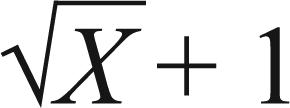Abstracts
This study aimed to evaluate the larval biology of Heliothis virescens in soybean MON 87701 x MON 89788 and its isogenic non-Bt. In general, the impact of soybean MON 87701 x MON 89788 on H. virescens was evidenced in all bioassays, 100% larval mortality, independent of the structure (leaf or pod) consumed by the pest. The small larvae (1st and 3rdinstar) demonstrated that they were unable to damage fresh pods of soybean, regardless of whether Bt or not Bt. The large larvae (5th instar) fed on soybean MON 87701 x MON 89788 soybeans consumed three times less compared to larvae fed on non-Bt soybeans, and resulted in reduced longevity and larval survival. When soybean plants were infested with 5th instar larvae, H. virescens caused injuries in the steams of the conventional soybean. It was recorded that the insects moves quickly to this region of the plant. However the soybean MON 87701 x MON 89788 was an effective tool in controlling H. virescens.
varietal resistance; tobacco budworm; soybean pests; lepidoptera pests; Heliothinae
Este estudo objetivou avaliar a biologia larval de Heliothis virescens em soja MON 87701 x MON 89788 e na soja convencional não Bt. De forma geral, o impacto da soja MON 87701 x MON 89788 sobre H. virescens foi evidenciado em todos os bioensaios, com 100% de mortalidade larval, independente da estrutura (folha ou vagem) consumida pela praga. As lagartas pequenas (1° e 3° ínstar) demonstraram que são incapazes de danificarem vagens verdes de soja, independente de ser Bt ou não Bt. As lagartas grandes (5° ínstar) que se alimentaram de soja MON 87701 x MON 89788 consumiram três vezes menos soja comparativamente às desenvolvidas em soja não Bt, além de resultar em menor longevidade e viabilidade larval. No último experimento, as lagartas grandes de H. virescens ocasionaram injúrias na ponteira da soja convencional, verificando-se que a praga se desloca rapidamente para esta região da planta. Entretanto, a soja MON 87701 x MON 89788 é uma eficiente ferramenta no controle de H. virescens.
resistência varietal; lagarta-da-maçã; pragas da soja; lepidóptero-praga; Heliothinae
INTRODUCTION
Genetically modified crop plants, expressing proteins from the cry genes of Bacillus thuringiensis Berliner, to control pests from both the order Lepidoptera (Yu et al. 2011Yu HL, Yun HL and Kong MW. 2011. Risk assessment and ecological effects of transgenic Bacillus thuringiensis crops on non-target organisms. J Integr Plant Biol 53: 520-538.) and Coleoptera (Reed et al. 2001Reed GL, Jensen AS, Riebe J, Head G and Duan JJ. 2001. Transgenic Bt potato and conventional insecticides for Colorado potato beetle management: comparative efficacy and non-target impacts. Entomol Exp Appl 100: 89-100.), are increasingly cropped worldwide (Bobrowski et al. 2003Bobrowski VL, Fiuza LM, Pasquali G and Bodanese-Zanettini MH. 2003. Genes de Bacillus thuringiensis: uma estratégia para conferir resistência a insetos em plantas (Genes from Bacillus thuringiensis: a strategy to confer insect resistance in plants). Cienc Rural 34: 843-850.) not only due to their high efficacy but also because they are very easy to be used. In this scenario, a Bt-soybean has recently been developed by Monsanto combining the transformation events MON 87701 (expressing Cry1Ac protein) and MON 89788 (glyphosate tolerance) and was first commercially released in Brazil in the 2013-14 season. Brazil is the first country in the world to crop Bt-soybean and its experience with this technology will certainly be important worldwide.
The Bt-soybean, MON 87701 x MON 89788, was developed to be efficient against pests from the genus Lepidoptera. However, soybean crop is usually injured by different caterpillar species that might differ in its susceptibility to the Bt technology (Bernardi et al. 2012Bernardi O, Malvestiti GS, Dourado PM, Oliveira WS, Martinelli S, Berger GU, Head GP and Omoto C. 2012. Assessment of the high-dose concept and level of control proveided by Mon 87701 x MON 89788 soybean against Anticarsia gemmatalis and Pseudoplusia includens (Lepidptera: Noctuidae) in Brazil. Pest Manag Sci 68: 1083-1091., 2013Bernardi O, Dourado PM, Carvalho RA, Martinelli S, Berger GU, Head GP and Omoto C. 2013. High levels of biological activity of Cry1Ac protein expressed on MON 87701 x MON 89788 soybean against Heliothis virescens (Lepidoptera: Noctuidae). Pest Manag Sci DOI: 10.1002/ps.3581.
https://doi.org/10.1002/ps.3581...
). Recently, Brazilian growers are facing several caterpillar outbreaks around the country. Species from the subfamily Heliothiinae, which includes different destructive caterpillars such as Heliothis virescens and Helicoverpa zea or Helicoverpa armigera, for example, used to be regarded as secondary pest or did not occur. However, they were observed in the 2012/2013 and already early in the 2013/2014 crop season in high population levels (Bueno et al. 2013Bueno AF, Hirose E and Sosa-Gómez DR. 2013. Manejo Racional. Cultivar Grandes Culturas 173: 26-28.). Not only do these budworms attack the plant leaves, but also the soybean pods (Degrande and Vivan 2007Degrande PE and Vivan LM. 2007. Pragas da Soja. In: Boletim de Pesquisa da Soja: Fundação MT, 274 p.). Despite the greater occurrence of these pest in today's soybean crops (Tomquelski and Maruyama 2009Tomquelski GV and Maruyama LCT. 2009. Lagarta-da-maçã em soja. Cultivar Grandes Culturas 117: 20-22.), the behavior of H. virescensbudworms in soybean plants in early stages and the effect of Bt-soybean on these species are still unclear. Studies on the larval development of pests that feed on soybean leaves and pods are, therefore, needed. It is important to point out, not only might it be triggering yield losses on soybean but also on other crops as well. Consequently, the present study aimed to evaluate the impact of Bt-soybean on the biological characteristics of H. virescens, as well as larval behavior when feeding on soybean plants at early stages.
MATERIALS AND METHODS
Experimental Conditions and Insect Colony
This study was carried out at Embrapa Soja, in BOD-type climatized chambers set at 25 ± 2°C, with a relative humidity (RH) of 70 ± 10%, and photophase of 14 h. Helicoverpa virescens was reared under laboratory-controlled environmental conditions [25 ± 2°C temperature, 70 ± 10% RH, and 14/10 h photoperiod (L/D)] for about 30 generations and fed on the artificial diet proposed by Greene et al. (1976)Greene GL, Leppla NC and Dickerson WA. 1976. Velvetbean caterpillar: a rearing procedure and artificial medium. J Econ Entomol 69: 487-488..
Preparation and Use of Soybean Plants in Bioassays
The soybean used in this study was developed by the company Monsanto. The Bt-soybean MON 87701 × MON 89788 (expressing the Cry1Ac protein) and its isogenic non-Bt A5547 were used in this study. Plants were sowed in plastic vases (5-L) and kept in a greenhouse. Fertilization with 0-20-20 was applied 15 days after plant emergence, and oidium control was performed with the application of a 0.5 g/L dose of a sulfur-based product (S) (Kumulus®) whenever required.
Plants were used in the trials at R6 development stage (Fehr and Caviness 1977Fehr WR and Caviness CE. 1977. Stages of soybean development. Ames: University of Sciense and Technology. 80v.). Before feeding the caterpillar with the plants, prophylaxis of the plant tissues was performed by submerging the leaves and pods in a solution of water + 5% sodium hypochlorite for 15 min. Subsequently, this material was rinsed and offered to the budworms once completely dried.
Bioassay 1: Larval Longevity and Survival of H. VirescensFED With Bt and Non-Bt Soybeans
The experiment was carried out under controlled environmental conditions (25 ± 2°C, RH of 70 ± 10%, and photoperiod of 14:10 [L:D] h) in a fully randomized design with six treatment and four replicates per treatment. Each replicate consisted of the average value of 12 individualized caterpillars. Two genetic soybean materials were used (MON 87701 × MON 89788 and A5547) and were offered ad libitum in three different ways: leaves only, pod and leaves, and only pods totaling six treatments. The insects were placed in an acrylic gerbox with its bottom lined with filter paper. This paper was previously dampened to maintain humidity within the box.
To reduce the loss of moisture in the food, a ball of cotton wool soaked in water was placed around the stalk of the offered leaf or pods. Two holes were made on the gerbox lids (25 cm in diameter) to allow the flow of air and to avoid the accumulation of excess moisture. Each box received the caterpillars, which were placed in the center of the box, and the duration of the larval phase was assessed (in days), as well as the survival (%) of the individuals.
Bioassays 2 and 3: Biological Characteristics of H. Virescens FED with Bt and Non-BtGreen soybean Pods
The experiments were carried out under controlled environmental conditions (25 ± 2°C, RH of 70 ± 10%, and photoperiod of 14:10 [L:D] h) in a fully randomized design with two treatment and five replicates per treatment. Each replicate consisted of the average value of six individualized caterpillars of 3rd instar for bioassay 2, and 5th instar for bioassay 3. The larval development of H. virescens fed with MON 87701 × MON 89788 green soybean pods was compared with caterpillar fed with the isogenic non-Btsoybean.
The pods offered to the budworms were collected from soybean plant at R6 phenological stage (Fehr and Caviness 1977Fehr WR and Caviness CE. 1977. Stages of soybean development. Ames: University of Sciense and Technology. 80v.) and green pods were collected from the middle of the plant. The pods went through a process of prophylaxis, in which they were immersed for 15 min in a solution of water and sodium hypochlorite (5%). Following this process, they were placed on a table that was lined with a paper towel, where they remained until the material lost its excess moisture.
The pods were weighed daily using analytical scales, and then offered to the insects. This operation was repeated daily until the budworms ceased feeding (pre-pupal phase) or died. In parallel, pods collected daily were kept in the same experimental conditions in order to correct the moisture naturally lost by the pod from one day to the next. The average rate of moisture loss was measured at approximately 5%, and the data was corrected prior to statistical analysis. Both studies (third and fifth instar caterpillars) were assessed for biological parameters, pod consumption rate (g), larval viability (%), and longevity (days). In each assessment, the insects were touched with a forceps and those that failed to move were classified as dead.
Bioassay 4: H. Virescens Feeding on Non-BtSoybean Plants at Early Development Stages
The experiment was carried out under greenhouse conditions in a fully randomized design with two treatments (Bt and non-Bt soybeans) and ten replicates per treatment. Each replicate consisted of a pot with 5 caterpillars (1 insect/plant). In total, 50 soybean plants were used (N = 5 plants/vase) and organized into five replicates with 10 plants (N = 1 insect/plant). In this experiment, the soybean plants were cultivated using the same methodology as previously described. The experiment started when the soybean plants reached the V3 stage (Fehr and Caviness 1977Fehr WR and Caviness CE. 1977. Stages of soybean development. Ames: University of Sciense and Technology. 80v.). The infestation occurred by placing a 5th instar budworm on the third soybean leaf. The assessments were conducted at five time intervals: at 4, 19, 24, 43, and 48 h after the infestation, to determine which plant structure was attacked (leaf or stem) and to identify the location of the caterpillar. The injury of the plant stem was classified as follows: no injury (whole), with injury (not total consumption), and consumed (when budworms consumed 100% of the material).
Statistical Analysis
Results obtained in the different bioassays were subjected to exploratory analyses to assess the assumptions of normality of residuals (Shapiro and Wilk 1965), homogeneity of variance of treatments and additivity of the model (Burr and Foster 1972) prior to application of ANOVA. Means were then compared by Student's t or Tukey test (p ≤ 0.05) (SAS Institute 2001SAS Institute. 2001. User's Guide: Statistics, 6th version, Cary, 2001.).
RESULTS
First instar of H. virescens presented 100% mortality in less than three days when fed on the foliage material and green pods of MON 87701 × MON 89788 soybean (Table I). When the insects fed on non-Bt soybean, caterpillar development stage lasted approximately 17 days, and maximum survival was achieved by insects fed on soybean leaves and pods (Table I).
It is important to point out that when the first and third instar caterpillars were given exclusively green soybean pods, 100% pest mortality was observed, regardless of MON 87701 × MON 89788 soybean consumption or isogenic non-Bt (Tables Iand II). First instar of H. virescens did not injure the pods and appeared to die from starvation when only this treatment was offered. On the other hand, the third instar insects managed to consume the pods, but only superficially (scratching the integument), which was not enough to survive (Table II). For the H. virescens budworms that started consumption of green pods at the fifth instar, the ingestion of MON 87701 × MON 89788 soybean affected pest development, reduced pod consumption (around three times less), reduced longevity, and caused 100% mortality (Table II). The non-Bt soybean consumption allowed for 86% budworm survival, indicating that only larger budworms were capable of damaging green pods.
Treatment Bioassay 3 Total consumption (grams) [% daily] Longevity (days) Survival (%) Bt-soybean pod 0.56 ± 0.09 b [8.00] 5.53 ± 0.60 b 0.00 ± 0.00 b Non-Bt soybean pod 1.72 ± 0.12 a [13.40] 9.60 ± 0.26 a 86.00 ± 0.08 a CV (%) 21.55 13.76 15.75 F 55.73 38.09 403.02 DFresidue 8.00 8.00 8.00 P < 0.001 < 0.001 < 0.001 1
1
When soybean plants were artificially infested by 5th instar caterpillars, the insects quickly moved to the terminal growth region of the soybean plant (Figures 1A and 1B). This feature was evident 19 hours after infestation, when approximately 75% of the insects were located at the soybean growing stems. At the end of the assessments (48 h), 90% of the plants presented injured steams (showing injury and/or consumption), and 50% of that total was completely consumed by the budworms (Figure 1B).
A) Proportion of caterpillars found on completely expanded soybean leaf or in the area of terminal growth (steams) of the young soybean plant (stage V3) conventional (non- Bt). Mean ± EPM followed by the same letter in the column of the same stage of insect development do not differ according to the student's t-test (Figure 1A) and Tukey's test (p≤0.05). (ns = no statistical difference). Analyses were performed on data transformed into (
 ).
).
DISCUSSION
The expression of Cry1Ac insecticidal protein in Btcotton has been proven to be very effective in the control of H. virescens (Terán-Vargas 2005Terán-Vargas AP. 2005. Bollgard Cotton and Resistance of Tobacco Budworm (Lepidoptera: Noctuidae) to Conventional Insecticides in Southern Tamaulipas, Mexico. J Econ Entomol 98: 2203-2209.). This is similar to the results of our study, which indicate the efficacy of MON 87701 × MON 89788 soybean in the control of H. virescens. This pest is currently a concern for soybean producers throughout many parts of Brazil, mainly because the insect's behavior is not well understood, which makes the adoption of an efficient control strategy difficult. Growers commonly face caterpillar's movement from weeds to the main crop and when the insect is in older instars, severe damage might occur (Bueno et al. 2013Bueno AF, Hirose E and Sosa-Gómez DR. 2013. Manejo Racional. Cultivar Grandes Culturas 173: 26-28.).
This study demonstrated that this pest is of greater importance than other defoliators that appear during the soybean vegetative phase, due to its peculiar behavior of feeding from the terminal growth region of the plant (steams). Interestingly, the caterpillars moved quickly towards the soybean apical region, reaching 30% of its height approximately 4 hours after infestation and 70% 19 hours later. This result suggests that new soybean plant tissue is probably more attractive to the pest, most likely due to being more palatable and having a greater nutritional valuable, since they prefer to feed from structures with greater water and nitrogen contents (Fitt 1989Fitt GP. 1989. The ecology of Heliothis species in relation to agroecossystems. Annu Rev Entomol 34: 17-52.). According to McWilliams and Beland (1977)McWilliams JM and Beland GL. 1977. Bollworm: Effect of soybean leaf and pod maturity on development in the laboratory. Ann Entomol Soc Am 70: 214-216., Helicoverpa zea(Lepidoptera: Noctuidae) worms exhibit better development when they feed on the leaves of the plant's terminal growth region, which results in the reduction of the duration of the early phase and greater larval viability. In this study, the authors recorded that the larval biology of pests is influenced by the age of the consumed leaves, with the percentage of mortality ranging from just 7% for new leaves (recently expanded) to 86% for older leaves. This could explain the relative low larval viability of H. virescens in this study (31.25 to 37.5%), as leaves from the middle third of the plant were always used.
One of the main concerns regarding the occurrence of H. virescens in soybean is the fact that the pest attacks reproductive structures of the plant such as the pods (Bueno et al. 2013Bueno AF, Hirose E and Sosa-Gómez DR. 2013. Manejo Racional. Cultivar Grandes Culturas 173: 26-28.). The comparison between pest larval development on green pods of soybean MON 87701 × MON 89788 and on its isogenic non-Bt showed once again that this technology is effective in the control of the pest. In general, the impact of Bt was greater on newborn budworms than on more developed individuals. However, this study demonstrated that MON 87701 × MON 89788 soybean caused 100% larval mortality even in large budworms (fifth instar). This result is similar to Bernardi et al. (2013)Bernardi O, Dourado PM, Carvalho RA, Martinelli S, Berger GU, Head GP and Omoto C. 2013. High levels of biological activity of Cry1Ac protein expressed on MON 87701 x MON 89788 soybean against Heliothis virescens (Lepidoptera: Noctuidae). Pest Manag Sci DOI: 10.1002/ps.3581.
https://doi.org/10.1002/ps.3581...
, although those authors used younger green pods before the start of pod filling, as opposed to this study, which used pods with full grains (stage R6). This result indicates that even in an older plant, the concentration of the insecticidal protein is sufficient and effective in the control of H. virescens budworms.
One interesting observation was that first and third instar budworms could not complete larval development when fed exclusively on green soybean pods, regardless of whether it was Bt or non-Bt. It is therefore likely that budworms need to feed on vegetative plant structures before migrating to the pods, although the pest may cause injury to this soybean structure as early as from the third instar (Table II). In cotton crops, H. virescens budworms remain in the upper region of plants during the first three instars, attacking the vegetative structures. However, when they reach the fifth instar, they move towards the lower areas of the plant and feed on the reproductive structures (Farrar and Bradley Jr 1985). This behavior was also demonstrated in soybean crops for the species Helicoverpa armigera (Lepidoptera: Noctuidae), which is a pest that is very closely related to the H. virescens (Rogers and Brier 2010Rogers DJ and Brier HB. 2010. Pest-damage relationships for Helicoverpa armigera (Hubner) (Lepidoptera: Noctuidae) on soybean (Glycine max) and dry bean (Phaseolus vulgaris) during pod-fill. Crop Protection 29: 47-57.).
In summary, this study demonstrated that MON 87701 × MON 89788 soybean may significantly contribute towards the effort to control H. virescens even the caterpillars that might come from other plants. Moreover, this study encourages additional research investigations to assess the impact of H. virescens at different soybean phenological stages because during the soybean vegetative stage, this species does not only feed on soybean leaves, but also attacks (and prefers) the growth region of the plant, which increases the damage caused to the culture. This behavior distinguishes this species from classic defoliators, such as A. gemmatalis and C. includens, which invalidates using only the pest's leaf consumption rate when establishing multiple control levels (Bueno et al. 2011Bueno RCOF, Bueno AF, Moscardi F, Parra JRP and Hoffmann-Campo CB. 2011. Lepidopteran larva consumption of soybean foliage: basis for developing multiple-species economic thresholds for pest management decisions. Pest Manag Sci 67: 160-164.).
CONCLUSIONS
The soybean MON 87701 × MON 89788 can be an important tool in the control of H. virescens based on its efficiency in pest control, resulting in insect death in the larval stage when it feeds on leaves or pods of resistant soybean.
The fifth instar larvae of H. virescens are characterized by attacking the soybean apices (steams), which can lead to serious damage in non-Bt soybean crops. Additional studies are required to clarify the impact of the pest on culture productivity and to establish economic thresholds to be used in integrated pest management.
The authors would like to thank Embrapa Soja and the funding agencies Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for their financial support. This paper was approved for publication by the Editorial Board of Embrapa Soja.
REFERENCES
- Bernardi O, Dourado PM, Carvalho RA, Martinelli S, Berger GU, Head GP and Omoto C. 2013. High levels of biological activity of Cry1Ac protein expressed on MON 87701 x MON 89788 soybean against Heliothis virescens (Lepidoptera: Noctuidae). Pest Manag Sci DOI: 10.1002/ps.3581.
» https://doi.org/10.1002/ps.3581 - Bernardi O, Malvestiti GS, Dourado PM, Oliveira WS, Martinelli S, Berger GU, Head GP and Omoto C. 2012. Assessment of the high-dose concept and level of control proveided by Mon 87701 x MON 89788 soybean against Anticarsia gemmatalis and Pseudoplusia includens (Lepidptera: Noctuidae) in Brazil. Pest Manag Sci 68: 1083-1091.
- Bobrowski VL, Fiuza LM, Pasquali G and Bodanese-Zanettini MH. 2003. Genes de Bacillus thuringiensis: uma estratégia para conferir resistência a insetos em plantas (Genes from Bacillus thuringiensis: a strategy to confer insect resistance in plants). Cienc Rural 34: 843-850.
- Bueno AF, Hirose E and Sosa-Gómez DR. 2013. Manejo Racional. Cultivar Grandes Culturas 173: 26-28.
- Bueno RCOF, Bueno AF, Moscardi F, Parra JRP and Hoffmann-Campo CB. 2011. Lepidopteran larva consumption of soybean foliage: basis for developing multiple-species economic thresholds for pest management decisions. Pest Manag Sci 67: 160-164.
- Degrande PE and Vivan LM. 2007. Pragas da Soja. In: Boletim de Pesquisa da Soja: Fundação MT, 274 p.
- Farrar RR and Bradley JR Jr. 1985 Within-plant distribution of Heliothis spp. (Lepidoptera: Noctuidae) eggs and larvae on cotton in North Carolina. Environ Entomol 14: 205-209.
- Fehr WR and Caviness CE. 1977. Stages of soybean development. Ames: University of Sciense and Technology. 80v.
- Fitt GP. 1989. The ecology of Heliothis species in relation to agroecossystems. Annu Rev Entomol 34: 17-52.
- Greene GL, Leppla NC and Dickerson WA. 1976. Velvetbean caterpillar: a rearing procedure and artificial medium. J Econ Entomol 69: 487-488.
- McWilliams JM and Beland GL. 1977. Bollworm: Effect of soybean leaf and pod maturity on development in the laboratory. Ann Entomol Soc Am 70: 214-216.
- Reed GL, Jensen AS, Riebe J, Head G and Duan JJ. 2001. Transgenic Bt potato and conventional insecticides for Colorado potato beetle management: comparative efficacy and non-target impacts. Entomol Exp Appl 100: 89-100.
- Rogers DJ and Brier HB. 2010. Pest-damage relationships for Helicoverpa armigera (Hubner) (Lepidoptera: Noctuidae) on soybean (Glycine max) and dry bean (Phaseolus vulgaris) during pod-fill. Crop Protection 29: 47-57.
- SAS Institute. 2001. User's Guide: Statistics, 6th version, Cary, 2001.
- Shapiro, SS and Wilk MB. 1965. An analysis of variance test for normality. Biometrika 52: 591-611.
- Terán-Vargas AP. 2005. Bollgard Cotton and Resistance of Tobacco Budworm (Lepidoptera: Noctuidae) to Conventional Insecticides in Southern Tamaulipas, Mexico. J Econ Entomol 98: 2203-2209.
- Tomquelski GV and Maruyama LCT. 2009. Lagarta-da-maçã em soja. Cultivar Grandes Culturas 117: 20-22.
- Yu HL, Yun HL and Kong MW. 2011. Risk assessment and ecological effects of transgenic Bacillus thuringiensis crops on non-target organisms. J Integr Plant Biol 53: 520-538.
Publication Dates
-
Publication in this collection
June 2014
History
-
Received
3 Dec 2013 -
Accepted
17 Feb 2014