Abstract
Abstract: Fragile X Syndrome (FXS) is a neurodevelopmental disorder caused by dynamic mutations of a CGG repetition segment in an X chromosome’s single gene. It is considered the leading hereditary cause of both Autism Spectrum Disorders and Intellectual Disability. Some authors suggest that all individuals diagnosed with some of these latter conditions to be clinically and molecularly trialled for FXS due to the high levels of comorbidity between both conditions and also due to the variable expressiveness of this syndrome. This study has focused on verifying the presence of FMR1 expanded alleles since there is a lack of information about this kind of mutation in autism patients from the northern region of Brazil. The presence of large alleles for this gene could offer new therapeutic or pharmacological methods for the treatment of these patients. Both the presence and the frequency of CGG expansions were verified in 90 autism males by molecular analysis. Four of them had intermediate alleles and four others presented premutated alleles. Premutation carriers are on the propensity of developing the late onset Fragile X-associated tremor/ataxia syndrome. No full mutation alleles were found. Further studies are necessary to obtain more accurate statistical data about this kind of dynamic mutation.
Key words
autism spectrum disorders; dynamic mutation; fragile X syndrome; molecular screening; premutation; trinucleotide repeats
INTRODUCTION
Fragile X-Syndrome (FXS), previously named Martin-Bell syndrome, was first identified by Martin and BellMARTIN JP and BELL J. 1943. A pedigree of mental defect showing sex-linkage. J Neurol Neurosurg Psychiatry 6: 154-157. (1943) when they observed eleven patients with intellectual deficiency and facial dysmorphia in a six-generation family. The authors noticed that this neurological-morphological condition was only present in males, but not in their mothers and sisters. FXS is a monogenic recessive neurodevelopmental condition linked to the X-chromosome, with low penetrance, variable expressiveness, caused by the dynamic expansion of non-coding unstable repetitions compose by cytosine-guanine-guanine (JinJIN P and WARREN ST. 2000. Understanding the molecular basis of Fragile X syndrome. Hum Mol Genet 9(6): 901-908. and Warren 2000, BagniBAGNI C, TASSONE F, NERI G and HAGERMAN R. 2012. Fragile X syndrome: causes, diagnosis, mechanisms, and therapeutics. J Clin Invest 122: 4314-4322. et al. 2012). This syndrome is a neurodevelopmental disorder considered the leading hereditary cause of both Autism Spectrum Disorders (ASD) and Intellectual Disability (ID) (ChoiCHOI CH et al. 2015. PDE-4 Inhibition Rescues Aberrant Synaptic Plasticity in Drosophila and Mouse Models of Fragile X Syndrome. J Neurosci 35: 396-408. et al. 2015, SaldarriagaSALDARRIAGA W et al. 2016. Phenobarbital use and neurological problems in FMR1 premutation carriers. Neurotoxicology 53: 141-147. et al. 2016).
The primary cause of more than 98% cases of FXS is a dynamic mutation of CGG expansion at the 5’-UTR of FMR1 gene (Fragile X Mental Retardation 1 gene), located at the FRAXA locus in Xq27.3 (ShermanSHERMAN S, PLETCHER BA and DRISCOLL DA. 2005. Fragile X syndrome: diagnostic and carrier testing. Genet Med 7: 584-587. et al. 2005, PeprahPEPRAH E. 2014. Understanding decreased fertility in women carriers of the FMR1 premutation: a possible mechanism for Fragile X-Associated Primary Ovarian Insufficiency (FXPOI). Reprod Health 11: 1-3. 2014, WinarniWINARNI TI, UTARI A, MUNDHOFIR FEP, HAGERMAN RJ and FARADZ SMH. 2013. Fragile X syndrome: clinical, cytogenetic and molecular screening among autism spectrum disorder children in Indonesia. Clin Genet 84: 577-580. et al. 2013). This gene is classified into four different forms, according to their CGG-repeat size: healthy individuals have normal alleles ranging from 5 to 44 copies (JangJANG JH, LEE K, CHO EH, LEE EH, KIM WS and KI CS. 2014. Frequency of FMR1 premutation carriers and rate of expansion to full mutation in a retrospective diagnostic FMR1 Korean sample. Clin Genet 85: 441-445. et al. 2014, SethnaSETHNA F, MOON C and WANG H. 2014. From FMRP function to potential therapies for Fragile X syndrome. Neurochem Res 39: 1016-1031. et al. 2014). Affected patients usually exhibit mutated alleles longer than 200 CGGs (DeDE ESCH CE, ZEIDLER S and WILLEMSEN R. 2014. Translational endpoints in Fragile X syndrome. Neurosci Biobehav Rev 46: 256-269. Esch et al. 2014, LathamLATHAM GJ, COPPINGER J, HADD AG and NOLIN SL. 2014. The role of AGG interruptions in Fragile X repeat expansions: a twenty-year perspective. Front Genet 5: 1-6. et al. 2014, MyrickMYRICK LK et al. 2015. Independent role for presynaptic FMRP revealed by an FMR1 missense mutation associated with intellectual disability and seizures. Proc Natl Acad Sci USA 112(4): 949-956. et al. 2015). This significant expansion, known as the full mutation, leads to hypermethylation of the promoter region and transcriptional silencing (Sethna et al. 2014). The deficiency or lack of expression of a protein called FMRP (Fragile X Mental Retardation Protein) is then related to the classical symptoms of FXS, such as behavioral disorders, cognitive impairments and facial dysmorphia (HeulensHEULENS I, SUTTIE M, POSTNOV A, DECLERCK N, PERROTTA CS, MATTINA T, FAVARELLI F, FORZANO F, KOOY RF and HAMMOND P. 2013. Craniofacial characteristics of Fragile X syndrome in mouse and man. Eur J Hum Genet 21(8): 816-823. et al. 2013, Winarni et al. 2013). The others two remaining classes for FMR1 gene are called intermediate or grey-zone alleles (45–54 triples) and premutation alleles (55–200 repeats), whose carriers are not affected but have increased risks for expansion to premutation or full mutation, respectively, in the next generation (Fernandez-CarvajalFERNANDEZ-CARVAJAL I, LOPEZ POSADAS B, PAN R, RASKE C, HAGERMAN PJ and TASSONE F. 2009. Expansion of an FMR1 grey-zone allele to a full mutation in two generations. J Mol Diagn 11: 306-310. et al. 2009, Jang et al. 2014).
Some authors (MandelMANDEL JL and BIANCALANA V. 2004. Fragile X mental retardation syndrome: from pathogenesis to diagnostic issues. Growth Horm IGF Res 14: 158-165. and Biancalana 2004, HagermanHAGERMAN R, HOEM G and HAGERMAN P. 2010. Fragile X and autism: Intertwined at the molecular level leading to targeted treatments. Mol Autism 1: 1-12. et al. 2010) suggest that clinical and molecular trials for FXS be performed in all individuals diagnosed with ASD and or ID due to the high levels of comorbidity between ASD and FXS and to the variable expressiveness of FXS. The co-occurrence of ASD and FXS has been estimated at 30 to 50% (KaufmannKAUFMANN WE, CORTELL R, KAU ASM, BUKELIS I, TIERNEY E, GRA RM, COX C, CAPONE GT and STANARD P. 2004. Autism spectrum disorder in Fragile X syndrome: communication, social interaction, and specific behaviors. Am J Med Genet A 129A(3): 225-234. et al. 2004, HarrisHARRIS SW, HESSL D, GOODLIN-JONES B, FERRANTI J, BACALM S, BARBATO S, TASSONE F, HAGERMAN PJ, HERMAN H and HAGERMAN RJ. 2008. Autism profiles of males with Fragile X syndrome. Am J Ment Retard 113: 427-438. et al. 2008, AbbedutoABBEDUTO L, MCDUFFIE A and THURMAN A. 2014. The Fragile X syndrome-autism comorbidity: what do we really know? Front Genet 5: 1-10. et al. 2014) and both neurological conditions exhibit common etiological relationship. Also, the clinical characteristics of affected FXS patients are nonspecific and are quite subtle, varying from mild to severe (Mandel and Biancalana 2004, Bagni et al. 2012). Individuals with FXS may present with anything from learning problems and a normal IQ to severe mental retardation and autistic behaviors (GarberGARBER KB, VISOOTSAK J and WARREN ST. 2008. Fragile X syndrome. Eur J Hum Genet 16: 666-672. et al. 2008). Therefore, the presence of FMR1 mutations in ASD patients could offer new therapeutic or pharmacological methods for their treatment.
The aim of this study is to verify the presence of expanded alleles for the FMR1 gene in ASD patients since there is a lack of information about this kind of mutation in the northern region of Brazil.
MATERIALS AND METHODS
This study was approved by the Committee of Ethics on Human Research of the Universidade do Estado do Amazonas (Nº CEP-UEA 363.912/2013). Genomic DNA of 90 male patients (ranging in age from 3 to 23 years old) was analysed for the polymorphism of locus FRAXA, located at the 5’UTR of FMR1 gene. All subjects included in the research were patients diagnosed with non-syndromic ASD, assisted by two specialized multidisciplinary institutions for the care of autistic people in the city of Manaus-Brazil (Centro de Educação Especial André Vidal de Araújo and Espaço de Atendimento Multidisciplinar ao Autista Amigo Ruy). All parents or legal guardians signed a free and informed consent form.
Cells from the jugal mucosa of each patient were collected using a SWAB stick and stored in Tris-EDTA solution (10mM / 0.1mM). Genomic DNA was extracted using CTAB 2% (DoyleDOYLE JJ and DOYLE JL. 1990. Isolation of plant DNA from fresh tissue. Focus 12: 13-15. and Doyle 1990) and then quantified in Eppendorf® BioSpectrometer instrument. Target locus was amplified by PCR using fluorescent primers developed by FuFU YH et al. 1991. Variation of the CGG repeat at the Fragile X site results in genetic instability: Resolution of the Sherman paradox. Cell 67(6): 1047-1058. et al. (1991): forward 5’-gctcagctccgtttcggtttcacttccggt-3’ and reverse 6-FAM-5’-agccccgcacttccaccaccagctcctcca-3’. Amplifications were performed in a SimpliAmpTM Applied Biosystems thermocycler programmed with the following conditions: initial denaturation at 94°C for 5’, 32 cycles of 94°C for 45’’, 65°C for 1’30’’ and 72°C for 2’’, and a final extension at 72°C for 10’. Reaction total volume was 24µL, with 2.4µL of DNA amplification buffer (Biotech Amazonia, Manaus, BRA); 0.85mM of each dNTP; 0.33µM of both forward and reverse primer; 0.63% DMSO (dimethylsulfoxide – Sigma-Aldrich, St Louis, MO, EUA); 1.65µM de BSA (bovine serum albumin acetylated) (Promega, Madison, WI, EUA); 1U de Platinum Pfx DNA polymerase (Invitrogen Life Technologies, Carlsbad, CA, EUA); and ~60ng of gDNA. Amplified DNA fragments were separated by electrophoresis on a 1% agarose gel, which was then photographed by a transilluminator UV L-PIX HE. Allele sizes were determined by capillary electrophoresis at an automatic sequencer ABI-3130XL using the polymer 3130 POP-7 (Applied Biosystems, Foster City, CA, EUA).
Allele sizes were determined by capillary electrophoresis at an automatic sequencer with an internal marker pUC19 ROX-labelled size standard. GeneMarker v.2.6.0 computer program performed the analysis of electropherograms. Expected allele size of the amplicon was 221bp, excluding the CGG repetition region itself. A formula developed by HamdanHAMDAN H, TYNAN J, FENWICK RA and LEON JA. 1997. Automated detection of trinucleotide repeats in Fragile X syndrome. J Mol Diagn 2(4): 259-269. et al. (1997) was used to calculate the number of CGG repetitions in the FMR1 gene. Allele frequency was calculated according to the ratio between the quantities of alleles of a particular allelic form and the total sample number. Modal value and arithmetic mean for CGG copies were obtained on Excel 2016® program. For the purposes of this paper, we will henceforward refer to the names of the four classes of alleles (normal, grey zone, premutation and full mutation) as NM, GZ, PM and FM, respectively.
RESULTS
Of 90 male patients analysed in this study, 75 presented NM alleles; four showed GZ alleles, and four showed PM alleles. FM alleles were not observed. Seven sample could not be amplified due to PCR issues. The frequency of each allele is shown in Table I.
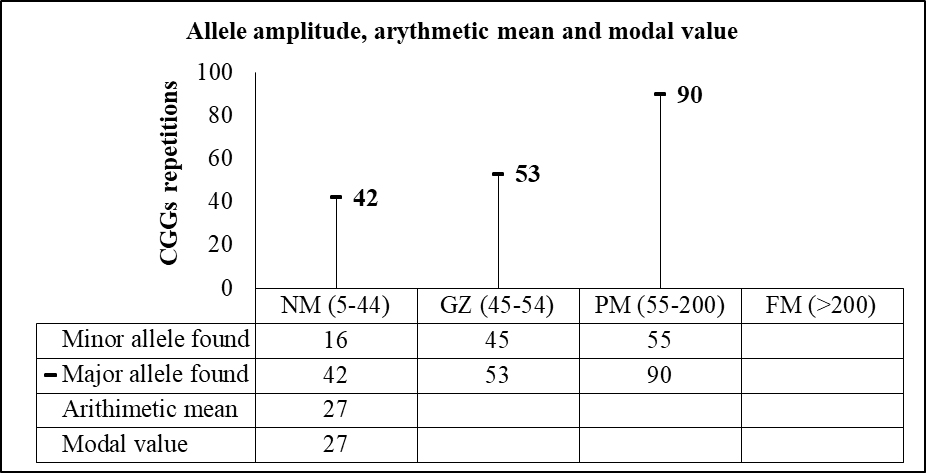
Both the arithmetic mean and the modal value for CCG repeats in the normal range were 27. The amplitude of CGG codons and the major and minor alleles for each allelic class are presented in Figure 1.
The higher CGG repeat size found for the normal range was 42. For the grey-zone and premutation range were 53 and 90, respectively. The arithmetic mean and the modal value were both only calculated for the normal allele type.
DISCUSSION
This study is the first one aiming the prevalence of dynamic mutations in the FMR1 gene among ASD patients in the northern region of Brazil. No full mutation alleles were found in this population. However, four patients showed GZ alleles and four others showed PM alleles.
Grey-zone (or intermediate) allele is believed to be a potential precursor for premutation alleles and both are unstably transmitted from mother to children (NolinNOLIN SL, GLICKSMAN A, ERSALESI N, DOBKIN C, BROWN T, CAO R, BLATT E, SAH S, LATHAN GJ and HADD AG. 2015. Fragile X full mutation expansions are inhibited by one or more AGG interruptions in premutation carriers. Genet Med 17: 358-364. et al. 2015). Intermediate-to-premutation expansions are rare and have not been observed in a single generation (CronisterCRONISTER A, TEICHER J, ROHLFS EM, DONNENFELD A and HALLAM S. 2008. Prevalence and instability of Fragile X alleles: implications for offering Fragile X prenatal diagnosis. Obstet Gynecol 111: 596-601. et al. 2008). Although, Fernandes-Carvajal et al. (2009) reported an intermediate-to-full mutation increasing in the CGG region within two generations when studying a family where a 52 CGGs grandfather transmitted to his grandson a ~538 repeats allele.
Premutation carriers (55-200 CGGs) are relatively common in the general population. About one in 260 females and one in 813 males are in this range (LeeheyLEEHEY MA. 2009. Fragile X-associated Tremor/Ataxia Syndrome (FXTAS): Clinical Phenotype, Diagnosis and Treatment. J Investig Med 57(8): 830-836. 2009). Premutation-to-full mutation expansion occurs exclusively from PM carriers mothers, given that, during maternal meiosis, premutation alleles are highly unstable (Sherman et al. 2005), which depends on their CGG length (Fernandez-Carvajal et al. 2009) and on the number and position of AGG (adenine-guanine-guanine) inside the CGG repeat (Latham et al. 2014). According to Nolin et al. (2015), the chances of a >90 CGGs allele undergo to full mutation exceed 94%. Paternal premutation alleles are stably transmitted to daughters and do not result in FM (Jang et al. 2014), although one case of such enlargement of the FMR1 gene has been related by Alvarez-MoraALVAREZ-MORA MI, GUITART M, RODRIGUEZ-REVENGA L, MADRIGAL I, GABAU E and MILÀ M. 2017. Paternal transmission of a FMR1 full mutation allele. Am J Med Genet A 173: 2795-2797. et al. (2017) when they showed a 16-year-old girl who inherited mosaic PM-FM alleles (175 repeats and >200 repeats) from her 88 CGG father.
The four patients with PM alleles found in this study were from 6 to 13 years old. According to HagermanHAGERMAN R and HAGERMAN P. 2013. Advances in clinical and molecular understanding of the FMR1 premutation and Fragile X-associated tremor/ataxia syndrome. Lancet Neurol 12: 786-798. and Hagerman (2013), boys with premutation in the early childhood present at higher rates of attention deficit hyperactivity disorder (ADHD), shyness, social deficits and intellectual disability. Thus, there should be given particular importance to these symptoms in these patients, given that they already have an ASD diagnosis. Male PM carriers, depending on their premutation range, are also on the propensity of developing Fragile X-associated tremor/ataxia syndrome (FXTAS) (JacquemontJACQUEMONT S, BERRY-KRAVIS E, HAGERMAN R, RAISON F, GASPARINI F, APOSTOL G, UFER M, DES PORTES V and GOMEZ-MANCILLA B. 2014. The challenges of clinical trials in Fragile X syndrome. Psychopharmacology 231(6): 1237-1250. et al. 2014), which is a late-onset neurodegenerative disorder that affects mostly men in the early seventh decade, causing cerebellar gait ataxia and intention tremor (Leehey 2009). Another point to bear in mind is that, due to the mecanisms of trinucleotide repeats expansion during matternal transmition, the mothers of the eight patients we found with FMR1 grey-zone and premutated alleles may also have enlarged regions in this gene, smaller ones, but still in the grey-zone or the premutation range or even in the border of a previous allele class. Premutation alleles, for example, undergo an expansion in almost all cases when transmitted by a female (Barasoian et al. 2016).
Those carries mothers of GZ or PM alleles have a 20% chance of experiencing Fragile X-associated premature ovarian insufficiency, which is a cessation of menses prior to 40 years old (Allingham-HawkinsALLINGHAM-HAWKINS DJ et al. 1999. Fragile X premutation is a significant risk factor for premature ovarian failure: The International Collaborative POF in Fragile X Study—Preliminary Data Europe PMC Funders Group. Am J Med Genet 83: 322-325. et al. 1999). They also have a 10% to 15% chance of suffering seizures (TassoneTASSONE F, HAGERMAN PJ and HAGERMAN RJ. 2014. Fragile x premutation. J Neurodev Disord 6: 1-4. et al. 2014), and anxiety symptoms (Jacquemont et al. 2014).
The arithmetic mean of CGG triplets observed within those patients who showed NM alleles was 27. Others studies performed in Indonesia (FaradzFARADZ SMH, PATTILHA MZ, LEIGH DA, JENKINS M, LEGGO J, BUCKLE MZ and HOLDEN JJ. 2000. Genetic diversity at the FMR1 locus in the Indonesian population. Ann Hum Genet 64: 329-339. et al. 2000), China (Faradz et al. 2000, ZhouZHOU Y, TANG K, LAW HY, NG IS, LEE CG and CHONG SS. 2006. FMR1 CGG Repeat Patterns and Flanking Haplotypes in Three Asian Populations and Their Relationship With Repeat Instability. Ann Hum Genet 70(6): 784-796. et al. 2006) and Mexico (Rosales-ReynosoROSALES-REYNOSO MA, MENDOZA-CARRERA F, TROYO-SANROMÁN R, MEDINA C and BARROS-NÚÑEZ P. 2005. Genetic diversity at the FMR1 locus in Mexican population. Arch Med Res 36: 412-417. et al. 2005) reported mean values of 29, 29 and 32 CGGs, respectively. The modal value we observed for CGG repetitions was also 27. Others studies performed with the Brazilian population found a modal value of 20 CGG repetitions (SucharovSUCHAROV CC, SILVA R, RONDINELLI E and MOURA-NETO RS. 1999. Fragile X trinucleotide repeats from a normal population in Rio de Janeiro, Brazil. Hereditas 130: 189-190. et al. 1999, Mingroni-NettoMINGRONI-NETTO RC et al. 2002. Distribution of CGG repeats and FRAXAC1/DXS548 alleles in South American populations. Am J Med Genet A 111: 243-252. et al. 2002). Although it is well-known that normal population present polymorphic alleles ranging from 4 to 55 repeats (Sethna et al. 2014), the importance of calculating the allelic frequency for FMR1 gene is that it is possible to verify the genetic instability at the FRAXA site and to identify the potential risks of allelic expansion, which can be attractive to medical geneticists and carriers of pre-mutated alleles at risk of increase for the full mutation (Peprah 2014).
There are several institutions for the care of autism children in the city of Manaus, Brazil. Here, we proposed a simple and free molecular test to only a small portion of the many parents who signed up to conduct the study with their children. Most parents informally reported that sometimes they blame themselves for their children neurological conditions, based on local beliefs. According to them, they are not properly informed about the possible causes of ASD. Only a small fraction of these parents are aware of the multifactors of autism spectrum disorders, including the genetic ones, but they relate that could not afford genetic tests for FXS requested by therapists. Such tests are quite expensive and often inaccessible and time-consuming in this region. Discovering genetic mutations in ASD patients is an important step not only to establish appropriate intervention and treatment but also to carry out genetic counselling that will inform the recurrent risk of FXS and Fragile X-associated disorders in the family. Once few molecular analysis have been carried out with the Brazilian population in order to verify the incidence of expanded alleles among the autistic population, more in-depht studies are necessary to obtain more accurate statistical data about this kind of dynamic mutation.
ACKNOWLEGMENTS
The authors wish to thank the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES-2014) for its financial support and thank all parents or legally responsible for ASD patients, as well as to the managers of the Centro de Educação Especial André Vidal de Araújo and Espaço de Atendimento Multidisciplinar ao Autista Amigo Ruy) for their kindness and accessibility to their care institutions.
REFERENCES
- ABBEDUTO L, MCDUFFIE A and THURMAN A. 2014. The Fragile X syndrome-autism comorbidity: what do we really know? Front Genet 5: 1-10.
- ALLINGHAM-HAWKINS DJ et al. 1999. Fragile X premutation is a significant risk factor for premature ovarian failure: The International Collaborative POF in Fragile X Study—Preliminary Data Europe PMC Funders Group. Am J Med Genet 83: 322-325.
- ALVAREZ-MORA MI, GUITART M, RODRIGUEZ-REVENGA L, MADRIGAL I, GABAU E and MILÀ M. 2017. Paternal transmission of a FMR1 full mutation allele. Am J Med Genet A 173: 2795-2797.
- BAGNI C, TASSONE F, NERI G and HAGERMAN R. 2012. Fragile X syndrome: causes, diagnosis, mechanisms, and therapeutics. J Clin Invest 122: 4314-4322.
- BARASOAIN M, BARRENETXEA G, HUERTA I, TÉLEZ M, CRIADO B and ARRIETA I. 2016. Study of the genetic etiology of Primary Ovarian Insufficiency: FMR1 gene. Genes 7: 1-18.
- CHOI CH et al. 2015. PDE-4 Inhibition Rescues Aberrant Synaptic Plasticity in Drosophila and Mouse Models of Fragile X Syndrome. J Neurosci 35: 396-408.
- CRONISTER A, TEICHER J, ROHLFS EM, DONNENFELD A and HALLAM S. 2008. Prevalence and instability of Fragile X alleles: implications for offering Fragile X prenatal diagnosis. Obstet Gynecol 111: 596-601.
- DE ESCH CE, ZEIDLER S and WILLEMSEN R. 2014. Translational endpoints in Fragile X syndrome. Neurosci Biobehav Rev 46: 256-269.
- DOYLE JJ and DOYLE JL. 1990. Isolation of plant DNA from fresh tissue. Focus 12: 13-15.
- FARADZ SMH, PATTILHA MZ, LEIGH DA, JENKINS M, LEGGO J, BUCKLE MZ and HOLDEN JJ. 2000. Genetic diversity at the FMR1 locus in the Indonesian population. Ann Hum Genet 64: 329-339.
- FERNANDEZ-CARVAJAL I, LOPEZ POSADAS B, PAN R, RASKE C, HAGERMAN PJ and TASSONE F. 2009. Expansion of an FMR1 grey-zone allele to a full mutation in two generations. J Mol Diagn 11: 306-310.
- FU YH et al. 1991. Variation of the CGG repeat at the Fragile X site results in genetic instability: Resolution of the Sherman paradox. Cell 67(6): 1047-1058.
- GARBER KB, VISOOTSAK J and WARREN ST. 2008. Fragile X syndrome. Eur J Hum Genet 16: 666-672.
- HAGERMAN R and HAGERMAN P. 2013. Advances in clinical and molecular understanding of the FMR1 premutation and Fragile X-associated tremor/ataxia syndrome. Lancet Neurol 12: 786-798.
- HAGERMAN R, HOEM G and HAGERMAN P. 2010. Fragile X and autism: Intertwined at the molecular level leading to targeted treatments. Mol Autism 1: 1-12.
- HAMDAN H, TYNAN J, FENWICK RA and LEON JA. 1997. Automated detection of trinucleotide repeats in Fragile X syndrome. J Mol Diagn 2(4): 259-269.
- HARRIS SW, HESSL D, GOODLIN-JONES B, FERRANTI J, BACALM S, BARBATO S, TASSONE F, HAGERMAN PJ, HERMAN H and HAGERMAN RJ. 2008. Autism profiles of males with Fragile X syndrome. Am J Ment Retard 113: 427-438.
- HEULENS I, SUTTIE M, POSTNOV A, DECLERCK N, PERROTTA CS, MATTINA T, FAVARELLI F, FORZANO F, KOOY RF and HAMMOND P. 2013. Craniofacial characteristics of Fragile X syndrome in mouse and man. Eur J Hum Genet 21(8): 816-823.
- JACQUEMONT S, BERRY-KRAVIS E, HAGERMAN R, RAISON F, GASPARINI F, APOSTOL G, UFER M, DES PORTES V and GOMEZ-MANCILLA B. 2014. The challenges of clinical trials in Fragile X syndrome. Psychopharmacology 231(6): 1237-1250.
- JANG JH, LEE K, CHO EH, LEE EH, KIM WS and KI CS. 2014. Frequency of FMR1 premutation carriers and rate of expansion to full mutation in a retrospective diagnostic FMR1 Korean sample. Clin Genet 85: 441-445.
- JIN P and WARREN ST. 2000. Understanding the molecular basis of Fragile X syndrome. Hum Mol Genet 9(6): 901-908.
- KAUFMANN WE, CORTELL R, KAU ASM, BUKELIS I, TIERNEY E, GRA RM, COX C, CAPONE GT and STANARD P. 2004. Autism spectrum disorder in Fragile X syndrome: communication, social interaction, and specific behaviors. Am J Med Genet A 129A(3): 225-234.
- LATHAM GJ, COPPINGER J, HADD AG and NOLIN SL. 2014. The role of AGG interruptions in Fragile X repeat expansions: a twenty-year perspective. Front Genet 5: 1-6.
- LEEHEY MA. 2009. Fragile X-associated Tremor/Ataxia Syndrome (FXTAS): Clinical Phenotype, Diagnosis and Treatment. J Investig Med 57(8): 830-836.
- MANDEL JL and BIANCALANA V. 2004. Fragile X mental retardation syndrome: from pathogenesis to diagnostic issues. Growth Horm IGF Res 14: 158-165.
- MARTIN JP and BELL J. 1943. A pedigree of mental defect showing sex-linkage. J Neurol Neurosurg Psychiatry 6: 154-157.
- MINGRONI-NETTO RC et al. 2002. Distribution of CGG repeats and FRAXAC1/DXS548 alleles in South American populations. Am J Med Genet A 111: 243-252.
- MYRICK LK et al. 2015. Independent role for presynaptic FMRP revealed by an FMR1 missense mutation associated with intellectual disability and seizures. Proc Natl Acad Sci USA 112(4): 949-956.
- NOLIN SL, GLICKSMAN A, ERSALESI N, DOBKIN C, BROWN T, CAO R, BLATT E, SAH S, LATHAN GJ and HADD AG. 2015. Fragile X full mutation expansions are inhibited by one or more AGG interruptions in premutation carriers. Genet Med 17: 358-364.
- PEPRAH E. 2014. Understanding decreased fertility in women carriers of the FMR1 premutation: a possible mechanism for Fragile X-Associated Primary Ovarian Insufficiency (FXPOI). Reprod Health 11: 1-3.
- ROSALES-REYNOSO MA, MENDOZA-CARRERA F, TROYO-SANROMÁN R, MEDINA C and BARROS-NÚÑEZ P. 2005. Genetic diversity at the FMR1 locus in Mexican population. Arch Med Res 36: 412-417.
- SALDARRIAGA W et al. 2016. Phenobarbital use and neurological problems in FMR1 premutation carriers. Neurotoxicology 53: 141-147.
- SETHNA F, MOON C and WANG H. 2014. From FMRP function to potential therapies for Fragile X syndrome. Neurochem Res 39: 1016-1031.
- SHERMAN S, PLETCHER BA and DRISCOLL DA. 2005. Fragile X syndrome: diagnostic and carrier testing. Genet Med 7: 584-587.
- SUCHAROV CC, SILVA R, RONDINELLI E and MOURA-NETO RS. 1999. Fragile X trinucleotide repeats from a normal population in Rio de Janeiro, Brazil. Hereditas 130: 189-190.
- TASSONE F, HAGERMAN PJ and HAGERMAN RJ. 2014. Fragile x premutation. J Neurodev Disord 6: 1-4.
- WINARNI TI, UTARI A, MUNDHOFIR FEP, HAGERMAN RJ and FARADZ SMH. 2013. Fragile X syndrome: clinical, cytogenetic and molecular screening among autism spectrum disorder children in Indonesia. Clin Genet 84: 577-580.
- ZHOU Y, TANG K, LAW HY, NG IS, LEE CG and CHONG SS. 2006. FMR1 CGG Repeat Patterns and Flanking Haplotypes in Three Asian Populations and Their Relationship With Repeat Instability. Ann Hum Genet 70(6): 784-796.
Publication Dates
-
Publication in this collection
23 Sept 2019 -
Date of issue
2019
History
-
Received
16 May 2018 -
Accepted
4 Oct 2018