Abstracts
The concept of microdomains in plasma membranes was developed over two decades, following observation of polarity of membrane based on clustering of specific membrane components. Microdomains involved in carbohydrate-dependent cell adhesion with concurrent signal transduction that affect cellular phenotype are termed "glycosynapse". Three types of glycosynapse have been distinguished: "type 1" having glycosphingolipid associated with signal transducers (small G-proteins, cSrc, Src family kinases) and proteolipids; "type 2" having O-linked mucin-type glycoprotein associated with Src family kinases; and "type 3" having N-linked integrin receptor complexed with tetraspanin and ganglioside. Different cell types are characterized by presence of specific types of glycosynapse or their combinations, whose adhesion induces signal transduction to either facilitate or inhibit signaling. E.g., signaling through type 3 glycosynapse inhibits cell motility and differentiation. Glycosynapses are distinct from classically-known microdomains termed "caveolae", "caveolar membrane", or more recently "lipid raft", which are not involved in carbohydrate-dependent cell adhesion. Type 1 and type 3 glycosynapses are resistant to cholesterol-binding reagents, whereas structure and function of "caveolar membrane" or "lipid raft" are disrupted by these reagents. Various data indicate a functional role of glycosynapses during differentiation, development, and oncogenic transformation.
clustering; carbohydrate-to-carbohydrate interaction; carbohydrate-binding protein; integrin; tetraspanin; growth factor receptor
O conceito de microdomínios em membrana plasmática foi desenvolvido há mais de duas décadas, após a observação da polaridade da membrana baseada no agrupamento de componentes específicos da membrana. Microdomínios envolvidos na adesão celular dependente de carboidrato, com transdução de sinal que afeta o fenótipo celular são denominados ''glicosinapses''. Três tipos de glicosinapse foram observados: ''tipo 1'' que possue glicoesfingolipídio associado com transdutores de sinal (proteínas G pequenas, cSrc, cinases da família Src) e proteolipídios; ''tipo 2'' que possue glycoproteínas O-ligadas tipo mucina associadas com cinases da família Scr; e ''tipo 3'' que possue receptor de integrina, com glycanas N-ligadas, complexado com tetraspanina e gangliosídio. Tipos celulares diferentes são caracterizados pela presença de tipos específicos de glicosinapse ou suas combinações, cuja adesão induz transdução de sinal para facilitar ou inibir a sinalização. Ex., a sinalização através da glicosinapse tipo 3 inibe mobilidade celular e a diferenciação. Glicosinapses são distintas dos microdomínios conhecidos classicamente como ''caveolas'', ''membrana caveolar'', ou mais recentemente ''rafts lipídicos'', os quais não estão envolvidos na adesão celular dependente de carboidrato. Glicosinapses tipo 1 e tipo 3 são resistentes a regentes que se ligam ao colesterol, enquanto a estrututa e função de ''membrana caveolar'' ou ''rafts lipídicos'' são sensíveis a esses reagentes. Vários dados sugerem um papel funcional para as glicosinapses durante a diferenciação, o desenvolvimento e a transformação oncogênica.
interação carboidrato-carboidrato; proteína que se liga ao carboidrato; integrina; tetraspanina; receptor de fator de crescimento
BIOMEDICAL AND MEDICAL SCIENCES
Glycosynapses: microdomains controlling carbohydrate-dependent cell adhesion and signaling
Senitiroh Hakomori
Pacific Northwest Research Institute, 720 Broadway, Seattle, WA 98122-4302 U.S.A. and Departments of Pathobiology and Microbiology, University of Washington, Seattle, WA 98195 U.S.A.
ABSTRACT
The concept of microdomains in plasma membranes was developed over two decades, following observation of polarity of membrane based on clustering of specific membrane components. Microdomains involved in carbohydrate-dependent cell adhesion with concurrent signal transduction that affect cellular phenotype are termed "glycosynapse". Three types of glycosynapse have been distinguished: "type 1" having glycosphingolipid associated with signal transducers (small G-proteins, cSrc, Src family kinases) and proteolipids; "type 2" having O-linked mucin-type glycoprotein associated with Src family kinases; and "type 3" having N-linked integrin receptor complexed with tetraspanin and ganglioside. Different cell types are characterized by presence of specific types of glycosynapse or their combinations, whose adhesion induces signal transduction to either facilitate or inhibit signaling. E.g., signaling through type 3 glycosynapse inhibits cell motility and differentiation. Glycosynapses are distinct from classically-known microdomains termed "caveolae", "caveolar membrane", or more recently "lipid raft", which are not involved in carbohydrate-dependent cell adhesion. Type 1 and type 3 glycosynapses are resistant to cholesterol-binding reagents, whereas structure and function of "caveolar membrane" or "lipid raft" are disrupted by these reagents. Various data indicate a functional role of glycosynapses during differentiation, development, and oncogenic transformation.
Key words: clustering, carbohydrate-to-carbohydrate interaction, carbohydrate-binding protein, integrin, tetraspanin, growth factor receptor.
RESUMO
O conceito de microdomínios em membrana plasmática foi desenvolvido há mais de duas décadas, após a observação da polaridade da membrana baseada no agrupamento de componentes específicos da membrana. Microdomínios envolvidos na adesão celular dependente de carboidrato, com transdução de sinal que afeta o fenótipo celular são denominados ''glicosinapses''. Três tipos de glicosinapse foram observados: ''tipo 1'' que possue glicoesfingolipídio associado com transdutores de sinal (proteínas G pequenas, cSrc, cinases da família Src) e proteolipídios; ''tipo 2'' que possue glycoproteínas O-ligadas tipo mucina associadas com cinases da família Scr; e ''tipo 3'' que possue receptor de integrina, com glycanas N-ligadas, complexado com tetraspanina e gangliosídio. Tipos celulares diferentes são caracterizados pela presença de tipos específicos de glicosinapse ou suas combinações, cuja adesão induz transdução de sinal para facilitar ou inibir a sinalização. Ex., a sinalização através da glicosinapse tipo 3 inibe mobilidade celular e a diferenciação. Glicosinapses são distintas dos microdomínios conhecidos classicamente como ''caveolas'', ''membrana caveolar'', ou mais recentemente ''rafts lipídicos'', os quais não estão envolvidos na adesão celular dependente de carboidrato. Glicosinapses tipo 1 e tipo 3 são resistentes a regentes que se ligam ao colesterol, enquanto a estrututa e função de ''membrana caveolar'' ou ''rafts lipídicos'' são sensíveis a esses reagentes. Vários dados sugerem um papel funcional para as glicosinapses durante a diferenciação, o desenvolvimento e a transformação oncogênica.
Palavras-chave: interação carboidrato-carboidrato, proteína que se liga ao carboidrato, integrina, tetraspanina, receptor de fator de crescimento.
1 INTRODUCTION AND HISTORICAL RETROSPECTIVE
The concept of microdomains in plasma membrane evolved from questions about the original "fluid mosaic" model of Singer and Nicolson (1972), in which membrane proteins are randomly distributed in the "sea" of homogeneous lipid bilayer. I.e., (i) whether the lipid bilayer is really homogeneous; (ii) whether distribution of membrane proteins is really random.
The "plate model", proposed by M.K. Jain and H.B. White (Jain and White 1977), which emphasized that the "biomembrane continuum is broken up into a number of relative rigid plates or patches which are in relative motion with respect to each other" may be the first alternative to the Singer and Nicolson model, addressing the above questions. According to this model, the ordered and rigid regions are separated from each other by fluid and disordered regions. Structural and functional units play a role in transport and cellular interaction, and show cooperativity among themselves in order to maintain overall function of biological membranes. The Jain/White plate model is illustrated in Fig. 1. This view was remarkably similar to the concept of microdomains elaborated recently [e.g., by D.A. Brown (Schroeder et al. 1994, Arreaza et al. 1994, Brown and London 1997)]. The idea of signal transduction through membrane microdomains was developed only when growth factor receptor tyrosine kinase was shown to be inhibited by surrounding gangliosides (Bremer et al. 1984), and when Src family kinase activity was found to be associated with GPI-anchored receptors (Stefanova et al. 1991) (see Fig. 2 and its legend).
Development of the microdomain concept, initiated by observation of (i) apical vs. basolateral difference in glycosphingolipid (GSL) and glycoprotein (GP) distribution patterns; (ii) clustering patterns of GSLs and GPs; and (iii) functional organization of clustered GSLs or GPs with cytoplasmic signal transducers, etc., is chronologically illustrated in Fig. 2 and its legend.
The concept of microdomains mediating cell adhesion, particularly carbohydrate-dependent adhesion coupled with signaling, was developed only recently, and was termed "glycosynapse" (see below) (Iwabuchi et al. 1998a, b, Hakomori et al. 1998, Hakomori 2000).
2 GLYCOSYNAPSES: MICRODOMAINS INVOLVED IN CARBOHYDRATE-DEPENDENT ADHESION COUPLED WITH SIGNAL TRANSDUCTION
Specific carbohydrate epitopes present in cell surface glycoconjugates, either GSLs or N- or O-linked glycoproteins, are recognized by carbohydrate-binding proteins or complementary carbohydrates. In each case, glycosyl epitopes, carried by GSLs or glycoproteins, are clustered to form microdomains, in which specific signal transducers, adhesion receptors, or growth factor receptors are organized. Through this organization, carbohydrate-dependent adhesion creates signals that are transmitted to the nucleus, leading to changes in cellular phenotype associated with cell adhesion.
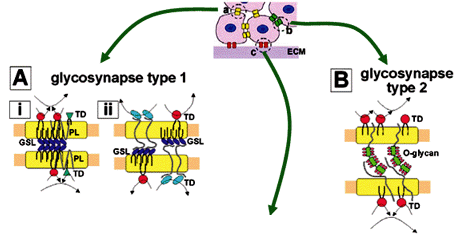
Microdomains involved in carbohydrate-dependent adhesion coupled with signal transduction were termed "glycosynapses" (Hakomori 2002, Hakomori and Handa 2002), in analogy to "immunological synapses" involved in immunocyte adhesion with concurrent signaling leading to activation of T-cells and B-cells (Bromley et al. 2001, Krummel and Davis 2002). The term "glycosynapse" is used to emphasize the point that the terms "caveolae" or "lipid raft" do not indicate cell adhesion, or signaling induced by cell adhesion. This conceptual distinction is illustrated in Fig. 3.
During the early 1990s, the concept of a membrane morphological unit termed "caveolae", characterized by presence of the scaffold protein caveolin and involved in signal transduction and endocytosis/exocytosis, received much attention (for review see Anderson 1998). Membrane microdomains with similar biochemical properties and cell biological functions, but lacking caveolin (Parton and Simons 1995), were frequently observed, to which the term "raft" or "lipid raft" was applied (for review see Simons and Ikonen 1997). Neither microdomain "caveolae" nor "raft" dealt with the essential function of cell adhesion, particularly carbohydrate-dependent adhesion. Furthermore, function of glycosynapse 1 and 3 (see below) is unaffected by or resistant to cholesterol-binding reagents, in contrast to function and structure of "caveolae" and "raft". Contrasting properties of these microdomains are summarized in Fig. 4.
Three types of glycosynapse are described in the following subsections.
2.1 GLYCOSYNAPSE 1: GSL MICRODOMAINS CONTROLLING GSL-DEPENDENT ADHESION AND SIGNALING
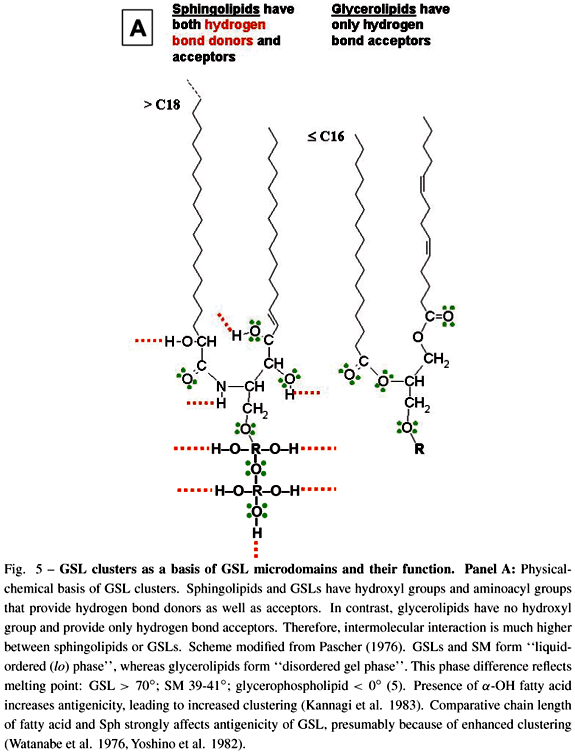
GSLs have strong ability to interact with each other (cis interaction) through hydrogen bonds, since GSL molecules have both hydrogen bond acceptors and donors. In contrast, glycerophospholipids have no hydroxyl group (i.e., no hydrogen bond donor), and therefore no ability to undergo cis interaction through hydrogen bonds (Fig. 5A). Under scanning EM with freeze-fracture technique, GSL clusters can be clearly seen on cell membranes as well as GSL-phosphatidylcholine liposomes (Fig. 5B). GSL microdomains were originally found to be detergent-insoluble (Okada et al. 1984), and were separated as buoyant, low-density membrane fraction containing cSrc, Src family kinases, small G-proteins (RhoA, Ras, Rac), FAK, and occasionally growth factor receptors (Liu et al. 1996, Mineo et al. 1996, Yamamura et al. 1997, Iwabuchi et al. 1998a, b). GSL microdomains from mouse B16 melanoma cells, highly enriched in GM3 ganglioside, cSrc, RhoA, were separated from "caveolar membrane" or "raft", which are enriched in cholesterol, sphingomyelin, and caveolin. Possible structure of such GSL microdomain is illustrated in Fig. 5C.
GSLs clusters in glycosynapse 1 are involved in adhesion between interfacing cells through carbohydrate-to-carbohydrate interaction, initially observed for teratocarcinoma F9 cell autoaggregation that mimics compaction of preimplantation embryo. This process is based on Lex self-recognition in the presence of Ca2+ (Eggens et al. 1989). Cell adhesion mediated by GSL clusters, coupled with initiation of signal transduction, is illustrated schematically in Fig. 5D.
The functional role of Lex GSL in glycosynapse 1 was compared to that of embryoglycan carrying multiple Lex determinants in mediating autoaggregation of F9 cells (Kojima et al. 1994). Clustered Lex carried by GSL or by embryoglycan functioned equally well, in the presence of Ca2+.
Another example of GSL-dependent adhesion through glycosynapse 1 is adhesion of mouse B16 melanoma cell (expressing high level of GM3) to mouse endothelial cells (ECs) (expressing high lactosylceramide (LacCer) or Gg3Cer). Such adhesion is the initial step in B16 cell metastasis (Kojima et al. 1992). This hypothesis was confirmed by the finding that in vivo B16 cell metastasis is inhibited by GM3-liposomes or Gg3-liposomes, but not by sialylparagloboside-liposomes (Otsuji et al. 1995).
The concept of GSL-dependent adhesion was extended to interaction of GM3 with Gg3 (Kojima and Hakomori 1991a, Matsuura et al. 2000), Gb4 with Gb5 or nLc4 (Yu et al. 1998), H with Ley (Zhu et al. 1995), and GalCer with sulfatide (Stewart and Boggs 1993, Menikh et al. 1997). Some of the structures involved in GSL-to-GSL trans interaction that induces GSL-dependent adhesion are shown in Fig. 6.
GSL-dependent adhesion through carbohydrate-binding proteins, e.g., siglec-4 or MAG (myelin-associated glycoprotein), which bind preferentially to GD1a, GT1b, or GQ1b (Yang et al. 1996, Schnaar et al. 1998, Vyas et al. 2002).
2.2 GLYCOSYNAPSE 2: ADHESION MEDIATED BY CARBOHYDRATE-TO-PROTEIN INTERACTION
Mucin-type glycoproteins have tandem repeat of defined peptide core with many Ser/Thr residues, through which multiple O-linked glycans are presented. Various glycosyl structures (sialyl-Lex, sialyl-Lea, sialyl-Tn) carried by O-linked glycans in mucin-type glycoproteins are the essential epitopes for selectins (Varki 1994, Kannagi 1997, McEver and Cummings 1997), siglecs (Crocker et al. 1994, Crocker and Varki 2001, Ito et al. 2001), and other carbohydrate-binding proteins that mediate adhesion of tumor cells, neutrophils, or peripheral blood mononuclear cells.
We found recently that MUC1 and other mucin-type glycoproteins (see below), together with Src family kinases, are expressed in low-density membrane fraction, prepared in Brij58 or Na2CO3, of T-cell lymphoma cell lines Jurkat and HUT78. MUC1 microdomains are characterized by having Src family kinases (Yes, Fyn, lck56), CD45, and P-selectin glycoprotein ligand-1 (PSGL-1). Importantly, both CD45 and PSGL-1 are mucin-type glycoproteins having structural unit similar to MUC1 (Handa et al. 2001). These preliminary data are shown in Fig. 7A-E. Coexistence of MUC1, PSGL-1, and CD45 in the same low-density membrane microdomain of the same T-cell line may not be coincidental, but its functional significance remains to be studied. The functional notion of MUC1 in T-cells has been studied recently, revealing that: (i) activated but not resting human T-cells express and secrete MUC1 (Agrawal et al. 1998a); (ii) activated T-cells express MUC1 at "leading edge" associated with gangliosides (Correa et al. 2003); (iii) soluble MUC-1 inhibits T-cell proliferation (Agrawal et al. 1998b, c). These observations suggest that MUC1 in T-cells may define T-cell activation and migration, and that excessive MUC1 expression may inhibit activation. A recent study indicates that MUC1 expression in Jurkat cells is closely associated with IL2 secretion, since "knock-down" of MUC1 by RNAi approach blocked IL2 secretion (Handa K, Andersen S, Hakomori S, unpubl. data). MUC1 may act as sensor of environmental change to transmit signals into cells to promote T-cell survival through IL2 production. The presence of mucin-type glycoproteins in glycosynapse 2 requires extensive further study.
Presence of mucin-type glycoproteins in T-cells was not known until very recently (Agrawal et al. 1998a, c). We found unexpectedly that three mucin-type glycoproteins, MUC1, PSGL-1, and CD45, are all resistant to 1% Brij 48 or 500 mM Na2CO3, and all present in low-density membrane fraction, associated with Src kinases (Handa et al. 2001). In contrast, mucin-type glycoproteins are classically well-known components of mucous membranes and their secretions. Many common human cancers are derived from secretory epithelial cells (endodermal epithelia) in which mucin-type glycoproteins are abundant, and carry typical tumor-associated carbohydrate antigens such as sialyl-Lex, sialyl-Lea, and sialyl-Tn. Our preliminary studies indicate that MUC1 and MUC4 expressed in mammary carcinoma cell line MCF7 are present in low-density membrane fraction, associated with cSrc, CD9, and FAK (Steelant et al. 2002). We expect great future interest in characterization of glycosynapse 2 in various types of human cancer, which is involved in adhesion of tumor cells to ECs to initiate metastasis. Thus, microdomains having mucin-type glycoproteins organized with Src family kinases, involved in carbohydrate-dependent cell adhesion and signaling, are collectively termed "glycosynapse 2".
2.3 GLYCOSYNAPSE 3: MICRODOMAINS CONTROLLING CARBOHYDRATE-DEPENDENT MODULATION OF CELL ADHESION THROUGH ADHESIVE PROTEIN RECEPTOR
The best-studied adhesion receptors are integrins, which are often associated with tetraspanins (TSPs). Cell adhesion to extracellular matrix (ECM) is mediated by various combinations of a and b subunits of integrins (Ruoslahti 1991, Hynes 1992), whose association with tetraspanins (for review see Hemler 2003) depends on N-glycosylation status of both integrins and TSPs, as well as surrounding gangliosides (Ono et al. 1999, 2000, 2001).
TSPs CD9 (Miyake et al. 1991) and CD82 (Dong et al. 1995) were identified as inhibitory factors on tumor cell motility and metastasis. Their effect depends highly on glycosylation status. In order to clarify such effect of glycosylation on cell motility mediated by integrin/TSP/ganglioside complex, we performed the following series of experiments.
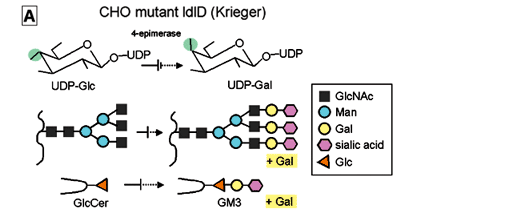
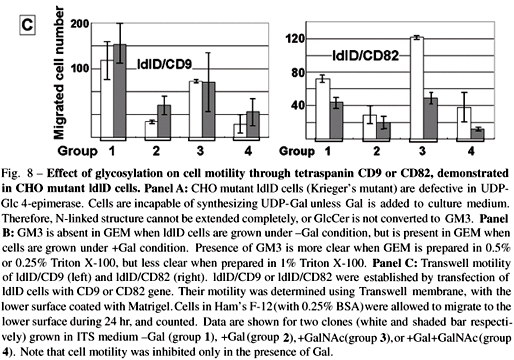
(i) ldlD cells defective in UDP-Gal 4-epimerase (Kingsley et al. 1986, Krieger et al. 1989 ), which cannot synthesize GM3 or complete N-glycosylation unless cultured in insulin-transferrin-selenium medium (ITS) medium with addition of galactose (+Gal condition), were transfected with CD9 or CD82 gene, giving rise to ldlD/CD9 and ldlD/CD82 cell lines respectively (Fig. 8A,B ). We found, unexpectedly, that motility of these CD9 and CD82 transfectants was greatly enhanced under -Gal condition, i.e., when GM3 is absent and N-glycosylation is incomplete. In contrast, motility was strongly inhibited under +Gal condition, whereby endogenous GM3 synthesis occurs and N-glycosylation of CD82 is completed (Ono et al. 1999) (Fig. 8C).
(ii) Because the ligand of integrin a3 is LN5, a3/ LN5-dependent ldlD cell motility was greatly inhibited when a3/CD9/GM3 complex was formed under +Gal condition, but the motility was greatly enhanced under -Gal condition, whereby complex appeared to be dissociated (Fig. 9A,B). The enhanced motility under -Gal condition was moderately reduced, while the suppressed motility under +Gal condition was greatly enhanced, when anti-a3 antibodies were added (Fig. 9B-b). These observations confirm that the motility-inhibitory effect of a3/CD9/GM3 complex occurs primarily at the cell surface, and that the effect is based on interaction of a3 with LN5.
(iii) The motility-inhibitory effect of CD9 or CD82 is suggested by their capability to form complex with integrin receptors (Mannion et al. 1996, Ono et al. 2000, 2001). Motility of three colorectal cancer lines expressing high CD9 level was reduced greatly only upon addition of exogenous GM3 (10, 50 mm) (Fig. 10A), possibly through CD9/GM3/integrin receptor complex, as evidenced by addition of photoactivatable GM3 to HRT18 cells (Fig. 10B).
3 PERSPECTIVES ON GLYCOSYNAPSES IN MOLECULAR CELL BIOLOGY
I would like to present a few perspectives based on the concept of glycosynapse.
PHENOTYPIC VARIABILITY AND GLYCOSYNAPSE
Current molecular cell biology has advanced greatly along the scenario of its "central dogma": DNA ® RNA ® protein. The total number of genes in genome of Caenorhabditis elegans was determined as 20,000 in 1998 (The C. elegans Sequencing Consortium 1998). Many molecular biologists expected that the number of genes in the human genome would be 4-10 times higher than this, because of the much greater complexity of phenotypes in human cells as compared to nematode. The number of human genes was clarified 3 years later, surprisingly, as 30,000 (Venter et al. 2001). How is this minor difference in gene number related to such a large difference in complexity of phenotypes between nematodes and humans? Variability in splicing of DNA? Variability in mode of transcription factor effect? Epigenetic effect, i.e., change of gene effect without change of DNA sequence (acetylation or methylation of DNA, or chromatin effect in nucleosome)? Recent studies indicate the presence of hitherto-unknown DNA located outside conventional genome; such "extraterritorial" DNA may encode unusual RNA (e.g., "riboswitch") which modulates messenger function or other functions involved in the "central dogma", e.g., (Gibbs 2003a, b).
While it is essential to consider these recent breakthrough developments in addition to classic genome/proteome concepts in order to understand the complexity of phenotypes, it is also important to recognize that post-translational modification of proteins increases complexity. 60% of proteins are modified by (i) N- or O-glycosylation; (ii) lipidation (i.e., palmitoylation or myristoylation); (iii) association with ganglioside or GSL leading to alteration of function. Processes i, ii, and iii are obviously not based simply on the ''central dogma'', but are controlled by assembly of secondary gene products. Variability of glycosylation in glycoproteins and GSLs is shown in Table I.
This variability in molecules suggests creation of further variability in molecular assembly of membranes, which is the theme of this article, and which I have illustrated in three examples of glycosynapse. To say that only three types exist is certainly an oversimplification. There are undoubtedly a much larger number, showing a wide range of flexibility in response to cellular environment.
CELL SOCIAL FUNCTION AND GLYCOSYNAPSE
Cell surfaces are enriched in carbohydrates, which are assumed to be involved in cell ''social functions'' (e.g., recognition, adhesion). This line of study is currently a major theme in cell biology, in contrast to classic molecular cell biology based on the ''central dogma''. Cell adhesion is associated with signal transduction to define cellular phenotype. The concept of glycosynapse helps explain the molecular mechanisms by which cells recognize each other and undergo phenotypic changes such as differentiation, development, oncogenic transformation, metastasis, apoptosis, etc.
Various disease processes can be elucidated through study of glycosynapse structure and function. A few examples are: (i) Oncogenic transformation and invasion through adhesion of tumor cells to ECs through glycosynapse 1 or 2. (ii) Enhanced or inhibited motility/invasiveness through interaction of ECM with integrin/tetraspanin/ganglioside complex (glycosynapse 3). (iii) Many infectious agents are targeted to GSLs or GPs in glycosynapses. Among these, GSL receptors are best elucidated so far. (iv) Accumulation of neutrophils or eosinophils at inflammatory lesion is the key event during inflammatory processes. Such accumulation is based on neutrophil or eosinophil binding to ECs mediated by myeloglycan, which is present in glycosynapse. (v) Neuronal cell function in brain or central nervous system is maintained by nerve growth factor (NGF) receptor or brain-derived growth factor (BDGF) receptor, whose functions are modulated by surrounding gangliosides or GSLs in glycosynapse (Mutoh et al. 1995, Sakakura et al. 1996, Prinetti et al. 1999).
Elucidation of structure and function of glycosynapses involved in these disease processes is expected to lead to methods for prevention and cure of specific diseases.
Manuscript received on April 1, 2004; accepted for publication on April 2, 2004
presented by LUCIA MENDONÇA PREVIATO
E-mail: hakomori@u.washington.edu
- AGRAWAL B, KRANTZ MJ, PARKER J AND LONGENECKER BM. 1998a. Expression of MUC1 mucin on activated human T cells: implications for a role of MUC1 in normal immune regulation. Cancer Res 58: 4079-4081.
- AGRAWAL B, GENDLER SJ AND LONGENECKER BM. 1998b. The biological role of mucins in cellular interactions and immune regulation: prospects for cancer immunotherapy. Mol Med Today 9: 397-403.
- AGRAWAL B, KRANTZ MJ, REDDISH MA AND LONGENECKER BM. 1998c. Cancer-associated MUC1 mucin inhibits human T-cell proliferation, which is reversible by IL-2. Nature Medicine 4: 43-49.
- ANDERSON RGW. 1998. The caveolae membrane system. Annu Rev Biochem 67: 199-225.
- ARREAZA G, MELKONIAN KA, LAFEVRE-BERNT M AND BROWN DA. 1994. Triton X-100-resistant membrane complexes from cultured kidney epithelial cells contain the Src family protein tyrosine kinase p62yes. J Biol Chem 269: 19123-19127.
- BREMER EG, HAKOMORI S, BOWEN-POPE DF, RAINES EW AND ROSS R. 1984. Ganglioside-mediated modulation of cell growth, growth factor binding, and receptor phosphorylation. J Biol Chem 259: 6818-6825.
- BREMER EG, SCHLESSINGER J AND HAKOMORI S. 1986. Ganglioside-mediated modulation of cell growth. Specific effects of GM3 on tyrosine phosphorylation of the epidermal growth factor receptor. J Biol Chem 261: 2434-2440.
- BROMLEY SK, BURACK WR, JOHNSON KG, SOMERSALO K, SIMS TN, SUMEN C, DAVIS MM, SHAW AS, ALLEN PM AND DUSTIN ML. 2001. The immunological synapse. Annu Rev Immunol 19: 375-396.
- BROWN DA AND LONDON E. 1997. Structure of detergent-resistant membrane domains: does phase separation occur in biological membranes? Biochem Biophys Res Commun 240: 1-7.
- CARTER WG AND HAKOMORI S. 1981. A new cell surface, detergent-insoluble glycoprotein matrix of human and hamster fibroblasts. The role of disulfide bonds in stabilization of the matrix. J Biol Chem 256: 6953-6960.
- CORREA I, PLUNKETT T, VLAD A, MUNGUL A, CANDELORA-KETTEL J, BURCHELL JM, TAYLOR-PAPADIMITRIOU J AND FINN OJ. 2003. Form and pattern of MUC1 expression on T cells activated in vivo or in vitro suggests a function in T-cell migration. Immunology 108: 32-41.
- CROCKER PR AND VARKI A. 2001. Siglecs in the immune system. Immunology 103: 137-145.
- CROCKER PR, MUCKLOW S, BOUCKSON V, MCWILLIAM A, WILLIS AC, GORDON S, MILON G, KELM S AND BRADFIELD P. 1994. Sialoadhesin, a macrophage sialic acid binding receptor for haemopoietic cells with 17 immunoglobulin-like domains. EMBO J 13: 4490-4503.
- DONG J-T, LAMB PW, RINKER-SCHAEFFER CW, VUKANOVIC J, ICHIKAWA T, ISAACS JT AND BARRETT JC. 1995. KAI1, a metastasis suppressor gene for prostate cancer on human chromosome 11p11.2. Science 268: 884-886.
- EGGENS I, FENDERSON BA, TOYOKUNI T, DEAN B, STROUD MR AND HAKOMORI S. 1989. Specific interaction between Lex and Lex determinants. A possible basis for cell recognition in preimplantation embryos and in embryonal carcinoma cells. J Biol Chem 264: 9476-9484.
- FORSTNER GG AND WHERRETT JR. 1973. Plasma membrane and mucosal glycosphingolipids in the rat intestine. Biochim Biophys Acta 306: 446-459.
- FORSTNER GG, TANAKA K AND ISSELBACHER KJ. 1968. Lipid composition of the isolated rat intestinal microvillus membrane. Biochem J 109: 51-59.
- GIBBS WW. 2003a. The unseen genome: gems among the junk. Sci Am 289: 46-53.
- GIBBS WW. 2003b. The unseen genome: beyond DNA. Sci Am 289: 106-113.
- HAKOMORI S. 2000. Cell adhesion/recognition and signal transduction through glycosphingolipid microdomain. Glycoconj J 17: 143-151.
- HAKOMORI S. 2002. Inaugural Article by a Recently Elected Academy Member: Inaugural Article: The glycosynapse Proc Natl Acad Sci USA 99: 225-232.
- HAKOMORI S AND HANDA K. 2002. Glycosphingolipid-dependent cross-talk between glycosynapses interfacing tumor cells with their host cells: essential basis to define tumor malignancy. FEBS Lett 531: 88-92.
- HAKOMORI S, HANDA K, IWABUCHI K, YAMAMURA S AND PRINETTI A. 1998. New insights in glycosphingolipid function: "glycosignaling domain", a cell surface assembly of glycosphingolipids with signal transducer molecules,involved in cell adhesion coupled with signaling. Glycobiology 8: xi-xviii.
- HANDA K, JACOBS F, LONGENECKER BM AND HAKOMORI S. 2001. Association of MUC-1 and PSGL-1 with Low-Density Microdomain in T-Lymphocytes: A Preliminary Note Biochem Biophys Res Commun 285: 788-794.
- HANSSON GC, SIMONS K AND VAN MEER G. 1986. Two strains of the Madin-Darby canine kidney (MDCK) cell line have distinct glycosphingolipid compositions. EMBO J 5: 483-489.
- HEMLER ME. 2003. Tetraspanin proteins mediate cellular penetration, invasion, and fusion events and define a novel type of membrane microdomain. Annu Rev Cell Dev Biol 19: 397-422.
- HYNES RO. 1992. Integrins: versatility, modulation, and signaling in cell adhesion. Cell 69: 11-25.
- ITO A, HANDA K, WITHERS DA, SATOH M AND HAKOMORI S. 2001. Binding specificity of siglec7 to disialogangliosides of renal cell carcinoma: possible role of disialogangliosides in tumor progression. FEBS Lett 498: 116-120.
- IWABUCHI K, YAMAMURA S, PRINETTI A, HANDA K AND HAKOMORI S. 1998a. GM3-enriched microdomain involved in cell adhesion and signal transduction through carbohydrate-carbohydrate interaction in mouse melanoma B16 cells. J Biol Chem 273: 9130-9138.
- IWABUCHI K, HANDA K AND HAKOMORI S. 1998b. Separation of "glycosphingolipid signaling domain" from caveolin-containing membrane fraction in mouse melanoma B16 cells and its role in cell adhesion coupled with signaling. J Biol Chem 273: 33766-33773.
- JAIN MK AND WHITE HB. 1977. Long-range order in biomembranes. Adv Lipid Res 15: 1-60.
- KANNAGI R. 1997. Carbohydrate-mediated cell adhesion involved in hematogenous metastasis of cancer. Glycoconj J 14: 577-584.
- KANNAGI R, STROUP R, COCHRAN NA, URDAL DL, YOUNG WWJ AND HAKOMORI S. 1983. Factors affecting expression of glycolipid tumor antigens: influence of ceramide composition and coexisting glycolipid on the antigenicity of gangliotriaosylceramide in murine lymphoma cells. Cancer Res 43: 4997-5005.
- KINGSLEY DM, KOZARSKY KF, HOBBIE L AND KRIEGER M. 1986. Reversible defects in O-linked glycosylation and LDL receptor expression in a UDP-Gal/ UDP-GalNAc 4-epimerase deficient mutant. Cell 44: 749-759.
- KOJIMA N AND HAKOMORI S. 1991a. Cell adhesion, spreading and motility of GM3-expressing cells based on glycolipid-glycolipid interaction. J Biol Chem 266: 17552-17558.
- KOJIMA N AND HAKOMORI S. 1991b. Synergistic effect of two cell recognition systems: glycosphingolipid-glycosphingolipid interaction and integrin receptor interaction with pericellular matrix protein. Glycobiology 1: 623-630.
- KOJIMA N, SHIOTA M, SADAHIRA Y, HANDA K AND HAKOMORI S. 1992. Cell adhesion in a dynamic flow system as compared to static system. Glycosphingolipid-glycosphingolipid interaction in the dynamic system predominates over lectin- or integrin-based mechanisms in adhesion of B16 melanoma cells to non-activated endothelial cells. J Biol Chem 267: 17264-17270.
- KOJIMA N, FENDERSON BA, STROUD MR, GOLDBERG RI, HABERMANN R, TOYOKUNI T AND HAKOMORI S. 1994. Further studies on cell adhesion based on Le(x)-Le(x) interaction, with new approaches: embryoglycan aggregation of F9 teratocarcinoma cells, and adhesion of various tumour cells based on Le(x) expression. Glycoconj J 11: 238-248.
- KRIEGER M, REDDY P, KOZARSKY K, KINGSLEY D, HOBBIE L AND PENMAN M. 1989. Analysis of the synthesis, intracellular sorting, and function of glycoproteins using a mammalian cell mutant with reversible glycosylation defects. Meth Cell Biol 32: 57-84.
- KRUMMEL MF AND DAVIS MM. 2002. Dynamics of the immunological synapse: finding, establishing and solidifying a connection. Curr Opin Immunol 14: 66-74.
- LIU P, YING Y, KO Y-G AND ANDERSON RGW. 1996. Localization of platelet-derived growth factor-stimulated phosphorylation cascade to caveolae. J Biol Chem 271: 10299-10303.
- MANNION BA, BERDITCHEVSKI F, KRAEFT S-K, CHEN LB AND HEMLER ME. 1996. Transmembrane-4 superfamily proteins CD81 (TAPA-1), CD82, CD63, and CD53 specifically associated with integrin alpha 4 beta 1 (CD49d/CD29). J Immunol 157: 2039-2047.
- MATLIN KS AND SIMONS K. 1984. Sorting of an apical plasma membrane glycoprotein occurs before it reaches the cell surface in cultured epithelial cells. J Cell Biol 99: 2131-2139.
- MATSUURA K, KITAKOUJI H, SAWADA N, ISHIDA H, KISO M, KITAJIMA K AND KOBAYASHI K. 2000. A Quantitative Estimation of Carbohydrate-Carbohydrate Interaction Using Clustered Oligosaccharides of Glycolipid Monolayers and of Artificial Glycoconjugate Polymers by Surface Plasmon Resonance J Am Chem Soc 122: 7406-7407.
- MCEVER RP AND CUMMINGS RD. 1997. Perspectives series: cell adhesion in vascular biology. Role of PSGL-1 binding to selectins in leukocyte recruitment. J Clin Invest 100: 485-491.
- MENIKH A, NYHOLM P-G AND BOGGS JM. 1997. Biochemistry 36: 3438-3447.
- MINEO C, JAMES GL, SMART EJ AND ANDERSON RGW. 1996. J Biol Chem 271: 11930-11935.
- MIYAKE M, KOYAMA M, SENO M AND IKEYAMA S.1991. Identification of the motility-related protein (MRP-1), recognized by monoclonal antibody M31-15, which inhibits cell motility. J Exp Med 174: 1347-1354.
- MUTOH T, TOKUDA A, MIYADA T, HAMAGUCHI M AND FUJIKI N. 1995. Ganglioside GM1 binds to the Trk protein and regulates receptor function. Proc Natl Acad Sci USA 92: 5087-5091.
- OKADA Y, MUGNAI G, BREMER EG AND HAKOMORI S. 1984. Glycosphingolipids in detergent-insoluble substrate attachment matrix (DISAM) prepared from substrate attachment material (SAM). Their possible role in regulating cell adhesion. Exp Cell Res 155: 448-456.
- ONO M, HANDA K, WITHERS DA AND HAKOMORI S. 1999. Motility inhibition and apoptosis are induced by metastasis-suppressing gene product CD82 and its analogue CD9, with concurrent glycosylation. Cancer Cancer Res 59: 2335-2339.
- ONO M, HANDA K, WITHERS DA AND HAKOMORI S. 2000. Glycosylation effect on membrane domain (GEM) involved in cell adhesion and motility: a preliminary note on functional alpha3, alpha5-CD82 glycosylation complex in ldlD 14 cells. Biochem Biophys Res Commun 279: 744-750.
- ONO M, HANDA K, SONNINO S, WITHERS DA, NAGAI H AND HAKOMORI S. 2001. GM3 ganglioside inhibits CD9-facilitated haptotactic cell motility: coexpression of GM3 and CD9 is essential in the downregulation of tumor cell motility and malignancy. Biochemistry 40: 6414-6421.
- OTSUJI E, PARK YS, TASHIRO K, KOJIMA N, TOYOKUNI T AND HAKOMORI S. 1995. Inhibition of B16 melanoma metastasis by administration of GM3- or Gg3-liposomes: Blocking adhesion of melanoma cells to endothelial cells (anti-adhesion therapy) via inhibition of GM3-Gg3Cer or GM3-LacCer interaction. Int J Oncol 6: 319-327.
- PARTON RG AND SIMONS K. 1995. Digging into caveolae. Science 269: 1398-1399.
- PASCHER I. 1976. Molecular arrangements in sphingolipids. Conformation and hydrogen bonding of ceramide and their implication on membrane stability and permeability. Biochim Biophys Acta 455: 433-451.
- PRINETTI A, IWABUCHI K AND HAKOMORI S. 1999. Glycosphingolipid-enriched signaling domain in mouse neuroblastoma Neuro2a cells. Mechanism of ganglioside-dependent neuritogenesis. J Biol Chem 274: 20916-20924.
- RAHMANN H, RÖSNER H, KÖRTJE K-H, BEITINGER H AND VEYBOLD V. 1994. in Biological function of gangliosides (Progress in Brain Research, Vol. 101) (SVENNERHOLM L, ASBURY AK, REISFELD RA, SANDHOFF K, SUZUKI K, TETTAMANTI G AND TOFFANO G, eds.), pp. 127-145, Elsevier, Amsterdam.
- ROCK P, ALLIETTA M, YOUNG WWJ, THOMPSON TE AND TILLACK TW. 1990. Organization of glycosphingolipids in phosphatidylcholine bilayers: use of antibody molecules and Fab fragments as morphologic markers. Biochemistry 29: 8484-8490.
- ROCK P, ALLIETTA M, YOUNG WWJ, THOMPSON TE AND TILLACK TW. 1991. Ganglioside GM1 and asialo-GM1 at low concentration are preferentially incorporated into the gel phase in two-component, two-phase phosphatidylcholine bilayers. Biochemistry 30: 19-25.
- RUOSLAHTI E. 1991. Integrins. J Clin Invest 87: 1-5.
- SAKAKURA C, IGARASHI Y, ANAND JK, SADOZAI KK AND HAKOMORI S. 1996. Plasmalopsychosine of human brain mimics the effect of nerve growth factor by activating its receptor kinase and mitogen-activated protein kinase in PC12 cells. Induction of neurite outgrowth and prevention of apoptosis. J Biol Chem 271: 946-952.
- SCHNAAR RL, COLLINS BE, WRIGHT LP, KISO M, TROPAK MB, RODER JC AND CROCKER PR. 1998. in Sphingolipids as signaling modulators in the nervous system (Ann NY Acad Sci, Vol. 845) (LEDEEN RW, HAKOMORI S, YATES AJ, SCHNEIDER JS AND YU RK., eds.), pp. 92-105, NY Acad Sci, New York, NY.
- SCHROEDER R, LONDON E AND BROWN D. 1994. Interactions between saturated acyl chains confer detergent resistance on lipids and glycosylphosphatidylinositol (GPI)-anchored proteins: GPI-anchored proteins in liposomes and cells show similar behavior. Proc Natl Acad Sci USA 91: 12130-12134.
- SIMONS K AND IKONEN E. 1997. Functional rafts in cell membranes. Nature 387: 569-572.
- SINGER SJ AND NICOLSON G. 1972. The fluid mosaic model of the structure of cell membranes. Science 185: 720-731.
- SORICE M, PAROLINI I, SANSOLINI T, GAROFALO T, DOLO V, SARGIACOMO M, TAI T, PESCHLE C, TORRISI MR AND PAVAN A. 1997. Evidence for the existence of ganglioside-enriched plasma membrane domains in human peripheral lymphocytes. J Lipid Res 38: 969-980.
- STEELANT WF, KAWAKAMI Y, ITO A, HANDA K, BRUYNEEL EA, MAREEL M AND HAKOMORI S. 2002. Monosialyl-Gb5 organized with cSrc andFAK in GEM of human breast carcinoma MCF-7 cells defines their invasive properties. FEBS Lett 531: 93-98.
- STEFANOVA I, HOREJSI V, ANSOTEGUI IJ, KNAPP W AND STOCKINGER H. 1991. GPI-anchored cell-surface molecules complexed to protein tyrosine kinases. Science 254: 1016-1019.
- STEWART RJ AND BOGGS JM. 1993. A carbohydrate-carbohydrate interaction between galactosylceramide-containing liposomes and cerebroside sulfate-containing liposomes: dependence on the glycolipid ceramide composition. Biochemistry 32: 10666-10674.
- THE C. elegans SEQUENCING CONSORTIUM. 1998. Science 282: 2012-2018.
- TILLACK TW, ALLIETTA M, MORAN RE AND YOUNG WWJ. 1983. Localization of globoside and Forssman glycolipids on erythrocyte membranes. Biochim Biophys Acta 733: 15-24.
- VARKI A. 1994. Selectin ligands. Proc Natl Acad Sci USA 91: 7390-7397.
- VENTER JC, ADAMS MD, MYERS EW, LI PW, MURAL RJ, SUTTON GG et al. 2001. The sequence of the human genome. Science 291: 1304-1351.
- VYAS AA, PATEL HV, FROMHOLT SE, HEFER-LAUC M, VYAS KA, DANG J, SCHACHNER M AND SCHNAAR RL. 2002. Gangliosides are functional nerve cell ligands for myelin-associated glycoprotein (MAG), an inhibitor of nerve regeneration. Proc Natl Acad Sci USA 99: 8412-8417.
- WATANABE K, MATSUBARA T AND HAKOMORI S. 1976. alpha-L-Fucopyranosylceramide, a novel glycolipid accumulated in some of the human colon tumors. J Biol Chem 251: 2385-2387.
- YAMAMURA S, HANDA K AND HAKOMORI S. 1997. A close association of GM3 with c-Src and Rho in GM3-enriched microdomains at the B16 melanoma cell surface membrane: a preliminary note. Biochem Biophys Res Commun 236: 218-222.
- YANG LJ-S, ZELLER CB, SHAPER NL, KISO M, HASEGAWA A, SHAPIRO RE AND SCHNAAR RL. 1996. Gangliosides are neuronal ligands for myelin-associated glycoprotein Proc Natl Acad Sci USA 93: 814-818.
- YATES AJ AND RAMPERSAUD A. 1998. in Sphingolipids as signaling modulators in the nervous system ( LEDEEN RW, HAKOMORI S, YATES AJ, SCHNEIDER JS AND YU RK., eds) Vol. 845, pp. 57-71, New York Acad Sci, New York, NY.
- YOSHINO T, WATANABE K AND HAKOMORI S. 1982. Chemical synthesis of alpha-L-fucopyranosylceramide and its analogues and preparation of antibodies directed to this glycolipid. Biochemistry 21: 928-934.
- YU S, WITHERS DA AND HAKOMORI S. 1998. Globoside-dependent adhesion of human embryonal carcinoma cells, based on carbohydrate-carbohydrate interaction, initiates signal transduction and induces enhanced activity of transcription factors AP1 and CREB. J Biol Chem 273: 2517-2525.
- ZHU Z, KOJIMA N, STROUD MR, HAKOMORI S AND FENDERSON BA. 1995. Monoclonal antibody directed to Le(y) oligosaccharide inhibits implantation in the mouse. Biol Repro 52: 903-912.
Publication Dates
-
Publication in this collection
20 Aug 2004 -
Date of issue
Sept 2004
History
-
Received
01 Apr 2004 -
Accepted
02 Apr 2004