Abstracts
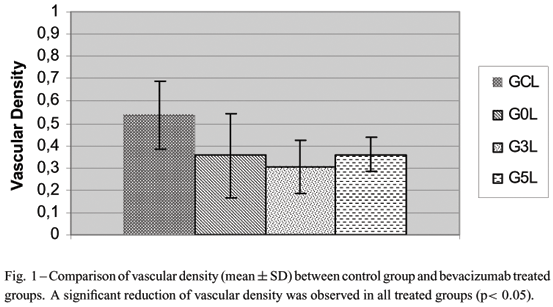
The purpose of this study was to evaluate the effects of the use of the subconjunctival injection of bevacizumab (Avastin®) on angiogenesis in the rat cornea. Corneas of 20 Wistar male rats were cauterized with silver nitrate crystal. Animals were divided in four groups: control group (GC) that received subconjunctivally 0.02 ml of 0.9% saline solution on the day of the lesion; group GO that received subconjunctivally 0.02 ml of bevacizumab just after the lesion; group G3 that received bevacizumab on day 3 and group G5 that received bevacizumab on day 5 after lesion. Animals were euthanized on day 7. The newly formed vessels were quantified after China Ink perfusion and photographs were obtained and analyzed in a computerized system (Image Pro-Plus®). In the control group, neovascularization covered 53.56% ± 15.11 (mean ± SD) of the corneal surface, compared with 35.57% ± 18.80 (mean ± SD) in the G0 group, 30.60%±11.82 (mean±SD) in the G3 and 35.86%±0.07 (mean±SD) in the G5. The results showed an inhibition of angiogenesis when the control group was compared with all treated groups. These results suggest that subconjunctival injection of bevacizumab is able to inhibit corneal angiogenesis independently of the day of treatment.
bevacizumab; cornea; neovascularization; angiogenesis
O objetivo deste estudo foi avaliar os efeitos da aplicação subconjuntival de bevacizumab (Avastin®) na angiogênese corneal em ratos. Vinte ratos Wistar, machos, foram submetidos a cauterização química com cristal de nitrato de prata. Os animais foram divididos em 4 grupos: O grupo controle (GC), recebeu injeção de 0,02 ml de solução fisiológica pela via subconjuntival no momento da lesão. O grupo G0 recebeu 0,02 ml de bevacizumab (Avastin®) imediatamente depois da lesão. O grupo G3 recebeu 0,02 ml de bevacizumab no terceiro dia após a lesão.O grupo G5 recebeu 0,02 ml de bevacizumab no quinto dia após a lesão. Os animais foram eutanasiados 7 dias após a cauterização. Os vasos neoformados foram quantificados após preenchimento do leito vascular com Tinta da China e imagens foram obtidas e analisadas em sistema computadorizado (Image Pro-Plus®). No grupo controle a neovascularização ocupou 53,56% ± 15,11 (média ± DP) da superfície corneal comparando a 35,57% ± 18,80 no grupo G0, 30,60% ± 11,82 (média ± DP) no G3 e 35,86% ± 0,07 (média ± DP) no G5. Os resultados mostram uma inibição da angiogênese quando se compara GC com os grupos tratados. Os resultados sugerem que a injeção subconjuntival de Bevacizumab é capaz de inibir a angiogênese corneal independentemente do dia de aplicação.
bevacizumab; córnea; neovascularização; angiogênese
BIOMEDICAL AND MEDICAL SCIENCES
The effects of the subconjunctival injection of bevacizumab (Avastin®) on angiogenesis in the rat cornea
Luiz F.M. BarrosI; Rubens Belfort-JrII, * * Member Academia Brasileira de Ciências
IDepartamento de Cirurgia, Faculdade de Medicina Veterinária e Zootecnia, Universidade de São Paulo, Av. Prof. Orlando Marques de Paiva, 87, Cidade Universitária, 05508-900 São Paulo, SP, Brasil
IIDepartamento de Oftalmologia, Universidade Federal de São Paulo, Rua Botucatu, 822, Vila Clementino, 04023-062 São Paulo, SP, Brasil
Correspondence to Correspondence to: Luiz Felipe de Moraes Barros E-mail: luizfelipemb@yahoo.com.br
ABSTRACT
The purpose of this study was to evaluate the effects of the use of the subconjunctival injection of bevacizumab (Avastin®) on angiogenesis in the rat cornea. Corneas of 20 Wistar male rats were cauterized with silver nitrate crystal. Animals were divided in four groups: control group (GC) that received subconjunctivally 0.02 ml of 0.9% saline solution on the day of the lesion; group GO that received subconjunctivally 0.02 ml of bevacizumab just after the lesion; group G3 that received bevacizumab on day 3 and group G5 that received bevacizumab on day 5 after lesion. Animals were euthanized on day 7. The newly formed vessels were quantified after China Ink perfusion and photographs were obtained and analyzed in a computerized system (Image Pro-Plus®). In the control group, neovascularization covered 53.56% ± 15.11 (mean ± SD) of the corneal surface, compared with 35.57% ± 18.80 (mean ± SD) in the G0 group, 30.60%±11.82 (mean±SD) in the G3 and 35.86%±0.07 (mean±SD) in the G5. The results showed an inhibition of angiogenesis when the control group was compared with all treated groups. These results suggest that subconjunctival injection of bevacizumab is able to inhibit corneal angiogenesis independently of the day of treatment.
Key words: bevacizumab, cornea, neovascularization, angiogenesis.
RESUMO
O objetivo deste estudo foi avaliar os efeitos da aplicação subconjuntival de bevacizumab (Avastin®) na angiogênese corneal em ratos. Vinte ratos Wistar, machos, foram submetidos a cauterização química com cristal de nitrato de prata. Os animais foram divididos em 4 grupos: O grupo controle (GC), recebeu injeção de 0,02 ml de solução fisiológica pela via subconjuntival no momento da lesão. O grupo G0 recebeu 0,02 ml de bevacizumab (Avastin®) imediatamente depois da lesão. O grupo G3 recebeu 0,02 ml de bevacizumab no terceiro dia após a lesão.O grupo G5 recebeu 0,02 ml de bevacizumab no quinto dia após a lesão. Os animais foram eutanasiados 7 dias após a cauterização. Os vasos neoformados foram quantificados após preenchimento do leito vascular com Tinta da China e imagens foram obtidas e analisadas em sistema computadorizado (Image Pro-Plus®). No grupo controle a neovascularização ocupou 53,56% ± 15,11 (média ± DP) da superfície corneal comparando a 35,57% ± 18,80 no grupo G0, 30,60% ± 11,82 (média ± DP) no G3 e 35,86% ± 0,07 (média ± DP) no G5. Os resultados mostram uma inibição da angiogênese quando se compara GC com os grupos tratados. Os resultados sugerem que a injeção subconjuntival de Bevacizumab é capaz de inibir a angiogênese corneal independentemente do dia de aplicação.
Palavras-chave: bevacizumab, córnea, neovascularização,angiogênese.
INTRODUCTION
Corneal neovascularization is a sequel of several inflammatory disease of the anterior segment, such as infections, degenerative and traumatic disorders, reaction to corneal transplantation and extended lens wear (Chang et al. 2001, Hosseini and Nejabat 2007).
VEGF (Vascular Endothelial Growth Factor) has been implicated in corneal neovascularization. The implantation of nylon discs impregnated with VEGF or bFGF (Basic Fibroblast Growth Factor) can promotegrossly prominent corneal neovascularization extending from the limbus to the implant 7 days after the implantation (Coxon et al.2002). The application of silver nitrate on the cornea induces corneal neovascularization and increases the levels of VEGF-C e VEGFR-3 three days after lesion (Edelman et al.1999, Mimura et al.2001). Other experimental models such as alkali burn and krypton laser photocoagulation can also induce neovascularization that is accompanied by increased levels of VEGF (Gan et al. 2004, Kvanta et al. 2000).
Inhibition of VEGF and, thereby, inhibiting angiogenesis can be an effective treatment for a variety of ocular diseases followed by neovascularization. Anti-VGEF antibodies, such as ranibizumab (Lucentis®) and pegaptanib (Macugen®) have been designed with the aiming to control neovascularizartion in neovascular age-related macular degeneration (AMD) (Heier et al. 2006, Rosenfeld et al.2005, 2006, Gragoudas et al.2004), proliferative diabetic retinopathy (PDR) (Macugen Diabetic Retinophathy Study Group 2006) and corneal neovascularization (Hosseini and Nejabat 2007, Manzano et al. 2006).
Bevacizumab, a humanized monoclonal anti-body to VEGF was designed for intravenous applications and approved for the treatment of colorectal cancer (Kabbinavar et al. 2003, Hurwitz et al. 2004, Emmanouilides et al. 2004). Recently bevacizumab has been used, with promising results, as a systemic or intra-vitreal treatmentfor exsudative AMD (Michels et al. 2005, Averyet al.2006a). Good results were also reported using bevacizumab for the treatment of diabetic retinopathy reducing retina and iris neovascularization (Avery et al. 2006b, Avery 2006). One case of neovascular glaucoma following central vein occlusion was treated with bevacizumab with markedly improvement of IOP and discomfort (Kahook et al. 2006). Bevacizumab seems to be an effective option to inhibit corneal neovascularization. A twice a day topic application of 4mg/ml bevacizumab for 7 days decreased corneal vascularization in 40% (Manzano et al.2006). Partial regression of new vessels was observed when Avastin was injected in the stroma of five eyes with corneal neovascularization related to corneal graft and limbic stem cell deficiency (Höfling-Lima et al.2006).
The subconjunctival injection is also a widely used delivery method of drugs in the eye. Liu et al. (2006) demonstrated that a single subconjuctival injection of a VEGF trap can promote a dose-dependent regression of newly formed vessels in a suture-induced model of corneal neovascularization.
The aim of this paper was to study the effect of the subconjunctival injection of bevacizumab on the experimental induced corneal neovascularization in rats after silver nitrate lesion.
MATERIALS AND METHODS
Twenty male Wistar rats, aging 8 to 10 weeks and weighting 250g to 300g were used. Under general anesthesia induced and maintained by isoflurane supplemented with topical anesthesia (0.5% proparacaine hydrochloride) corneas were cauterized with silver nitrate to induce neovascularization. Both corneas of each animal were cauterized by pressing a crystal of silver nitrate, 2mm far from the limbus, under surgical microscope. The eyes were than carefully rinsed with approximately 10 ml of saline solution. The experiment was performed according to the ARVO animal care regulation and approval of the local animal research ethics committee.
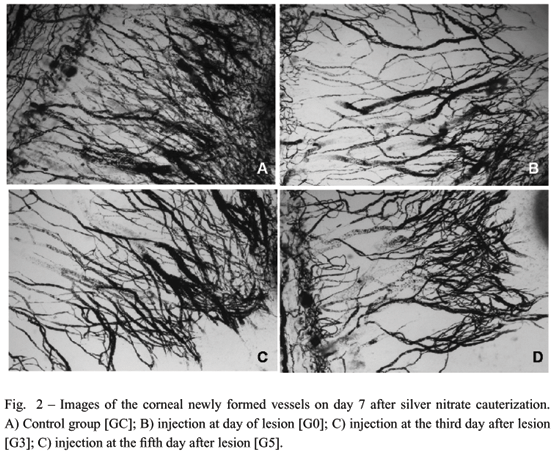
Following cauterization, animals were divided into four groups: Group GC (n=10) received a subconjunctival injection of 0,02 ml of 0,9% saline solution; group GO (n=10) received a subconjunctival injection of 0,02 ml of bevacizumab (Avastin®) just after the lesion; group G3 (n=10) received a subconjunctival injection of 0,02ml of bevacizumab (Avastin®) at day 3 after lesion and group G5 (n=10) received a subconjunctival injection of 0,02 ml of bevacizumab (Avastin®) at day 5 after lesion. Seven days after lesion, animals were euthanized by hyper inhalation of isoflurane and corneas were perfused with China ink. Eyes were fixed in paraformoldehyde for 24 hours, dehydrated with increasing concentrations of ethanol and diaphanized with benzene. Corneas were dissected and mounted onto slides. Under microscopy photographs were taken and the newly formed vessels were quantified and analyzed by computerized system (Image Pro-Plus®). Three standardized areas (1350 ×1020 µm) were examined and an average value was taken from each cornea. The Kruskal-Wallis test followed by the Dunn test with P < 0.05 was used for comparisons.
RESULTS
In the bevacizumab-treated eyes the vascular density of new blood vessels was lower than in control eyes, independently of the injection day. In the control group, neovascularization covered 53.56% ± 15.11 (mean ± standard deviation [SD]) of the corneal surface, compared with 35.57% ± 18.80 (mean ± SD) in the G0 group, 30.60% ± 11.82 (mean ± SD) in the G3 and 35.86% ± 0.07 (mean ± SD) in the G5 (Figs. 1 and 2). When vascular density is compared between treated groups no statistical differences were observed. No adverse effects related to bevacizumab injection were observed in all treated animals.
DISCUSSION
Many drugs as Hyperycin, Rapamycin and non-steroidal anti-inflammatory drugs with cycloxigenase inhibitory activity have been experimentally evaluated for corneal neovascularization inhibition (Lavie et al. 2005, Kwon and Kim 2006, Castro et al. 2004). Among these drugs, steroids seem to the best therapy to inhibit corneal neovascularization (Riazi-Esfahani et al. 2006) and remain the mainstay of therapy to prevent corneal graft rejection (Randleman and Stulting 2006).
Our results suggest that bevacizumab can inhibit corneal neovascularization in this rat model in all tested groups.Although our data showed statistical significance (p < 0,05), the inhibition of corneal neovascularization was far from complete. Some possibilities can explain this incomplete inhibition, as insufficient dose and/or diffusion and absorption of bevacizumab through the conjunctiva with partial inhibition of VEGF activity. Besides that, other cytokines, as FGF (Fibroblast Growth Factor) contributes to the angiogenic process and cannot be inhibited by Bevacizumb (Gaudric et al. 1992).
Bevacizumab has been successfully used systemically and intravitreally in humans with exsudative age related macular degeneration, and diabetic retinopathy controlling retina, choroidal and iris neovascularization with minimal or no adverse effects (Michels et al. 2005, Avery et al. 2006b, Avery 2006, Feiner et al. 2006). Topical application of bevacizumab (4mg/ml) was effective to control experimentally induced corneal neovascularization in rats (Manzano et al. 2006). Two intrastromal injections of 0.05 ml of Avastin® with a month interval were also effective to promote regression of newly formed vessels in all studied patients (Höfling-Lima et al. 2006).
It is interesting to observe that these findings demonstrate the effect of the bevacizumab inhibition in a shorttime period. There are clinical data that reveal that bevacizumab intravitreous and intrastromal application can be transitory and persist only for few weeks. The observation of the effects of bevacizumab longer than our study could be done to investigate how long the inhibition of neovascularization can persist.
The subconjuctival injection seems to be a goodoption to inhibit corneal neovascularization. This deliverymethod is easy and simple to be performed, and has minimal related complications. The possible systemic absorption and extra ocular side effects need thought to beadequately addressed to avoid potential complications.
We have shown that subconjuntival injection ofbevacizumab (Avastin®) is effective in controlling corneal neovascularization in this experimental animal model. Controlled clinical trials must be performed to demonstrate the efficacy of bevacizumab in the treatment of corneal neovascularization or corneal graft rejection.
ACKNOWLEDGMENTS
We are grateful to Dr.Carolina Scarpa for kindly helping with the preparation of slides, Dr.Maria Lucia Zaidan Dagli, professor of Departamento de Patologia, Faculdade de Medicina Veterinária e Zootecnia, Universidade de São Paulo, for permitting the use of the imageanalyzer equipment and Dr.Acacio Souza Lima, chief of the Setor de Farmacologia Ocular, Departamento de Oftalmologia, Universidade Federal de São Paulo for providing the Avastin and to Dr.Fernando Ferreira, Departamento de Medicina Veterinária Preventiva e Saúde Animal, Faculdade de Medicina Veterinária e Zootecnia, Universidade de São Paulo, for assistance with statistical analysis.
Manuscript received on February 12, 2007; accepted for publication on April 17, 2007; contributed by RUBENS BELFORT JR.
- AVERY RL. 2006. Regression of retinal and iris neovascularization after intravitreal bevacizumab (Avastin) treatment. Retina 26: 352-354.
- AVERY RL, PIERAMICINI DJ, RABENA MD, CASTELLARIN AC, NASIR MA AND GIUST MJ. 2006a. Intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmology 113: 363-372.
- AVERY RL, PEARLMAN J, PIERAMICI DJ, RABENA MD, CASTELLARINI AA, NASIR MA, GIUST MJ, WENDEL R AND PATEL A. 2006b. Intravitreal bevacizumab (Avastin) in the treatment of proliferative diabetic retinopathy. Ophthalmology 113: 1695.
- CASTRO MR, LUTZ D AND EDELMAN JL. 2004. Effect of COX inhibitors on VEGF-induced retinal vascular leakage and experimental corneal and choroidal neovascularization. Exp Eye Res 79: 275-285.
- CHANG JH, GABISON EE, KATP T AND AZAR DT. 2001. Corneal neovascularization. Curr Opin Ophthalmol 12: 242-249.
- COXON A ET al. 2002. Inhibition of interleukin-1 butnot tumor necrosis factor suppresses neovascularization in rat models of corneal angiogenesis and adjuvant arthritis.Arthritis Rheum 46: 2604-2612.
- EDELMAN JL, CASTRO MR AND WEN Y. 1999. Correlation of VEGF expression by leukocytes with the growth and regression of blood vessels in the rat cornea. Invest Ophthalmol Vis Sci 40: 1112-1123.
- EMMANOUILIDES C, PEGRAN M, ROBINSON R, HECHT R AND ISACOFF W. 2004. Anti-VEGF antibody bevacizumab (Avastin) with 5FU/LV as third line treatment for colorectal cancer. Tech Coloproctol 8: s50-s52.
- FEINER L, BARR EE, SHUI YB, HOLEKAMP NM AND BRANTLEY-JR MA. 2006. Safety of intravitreal injection of bevacizumab in rabbit's eye. Retina 26: 882-888.
- GAN L, FAGERHOLM P AND PALMBLAD J. 2004. Vascular endothelial growth factor (VEGF) and its receptor VEGFR-2 in the regulation of corneal neovascularization and wound healing. Acta Ophthalmol Scand 82: 557-563.
- GAUDRIC A, N'GUYEN T, MOENNER M, GLACET-BERNARD A AND BARRITAULT D. 1992. Quantification of angiogenesis due to basic fibroblast growth factor in a modified rabbit corneal model. Ophthalmic Res 24: 181-188.
- GRAGOUDAS ES, ADAMIS AP, CUNNINGHAN ET, FEISOND M AND GUYER DR. 2004. Pegaptanib for neovascular age-related macular degeneration. N Engl J Med 351: 2805-2816.
- HEIER JS ET AL. 2006. Ranibizumab for treatment of neovascular age-related macular degeneration. Ophthalmology 113: 633-642.
- HÖFLING-LIMA AL, BELFORT-JR R, FREITAS D AND FARAH ME. 2006. Intrastromal Injection of Bevacizumab (Avastin) for Treatment of Corneal Neovascularization. American Academy of Ophthalmology Annual Meeting, Las Vegas, Nov.
- HOSSEINI H AND NEJABAT MA. 2007. Potential therapeutic strategy for inhibition of corneal neovascularization with new anti-VEGF agents. Med Hypotheses 68: 799-801.
- HURWITZ H ET AL. 2004. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 350: 2335-2342.
- KABBINAVAR FI, MEROPOL NJ, LIEBERMAN G, GRIFFING S AND BERGSLAND E. 2003. Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/Leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J Clin Oncol 21: 60-65.
- KAHOOK MY, SHUMAN JS AND NOECKER RJ. 2006. Intravitreal bevacizumab in a patient with neovascular glaucoma. Ophthalmic Surg Lasers Imaging 37: 144-146.
- KWON YS AND KIM JC. 2006. Inhibition of corneal neovascularization by rapamycin. Exp Mol Med 38: 173-179.
- KVANTA A, SARMAN S, FAGERHOLM P, SEREGARD S AND STEEN B. 2000. Expression of matrix metalloproteinase-2 (MMP-2) and vascular endothelial growth factor (VEGF) in inflammation-associated corneal neovascularization. Exp Eye Res 70: 419-428.
- LAVIE G, MANDEL M, HAZAN S, BARLIYA T, BLANK M, GRUNBAUM A, MERUELO D AND SOLOMON A. 2005. Anti-angiogenic activities of hypericin in vivo: potential for ophthalmologic applications. Angiogenesis 8: 35-42.
- LIU Y, CAO J, RENARD RA, SONG H, HYLTON H, RUDGE JS, PAPADOPOULOS N, YANCOPOULOS GD AND WIEGAND SJ. 2006. Low dose, subconjuntival administration of VEGF trap inhibits suture-induced corneal neovascularization and inflammation, In: ARVO Annual Meeting, 2006, Fortlauderdale. Proceedings of the Arvo Annual Meeting, Rockville: Association for Research on Vision and Ophthalmology, Res 1626.
- MACUGEN DIABETIC RETINOPATHY STUDY GROUP.. 2006. Changes in retinal neovascularization after pegaptanib(Macugen) therapy in diabetic individuals. Ophthalmology 113: 23-28.
- MANZANO R, PEYMAN G, KHAN P, CARVOUNIS P, KIVILCIM M, REN M, LAKE J AND CHEVEZ-BARRIOS P. 2006. Inhibition of experimental corneal neovascularization by bevacizumab (AVASTIN). Br J Ophthalmol. http://bjo.bmj.com/cgi/content/abstract/bjo 2006.107912v1
- MICHELS S, ROSENFELD PJ, PUÇOAFITO CA, MARCUS EN AND VENKATRAMAN AS. 2005. Systemic bevacizumab (Avastin) therapy for neovascular age-related macular degeneration twelve-week results of an uncontrolled open-label clinical study. Ophthalmology 112: 1035-1047.
- MIMURA T, AMANO S, USUI T, KAJI Y, OSHIKA T AND ISHII Y. 2001. Expression of vascular endothelial growth factor C and vascular endothelial growth factor receptor 3 in corneal lymphangiogenesis. Exp Eye Res 72: 71-78.
- RANDLEMAN JB AND STULTING RD. 2006. Prevention and treatment of corneal graft rejection: current practice patterns (2004). Cornea 25: 286-290.
- RIAZI-ESFAHANI M, PEYMAN GA, AYDIN E, KAZI AA, KIVILCIM M AND SANDERS DR. 2006. Prevention of corneal neovascularization: evaluation of various commercially available compounds in an experimental ratmodel. Cornea 25: 801-805.
- ROSENFELD PJ, SCHWARTZ SD, BLUMENKRANZ MS, MILLER JW, HALLER JA, REIMANN JD, GREENE WL AND SHAMS N. 2005. Maximum tolerated dose of a humanized anti-vascular endothelial growth factor antibody fragment for treating neovascular age-related macular degeneration. Ophthalmology 112: 1048-1053.
- ROSENFELD PJ, HEIER JS, HANTSBARGER G AND SHAMSN. 2006. Tolerability and efficacy of multiple escalating doses of ranibizumab (Lucentis) for neovascular age-related macular degeneration. Ophthalmology 113: 623-632.
Publication Dates
-
Publication in this collection
27 Aug 2007 -
Date of issue
Sept 2007
History
-
Received
12 Feb 2007 -
Accepted
17 Apr 2007