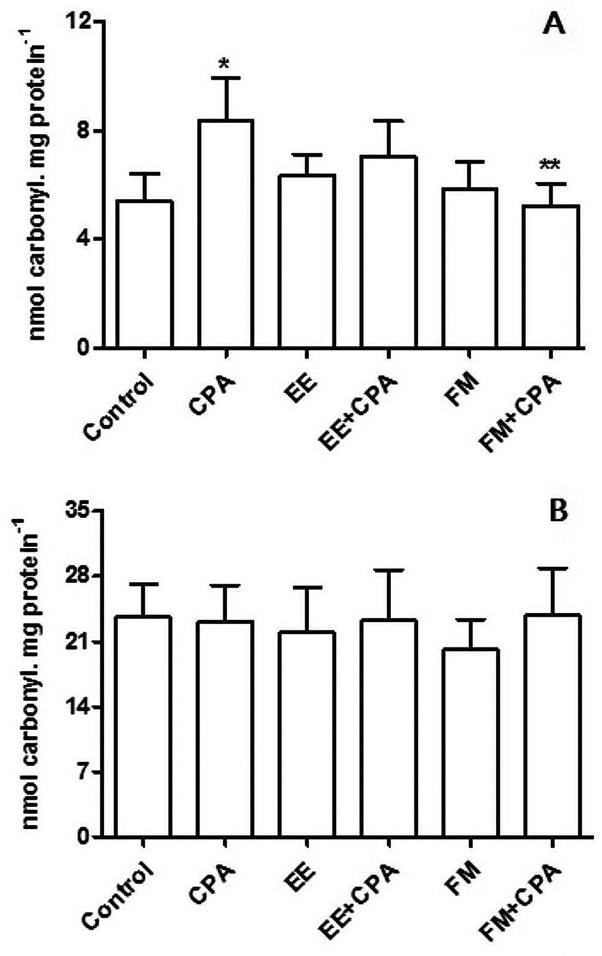
Regarding the in vivo experiment, there was a significant increase (p < 0.05) in the levels of carbonylated proteins in the liver tissue of the positive control group (that received only CP) when compared to the negative control group, and a significant decrease in the FM+CP group when compared to the positive control (Figure 2A). There was no significant difference among treatments for the levels of carbonylated proteins in renal tissue (Figure 2B), nor for TBARS in hepatic tissue, or for ascorbic acid and catalase in liver and kidney.
Levels of carbonylated proteins of the liver (A) and kidney (B) of Swiss mice treated with the ethanolic extract (EE) and hydrometanolic fraction (FM) of Capirona decorticans leaves and cyclophosphamide (CPA) (n = 8 per treatment). Treatment specifications are available in Table 1. Control = negative control; CPA = positive control; CPA dosage at 25 mg kg-1; EE and FM dosage at 250 mg kg-1. Asterisks indicate significant differences at p < 0.0001 (* relative to the control; ** relative to CPA).
Regarding CP-induced damage to DNA, the EE+CP and FM+CP groups showed a reduction of 96% and 71% (p < 0.001), respectively, in the frequency of MNPCE in the bone marrow when compared to the positive control group (Table 4). The FM group did not present any mutagenic potential, but the EE group was mutagenic when compared with the negative control group.
Frequency of micronucleated polychromatic erythrocytes (MNPCE) in bone marrow of male Swiss mice after pre-treatment with ethanolic extract (EE) and hydromethanolic fraction (FM) of Capirona decorticans leaves and cyclophosphamide (CP). N analyzed cells = number of analyzed cells (1000 per individual); N = number of MNPCE; % = frequency of MNPCE.
DISCUSSION
The antiradical activity of C. decorticans detected in this study was comparable to that of the analyzed standards (ascorbic acid and rutin). Antiradical activity has been related to polyphenol action in the sequestration of free radicals and the prevention of mutagenic processes related to diverse human diseases (Yasir et al. 2016Yasir, M.; Sultana, B.; Nigam, P.S.; Owusu-Apenten, R. 2016. Antioxidant and genoprotective activity of selected cucurbitaceae seed extracts and LC-ESIMS/MS identification of phenolic components. Food Chemistry, 199: 307-313.).
Compound 1 presented molecular ion at 609.52 [m/z-H] and fragment at 300.20 (m/z-309.32), which corresponds to the loss of two glycans, being similar to that obtained by Simirgiotis et al. (2016Simirgiotis, M.J.; Quispe, C.; Areche, C.; Sepúlveda, B. 2016. Phenolic compounds in chilean mistletoe (quintral, Tristerix tetrandus) analyzed by UHPLC-Q/Orbitrap/MS/MS and its antioxidant properties. Molecules, 21: 245.). Compound 2 presented molecular ion at 463.38 [m/z-H] and fragment at 300.00 [m/z-163.38] indicating the breakdown of the glycoside grouping of this aglycone. For Compound 3, molecular ion was identified at 447.38 [m/z-H] and fragment at 301.00 [m/z-146.38], corresponding to the disruption of the glycone moiety (rhamnose), with a similar fragmentation pattern to that described by He et al. (2014He, C.Y.; Fu, J.; Ma, J.Y.; Feng, R.; Tan, X.S.; Huang, M. 2014. Biotransformation and in vitro metabolic profile of bioactive extracts from a traditional miao-nationality herbal medicine, polygonum capitatum. Molecules, 19: 10291-10308.). For compound 4 the molecular ion was identified at 317.24 [m/z-H] and fragment at 150.90 [m/z-166], that corresponds to the break in ring C, the same pattern obtained by Sun et al. (2014Sun, Z.; Zhao, L.; Zuo, L.; Qi, C.; Zhao, P.; Hou, X. 2014. A UHPLC-MS/MS method for simultaneous determination of six flavonoids, gallic acid and 5,8-dihydroxy-1,4-naphthoquinone in rat plasma and its application to a pharmacokinetic study of Cortex juglandis Mandshuricae extract. Journal of Chromatography B, 958: 55-62.). On the other hand, compound 5 had a molecular ion at 301.24 [m/z-H] and the fragment at 151.00 [m/z-150.24], a similar fragmentation to that found by Yasir et al. (2016Yasir, M.; Sultana, B.; Nigam, P.S.; Owusu-Apenten, R. 2016. Antioxidant and genoprotective activity of selected cucurbitaceae seed extracts and LC-ESIMS/MS identification of phenolic components. Food Chemistry, 199: 307-313.)the yield of seeds extract was 20-41% (w/w and Sun et al. (2014), representing the C-ring rupture between carbon 2 and carbonyl. For compound 6 the molecular ion was at 269.24 [m/z-H] and fragment at 116.80 [m/z-155.44], which corresponds to the connection break between carbons 2 and 3 of ring C. The fragmentation profiles of compounds 3 and 7 were similar to those described by Dai et al. (2015Dai, B.; Hu, Z.; Li, H.; Yan, C.; Zhang, L. 2015. Simultaneous determination of six flavonoids from Paulownia tomentosa flower extract in rat plasma by LC-MS/MS and its application to a pharmacokinetic study. Journal of Chromatography B, 26: 54-61.). Compound 7 presented the molecular ion at 285.24 [m/z-H] and fragment at 133.00 [m/z-152.24], representing the C-ring breaking benzene diol; similar to the fragmentation in negative ionization mode described by Yasir et al. (2016)the yield of seeds extract was 20-41% (w/w.
The significantly lower level of carbonylated proteins in the liver of the FM+CP group relative to the CP control indicated that FM promoted a reduction of the damage caused by CP in this organ. No such effect was observed in the kidneys, possibly because the dose of CP was too low to cause renal damage. A dose of 27 mg kg-1 CP every three weeks over 10 weeks promoted oxidative stress in renal tissue of rats (Kocahan et al. 2017Kocahan, S.; Dogan, Z.; Erdemli, E.; Taskin, E. 2017. Protective effect of quercetin against oxidative stress-induced toxicity associated with doxorubicin and cyclophosphamide in rat kidney and liver tissue. Iranian Journal of Kidney Diseases, 11: 124-131. ). Protein carbonylation is characteristic of damage caused by oxidative stress and its occurrence can lead to changes in organ structure, decrease in enzymatic activity and destruction of proteins, being an important marker in toxicological studies (Qiu et al. 2016Qiu, C.; Zhu, T.; Lan, L.; Zeng, Q.; Du, Z. 2016. Analysis of maceaene and macamide contents of petroleum ether extract of black, yellow, and purple Lepidium meyenii (maca) and their antioxidant effect on diabetes mellitus rat model. Brazilian Archives Biology and Technology, 59: 1-9.; Ramkissoon et al. 2013Ramkissoon, J.S.; Mahomoodally, M.F.; Ahmed, N.; Subratty, A.H. 2013. Antioxidant and anti-glycation activities correlates with phenolic composition of tropical medicinal herbs. Asian Pacific Journal of Tropical Medicine, 6: 561-569.). Our differing results may be related to the CP dose and treatment duration (Horn et al. 2016Horn, R.C.; Magni, M.P.; Mori, N.C.; Junges, L.; Golle, D.P.; Koefender, J.; Manfio, C.E.; Gelatti, G.T.; Felippin, T. 2016. Avaliação “in vitro” do efeito da infusão de Cunila microcephala Benth sobre a atividade da enzima acetilcolinesterase e biomarcadores de estresse oxidativo em eritrócitos de agricultores. Revista Brasileira de Plantas Medicinais, 18: 341-348.; Qiu et al. 2016).
An increase in TBARS may result from oxidative processes that compromise antioxidant enzymes, leading to damage to cellular membranes and their functions (Borges et al. 2011Borges, L.L.; Lúcio, T.C.; Gil, E.D.S.; Barbosa, E.F. 2011. Uma abordagem sobre Métodos analíticos para determinação da atividade antioxidante em produtos naturais. Enciclopédia Biosfera, 7: 1-20.). We observed no effect of CP and C. decorticans extract on TBARS in hepatic and renal tissues. TBARS results are dose-dependent (Horn et al. 2016Horn, R.C.; Magni, M.P.; Mori, N.C.; Junges, L.; Golle, D.P.; Koefender, J.; Manfio, C.E.; Gelatti, G.T.; Felippin, T. 2016. Avaliação “in vitro” do efeito da infusão de Cunila microcephala Benth sobre a atividade da enzima acetilcolinesterase e biomarcadores de estresse oxidativo em eritrócitos de agricultores. Revista Brasileira de Plantas Medicinais, 18: 341-348.). TBARS was significantly increased in mice liver exposed to 25 mg kg-1 of CP for 10 days (Zarei and Shivanandappa 2013Zarei, M.; Shivanandappa, T. 2013. Amelioration of cyclophosphamide-induced hepatotoxicity by the root extract of Decalepis hamiltonii in mice. Food Chemistry Toxicology, 57: 179-184.), also in mice 24 hours after having been administrated one dose of 200 mg kg-1 CP (Valadares et al. 2010Valadares, M.C.; Pereira, E.R.T.; Benfica, P.L; Paula, J.R. 2010. Assessment of mutagenic and antimutagenic effects of Punica granatum in mice. Brazilian Journal of Pharmaceutical Sciences, 46: 121-127.), and in the kidney of rats exposed to 27 mg kg-1 CP administered four times in ten weeks (Kocahan et al. 2017Kocahan, S.; Dogan, Z.; Erdemli, E.; Taskin, E. 2017. Protective effect of quercetin against oxidative stress-induced toxicity associated with doxorubicin and cyclophosphamide in rat kidney and liver tissue. Iranian Journal of Kidney Diseases, 11: 124-131. ). In a test of rutin as a potential inhibitor of the oxidative stress caused by CP, the levels of lipid peroxidation also remained unchanged (Nafees et al. 2015Nafees, S.; Rashid, S.; Ali, N.; Hasan, S.K.; Sultana, S. 2015. Rutin ameliorates cyclophosphamide induced oxidative stress and inflammation in Wistar rats: Role of NFkB/MAPK pathway. Chemico-Biological Interactions, 231: 98-107.).
Ascorbic acid levels did not vary significantly among treatments, indicating that CP, EE and FM were inert in this test for oxidative stress. This is an important non-enzymatic antioxidant agent, since ascorbic acid acts in the defense of numerous cellular structures, and in low concentrations it prevents oxidation and consequent apoptosis (Head 1998Head, K.A. 1998. Ascorbic acid in the prevention and treatment of cancer. Alternative Medicine Review, 3: 174-86.). Similarly, the antioxidant activity of catalase did not change, indicating that our treatments caused no harmful effects involving a response of this enzyme. The catalase is an enzymatic antioxidant that converts H2O2 (considered a reactive oxygen species) to water and oxygen, and is important in the inactivation processes of oxidative and/or xenobiotic agents. It is increased in response to liver damage and also by enzyme synthesis during oxidative stress (Bonfanti et al. 2016Bonfanti, G.; Eliete, P.; Bitencourt, R.; Bona, K.S. De; Ricardo, L.; Cargnelutti, L.O.; et al. 2016. Safety assessment and behavioral effects of leaf extract of Solanum guaraniticum in rats. Brazilian Journal Pharmaceutical Sciences, 52: 45-57.). Catalase activity can be increased or decreased in the presence of plant extracts (Olaleyeet al. 2014Olaleye, M.T.; Amobonye, A.E.; Komolafe, K.; Akinmoladun, A.C. 2014. Protective effects of Parinari curatellifolia flavonoids against acetaminophen-induced hepatic necrosis in rats. Saudi Journal of Biological Sciences, 21: 486-492.), and also responds to the effects of CP, depending on dose and exposure time (Haque et al. 2001Haque, R.; Bin-Hafeez, B.; Ahmad, I.; Parvez, S.; Pandey, S.; Raisuddin, S. 2001. Protective effects of Emblica officinalis Gaertn. in cyclophosphamide-treated mice. Human & Experimental Toxicology, 20: 643 -650; Zarei and Shivanandappa 2013Zarei, M.; Shivanandappa, T. 2013. Amelioration of cyclophosphamide-induced hepatotoxicity by the root extract of Decalepis hamiltonii in mice. Food Chemistry Toxicology, 57: 179-184.; Kocahan et al. 2017Kocahan, S.; Dogan, Z.; Erdemli, E.; Taskin, E. 2017. Protective effect of quercetin against oxidative stress-induced toxicity associated with doxorubicin and cyclophosphamide in rat kidney and liver tissue. Iranian Journal of Kidney Diseases, 11: 124-131. ).
The known effects of CP, such as hepatotoxicity, nephrotoxicity, cardiotoxicity, oncogenic potential of secondary neoplasias, and generation of oxygen reactive species, are dependent on the administration dose and time of exposure (Mansour et al. 2015Mansour, H.H.; El Kiki, S.M.; Hasan, H.F. 2015. Protective effect of N-acetylcysteine on cyclophosphamide-induced cardiotoxicity in rats. Environmental Toxicology and Pharmacology, 40: 417-422.; Fahmy et al. 2016Fahmy, S.R.; Amien, A.I.; Abd-Elgleel, F.M.; Elaskalany, S.M. 2016. Antihepatotoxic efficacy of Mangifera indica L. polysaccharides against cyclophosphamide in rats. Chemico-Biological Interactions, 244: 113-120.). In this study, CP had no significant effect on the studied parameters, which was also observed by Basu et al. (2015Basu, A.; Bhattacharjee, A.; Samanta, A.; Bhattacharya, S. 2015. Prevention of cyclophosphamide-induced hepatotoxicity and genotoxicity: Effect of an l-cysteine based oxovanadium (IV) complex on oxidative stress and DNA damage. Environmental Toxicology and Pharmacoogy, 40: 747-757.viz., oxovanadium(IV). Probably the dose of 25 mg kg-1 CP was inadequate in our experimental model and therefore the effects of the extracts on the evaluated parameters could not be clearly observed.
The reduction in the frequency of MNPCE in the groups treated with EE and FM indicated a potential for C. decorticans extracts to have a protective effect against in vivo DNA damage caused by CP, which acts indirectly as an alkylating agent (IARC 2012IARC. 2012. International Agency for Research on Cancer. Working group on the evaluation of carcinogenic risks to humans. Pharmaceuticals, v. 100A. A review of human carcinogens. IARC, Lyon, France, p.63-90. ). These results suggest a possible concentration of antimutagenic substances in EE and FM or an adjuvant effect of the extracts, probably due to their content of phenolic compounds. In this context, the activity profile of the flavonoids identified in the extracts is indicative of antimutagenic potential. Rutin showed antioxidant and anti-inflammatory capacity against CP, preventing tissue and metabolic injuries (Nafees et al. 2015Nafees, S.; Rashid, S.; Ali, N.; Hasan, S.K.; Sultana, S. 2015. Rutin ameliorates cyclophosphamide induced oxidative stress and inflammation in Wistar rats: Role of NFkB/MAPK pathway. Chemico-Biological Interactions, 231: 98-107.). Quercitrin also has antioxidant properties and acts in preventing liver damage (Hong et al. 2013Hong, C.O.; Rhee, C.H.; Won, N.H.; Choi, H.D.; Lee, K.W. 2013. Protective effect of 70% ethanolic extract of Lindera obtusiloba Blume on tert-butyl hydroperoxide-induced oxidative hepatotoxicity in rats. Food and Chemical Toxicology, 53: 214-220.). Luteolin is cited as having anticancer and antioxidant properties (Samy et al., 2006Samy, R.P.; Gopalakrishnakone, P.; Ignacimuthu, S. 2006. Anti-tumor promoting potential of luteolin against 7,12-dimethylbenz(a)anthracene-induced mammary tumors in rats. Chemico-Biological Interactions, 164: 1-14.). Apigenin has been suggested to have protective action against genotoxic effects (Siddique et al. 2010Siddique, Y.H.; Ara, G.; Beg, T.; Afzal, M. 2010. Anticlastogenic effect of apigenin in human lymphocytes treated with ethinylestradiol. Fitoterapia, 81: 590-594.).
There was no indication of mutagenic potential for the FM extract, yet the EE extract showed mutagenic activity. Many substances with reported antimutagenic and anticancer activitiy may also show mutagenic and carcinogenic activity, mainly through their pro-oxidant effect (Zeiger 2003Zeiger, E. 2003. Illusions of safety: antimutagens can be mutagens, and anticarcinogens can be carcinogens. Mutation Research: Reviews in Mutation Research, 543: 191-194.). Phenolic compounds such as flavonoids have genotoxic potential (León-González et al. 2015León-González, A.J.; Auger, C.; Schini-Kerth, V.B. 2015. Pro-oxidant activity of polyphenols and its implication on cancer chemoprevention and chemotherapy. Biochemical Pharmacology, 98: 371-380.). For example, ascorbic acid can synthesize reactive oxygen species, which can promote mutagenic events when uncontrolled (Kramarenko et al. 2006Kramarenko, G.G.; Wilke, W.W.; Dayal, D.; Buettner, G.R.; Schafer, F.Q. 2006. Ascorbate enhances the toxicity of the photodynamic action of Verteporin in HL-60 cells. Free Radical Biology and Medicine, 40: 1615-1627.; León-González et al. 2015). Rutin and quercetin have deleterious mutagenic effects when used in high and non-feasible dosages (Silva et al. 2002Silva, J.D.; Herrmann, S.M.; Heuser, V.; Peres, W.; Possa Marroni, N.; González-Gallego, J.; Erdtmann, B. 2002. Evaluation of the genotoxic effect of rutin and quercetin by comet assay and micronucleus test. Food Chemistry Toxicology, 40: 941-947.). Flavonoids may act as genotoxic substances depending on variation in pH, presence of antioxidants and metabolic factors, and their antioxidant or pro-oxidant activity is dose-dependent, mainly in relation to myricetin (Hobbs et al. 2015Hobbs, C.A.; Swartz, C.; Maronpot, R.; Davis, J.; Recio, L.; Koyanagi, M.; Hayashi, S.M. 2015. Genotoxicity evaluation of the flavonoid, myricitrin, and its aglycone, myricetin. Food and Chemical Toxicology, 83: 283-292.). The antimutagenic and mutagenic effects observed in the treatment with C. decorticans extracts may be attributed to the presence of secondary metabolites (Gobbo-Neto and Lopes 2007Gobbo-Neto, L.; Lopes, N.P. 2007. Plantas medicinais: fatores de influência no conteúdo de metabólitos secundários. Química Nova, 30: 374-81.; Sampaio et al. 2016Sampaio, B.L.; Edrada-Ebel, R.; Da Costa, F.B. 2016. Effect of the environment on the secondary metabolic profile of Tithonia diversifolia: a model for environmental metabolomics of plants. Nature Scientific Reports, 6: 2926.).
CONCLUSIONS
Total phenol content, flavonoid compounds and antiradical activity determined in the ethanolic extract (EE) and hydromethanolic fraction (FM) of Capirona decorticans leaves supported the results obtained in vivo. EE and FM did not generate pathological alterations and prevented protein carbonylation caused by cyclophosphamide (CP) in hepatic tissue of mice. Seven flavonoids with biological activities were identified by LC-MS/MS. The reduction in MNPCE in mice treated with EE and FM plus CP indicated an antimutagenic effect, yet a mutagenic effect was also observed in mice treated only with EE, which may be related to the presence of mutagenic substances in the extract. The results suggest that C. decorticans leaf extract contains compounds with protective effect against pathological alterations in liver tissue, and that flavonoids may be involved in the observed antimutagenic action. The chemoprotective property of C. decorticans may be related to a possible anticarcinogenic effect.
ACKNOWLEDGMENTS
The authors acknowledge Universidade Federal de Mato Grosso for logistic support, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Amparo à Pesquisa do Estado de Mato Grosso (FAPEMAT) (Process number 159443/2015) for the financial support that made this work possible.
- Arafa, H.M. 2009. Uroprotective effects of curcumin in cyclophosphamide-induced haemorrhagic cystitis paradigm. Basic Clinical Pharmacology and Toxicology, 104: 393-399.
- Ares, A.M.; Valverde, S.; Nozal, M.J.; Bernal, J.L.; Bernal, J. 2016. Development and validation of a specific method to quantify intact glucosinolates in honey by LC-MS/MS. Journal Food Composition Analysis, 46: 114-122.
- Barrabé, L.; Maggia, L.; Pillon, Y., Rigault, F.F.; Mouly, A.; Davis, A.P.; Buerki, S. 2014. New Caledonian lineages of Psychotria (Rubiaceae) reveal different evolutionary histories and the largest documented plant radiation for the archipelago. Molecular Phylogenetics Evolution, 71: 15-35.
- Basu, A.; Bhattacharjee, A.; Samanta, A.; Bhattacharya, S. 2015. Prevention of cyclophosphamide-induced hepatotoxicity and genotoxicity: Effect of an l-cysteine based oxovanadium (IV) complex on oxidative stress and DNA damage. Environmental Toxicology and Pharmacoogy, 40: 747-757.
- Bonfanti, G.; Eliete, P.; Bitencourt, R.; Bona, K.S. De; Ricardo, L.; Cargnelutti, L.O.; et al. 2016. Safety assessment and behavioral effects of leaf extract of Solanum guaraniticum in rats. Brazilian Journal Pharmaceutical Sciences, 52: 45-57.
- Borges, L.L.; Lúcio, T.C.; Gil, E.D.S.; Barbosa, E.F. 2011. Uma abordagem sobre Métodos analíticos para determinação da atividade antioxidante em produtos naturais. Enciclopédia Biosfera, 7: 1-20.
- Bourgaud, F.; Gravot, A.; Milesi, S.; Gontier, E. 2001. Production of plant secondary metabolites: A historical perspective Plant Science, 161: 839-851.
- Bradford, M.M. 1976. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72: 248-254.
- Buege, J.A.; Aust, S.D. 1978. Microsomal lipid peroxidation. Methods in Enzymology, 52: 302-309.
- Cândido, T.L.N.; Silva, M.R.; Agostini-Costa, T.S. 2015. Bioactive compounds and antioxidant capacity of buriti (Mauritia flexuosa L.f.) from the Cerrado and Amazon biomes. Food Chemistry, 177: 313-319.
- Cao, Y.; Cao, R. 1999. Angiogenesis inhibited by drinking tea. Nature, 398: 381.
- Castillo, D.; Arevalo, J.; Herrera, F.; Ruiz, C.; Rojas, R.; Rengifo, E.; et al 2007. Spirolactoneiridoids might be responsible for the antileishmanial activity of a Peruvian traditional remedy made with Himatanthus sucuuba (Apocynaceae). Journal of Ethnopharmacology, 112: 410-414.
- Costa, D.A.; Chaves, M.H.; Silva, W.C.S.; Costa, C.L.S. 2010. Chemical constituents, total phenolics and antioxidant activity of Sterculia striata St. Hil. et Naud. Acta Amazonica, 40: 207-212.
- Coulibaly, A.Y.; Hashim, R.; Sulaiman, S.F.; Sulaiman, O.; Ang, L.Z.P.; Ooi, K.L. 2014. Bioprospecting medical plants for antioxidant components. Asian Pacific Journal of Tropical Medicine, 7: S553-S559.
- Dai, B.; Hu, Z.; Li, H.; Yan, C.; Zhang, L. 2015. Simultaneous determination of six flavonoids from Paulownia tomentosa flower extract in rat plasma by LC-MS/MS and its application to a pharmacokinetic study. Journal of Chromatography B, 26: 54-61.
- Delmanto, R.D.; de Lima, P.L.A.; Sugui, M.M.; da Eira, A.F.; Salvadori, D.M.F.; Speit, G.; Ribeiro, L.R. 2001. Antimutagenic effect of Agaricus blazei Murrill mushroom on the by cyclophosphamide. Mutation Research, 496: 15-21.
- Fahmy, S.R.; Amien, A.I.; Abd-Elgleel, F.M.; Elaskalany, S.M. 2016. Antihepatotoxic efficacy of Mangifera indica L. polysaccharides against cyclophosphamide in rats. Chemico-Biological Interactions, 244: 113-120.
- Garcia-Nino, W.R.; Pedraza-Chaverri, J. 2014. Protective effect of curcumin against heavy metals-induced liver damage. Food and Chemistry Toxicology, 69: 182-201.
- Gobbo-Neto, L.; Lopes, N.P. 2007. Plantas medicinais: fatores de influência no conteúdo de metabólitos secundários. Química Nova, 30: 374-81.
- Haque, R.; Bin-Hafeez, B.; Ahmad, I.; Parvez, S.; Pandey, S.; Raisuddin, S. 2001. Protective effects of Emblica officinalis Gaertn. in cyclophosphamide-treated mice. Human & Experimental Toxicology, 20: 643 -650
- He, C.Y.; Fu, J.; Ma, J.Y.; Feng, R.; Tan, X.S.; Huang, M. 2014. Biotransformation and in vitro metabolic profile of bioactive extracts from a traditional miao-nationality herbal medicine, polygonum capitatum. Molecules, 19: 10291-10308.
- Head, K.A. 1998. Ascorbic acid in the prevention and treatment of cancer. Alternative Medicine Review, 3: 174-86.
- Hobbs, C.A.; Swartz, C.; Maronpot, R.; Davis, J.; Recio, L.; Koyanagi, M.; Hayashi, S.M. 2015. Genotoxicity evaluation of the flavonoid, myricitrin, and its aglycone, myricetin. Food and Chemical Toxicology, 83: 283-292.
- Hong, C.O.; Rhee, C.H.; Won, N.H.; Choi, H.D.; Lee, K.W. 2013. Protective effect of 70% ethanolic extract of Lindera obtusiloba Blume on tert-butyl hydroperoxide-induced oxidative hepatotoxicity in rats. Food and Chemical Toxicology, 53: 214-220.
- Horn, R.C.; Magni, M.P.; Mori, N.C.; Junges, L.; Golle, D.P.; Koefender, J.; Manfio, C.E.; Gelatti, G.T.; Felippin, T. 2016. Avaliação “in vitro” do efeito da infusão de Cunila microcephala Benth sobre a atividade da enzima acetilcolinesterase e biomarcadores de estresse oxidativo em eritrócitos de agricultores. Revista Brasileira de Plantas Medicinais, 18: 341-348.
- IARC. 2012. International Agency for Research on Cancer. Working group on the evaluation of carcinogenic risks to humans. Pharmaceuticals, v. 100A. A review of human carcinogens. IARC, Lyon, France, p.63-90.
- Kocahan, S.; Dogan, Z.; Erdemli, E.; Taskin, E. 2017. Protective effect of quercetin against oxidative stress-induced toxicity associated with doxorubicin and cyclophosphamide in rat kidney and liver tissue. Iranian Journal of Kidney Diseases, 11: 124-131.
- Korkmaz, A.; Topal, T.; Obter, S. 2007. Pathophysiological aspects of cyclophosphamide and ifosfamide induced hemorrhagic cystitis; implication of reactive oxygen and nitrogen species as well as PARP activation. Cell Biology and Toxicology, 23: 303-12.
- Kramarenko, G.G.; Wilke, W.W.; Dayal, D.; Buettner, G.R.; Schafer, F.Q. 2006. Ascorbate enhances the toxicity of the photodynamic action of Verteporin in HL-60 cells. Free Radical Biology and Medicine, 40: 1615-1627.
- Kren, V.; Walterova, D. 2005. Silybin and silymarin - new effects and applications. Biomedical Papers, 149: 29-41.
- León-González, A.J.; Auger, C.; Schini-Kerth, V.B. 2015. Pro-oxidant activity of polyphenols and its implication on cancer chemoprevention and chemotherapy. Biochemical Pharmacology, 98: 371-380.
- MacGregor, J.T.; Heddle, J.A.; Hite, M.; Marcolin, B.H.; Ramel, C.; Salamone, M.F.; Tice, R.R.; Wild, D. 1987. Guidelines for the conduct of micronucleus assays in mammalian bone marrow erythrocytes. Mutation Research, 189: 103-112.
- Malone, M.H. 1983. The pharmacological evaluation of natural products - general and specific approachs to screening ethnopharmaceuticals. Journal of Ethnopharmacology, 8: 127-147.
- Manoharan, K.; Banerjee, M. 1985. β-Carotene reduces sister chromatid exchange induce chemical carcinogens in mouse mammary cells in organ culture. Cell Biology International Reports, London, 9: 783-789.
- Martins, D.; Nunez, C.V. 2015. Secondary metabolites from Rubiaceae species.Molecules, 20: 13422-95.
- Mansour, H.H.; El Kiki, S.M.; Hasan, H.F. 2015. Protective effect of N-acetylcysteine on cyclophosphamide-induced cardiotoxicity in rats. Environmental Toxicology and Pharmacology, 40: 417-422.
- Morais, M.G.; da Costa., G.A.F.; Aleixo, A.A.; de Oliveira, G.T.; Alves, L.F.; Duarte-Almeida, J.M.; Ferreira, J.M.S.; dos Santos Lima, L.A.R. 2015. Antioxidant, antibacterial and cytotoxic potential of the ripe fruits ofSolanum lycocarpumA. St. Hil. (Solanaceae). Natural Product Research, 29: 480-483
- Nafees, S.; Rashid, S.; Ali, N.; Hasan, S.K.; Sultana, S. 2015. Rutin ameliorates cyclophosphamide induced oxidative stress and inflammation in Wistar rats: Role of NFkB/MAPK pathway. Chemico-Biological Interactions, 231: 98-107.
- Nelson, D.P.; Kiesow, L.A. 1972. Enthalphy of decomposition of hydrogen peroxide by catalase at 25 °C (with molar extinction coefficients of H2O2 solution in the UV). Analytical Biochemistry, 49: 474-478.
- Olaleye, M.T.; Amobonye, A.E.; Komolafe, K.; Akinmoladun, A.C. 2014. Protective effects of Parinari curatellifolia flavonoids against acetaminophen-induced hepatic necrosis in rats. Saudi Journal of Biological Sciences, 21: 486-492.
- Pereira, C.A.B. 1991. Teste estatístico para comparar proporções em problemas de citogenética. In: Rabello-Gay, M.N.; Rodrigues, M.A.; Montelleone-Neto, L.A.R. (Ed.) Mutagênese, teratogênese e carcinogênese: métodos e critérios de avaliação Ed. FCA, São Paulo, p.113-21.
- Qiu, C.; Zhu, T.; Lan, L.; Zeng, Q.; Du, Z. 2016. Analysis of maceaene and macamide contents of petroleum ether extract of black, yellow, and purple Lepidium meyenii (maca) and their antioxidant effect on diabetes mellitus rat model. Brazilian Archives Biology and Technology, 59: 1-9.
- Ramkissoon, J.S.; Mahomoodally, M.F.; Ahmed, N.; Subratty, A.H. 2013. Antioxidant and anti-glycation activities correlates with phenolic composition of tropical medicinal herbs. Asian Pacific Journal of Tropical Medicine, 6: 561-569.
- Ribeiro, L.R.; Salvadori, D.M.F.; Marques, E.K. 2003. Mutagênese ambiental Editora da ULBRA, Canoas-RS, 356p.
- Roe, J.H. 1954. Chemical determination of ascorbic, dehydroascorbic, and diketogulonic acids. In: Glick, D. (Ed.). Methods of Biochemical Analysis, v.1. Interscience, New York, p.115.
- Sampaio, B.L.; Edrada-Ebel, R.; Da Costa, F.B. 2016. Effect of the environment on the secondary metabolic profile of Tithonia diversifolia: a model for environmental metabolomics of plants. Nature Scientific Reports, 6: 2926.
- Samy, R.P.; Gopalakrishnakone, P.; Ignacimuthu, S. 2006. Anti-tumor promoting potential of luteolin against 7,12-dimethylbenz(a)anthracene-induced mammary tumors in rats. Chemico-Biological Interactions, 164: 1-14.
- Siddique, Y.H.; Ara, G.; Beg, T.; Afzal, M. 2010. Anticlastogenic effect of apigenin in human lymphocytes treated with ethinylestradiol. Fitoterapia, 81: 590-594.
- Silva, J.D.; Herrmann, S.M.; Heuser, V.; Peres, W.; Possa Marroni, N.; González-Gallego, J.; Erdtmann, B. 2002. Evaluation of the genotoxic effect of rutin and quercetin by comet assay and micronucleus test. Food Chemistry Toxicology, 40: 941-947.
- Simirgiotis, M.J.; Quispe, C.; Areche, C.; Sepúlveda, B. 2016. Phenolic compounds in chilean mistletoe (quintral, Tristerix tetrandus) analyzed by UHPLC-Q/Orbitrap/MS/MS and its antioxidant properties. Molecules, 21: 245.
- Sun, Z.; Zhao, L.; Zuo, L.; Qi, C.; Zhao, P.; Hou, X. 2014. A UHPLC-MS/MS method for simultaneous determination of six flavonoids, gallic acid and 5,8-dihydroxy-1,4-naphthoquinone in rat plasma and its application to a pharmacokinetic study of Cortex juglandis Mandshuricae extract. Journal of Chromatography B, 958: 55-62.
- Taylor, A.; Campos, M.; Zappi, D. 2007. Flora da Reserva Ducke, Amazonas, Brasil: Rubiaceae Rodriguesia, 58: 549-616.
- Terpinc, P.; Cigic, B.; Polak, T.; Hribar, J.; Porl, T. 2016. LC-MS analysis of phenolic compounds and antioxidant activity of buckwheat at different stages of malting. Food Chemistry, 210: 9-17.
- Valadares, M.C.; Pereira, E.R.T.; Benfica, P.L; Paula, J.R. 2010. Assessment of mutagenic and antimutagenic effects of Punica granatum in mice. Brazilian Journal of Pharmaceutical Sciences, 46: 121-127.
- Waters, M.D.; Brady, A.L.; Stack, H.F.; Brockman, H.E. 1990. Antimutagenicity profiles for some model compounds. Mutation Research: Reviews in Genetic Toxicology, 238: 57-85.
- Yan, L.J.; Traber, M.G.; Packer, L. 1995. Spectrophotometric method for determination of carbonyls in oxidatively modified apolipoprotein B of human low-density lipoproteins. Analytical Biochemistry, 228: 349-351.
- Yasir, M.; Sultana, B.; Nigam, P.S.; Owusu-Apenten, R. 2016. Antioxidant and genoprotective activity of selected cucurbitaceae seed extracts and LC-ESIMS/MS identification of phenolic components. Food Chemistry, 199: 307-313.
- Zarei, M.; Shivanandappa, T. 2013. Amelioration of cyclophosphamide-induced hepatotoxicity by the root extract of Decalepis hamiltonii in mice. Food Chemistry Toxicology, 57: 179-184.
- Zeiger, E. 2003. Illusions of safety: antimutagens can be mutagens, and anticarcinogens can be carcinogens. Mutation Research: Reviews in Mutation Research, 543: 191-194.
-
CITE AS:
Barbosa, F.G.; Sugui, M.M.; Sinhorin, V.D.G.; Bicudo, R. de C.; Moura, F.R. de; Sinhorin, A.P. 2018. First phytochemical and biological study of the ethanolic extract from leaves of Capirona decorticans (Rubiaceae). Acta Amazonica 48: 338-346.
Edited by
ASSOCIATE EDITOR:
Publication Dates
-
Publication in this collection
Oct-Dec 2018
History
-
Received
03 Oct 2017 -
Accepted
08 Aug 2018