ABSTRACT
Mori folium, the leaf of Morus alba L. (Moraceae), has been traditionally used for various medicinal purposes from ancient times to the present. In this study, we examined the effects of water extract of Mori folium (WEMF) on the production of inflammatory mediators, such as nitric oxide (NO) and prostaglandin E2 (PGE2), and reactive oxygen species (ROS) in lipopolysaccharide (LPS)-stimulated murine RAW 264.7 macrophages. Our data indicated that WEMF significantly suppressed the secretion of NO and PGE2 in RAW 264.7 macrophages without any significant cytotoxicity. The protective effects were accompanied by a marked reduction in their regulatory gene expression at the transcription level. WEMF attenuated LPS-induced intracellular ROS production in RAW 264.7 macrophages. It inhibited the nuclear translocation of the nuclear factor-kappa B p65 subunit and the activation of mitogen-activated protein kinases in LPS-treated RAW 264.7 macrophages. Furthermore, WEMF reduced LPS-induced NO production and ROS accumulation in zebrafish. Although more efforts are needed to fully understand the critical role of WEMF in the inhibition of inflammation, the findings of the present study may provide insights into the approaches for Mori folium as a potential therapeutic agent for inflammatory and antioxidant disorders.
Key words:
Mori folium; inflammation; ROS; macrophage; zebrafish
INTRODUCTION
Inflammation is a primary protective response of the body involving the activation of immune system processes. The inflammatory response is a highly regulated self-limiting process for identifying and destroying invading pathogens and restoring normal tissue structure and function (Conti et al. 2004CONTI B, TABAREAN I, ANDREI C AND BARFAI T. 2004. Cytokines and fever. Front Biosci 9: 1433-1449., Freire and Van Dyke 2013FREIRE MO AND VAN DYKE TE. 2013. Natural resolution of inflammation. Periodontol 2000 63: 149-164.). However, an excessive inflammatory response has been recognized as the main cause of chronic inflammation, such as in cardiovascular disease, rheumatoid arthritis, inflammatory bowel disease, Alzheimer's disease, and even cancer (Amin et al. 1999AMIN AR, ATTUR M AND ABRAMSON SB. 1999. Nitric oxide synthase and cyclooxygenases: distribution, regulation, and intervention in arthritis. Curr Opin Rheumatol 11: 202-209., Freire and Van Dyke 2013).
When macrophages are over-activated by inflammatory stimulants, including the gram-negative bacterial endotoxin lipopolysaccharides (LPS), the cells induce the production of inflammatory mediators, including nitric oxide (NO) and prostaglandin E2 (PGE2), and inflammatory cytokines along with the activation of several signaling pathways, such as nuclear factor-kappa B (NF-κB) and mitogen-activated protein kinases (MAPKs) signaling (Kaminska 2005KAMINSKA B. 2005. MAPK signalling pathways as molecular targets for anti-inflammatory therapy-from molecular mechanisms to therapeutic benefits. Biochim Biophys Acta 1754: 253-262., Lu et al. 2011LU YC, JAYAKUMAR T, DUANN YF, CHOU YC, HSIEH CY, YU SY, SHEU JR AND HSIAO G. 2011. Chondroprotective role of sesamol by inhibiting MMPs expression via retaining NF-κB signaling in activated SW1353 cells. J Agric Food Chem 59: 4969-4978., Rigoglou and Papavassiliou 2013RIGOGLOU S AND PAPAVASSILIOU AG. 2013. The NF-κB signalling pathway in osteoarthritis. Int J Biochem Cell Biol 45: 2580-2584., Muralidharan and Mandrekar 2013MURALIDHARAN S AND MANDREKAR P. 2013. Cellular stress response and innate immune signaling: integrating pathways in host defense and inflammation. J Leukoc Biol 94: 1167-1184.). Excessive production of these inflammatory mediators and cytokines further provoke deleterious consequences in the pathogenesis of many inflammatory diseases (McDaniel et al. 1996, Muralidharan and Mandrekar 2013).
Another important component of inflammation is oxidative stress, which reflects the imbalance between the production of reactive oxygen species (ROS) and the ability of the biological system to remove them (Brüne et al. 2013BRÜNE B, DEHNE N, GROSSMANN N, JUNG M, NAMGALADZE D, SCHMID T, VON KNETHEN A AND WEIGERT A. 2013. Redox control of inflammation in macrophages. Antioxid Redox Signal 19: 595-637., Mills and O'Neill 2016MILLS EL AND O'NEILL LA. 2016. Reprogramming mitochondrial metabolism in macrophages as an anti-inflammatory signal. Eur J Immunol 46: 13-21.). The overproduced ROS by activated macrophages acts as an important contributor to the manifestation of inflammation (Varga et al. 2013VARGA A, BUDAI MM, MILESZ S, BACSI A, TOZER J AND BENKO S. 2013. Ragweed pollen extract intensifies lipopolysaccharide-induced priming of NLRP3 inflammasome in human macrophages. Immunology 138: 392-401., Mills and O'Neill 2016), and it is also involved in the production of inflammatory mediators in LPS-stimulated macrophages (Haddad and Land 2002HADDAD JJ AND LAND SC. 2002. Redox signaling-mediated regulation of lipopolysaccharide-induced proinflammatory cytokine biosynthesis in alveolar epithelial cells. Antioxid Redox Signal 4: 179-193.). Consequently, the suppression of the production of inflammatory factors by blocking macrophage activation emerges as a potential therapeutic approach to relieve the progression of inflammatory and oxidative disorders (Cunha et al. 2008CUNHA TM, VERRI WA JR, SCHIVO IR, NAPIMOGA MH, PARADA CA, POOLE S, TEIXEIRA MM, FERREIRA SH AND CUNHA F. 2008. Crucial role of neutrophils in the development of mechanical inflammatory hypernociception. J Leukoc Biol 83: 824-832., Zhang and Wang 2014ZHANG L AND WANG CC. 2014. Inflammatory response of macrophages in infection. Hepatobiliary Pancreat Dis Int 13: 138-152.). For this reason, the development of anti-inflammatory and/or antioxidant agents, which can control the activation of macrophages, is necessary for the prevention and treatment of various diseases.
Recent data have convincingly pointed out that natural resources have been widely and safely consumed over centuries and that most of them have a wide range of diverse biological activities with few side effects (Bocanegra et al. 2009BOCANEGRA A, BASTIDA S, BENEDI J, RODENAS S AND SANCHEZ-MUNIZ FJ. 2009. Characteristics and nutritional and cardiovascular-health properties of seaweeds. J Med Food 12: 236-258. , Abuajah et al. 2015ABUAJAH CI, OGBONNA AC AND OSUJI CM. 2015. Functional components and medicinal properties of food: a review. J Food Sci Technol 52: 2522-2529.). Among these natural resources, Morus alba L. that belongs to the Moraceae family, is one of the most valuable and rich in natural ingredients plant. This tree is widely distributed in Eastern Asia countries including Korea, China and Japan but now is cultivated throughout worldwide. The leaf of plant, Mori folium (Supplementary Material, Figure S1), has been used in traditional medicine for the treatment of various diseases (Yang et al. 2014YANG L, BU L, SUN W, HU L AND ZHANG S. 2014. Functional characterization of mannose-binding lectin in zebrafish: implication for a lectin-dependent complement system in early embryos. Dev Comp Immunol 46: 314-322., Chan et al. 2016CHAN EW, LYE PY AND WONG SK. 2016. Phytochemistry, pharmacology, and clinical trials of Morus alba. Chin J Nat Med 14: 17-30.). Mori folium exhibits a variety of pharmacological activities, such as anti-microbial (Tirupathi et al. 2011TIRUPATHI RG, SURESH BK, UJWAL KJ, SUJANA P, RAOA AV AND SREEDHAR AS. 2011. Anti-microbial principles of selected remedial plants from Southern India. Asian Pac J Trop Biomed 1: 298-305.), anti-tumor (Deepa et al. 2013DEEPA M, SURESHKUMAR T, SATHEESHKUMAR PK AND PRIYA S. 2013. Antioxidant rich Morus alba leaf extract induces apoptosis in human colon and breast cancer cells by the downregulation of nitric oxide produced by inducible nitric oxide synthase. Nutr Cancer 65: 305-310.), anti-obesity (Sugimoto et al. 2009SUGIMOTO M ET AL. 2009. Mulberry leaf ameliorates the expression profile of adipocytokines by inhibiting oxidative stress in white adipose tissue in db/db mice. Atherosclerosis 204: 388-394., Ann et al. 2015ANN JY, EO H AND LIM Y. 2015. Mulberry leaves (Morus alba L.) ameliorate obesity-induced hepatic lipogenesis, fibrosis, and oxidative stress in high-fat diet-fed mice. Genes Nutr 10: 46.), anti-hypotensive (Kobayashi et al. 2010KOBAYASHI Y, MIYAZAWA M, KAMEI A, ABE K AND KOJIMA T. 2010. Ameliorative effects of mulberry (Morus alba L.) leaves on hyperlipidemia in rats fed a high-fat diet: induction of fatty acid oxidation, inhibition of lipogenesis, and suppression of oxidative stress. Biosci Biotechnol Biochem 74: 2385-2395.), neuroprotective (Xiang et al. 2010XIANG J, TANG YP, ZHOU ZY, WU P, WANG Z, MORI M AND CAI DF. 2010. Apocynum venetum leaf extract protects rat cortical neurons from injury induced by oxygen and glucose deprivation in vitro. Can J Physiol Pharmacol 88: 907-917.), anti-diabetic (Naowaboot et al. 2009NAOWABOOT J, PANNANGPETCH P, KUKONGVIRIYAPAN V, KUKONGVIRIYAPAN U, NAKMAREONG S AND ITHARAT A. 2009. Mulberry leaf extract restores arterial pressure in streptozotocin-induced chronic diabetic rats. Nutr Res 29: 602-608. ), and immunomodulatory potentials (Kwon et al. 2016KWON DH ET AL. 2016. The immunomodulatory activity of Mori folium, the leaf of Morus alba L., in RAW 264.7 macrophages in vitro. J Cancer Prev 21: 144-151.). In addition, Mori folium possesses free radical-scavenging activities (Kim and Jang 2011KIM GN AND JANG HD. 2011. Flavonol content in the water extract of the mulberry (Morus alba L.) leaf and their antioxidant capacities. J Food Sci 76: C869-873. , Iqbal et al. 2012, Raman et al. 2016RAMAN ST, GANESHAN AK, CHEN C, JIN C, LI SH, CHEN HJ AND GUI Z. 2016. In vitro and in vivo antioxidant activity of flavonoid extracted from mulberry fruit (Morus alba L.). Pharmacogn Mag 12: 128-133.), which may explain its antioxidant ability (Khan et al. 2013KHAN MA ET AL. 2013. A comparative study on the antioxidant activity of methanolic extracts from different parts of Morus alba L. (Moraceae). BMC Res Notes 6: 24., Kim et al. 2014, Raman et al. 2016). Previous studies, including our recent data, indicated that the extracts and components of Mori folium have strong anti-inflammatory properties (Hong et al. 2002HONG CH, HUR SK, OH OJ, KIM SS, NAM KA AND LEE SK. 2002. Evaluation of natural products on inhibition of inducible cyclooxygenase (COX-2) and nitric oxide synthase (iNOS) in cultured mouse macrophage cells. J Ethnopharmacol 83: 153-159., Shibata et al. 2007SHIBATA Y ET AL. 2007. Mulberry leaf aqueous fractions inhibit TNF-alpha-induced nuclear factor kappaB (NF-kappaB) activation and lectin-like oxidized LDL receptor-1 (LOX-1) expression in vascular endothelial cells. Atherosclerosis 193: 20-27., Chao et al. 2009CHAO WW, KUO YH, LI WC AND LIN BF. 2009. The production of nitric oxide and prostaglandin E2 in peritoneal macrophages is inhibited by Andrographis paniculata, Angelica sinensis and Morus alba ethyl acetate fractions. J Ethnopharmacol 122: 68-75., Jeong et al. 2016JEONG JW ET AL. 2016. Mori folium inhibits interleukin-1β-induced expression of matrix metalloproteinases and inflammatory mediators by suppressing the activation of NF-κB and p38 MAPK in SW1353 human chondrocytes. Int J Mol Med 37: 452-460.). Despite these encouraging studies, the effects and molecular mechanisms responsible for the anti-inflammatory and antioxidant potentials of Mori folium have remained elusive. Therefore, we investigated in this study the anti-inflammatory and antioxidant actions of the water extract of Mori folium (WEMF) in LPS-stimulated RAW 264.7 macrophage cells by measuring its ability to inhibit NO, PGE2, and ROS production. Moreover, we confirmed the protective effects of WEMF on NO and ROS generation in zebrafish larvae.
MATERIALS AND METHODS
PREPARATION AND FINGERPRINTING OF WEMF
The dried leaves of M. alba were obtained from Bio-Port Korea, Inc. (Busan, Republic of Korea), and WEMF was prepared as previous described (Jeong et al. 2016JEONG JW ET AL. 2016. Mori folium inhibits interleukin-1β-induced expression of matrix metalloproteinases and inflammatory mediators by suppressing the activation of NF-κB and p38 MAPK in SW1353 human chondrocytes. Int J Mol Med 37: 452-460.). WEMF was dissolved in a 100 mg/ml concentration with distilled water, and the stock solution was then diluted with culture medium to the desired concentration prior to use. To confirmation the reproducibility of WEMF, we conducted fingerprinting using a high-performance liquid chromatography (HPLC)-based compositional analysis with two main reference compounds, namely, rutin hydrate and astragalin. All analyses were performed using an Agilent 1100 series HPLC instrument (Agilent Technologies, San Jose, CA, USA) as previously reported (Lee et al. 2014LEE JS, KIM HG, HAN JM, KIM DW, YI MH, SON SW, KIM YA, LEE JS, CHOI MK AND SON CG. 2014. Ethanol extract of Astragali Radix and Salviae Miltiorrhizae Radix, Myelophil, exerts anti-amnesic effect in a mouse model of scopolamine-induced memory deficits. J Ethnopharmacol 153: 782-792.). The representative sample chromatogram and quantitative analysis are illustrated in Figure 1.
Fingerprint analysis of WEMF using HPLC. The two reference components (a) and WEMF (b) were analyzed by HPLC analysis. The quantitative data of WEMF (c) for rutin hydrate and astragalin were presented.
CELL CULTURE AND CELL VIABILITY ASSAY
The RAW 264.7 murine macrophage cell line was obtained from the Korean Cell Line Bank (Seoul, Republic of Korea) and cultured at 37°C in 5% CO2 containing Dulbecco s modified Eagle's medium (WelGENE Inc., Daegu, Republic of Korea) supplemented with 10% fetal bovine serum (WelGENE Inc.), 100 U/ml of penicillin, and 100 mg/ml of streptomycin (Sigma-Aldrich Chemical Co., St. Louis, MO, USA). A colorimetric 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT, Sigma-Aldrich Chemical Co.) assay was performed to measure cell viability. In brief, RAW 264.7 cells were treated with various concentrations of WEMF for 24 h or pretreated with WEMF for 1 h before stimulation with 500 ng/ml LPS (Sigma-Aldrich Chemical Co.) for 24 h. After incubation, the medium was discarded, and MTT solution (5 mg/mL in phosphate-buffered saline, PBS) was added to each well and incubated for another 3 h at 37°C. The medium was removed and dimethyl sulfoxide (Sigma-Aldrich Chemical Co.) was added to dissolve the formazan dye. The optical density was then read at 560 nm using a microplate spectrophotometer (Molecular Devices, Sunnyvale, CA, USA) to determine cell viability (Oh et al. 2015OH K, MOON HG, LEE DS AND YOO YB. 2015. Tissue transglutaminase-interleukin-6 axis facilitates peritoneal tumor spreading and metastasis of human ovarian cancer cells. Lab Anim Res 31: 188-197.).
MEASUREMENT OF NO PRODUCTION IN RAW 264.7 MACROPHAGES
The production of NO in culture supernatants was assayed using Griess reagent (Sigma-Aldrich Chemical Co.). For this assay, the supernatant was collected and mixed with the same volume of Griess reagent for 10 min at room temperature in the dark. Absorbance was measured at 540 nm on a microplate reader, and NO concentrations were calculated by referencing a standard curve generated by known concentrations of sodium nitrite (Lee et al. 2015aLEE H, PYO MJ, BAE SK, HEO Y, KIM CG, KANG C AND KIM E. 2015a. Improved therapeutic profiles of PLA2-free bee venom prepared by ultrafiltration method. Toxicol Res 31: 33-40.).
MEASUREMENT OF PGE2 PRODUCTION IN RAW 264.7 MACROPHAGES
To measure the production of PGE2, the cells were cultured under the same conditions as those for the NO measurement assay. The levels of PGE2 concentrations in cultured media were determined by an enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems, Minneapolis, MN, USA), according to the manufacturer's instructions (Wang et al. 2015WANG L, XU ML, LIU J, WANG Y, HU JH AND WANG MH. 2015. Sonchus asper extract inhibits LPS-induced oxidative stress and pro-inflammatory cytokine production in RAW264.7 macrophages. Nutr Res Pract 9: 579-585.).
RNA ISOLATION AND REVERSER TRANSCRIPTION POLYMERASE CHAIN REACTION (RT-PCR)
Total RNA was isolated from cells using TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA, USA) according to the manufacturer's instructions and reverse transcribed using the M-MLV reverse transcriptase kit (BioNEER, Daejeon, Republic of Korea) to produce cDNAs. RT-generated cDNAs encoding iNOS and COX-2 genes were amplified by PCR using the desired primers (BioNEER). Following amplification, the PCR products were separated by 1.5% agarose gel electrophoresis, stained with ethidium bromide, and visualized by ultraviolet illumination. In a parallel experiment, glyceraldehyde 3-phosphate dehydrogenase was used as an internal control.
PROTEIN EXTRACTION AND WESTERN BLOT ANALYSIS
The cells were collected and resuspended in an extraction lysis buffer [25 mM Tris-Cl (pH 7.5), 250 mM NaCl, 5 mM ethylene diaminetetra acetic acid, 1% NP-40, 1 mM pheny-methylsulfonyl fluoride, and 5 mM dithiothreitol] for 30 min at 4°C. In a parallel experiment, nuclear and cytosolic proteins were separated using NE-PER nuclear and cytosolic extraction reagents (Pierce Biotechnology, Rockford, IL, USA) according to the manufacturer's protocol. Equal amounts of protein from each sample were separated by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis at 90 V for 2 h and transferred onto polyvinylidene fluoride membranes (Millipore, Bedford, MA, USA). Thereafter, the membranes were incubated overnight at 4°C with the corresponding primary antibodies purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA) and Cell Signaling Technology, Inc. (Boston, MA, USA). Then, the membranes were incubated with the appropriate secondary antibodies conjugated to horseradish peroxidase (Amersham Co., Arlington Heights, IL, USA)) at room temperature for 2 h. Using an enhanced chemiluminescence (ECL, Amersham Co.) detection system, immunoreactive bands were detected.
IMMUNOFLUORESCENT STAINING FOR NF-ΚB P65 IN RAW 264.7 MACROPHAGES
The NF-κB p65 nuclear translocalization was detected by an immunofluorescence assay using a fluorescence microscope. For this study, RAW 246.7 cells were pretreated with WEMF for 1 h and then stimulated with LPS for 1 h. The cells were fixed with 3.7% paraformaldehyde (Sigma-Aldrich Chemical Co.) in PBS for 10 min at 4°C, permeabilized with 0.4% Triton X-100 in PBS for 10 min, and blocked with 5% bovine serum albumin for 1 h. The cells were probed with anti-p65 NF-κB antibody (Santa Cruz Biotechnology, Inc.) overnight at 4°C and then incubated with fluorescein isothiocyanate-conjugated donkey anti-rabbit IgG (Jackson ImmunoResearch Laboratories Inc., West Grove, PA, USA) for 2 h at room temperature. The position of the cell nucleus was determined with 4,6-diamidino-2-phenylindole (DAPI, Sigma-Aldrich Chemical Co.) solution (1 mg/ml) for 15 min. After washing the cells with PBS, fluorescence was visualized using a fluorescence microscope (Carl Zeiss, Oberkochen, Germany).
MEASUREMENT OF ROS GENERATION IN RAW 264.7 MACROPHAGES
To measure the ROS levels, the cells were washed twice with PBS and lysed with 1% Triton X-100 in PBS for 10 min at 37°C. The cells were then stained with 10 μM 2',7'-dichlorofluorescein diacetate (DCF-DA, Molecular Probes, Eugene, OR, USA) for 20 min at room temperature in the dark. The green fluorescence emitted by DCF was recorded at 515 nm using a flow cytometer (Becton Dickinson, San Jose, CA, USA), and 10,000 events were counted per sample (Eom et al. 2015EOM SA ET AL. 2015. Protective effects of PEP-1-Catalase on stress-induced cellular toxicity and MPTP-induced Parkinson's disease. BMB Rep 48: 395-400.). Image analysis for the generation of intracellular ROS was acquired using a fluorescence microscope.
ZEBRAFISH EMBRYO AND LARVAE MAINTENANCE
Adult zebrafish were obtained from Dr. Hyo-Jong Lee, College of Pharmacy, Inje University (Gimhae, Republic of Korea) and maintained at 28.5°C with a 14:10 h light/dark cycle in a recirculating tank system using local tap water (pH 7.2-7.6, salinity 0.03%-0.04%). The embryos were obtained from natural spawning within 30 min and maintained at a density of about 50 embryos per 100 mm2 in a Petri dish containing media, as previously reported (Wijesinghe et al. 2014WIJESINGHE WA, KIM EA, KANG MC, LEE WW, LEE HS, VAIRAPPAN CS AND JEON YJ. 2014. Assessment of anti-inflammatory effect of 5β-hydroxypalisadin B isolated from red seaweed Laurencia snackeyi in zebrafish embryo in vivo model. Environ Toxicol Pharmacol 37: 110-117.). The entire study design and experimental procedures were approved by the Dongeui University Animal Care and Use Committee (Busan, Republic of Korea).
MEASUREMENT OF NO AND ROS PRODUCTION IN ZEBRAFISH LARVAE
Approximately three days post-fertilization (dpf), embryos (n = 25) were transferred to individual wells of a 24-well plate and maintained in embryo media containing sterile distilled water (vehicle control), 800 μg/ml WEMF (final concentration), 10 μg/ml LPS (final concentration), or 800 μg/ml WEMF for 1 h followed by treatment with 10 μg/ml LPS, except the larvae in the control group, for up to 4 dpf. The generation of NO and ROS in zebrafish larvae was analyzed using fluorescent probe dyes, 4-amino-5-methylamino-2'7' difluorofluorescein diacetate (DAF-FM-DA, Molecular Probes) and DCF-DA, respectively. After 4 dpf, the larvae were transferred into 24-well plates and incubated with DAF-FM DA (5 μM) and DCF-DA (20 μg/ml) solution for 1 h in the dark at 28.5°C, and then anaesthetized using 1-phenoxy-2-propanol (1/500 dilution, Acros Organics, Morris Plains, NJ, USA). The images of stained larvae were observed for NO and ROS generation under a fluorescence microscope, and fluorescence intensity of individual larvae was quantified at an excitation wavelength of 485 nm and an emission wavelength of 535 nm using a spectrophotometer and ImageJ 1.46r software (Wayne Rasband, National Institutes of Health, Bethesda, MD, USA), respectively. The generation of NO and ROS was calculated by comparing the fluorescence intensity of treatment larvae with that of the controls (Wijesinghe et al. 2014WIJESINGHE WA, KIM EA, KANG MC, LEE WW, LEE HS, VAIRAPPAN CS AND JEON YJ. 2014. Assessment of anti-inflammatory effect of 5β-hydroxypalisadin B isolated from red seaweed Laurencia snackeyi in zebrafish embryo in vivo model. Environ Toxicol Pharmacol 37: 110-117.).
STATISTICAL ANALYSIS
All data are presented as mean ± standard deviation (SD). Significant differences among groups were determined using the unpaired Student's t-test. A value of p<0.05 was accepted as an indication of statistical significance. All the figures shown here reflect the data obtained from at least three independent experiments.
RESULTS
CYTOTOXIC EFFECTS OF WEMF AND LPS ON RAW 264.7 MACROPHAGES
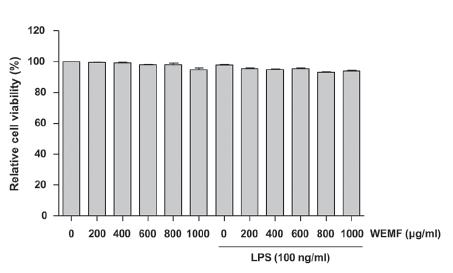
To exclude the cellular toxicity caused by WEMF treatment, RAW 264.7 cells were treated with WEMF and/or LPS for 24 h. The MTT assay showed that WEMF of up to 1,000 μg/ml in the presence or absence of 100 ng/ml LPS was not cytotoxic (Figure 2). Therefore, we selected 800 μg/ml WEMF as the maximum concentration for further experiments in RAW 264.7 cells.
Effects of WEMF on the cell viability of RAW 264.7 macrophages. The cells were treated with various concentrations of WEMF for 24 h or pretreated with the indicated concentrations of WEMF for 1 h prior to LPS (100 ng/ml) treatment for 24 h. Cell viability was assessed with an MTT reduction assay, and the results were expressed as the percentage of surviving cells over control cells (no addition of WEMF and LPS). Values represent the means ± SD of three independent experiments.
WEMF SUPPRESSED LPS-INDUCED NO AND PGE2 PRODUCTION IN RAW 264.7 MACROPHAGES
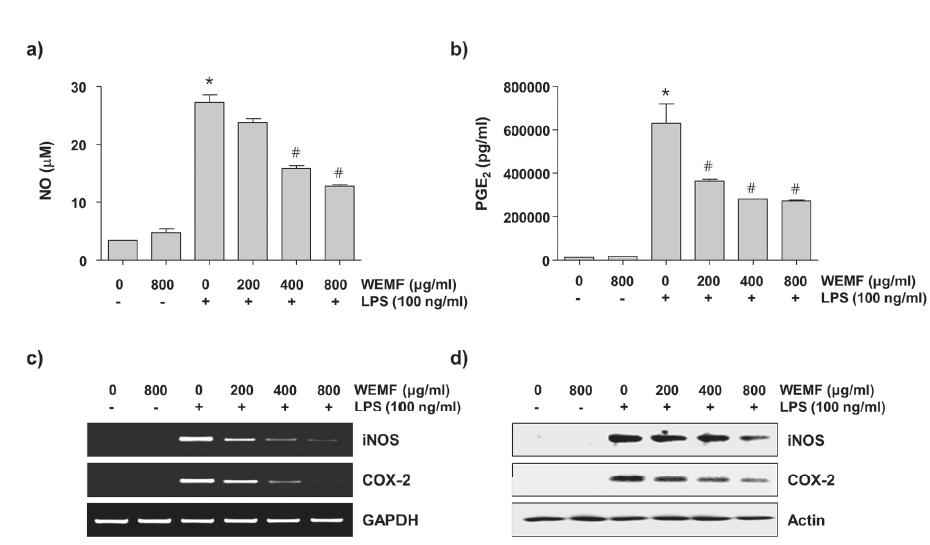
To determine the inhibitory properties of WEMF on LPS-induced NO and PGE2 production in RAW 264.7 cells, the cells were pretreated with the indicated concentrations of WEMF for 1 h and then stimulated with 100 ng/ml LPS for another 24 h. The levels of NO and PGE2 in the culture supernatants were determined by Griess reaction assay and ELISA, respectively. As indicated in Figure 3a and b, stimulation with LPS markedly induced the production of NO and PGE2 compared with not stimulating with LPS. However, WEMF significantly inhibited NO and PGE2 secretion in RAW 264.7 cells in a concentration-dependent manner.
Inhibition of NO and PGE2 production by WEMF in LPS-stimulated RAW 264.7 macrophages. The cells were pretreated with the indicated concentrations of WEMF for 1 h prior to incubation with 100 ng/ml LPS for 24 h. The levels of NO (a) and PGE2 (b) in culture media were measured by Griess assay and a commercial ELISA kit, respectively. Each value indicates the mean ± SD and is representative of the results obtained from three independent experiments (#p<0.05 compared with the control; *p<0.05 compared with cells cultured with 100 ng/ml LPS). (c) The total RNAs were isolated from cells grown under the same conditions as those in Figure 3 and prepared for the RT-PCR analysis of the iNOS and COX-2 mRNA expression using the indicated primers. (d) Cell lysates were prepared for Western blot analysis, with antibodies specific for murine iNOS and COX-2, and for an ECL detection system. The experiment was repeated three times and similar results were obtained. GAPDH and actin were used as the internal controls for the RT-PCR and Western blot analysis, respectively.
WEMF ATTENUATES LPS-INDUCED INOS AND COX-2 EXPRESSION IN RAW 264.7 MACROPHAGES
We next investigated if the inhibitory effects of WEMF on NO and PGE2 production were related to the regulation of the expression of their synthesis enzymes, iNOS and COX-2, respectively. As shown in Figure 3c and d, WEMF concentration-dependently inhibited the protein and mRNA expression of iNOS and COX-2 in the LPS-stimulated RAW 264.7 cells. These data indicate that WEMF suppresses NO and PGE2 production by reducing the expression of their encoding genes.
WEMF BLOCKS LPS-INDUCED NF-ΚB NUCLEAAR TRANSLOCATION IN RAW 264.7 MACROPHAGES
As active NF-κB translocates to the nucleus where it activates its target genes including, iNOS, COX-2, and pro-inflammatory cytokines, by binding to their promoter regions (Lu et al. 2011LU YC, JAYAKUMAR T, DUANN YF, CHOU YC, HSIEH CY, YU SY, SHEU JR AND HSIAO G. 2011. Chondroprotective role of sesamol by inhibiting MMPs expression via retaining NF-κB signaling in activated SW1353 cells. J Agric Food Chem 59: 4969-4978., Rigoglou and Papavassiliou 2013RIGOGLOU S AND PAPAVASSILIOU AG. 2013. The NF-κB signalling pathway in osteoarthritis. Int J Biochem Cell Biol 45: 2580-2584.), we investigated whether or not WEMF attenuates the LPS-induced nuclear translocation of NF-κB in RAW 264.7 cells. The immunoblotting data using cytoplasmic and nuclear extracts indicated that WEMF pretreatment inhibited the NF-κB p65 subunit nuclear accumulation, which was associated with the attenuation of IκBα degradation in LPS-stimulated RAW 264.7 cells (Figure 4a). Consistent with these results, immunocytochemistry analysis also indicated that NF-κB p65 was normally sequestered in the cytoplasm following stimulation with LPS. However, the LPS-mediated nuclear translocation of NF-κB was considerably blocked by pretreatment with WEMF (Figure 4b). These results indicated that WEMF attenuated the transcriptional activation of NF-κB, which controls the expression of pro‐inflammatory genes in LPS-treated RAW 264.7 cells.
Inhibition of NF-κB nuclear translocation by WEMF in LPS-stimulated RAW 264.7 macrophages. (a) The cells were pretreated with 800 μg/ml WEMF for 1 h before 100 ng/ml LPS treatment for 1 h. The nuclear and cytosolic proteins were prepared for Western blot analysis using anti-NF-κB p65 and anti-IκB-α antibodies and an ECL detection system. Lamin B and actin were used as internal controls for the nuclear and cytosolic fractions, respectively. (b) The cells were pretreated with 800 μg/ml WEMF for 1 h before 100 ng/ml LPS treatment. After 1 h of incubation, the localization of NF-κB p65 was visualized with fluorescence microscopy after immunofluorescence staining with anti-NF-κB p65 antibody (green). The cells were also stained with DAPI to visualize the nuclei (blue). The results are representative of those obtained from three independent experiments.
WEMF REDUCES THE ACTIVATION OF MAPKS IN LPS-STIMULATED RAW 264.7 MACROPHAGES
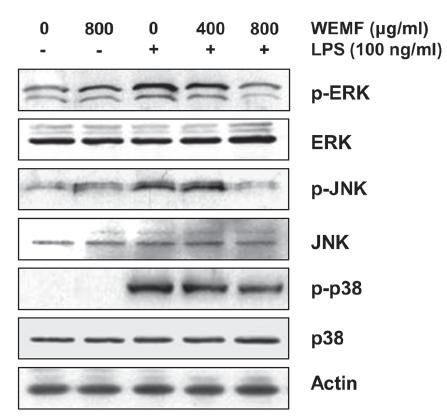
As the activation of MAPKs is crucial for LPS-stimulated NF-κB activation and the subsequent transcriptional activation of inflammatory gene expression (Kaminska 2005KAMINSKA B. 2005. MAPK signalling pathways as molecular targets for anti-inflammatory therapy-from molecular mechanisms to therapeutic benefits. Biochim Biophys Acta 1754: 253-262., Muralidharan and Mandrekar 2013MURALIDHARAN S AND MANDREKAR P. 2013. Cellular stress response and innate immune signaling: integrating pathways in host defense and inflammation. J Leukoc Biol 94: 1167-1184.), we analyzed the phosphorylation of MAPKs, such as extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK), and p38 MAPK, by Western blot analysis. As shown in Figure 5, stimulation with LPS resulted in the marked phosphorylation of ERK, JNK, and p38 MAPK, and their total expressions did not have any significant change. However, WEMF concentration-dependently blocked the LPS-induced phosphorylation of three MAPKs. The result showed that WEMF suppressed MAPKs signaling pathway to reduce inflammatory responses in LPS-induced RAW 264.7 cells.
Effects of WEMF on the LPS-induced phosphorylation of MAPKs in RAW 264.7 macrophages. The cells were treated with the indicated concentrations of WEMF for 1 h prior to treatment with 100 ng/ml LPS for 30 min. Total proteins were isolated and subjected to SDS-polyacrylamide gels, followed by Western blot analysis using the indicated antibodies. Proteins were visualized using the ECL detection system.
WEMF SUPPRESSES LPS-INDUCED ACCUMULATION OF ROS IN RAW 264.7 MACROPHAGES
As oxidative stress is partly involved in the initiation of inflammation (Brüne et al. 2013BRÜNE B, DEHNE N, GROSSMANN N, JUNG M, NAMGALADZE D, SCHMID T, VON KNETHEN A AND WEIGERT A. 2013. Redox control of inflammation in macrophages. Antioxid Redox Signal 19: 595-637., Mills and O'Neill 2016MILLS EL AND O'NEILL LA. 2016. Reprogramming mitochondrial metabolism in macrophages as an anti-inflammatory signal. Eur J Immunol 46: 13-21.), we examined whether or not WEMF could reduce the LPS-induced generation of ROS in RAW 264.7 cells using DCF-DA staining. The results of the flow cytometric assay indicated that the accumulation of intracellular ROS was observed at 0.5 h, and that the levels continued to increase up to 2 h by LPS treatment (Figure 6a). However, the increase in LPS-stimulated ROS production was markedly attenuated by pretreatment with WEMF (Figure 6b and c). As a positive control, the ROS scavenger N-acetyl-l-cysteine (NAC) also effectively attenuated LPS-induced ROS generation, but WEMF itself did not contribute to the ROS generation. This finding suggests that the anti-inflammatory potential of WEMF may be associated with its antioxidant effects on RAW 264.7 cells.
Effects of WEMF on LPS-induced ROS production in RAW 264.7 macrophages. The cells were treated with 100 ng/ml LPS for the indicated time (a) or pre-incubated with or without 800 μg/ml WEMF or 10 mM NAC for 1 h and then stimulated with 100 ng/ml LPS for 2 h (b and c). The cells were incubated with 10 μM DCF-DA for 30 min at 37°C. The cells were collected and DCF fluorescence was measured by a flow cytometry. Values represent the means ± SD of two independent experiments. (c) Images were obtained using a fluorescence microscope, presented from one experiment, and were representative of at least three independent experiments.
WEMF DOWNREGULATES LPS-INDUCED NO AND ROS PRODUCTION IN ZEBRAFISH
To confirm the in vivo protective effects of WEMF on LPS-induced NO generation, we visualized DAF-FM-DA staining in a zebrafish model. As shown in Figure 7a and b, the control, which was not treated with LPS or WEMF, and WEMF alone groups generated a clear image, thus indicating that WEMF alone did not affect the basal NO levels. However, stimulation of the zebrafish larvae with LPS markedly generated fluorescence image, thus suggesting that NO generation took place in the presence of LPS and WEMF reduced the LPS-stimulated elevation of NO production. We also evaluated the inhibitory effect of WEMF on LPS-induced ROS accumulation. Microphotographs of DCF-DA staining revealed excessive ROS accumulation after LPS stimulation. By contrast, when the zebrafish larvae were treated with WEMF prior to LPS administration, an effective reduction in the generation of ROS was observed (Figure 7c and d), thus suggesting that WEMF also inhibited LPS-stimulated ROS production in vivo.
Protective effect of WEMF on LPS-induced NO and ROS generation in zebrafish larvae. The zebrafish larvae were treated with 800 μg/ml WEMF and 10 μg/ml LPS for 24 h or pretreated with 800 μg/ml WEMF for 1 h prior to incubation with 10 μg/ml LPS for 24 h (a and b). The levels of NO and ROS generation were observed under a fluorescence microscope after staining with DAF-FM-DA and DCF-DA, respectively (c and d). The fluorescence intensities of NO and ROS levels in individual zebrafish larvae were quantified. The values represented the means ± SD of three independent experiments (#p<0.05 compared with the control; *p<0.05 compared with the LPS-treated group).
DISCUSSION
Inflammation is a host defense mechanism against pathogenic challenges, and multiple events are involved in the development of inflammation. During infection by gram-negative bacterial LPS, membrane-bound pattern recognition receptor Toll-like receptor 4 (TLR4) is a critical driver of immune responses (Aderem and Ulevitch 2000ADEREM A AND ULEVITCH RJ. 2000. Toll-like receptors in the induction of the innate immune response. Nature 406: 782-787., Nikaido 2003NIKAIDO H. 2003. Molecular basis of bacterial outer membrane permeability revisited. Microbiol Microbiol Mol Biol Rev 67: 593-656. ). The activation of TLR4 pathway leads to intracellular signaling pathways that culminate in the activation of several intracellular signaling pathways, including NF-κB and MAPKs. The consequent activation of macrophages promotes inflammation through the aberrant production of pro-inflammatory mediators and cytokines that recruit additional immune cells to sites of infection or tissue injury (Aderem and Ulevitch 2000, Nikaido 2003). Regarding the importance of pro-inflammatory cytokines in inflammatory responses, the pro-inflammatory mediators of NO and PGE2 play crucial roles in the development of chronic inflammatory disease (Rocca and FitzGerald 2002ROCCA B AND FITZGERALD GA. 2002. Cyclooxygenases and prostaglandins: shaping up the immune response. Int Immunopharmacol 2: 603-630., Kim et al. 2005KIM SF, HURI DA AND SNYDER SH. 2005. Inducible nitric oxide synthase binds, S-nitrosylates, and activates cyclooxygenase-2. Science 310: 1966-1970.). In addition, LPS exerts its inflammatory effects by inducing the expression of iNOS and COX-2, which directly stimulates the high production of NO and PGE2, respectively. Therefore, a compound capable of preventing the release of pro-inflammatory mediators or downregulating iNOS or COX-2 expression by the inactivation of macrophages may possess anti-inflammatory activities. In this study, we observed that WEMF significantly attenuated the LPS-induced increase in NO and PGE2, which are representative pro-inflammatory mediators, in RAW 264.7 macrophages by down-regulating iNOS and COX-2 expression at both the protein and mRNA levels without cytotoxicity. These indicated the inhibitory effect of WEMF on NO and PGE2 production was mainly due to the inhibitions of iNOS and COX-2 mRNA and protein expressions. Thus, the results support WEMF being a promising target for inhibiting the early steps in inflammatory pathways.
NF-κB has been shown to play an important role in various inflammatory states as a key transcription factor for many inflammation-associated enzymes and cytokine genes, which contain NF-κB binding motifs within their respective promoters (Lu et al. 2011LU YC, JAYAKUMAR T, DUANN YF, CHOU YC, HSIEH CY, YU SY, SHEU JR AND HSIAO G. 2011. Chondroprotective role of sesamol by inhibiting MMPs expression via retaining NF-κB signaling in activated SW1353 cells. J Agric Food Chem 59: 4969-4978., Rigoglou and Papavassiliou 2013RIGOGLOU S AND PAPAVASSILIOU AG. 2013. The NF-κB signalling pathway in osteoarthritis. Int J Biochem Cell Biol 45: 2580-2584.). NF-κB, a dimer of p65 and p50 subunits, is normally retained in the cytoplasm because of its association with its endogenous inhibitor, IκB-α. Once activated by inflammatory stimulants, including LPS, IκB-α is rapidly phosphorylated and degraded through a proteasome-mediated pathway, followed by a nuclear translocation of NF-κB, thus resulting in the transcriptional induction of inflammation-associated genes (Nikaido 2003NIKAIDO H. 2003. Molecular basis of bacterial outer membrane permeability revisited. Microbiol Microbiol Mol Biol Rev 67: 593-656. , Rigoglou and Papavassiliou 2013). MAPKs, a family of serine/threonine protein kinases including ERK, JNK, and p38 MAPK, are also directly involved in controlling signaling events that contribute to the production of pro-inflammatory factors in activated macrophages through the activation of NF-κB (Kaminska 2005KAMINSKA B. 2005. MAPK signalling pathways as molecular targets for anti-inflammatory therapy-from molecular mechanisms to therapeutic benefits. Biochim Biophys Acta 1754: 253-262., Muralidharan and Mandrekar 2013MURALIDHARAN S AND MANDREKAR P. 2013. Cellular stress response and innate immune signaling: integrating pathways in host defense and inflammation. J Leukoc Biol 94: 1167-1184.). Therefore, pharmacologic agents that effectively modulate NF-κB and MAPKs activation are promising candidates for treating various inflammatory diseases. To investigate the molecular mechanism of WEMF-mediated inhibition of inflammatory substances, its effect on the activation of MAPKs and NF-κB was evaluated. In the present study, we found that WEMF strongly suppressed the translocation of activated NF-κB to the nucleus and degradation of IκB-α was also inhibited. These findings indicate that WEMF inhibits NF-κB activation by suppressing IκB-α degradation and translocation of NF-κB from the cytosol into the nucleus in LPS-induced RAW 264.7 macrophages. The current study also demonstrated that WEMF concentration-dependently diminished the phosphorylation of ERK, JNK, and p38 MAPK by LPS treatment. These data suggest that suppression of MAPKs phosphorylation by WEMF might also be involved in the inhibition of the LPS-induced production of proinflammatory substances by RAW 264.7 macrophages. Taken together, our results indicate that WEMF suppresses inflammatory mediators, NO and PGE2 by inhibiting NF-κB B and MAPKs signaling pathways.
Oxidative stress, which represents the over-production of ROS, is strongly associated with other pathological statuses, including inflammation (Brüne et al. 2013BRÜNE B, DEHNE N, GROSSMANN N, JUNG M, NAMGALADZE D, SCHMID T, VON KNETHEN A AND WEIGERT A. 2013. Redox control of inflammation in macrophages. Antioxid Redox Signal 19: 595-637., Mills and O'Neill, 2016MILLS EL AND O'NEILL LA. 2016. Reprogramming mitochondrial metabolism in macrophages as an anti-inflammatory signal. Eur J Immunol 46: 13-21.). Moreover, during chronic inflammation, ROS amplifies inflammatory signals in macrophages through the activation of NF-κB signaling pathway and the over-expression of inflammation-associated genes (Kauppinen et al. 2013KAUPPINEN A, SUURONEN T, OJALA J, KAARNIRANTA K AND SALMINEN A. 2013. Antagonistic crosstalk between NF-κB and SIRT1 in the regulation of inflammation and metabolic disorders. Cell Signal 25: 1939-1948., Tan et al. 2016TAN HY, WANG N, LI S, HONG M, WANG X AND FENG Y. 2016. The reactive oxygen species in macrophage polarization: Reflecting its dual role in progression and treatment of human diseases. Oxid Med Cell Longev 2016: 2795090. ). Furthermore, MAPKs are also redox sensitive, and ROS targets the cysteines within the proteins and alters the kinase activation, which further activates redox-sensitive MAPKs (Rahman and MacNee 1998RAHMAN I AND MACNEE W. 1998. Role of transcription factors in inflammatory lung diseases. Thorax 53: 601-612., Fu et al. 2009). Thus, we investigated the inhibitory effect of WEMF on LPS-induced ROS generation and found that ROS accumulation was significantly reduced after pretreatment with WEMF in LPS-stimulated RAW 264.7 macrophages. Therefore, the WEMF-mediated inhibition of ROS generation might be attributed to its ability to scavenge free radicals, and could potentially inhibit the NF-κB and MAPKs-dependent expression of pro-inflammatory mediators, thereby resulting in an anti-inflammatory efficacy. We further investigated the protective effect of WEMF against LPS-induced NO and ROS generation using DAF-FM-DA and DCF-DA staining in zebrafish as an alternative in vivo animal model system. Consistent with previous results (Wijesinghe et al. 2014WIJESINGHE WA, KIM EA, KANG MC, LEE WW, LEE HS, VAIRAPPAN CS AND JEON YJ. 2014. Assessment of anti-inflammatory effect of 5β-hydroxypalisadin B isolated from red seaweed Laurencia snackeyi in zebrafish embryo in vivo model. Environ Toxicol Pharmacol 37: 110-117., Lee et al. 2015bLEE SH, YANG HW, DING Y, WANG Y, JEON YJ, MOON SH, JEON BT AND SUNG SH. 2015b. Anti-inflammatory effects of enzymatic hydrolysates of velvet antler in RAW 264.7 cells in vitro and zebrafish model. EXCLI J 14: 1122-1132., Cheong et al. 2016CHEONG SH, YANG HW, KO EY, AHN G, LEE W, KIM D, JEON YJ AND KIM K. 2016. Anti-inflammatory effects of trans-1,3-diphenyl-2,3-epoxypropane-1-one in zebrafish embryos in vivo model. Fish Shellfish Immunol 50: 16-20.), a dramatic increase in the fluorescence signals was observed in the LPS-exposed group unlike in the unstimulated control group, thus indicating that NO and ROS generation took place during LPS treatment in the zebrafish larvae. However, similar to our in vitro results, a significant reduction in the amount of NO and ROS was observed in zebrafish treated with WEMF prior to LPS treatment, thus indicating the strong anti-inflammatory and antioxidant potentials of WEMF.
CONCLUSIONS
The present results demonstrated that WEMF exerted potent anti-inflammatory and antioxidant effects in RAW 264.7 macrophages and zebrafish. In LPS-stimulated RAW 264.7 macrophages, WEMF markedly attenuated the production of pro-inflammatory mediators and accumulation of ROS. These effects of WEMF were associated with the suppression of LPS-induced nuclear translocalization of NF-κB and MAPKs activation. WEMF also significantly prevented the elevation of NO and ROS levels in an LPS-stimulated zebrafish model. Based on the results of this study, WEMF could have a beneficial effect to enhance anti-inflammatory and antioxidant treatment.
ACKNOWLEDGMENTS
This work was supported by the High Value-added Food Technology Development Program (314043-3), Ministry of Agriculture, Food and Rural Affairs, Republic of Korea.
REFERENCES
- ABUAJAH CI, OGBONNA AC AND OSUJI CM. 2015. Functional components and medicinal properties of food: a review. J Food Sci Technol 52: 2522-2529.
- ADEREM A AND ULEVITCH RJ. 2000. Toll-like receptors in the induction of the innate immune response. Nature 406: 782-787.
- AMIN AR, ATTUR M AND ABRAMSON SB. 1999. Nitric oxide synthase and cyclooxygenases: distribution, regulation, and intervention in arthritis. Curr Opin Rheumatol 11: 202-209.
- ANN JY, EO H AND LIM Y. 2015. Mulberry leaves (Morus alba L.) ameliorate obesity-induced hepatic lipogenesis, fibrosis, and oxidative stress in high-fat diet-fed mice. Genes Nutr 10: 46.
- BOCANEGRA A, BASTIDA S, BENEDI J, RODENAS S AND SANCHEZ-MUNIZ FJ. 2009. Characteristics and nutritional and cardiovascular-health properties of seaweeds. J Med Food 12: 236-258.
- BRÜNE B, DEHNE N, GROSSMANN N, JUNG M, NAMGALADZE D, SCHMID T, VON KNETHEN A AND WEIGERT A. 2013. Redox control of inflammation in macrophages. Antioxid Redox Signal 19: 595-637.
- CHAN EW, LYE PY AND WONG SK. 2016. Phytochemistry, pharmacology, and clinical trials of Morus alba. Chin J Nat Med 14: 17-30.
- CHAO WW, KUO YH, LI WC AND LIN BF. 2009. The production of nitric oxide and prostaglandin E2 in peritoneal macrophages is inhibited by Andrographis paniculata, Angelica sinensis and Morus alba ethyl acetate fractions. J Ethnopharmacol 122: 68-75.
- CHEONG SH, YANG HW, KO EY, AHN G, LEE W, KIM D, JEON YJ AND KIM K. 2016. Anti-inflammatory effects of trans-1,3-diphenyl-2,3-epoxypropane-1-one in zebrafish embryos in vivo model. Fish Shellfish Immunol 50: 16-20.
- CONTI B, TABAREAN I, ANDREI C AND BARFAI T. 2004. Cytokines and fever. Front Biosci 9: 1433-1449.
- CUNHA TM, VERRI WA JR, SCHIVO IR, NAPIMOGA MH, PARADA CA, POOLE S, TEIXEIRA MM, FERREIRA SH AND CUNHA F. 2008. Crucial role of neutrophils in the development of mechanical inflammatory hypernociception. J Leukoc Biol 83: 824-832.
- DEEPA M, SURESHKUMAR T, SATHEESHKUMAR PK AND PRIYA S. 2013. Antioxidant rich Morus alba leaf extract induces apoptosis in human colon and breast cancer cells by the downregulation of nitric oxide produced by inducible nitric oxide synthase. Nutr Cancer 65: 305-310.
- EOM SA ET AL. 2015. Protective effects of PEP-1-Catalase on stress-induced cellular toxicity and MPTP-induced Parkinson's disease. BMB Rep 48: 395-400.
- FREIRE MO AND VAN DYKE TE. 2013. Natural resolution of inflammation. Periodontol 2000 63: 149-164.
- FU P, BIRUKOVA AA, XING J, SAMMANI S, MURLEY JS, GARCIA JG, GRDINA DJ AND BIRUKOV KG. 2009. Amifostine reduces lung vascular permeability via suppression of inflammatory signalling. Eur Respir J 33: 612-624.
- HADDAD JJ AND LAND SC. 2002. Redox signaling-mediated regulation of lipopolysaccharide-induced proinflammatory cytokine biosynthesis in alveolar epithelial cells. Antioxid Redox Signal 4: 179-193.
- HONG CH, HUR SK, OH OJ, KIM SS, NAM KA AND LEE SK. 2002. Evaluation of natural products on inhibition of inducible cyclooxygenase (COX-2) and nitric oxide synthase (iNOS) in cultured mouse macrophage cells. J Ethnopharmacol 83: 153-159.
- IQBAL S, YOUNAS U, SIRAJUDDIN, CHAN KW, SARFRAZ RA , UDDIN K. 2012. Proximate composition and antioxidant potential of leaves from three varieties of Mulberry (Morus sp.): a comparative study. Int J Mol Sci 13: 6651-6664.
- JEONG JW ET AL. 2016. Mori folium inhibits interleukin-1β-induced expression of matrix metalloproteinases and inflammatory mediators by suppressing the activation of NF-κB and p38 MAPK in SW1353 human chondrocytes. Int J Mol Med 37: 452-460.
- KAMINSKA B. 2005. MAPK signalling pathways as molecular targets for anti-inflammatory therapy-from molecular mechanisms to therapeutic benefits. Biochim Biophys Acta 1754: 253-262.
- KAUPPINEN A, SUURONEN T, OJALA J, KAARNIRANTA K AND SALMINEN A. 2013. Antagonistic crosstalk between NF-κB and SIRT1 in the regulation of inflammation and metabolic disorders. Cell Signal 25: 1939-1948.
- KHAN MA ET AL. 2013. A comparative study on the antioxidant activity of methanolic extracts from different parts of Morus alba L. (Moraceae). BMC Res Notes 6: 24.
- KIM DS, KANG YM, JIN WY, SUNG YY, CHOI G AND KIM HK. 2014. Antioxidant activities and polyphenol content of Morus alba leaf extracts collected from varying regions. Biomed Rep 2: 675-680.
- KIM GN AND JANG HD. 2011. Flavonol content in the water extract of the mulberry (Morus alba L.) leaf and their antioxidant capacities. J Food Sci 76: C869-873.
- KIM SF, HURI DA AND SNYDER SH. 2005. Inducible nitric oxide synthase binds, S-nitrosylates, and activates cyclooxygenase-2. Science 310: 1966-1970.
- KOBAYASHI Y, MIYAZAWA M, KAMEI A, ABE K AND KOJIMA T. 2010. Ameliorative effects of mulberry (Morus alba L.) leaves on hyperlipidemia in rats fed a high-fat diet: induction of fatty acid oxidation, inhibition of lipogenesis, and suppression of oxidative stress. Biosci Biotechnol Biochem 74: 2385-2395.
- KWON DH ET AL. 2016. The immunomodulatory activity of Mori folium, the leaf of Morus alba L., in RAW 264.7 macrophages in vitro. J Cancer Prev 21: 144-151.
- LEE JS, KIM HG, HAN JM, KIM DW, YI MH, SON SW, KIM YA, LEE JS, CHOI MK AND SON CG. 2014. Ethanol extract of Astragali Radix and Salviae Miltiorrhizae Radix, Myelophil, exerts anti-amnesic effect in a mouse model of scopolamine-induced memory deficits. J Ethnopharmacol 153: 782-792.
- LEE H, PYO MJ, BAE SK, HEO Y, KIM CG, KANG C AND KIM E. 2015a. Improved therapeutic profiles of PLA2-free bee venom prepared by ultrafiltration method. Toxicol Res 31: 33-40.
- LEE SH, YANG HW, DING Y, WANG Y, JEON YJ, MOON SH, JEON BT AND SUNG SH. 2015b. Anti-inflammatory effects of enzymatic hydrolysates of velvet antler in RAW 264.7 cells in vitro and zebrafish model. EXCLI J 14: 1122-1132.
- LU YC, JAYAKUMAR T, DUANN YF, CHOU YC, HSIEH CY, YU SY, SHEU JR AND HSIAO G. 2011. Chondroprotective role of sesamol by inhibiting MMPs expression via retaining NF-κB signaling in activated SW1353 cells. J Agric Food Chem 59: 4969-4978.
- MCDANIEL ML, KWON G, HILL JR, MARSHALL CA AND CORBETT JA. 1996. Cytokines and nitric oxide in islet inflammation and diabetes. Proc Soc Exp Biol Med 211: 24-32.
- MILLS EL AND O'NEILL LA. 2016. Reprogramming mitochondrial metabolism in macrophages as an anti-inflammatory signal. Eur J Immunol 46: 13-21.
- MURALIDHARAN S AND MANDREKAR P. 2013. Cellular stress response and innate immune signaling: integrating pathways in host defense and inflammation. J Leukoc Biol 94: 1167-1184.
- NAOWABOOT J, PANNANGPETCH P, KUKONGVIRIYAPAN V, KUKONGVIRIYAPAN U, NAKMAREONG S AND ITHARAT A. 2009. Mulberry leaf extract restores arterial pressure in streptozotocin-induced chronic diabetic rats. Nutr Res 29: 602-608.
- NIKAIDO H. 2003. Molecular basis of bacterial outer membrane permeability revisited. Microbiol Microbiol Mol Biol Rev 67: 593-656.
- OH K, MOON HG, LEE DS AND YOO YB. 2015. Tissue transglutaminase-interleukin-6 axis facilitates peritoneal tumor spreading and metastasis of human ovarian cancer cells. Lab Anim Res 31: 188-197.
- RAHMAN I AND MACNEE W. 1998. Role of transcription factors in inflammatory lung diseases. Thorax 53: 601-612.
- RAMAN ST, GANESHAN AK, CHEN C, JIN C, LI SH, CHEN HJ AND GUI Z. 2016. In vitro and in vivo antioxidant activity of flavonoid extracted from mulberry fruit (Morus alba L.). Pharmacogn Mag 12: 128-133.
- RIGOGLOU S AND PAPAVASSILIOU AG. 2013. The NF-κB signalling pathway in osteoarthritis. Int J Biochem Cell Biol 45: 2580-2584.
- ROCCA B AND FITZGERALD GA. 2002. Cyclooxygenases and prostaglandins: shaping up the immune response. Int Immunopharmacol 2: 603-630.
- SHIBATA Y ET AL. 2007. Mulberry leaf aqueous fractions inhibit TNF-alpha-induced nuclear factor kappaB (NF-kappaB) activation and lectin-like oxidized LDL receptor-1 (LOX-1) expression in vascular endothelial cells. Atherosclerosis 193: 20-27.
- SUGIMOTO M ET AL. 2009. Mulberry leaf ameliorates the expression profile of adipocytokines by inhibiting oxidative stress in white adipose tissue in db/db mice. Atherosclerosis 204: 388-394.
- TAN HY, WANG N, LI S, HONG M, WANG X AND FENG Y. 2016. The reactive oxygen species in macrophage polarization: Reflecting its dual role in progression and treatment of human diseases. Oxid Med Cell Longev 2016: 2795090.
- TIRUPATHI RG, SURESH BK, UJWAL KJ, SUJANA P, RAOA AV AND SREEDHAR AS. 2011. Anti-microbial principles of selected remedial plants from Southern India. Asian Pac J Trop Biomed 1: 298-305.
- VARGA A, BUDAI MM, MILESZ S, BACSI A, TOZER J AND BENKO S. 2013. Ragweed pollen extract intensifies lipopolysaccharide-induced priming of NLRP3 inflammasome in human macrophages. Immunology 138: 392-401.
- WANG L, XU ML, LIU J, WANG Y, HU JH AND WANG MH. 2015. Sonchus asper extract inhibits LPS-induced oxidative stress and pro-inflammatory cytokine production in RAW264.7 macrophages. Nutr Res Pract 9: 579-585.
- WIJESINGHE WA, KIM EA, KANG MC, LEE WW, LEE HS, VAIRAPPAN CS AND JEON YJ. 2014. Assessment of anti-inflammatory effect of 5β-hydroxypalisadin B isolated from red seaweed Laurencia snackeyi in zebrafish embryo in vivo model. Environ Toxicol Pharmacol 37: 110-117.
- XIANG J, TANG YP, ZHOU ZY, WU P, WANG Z, MORI M AND CAI DF. 2010. Apocynum venetum leaf extract protects rat cortical neurons from injury induced by oxygen and glucose deprivation in vitro. Can J Physiol Pharmacol 88: 907-917.
- YANG L, BU L, SUN W, HU L AND ZHANG S. 2014. Functional characterization of mannose-binding lectin in zebrafish: implication for a lectin-dependent complement system in early embryos. Dev Comp Immunol 46: 314-322.
- ZHANG L AND WANG CC. 2014. Inflammatory response of macrophages in infection. Hepatobiliary Pancreat Dis Int 13: 138-152.
-
*
Contribution to the centenary of the Brazilian Academy of Sciences.
Publication Dates
-
Publication in this collection
2017
History
-
Received
01 Dec 2016 -
Accepted
18 Jan 2017