Abstract
The common bottlenose dolphin, Tursiops truncatus, is widely distributed. However, information about its ecology and behavior in Brazilian waters is scarce especially about the ‘offshore’ ecotype, an Evolutionarily Significant Unit in the Southwest Atlantic. We report for the first time the occurrence, behavior and habitat use of bottlenose dolphins Tursiops truncatus truncatus, in two Marine Protected Areas (MPA) in Cabo Frio coast, Brazil. There were fifteen sightings of different groups throughout the year. An overall of 429 individuals were photo-identified. 90.7% dolphins did not present a degree of residence, 1.4% dolphins were considered with high residency to the area, 5.8% medium and 2.1% low. Our habitat use map indicated dolphins were more common off ~10km from Cabo Frio municipality and between depths around 20-70m. Bottlenose dolphins on Cabo Frio coast were more frequently observed performing travelling, followed by foraging and socio-sexual behavior. Group sizes varied from three to 120 individuals. Larger groups were observed when travelling and foraging. Despite the existence of two local Marine Protected Areas, the fast human development in Cabo Frio may threaten this important area for bottlenose dolphins in terms of food resources and shelter from predators.
Key words
Behavioral ecology; Cabo Frio; cetacean; distribution; group size
INTRODUCTION
The knowledge concerning a species habitat preference is a central question in ecology. A specific habitat may be chosen due to several reasons, including biotic and abiotic factors (Redfern et al. 2006REDFERN JV ET AL. 2006. Techniques for cetacean–habitat modeling. Mar Ecol Prog Ser 310(1): 271-295., Torres et al. 2008TORRES LG, READ AJ & HALPIN PN. 2008. Fine-scale habitat modeling of a top marine predator: do prey data improve predictive capacity. Ecol Appl 18(7): 1702-1717., Tardin et al. 2013TARDIN RH, SIMÃO SM & ALVES MAS. 2013. Distribution of Tursiops truncates in Southeastern Brazil: a modeling approach for summer sampling. Nat Conserv 11(1): 1-10.) and many species displays different residency patterns, which may temporally and related to a specific behavior (Simões-Lopes & Fabian 1999SIMÕES-LOPES PC & FABIÁN ME. 1999. Residence patterns and site fidelity in bottlenose dolphins, Tursiops truncatus (Montagu) (Cetacea, Delphinidae) of Southern Brazil. RBZool 16(1): 1017-1024., Hoffmann et al. 2008HOFFMANN LS, TOLEDO FL & FREITAS TRO. 2008. Contribution to a behavioral data bank: association patterns and habitat use of a small group of coastal bottlenose dolphins Tursiops truncatus (Montagu, 1821) (Cetacea, Delphinidae) in southern Brazil. In: Braga ES (Ed), Oceanografia e mudanças globais, Instituto Oceanográfico da Universidade de São Paulo, 1st ed., São Paulo, p. 88-102., Dinis et al. 2016DINIS A, ALVES F, NICOLAU C, RIBEIRO C, KAUFMANN M, CAÑADAS A & FREITAS L. 2016. Bottlenose dolphin Tursiops truncatus group dynamics, site fidelity, residency and movement patterns in the Madeira Archipelago (North-East Atlantic). Afr J Mar Sci 38(2): 1-10., Di Giacomo & Ott 2016DI GIACOMO AB & OTT PH. 2016. Long-term site fidelity and residency patterns of bottlenose dolphins (Tursiops truncatus) in the Tramandaí Estuary, southern Brazil. LAJAM 11(1-2): 155-161.). In general, a species may choose a habitat based on the availability and quality of food sources and possible mates. The comprehension of these relationships in highly mobile marine species, such as cetaceans, is a challenging task. These species are long-lived, spend only part of their lives in the surface, use vast areas and exhibit complex social systems (Connor et al. 2000CONNOR RC, READ AJ & WRANGHAM R. 2000. Male reproductive strategies and social bonds. In: Mann J et al. (Eds), Cetacean societies: Field studies of dolphins and whales. The University of Chicago Press, 1st ed., Chicago, 448 p.). Therefore, investigating important ecological patterns such as occurrence and habitat use, in general, demand a large amount of funding and time which constrains our knowledge about these species ecology, especially on regional scale. On developing countries, such as Brazil, most research effort is concentrated on the Guiana dolphin, Sotalia guianensis, a coastal dolphin species that continually occurs from Southern Brazil (Florianópolis) to Honduras (Simões-Lopes 1988SIMÕES-LOPES PC. 1988. Ocorrência de uma população de Sotalia fluviatilis (Gervais, 1853) (Cetácea, Delphinidae) no limite sul da sua distribuição, Santa Catarina. Biotemas 1(1): 57-62., Edwards & Schnell 2001EDWARDS HH & SCHNELL GD. 2001. Status and ecology of Sotalia fluviatilis in the Cayos Miskito Reserve, Nicarágua. Mar Mamm Sci 17(3): 445-472.). In contrast, some worldwide well-known cetacean species are poorly known in Brazil, such as the common bottlenose dolphin, Tursiops truncatus.
Tursiops truncatus is widely distributed and occurs in tropical, sub-tropical, and temperate habitats between 45°N and 45°S (Wells & Scott 2008WELLS RS & SCOTT M. 2008. Common bottlenose dolphin. In: Perrin WF et al. (Eds), Encyclopedia of Marine Mammals. Academic Press, Chicago, 1352 p.). Some population have been continuously monitored over three decades [e.g., Shark Bay (Connor et al. 2000CONNOR RC, READ AJ & WRANGHAM R. 2000. Male reproductive strategies and social bonds. In: Mann J et al. (Eds), Cetacean societies: Field studies of dolphins and whales. The University of Chicago Press, 1st ed., Chicago, 448 p.) and Sarasota Bay (Wells 1991WELLS RS. 1991. The role of long-term study in understanding the social structure of a bottlenose community. In: Pryor K & Norris KS (Eds), Dolphin societies: Discoveries and puzzles. University of California Press, 1st ed., Los Angeles, 405p .)], however, in Brazilian waters, it is considered a data deficient species (ICMBio 2018ICMBIO - INSTITUTO CHICO MENDES DE CONSERVAÇÃO DA BIODIVERSIDADE. 2018. Livro Vermelho da Fauna Brasileira Ameaçada de Extinção: Brasília: ICMBio/MMA. Volume II – Mamíferos. 1st ed., ICMBio/MMA, Brasília, 625 p.). Recent genetic and morphometric findings identified two forms of T. truncatus in Brazil: “coastal” and “offshore” ecotypes. However, there is an intense debate whether they are different species or sub-species (Fruet et al. 2014FRUET PF ET AL. 2014. Remarkably low genetic diversity and strong population structure in common bottlenose dolphins (Tursiops truncatus) from coastal waters of the Southwestern Atlantic Ocean. Conserv Genet 15(4): 879-895., Costa et al. 2016COSTA APB, ROSEL PE, DAURA-JORGE FG & SIMÕES-LOPES PC. 2016. Offshore and coastal common bottlenose dolphins of the western South Atlantic face-to-face: What the skull and the spine can tell us. Mar Mamm Sci 32(4): 1433-1457., Wickert et al. 2016WICKERT JC, VON-EYE SM, OLIVEIRA LR & MORENO IB. 2016. Revalidation of Tursiops gephyreus Lahille 1908 (Cetartiodactyla: Delphinidae) from the southwestern Atlantic Ocean. J Mammal 97(6): 1728-1737.). On a recent study, a combination of mtDNA control region sequences and microsatellite genotypes support ‘coastal’ ecotype as a distinct Evolutionarily Significant Unit (ESU) than ‘offshore’ ecotype in the South Western Atlantic (SWA) (Fruet et al. 2017FRUET PF, DALLA-ROSA L, GENOVES RC, VALIATI VH, DE-FREITAS TR & MÖLLER LM. 2017. Biopsy darting of common bottlenose dolphins (Tursiops truncatus) in southern Brazil: evaluating effectiveness, short-term responses and wound healing. Lat Americ J Aquat Mamm 11(1-2): 121-132.). In our study, we follow Costa et al. (2016)COSTA APB, ROSEL PE, DAURA-JORGE FG & SIMÕES-LOPES PC. 2016. Offshore and coastal common bottlenose dolphins of the western South Atlantic face-to-face: What the skull and the spine can tell us. Mar Mamm Sci 32(4): 1433-1457. by considering the ‘offshore’ ecotype as a subspecies, Tursiops truncatus truncatus, and a distinct ESU (Fruet et al. 2017FRUET PF, DALLA-ROSA L, GENOVES RC, VALIATI VH, DE-FREITAS TR & MÖLLER LM. 2017. Biopsy darting of common bottlenose dolphins (Tursiops truncatus) in southern Brazil: evaluating effectiveness, short-term responses and wound healing. Lat Americ J Aquat Mamm 11(1-2): 121-132.).
Most of the known ecological information concerning T. truncatus was obtained from ‘coastal’ ESU mainly in Southern Brazil including Norte bay, Florianópolis – Santa Catarina (e.g. Flores & Fontoura 2006FLORES PAC & FONTOURA NF. 2006. Ecology of marine tucuxi, Sotalia guianensis, and bottlenose dolphin, Tursiops truncatus, in Baía Norte, Santa Catarina state, Southern Brazil. Lat Americ J Aquat Mamm 5(2): 105-115., Wedekin et al. 2008WEDEKIN LL, DAURA-JORGE FG, ROSSI-SANTOS MR & SIMÕES-LOPES PC. 2008. Notas sobre a distribuição, tamanho de grupo e comportamento do golfinho Tursiops truncatus (Cetacea: Delphinidae) na Ilha de Santa Catarina, sul do Brasil. Biota 8(4): 225-229.), Laguna – Santa Catarina (e.g. Simões-Lopes & Fabian 1999, Daura-Jorge et al. 2013aDAURA-JORGE FG, CANTOR M, INGRAM SN, LUSSEAU D & SIMÕES-LOPES PC. 2013a. The structure of a bottlenose dolphin society is coupled to a unique foraging cooperation with artisanal fishermen. Biol Lett 8(1): 702-705., 2016), Tramandaí (e.g. Di Giacomo & Ott 2016DI GIACOMO AB & OTT PH. 2016. Long-term site fidelity and residency patterns of bottlenose dolphins (Tursiops truncatus) in the Tramandaí Estuary, southern Brazil. LAJAM 11(1-2): 155-161.) and Patos Lagoon estuary, both located at Rio Grande do Sul (e.g. Fruet et al. 2011FRUET PF, SECCHI ER, DI TULLIO JC & KINAS PG. 2011. Abundance estimation of bottlenose dolphins, Tursiops truncatus (Cetacea, Delphinidae), inhabiting the Patos Lagoon estuary, southern Brazil: implications for conservation. RBZool 28(1): 23-30., 2012, Di Tullio et al. 2015DI TULLIO JC, FRUET PF & SECCHI ER. 2015. Identifying critical areas to reduce bycatch of coastal common bottlenose dolphins Tursiops truncatus in artisanal fisheries of the subtropical western South Atlantic. Endanger Species Res 29(1): 35-50.). The information about ‘offshore’ ESU comes sporadically from wide range offshore surveys (e.g. Rossi-Santos et al. 2006ROSSI-SANTOS M, WEDEKIN LL & SOUSA-LIMA RS. 2006. Distribution and habitat use of small cetaceans off Abrolhos Bank, eastern Brazil. Lat Americ J Aquat Mamm 5(1): 23-28., Carvalho & Rossi-Santos 2011CARVALHO MS & ROSSI-SANTOS MR. 2011. Sightings of the bottlenose dolphin (Tursiops truncatus) in Trindade island, South Atlantic Ocean. Mar Biodivers Rec 4(e15): 1-3., Di Tulio et al. 2016, Oliveira et al. 2017OLIVEIRA LR, OTT PH, MORENO IB, TAVARES M, SICILIANO S & BONATTO SL. 2017. Effective population size of an offshore population of bottlenose dolphins, Tursiops truncatus, from the São Pedro and São Paulo Archipelago, Brazil. Lat Americ J Americ Mamm 11(1-2): 162-169.) with few exceptions in Southeastern Brazil, where the ‘offshore’ ESU inhabit coastal areas (e.g. < 5km from the coast) (e.g. Lodi et al. 2009LODI L, MAYERHOFER LC & MONTEIRO-NETO CA. 2009. Evaluation of the video-identification technique applied to bottlenose dolphins (Tursiops truncatus) in Cagarras Archipelago, Rio de Janeiro, Brazil. J Mar Biolog Assoc UK 89(5): 1077-1081., Tardin et al. 2013TARDIN RH, SIMÃO SM & ALVES MAS. 2013. Distribution of Tursiops truncates in Southeastern Brazil: a modeling approach for summer sampling. Nat Conserv 11(1): 1-10., Lodi et al. 2014LODI L, CANTOR M, DAURA-JORGE FG & MONTEIRO-NETO CA. 2014. A missing piece from a bigger puzzle: declining occurrence of a transient group of bottlenose dolphins off Southeastern Brazil. Mar Ecol 35(4): 516-527., Lodi 2016).
The Plano de Ação Nacional de Pequenos Cetáceos organized and published by Instituto Chico Mendes de Conservação da Biodiversidade - ICMBio, reports that an important goal for the T. truncatus conservation is a detailed investigation concerning distribution patterns and behavior of dolphins in Brazilian waters (Barreto 2011BARRETO AS. 2011. Golfinho nariz de garrafa. In: Rocha-Câmara CC et al. (Eds), Plano Nacional de Ação de Pequenos Cetáceos. Instituto Chico Mendes de Conservação da Biodiversidade, 1st ed., Brasília, 129 p.). Since the ecology of the T. truncatus truncatus is poorly understood along Brazilian coast, we provide baseline ecological information about their occurrence, group size, composition, behavior, residence patterns and habitat use in Cabo Frio, Rio de Janeiro, Brazil.
MATERIALS AND METHODS
The study area (~500.7km2) is located in Cabo Frio coast (22°50’21”S; 41°54’37”W - 23°00’18”S; 42°05’53”W), northeastern of Rio de Janeiro State, including Arraial do Cabo, Cabo Frio and Armação dos Búzios municipalities (Fig. 1). The Cabo Frio coast is marked by a change in the shoreline orientation from a north-south to a southwest-northeast orientation, and it has a narrow continental shelf, forming a steep slope (De Leo & Pires-Vanin 2006DE LEO FC & PIRES-VANIN AMS. 2006. Benthic megafauna communities under influence of the SACW (South Atlantic Central Water) intrusion onto the Brazilian Southeastern shelf: a comparison between an upwelling and a non-upwelling ecosystem. J Mar Syst 60(3-4): 268-284., Reis et al. 2013REIS AT ET AL. 2013. Origin of step-like and lobate seafloor features along the continental shelf off Rio de Janeiro State, Santos basin-Brazil. Geomorph 203(1): 25-45.) (Fig. 1). Throughout a year, the mixture of the Brazil Current and the South Atlantic Central Water is strongly influenced by north-northeastern winds and by meanders and eddies in the Brazil Current which causes an upwelling phenomenon (Carbonel 1998CARBONEL C. 1998. Modelling of upwelling in the coastal area of Cabo Frio (Rio de Janeiro – Brazil). Rev Bras Oceanogr 46(1): 1-17., Coelho-Souza et al. 2012COELHO-SOUZA SA, LOPEZ MS, GUIMARÃES JRD, COUTINHO R & CANDELLA RN. 2012. Biophysical interactions in the Cabo Frio upwelling system, southeastern Brazil. Braz J Oceanogr 60(3): 353-365.). This upwelling is especially prevalent during spring and summer (Carbonel 1998CARBONEL C. 1998. Modelling of upwelling in the coastal area of Cabo Frio (Rio de Janeiro – Brazil). Rev Bras Oceanogr 46(1): 1-17., Coelho-Souza et al. 2012COELHO-SOUZA SA, LOPEZ MS, GUIMARÃES JRD, COUTINHO R & CANDELLA RN. 2012. Biophysical interactions in the Cabo Frio upwelling system, southeastern Brazil. Braz J Oceanogr 60(3): 353-365.). In general, upwelling results in high primary productivity and high fish abundance, generating a favorable condition for the occurrence of different cetacean species (Silva et al. 2003SILVA MA, PRIETO R, MAGALHÃES S, CABECINHAS R, CRUZ A, GONÇALVES JM & SANTOS RS. 2003. Occurrence and distribution of cetaceans in the waters around the Azores (Portugal), Summer and Autumn 1999-2000. Aquat Mamm 29(1): 77-83., Keiper et al. 2005KEIPER CA, AINLEY DG, ALLEN SG & HARVEY JT. 2005. Marine mammal occurrence and ocean climate off central California; 1986 to 1994 and 1997 to 1999. Mar Ecol Prog Ser 289(1): 285-306.). This phenomenon provides an important nutritional resource for cetaceans (Costa 2008COSTA D. 2008. Energetics. In: Perrin WF et al. (Eds), Encyclopedia of Marine Mammals. Academic Press, 2nd ed., Chicago, 1352 p.).
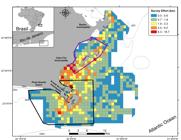
Study area located in Southeastern Brazil, Rio de Janeiro, showing isobaths. Survey effort is shown in map for each 1x1 km2 grid. Continuous blue line indicates Marine Protected Area (MPA) Reserva Extrativista Marinha do Arraial do Cabo and purple line MPA Pau-Brasil.
The Marine Protected Area (MPA - IUCN category V), located at the southern portion of the study area, was originally set to protect local fishermen lifestyle (Reserva Extrativista Marinha Arraial do Cabo) in 1997 (Unnumb. law, January 03rd 1997, ICMBio). Despite its creation, no management plan has been developed up to now. Therefore, this area has been intensively used for fisheries, tourism and diving boats, as well as military and petroleum activities (Gandra 2009GANDRA A. 2009. Comitê Gestor garantirá funcionamento de reserva marinha no estado do Rio de Janeiro. Disponível em https://arquivo.correiodobrasil.com.br/comite-gestor-garantira-funcionamento-de-reserva-marinha-no-rio/ . Acessado em 25 de abril de 2019.
https://arquivo.correiodobrasil.com.br/c...
). At the northern portion, a sustainable terrestrial Conservation Unit (IUCN category VI – Area de Proteção Ambiental do Pau Brasil) was created in 2002 (State decree numb. 31.346, June 06th 2002, INEA) to protect Brazilwood, Caesalpinia echinata, and part of its territory extends towards the sea (Fig. 1).
Between December 2010 and November 2012 and from February to August 2014, we conducted monthly boat surveys onboard a 6.5m inflatable boat equipped with a 150-hp engine (mean duration 5.7 h, minimum = 3.25 h, maximum = 8.00 h). The surveys followed non-systematic routes (mean speed = 20km/h) due to logistical and climatic constraints and to maximize dolphin encounters (Fig. 1). When spotting a group of dolphins, the boat followed each group of dolphins at a reduced speed (mean speed = 10km/h).
Residency patterns of bottlenose dolphins, at each sampling day, were investigated by means of photo-identification. A maximum number of individuals, in each group, were photographed using a CANON EOS 40D® camera equipped with a 75-300mm lens. Individuals could be recognized through natural marks, such as nicks and notches on their dorsal fins (Espécie et al. 2010ESPÉCIE MA, TARDIN RHO & SIMÃO SM. 2010. Degrees of residence of Guiana dolphins (Sotalia guianensis) in Ilha Grande Bay, south-eastern Brazil: a preliminary assessment. J Mar Biolog Assoc UK 90(8): 1633-1639.).
We recorded dolphin locations using a GARMIN VISTA CX GPS device continuously at every 500m, based on the focal group procedure (Lehner 1996LEHNER PN. 1996. Handbook of ethological methods, 2nd ed., Cambridge University Press, Cambridge, 403 p.). As we recorded these locations for dolphin groups, multiple GPS locations for each group can be available in a single day. For example, on July 14th 2011, one common bottlenose dolphin group was observed for 1.5 hours in which six GPS locations were recorded in distinct regions of the study area.
We recorded dolphin’s behavior and group size using a SONY Dcr30® video-camera following the focal group methodology with continuous sampling (Lehner 1996LEHNER PN. 1996. Handbook of ethological methods, 2nd ed., Cambridge University Press, Cambridge, 403 p.). We defined a group as individuals 100m apart from each other displaying the same behavior (Shane 1990SHANE SH. 1990. Behavior and ecology of the bottlenose dolphin at Sanibel Island, Florida. In: Leatherwood S & Reeves RR (Eds), The bottlenose dolphin. Academic Press, San Diego, 1st ed., 653 p.). Immatures were considered as individuals reaching up to half of adult’s size (Shane 1990SHANE SH. 1990. Behavior and ecology of the bottlenose dolphin at Sanibel Island, Florida. In: Leatherwood S & Reeves RR (Eds), The bottlenose dolphin. Academic Press, San Diego, 1st ed., 653 p.). Behaviors recorded were categorized as following:
-
1) Feeding – when individuals did not show directional movements and dove frequently in asynchronous fashion (Karczmarski et al. 2000KARCZMARSKI L, COCKCROFT VC & MCLACHLAN A. 2000. Habitat use and preferences of Indo-Pacific humpback dolphins Sousa chinensis in Algoa Bay, South Africa. Mar Mamm Sci 16(1): 65-79.);
-
2) Travelling – directional and persistent movements (Karczmarski et al. 2000KARCZMARSKI L, COCKCROFT VC & MCLACHLAN A. 2000. Habitat use and preferences of Indo-Pacific humpback dolphins Sousa chinensis in Algoa Bay, South Africa. Mar Mamm Sci 16(1): 65-79.);
-
3) socio-sexual – socio-sexual behavior occurred when individuals focused on each other, and the belly-to-belly position was frequently observed (Slooten 1994).
Data analyses
We defined residency patterns based on Ballance (1990)BALLANCE LT. 1990. Residence patterns, group organization and surface association of bottlenose dolphins in Kino Bay, Gulf of California, Mexico. In: Leatherwood S & Reeves RR (Eds), The bottlenose dolphin,1st ed., Academic Press, San Diego, 653p.: individuals sighted only once were classified as non-residents, while those sighted more than once were defined as residents. For residents, three degrees of residency were calculated (low, medium and high) based on the following: number of times a dolphin was sighted in the area; time (in days) between first and last sightings of each animal; and periodicity – average of days between recaptures. For example, a high degree of residency may be obtained by a high number of recaptures, a long interval between first and last sightings and a short time between recaptures. However, each measure must be interpreted with caution (Ballance 1990BALLANCE LT. 1990. Residence patterns, group organization and surface association of bottlenose dolphins in Kino Bay, Gulf of California, Mexico. In: Leatherwood S & Reeves RR (Eds), The bottlenose dolphin,1st ed., Academic Press, San Diego, 653p.) because animals sighted few times on consecutive days can present a relatively low degree of residency although they can have a high number of recaptures.
For habitat use analysis, we first divided the study area in 718 1-km2 cells using the ArcGIS-compatible Marine Geospatial Ecology Tools 0.8a64 (Roberts et al. 2010ROBERTS JJ, BEST BD, DUNN DC, TREML EA & HAPLIN PN. 2010. Marine Geospatial Ecology Tools: An integrated framework for ecological geoprocessing with ArcGIS, Python, R, MATLAB, and C++. Environ Model Softw 25(10): 1197-1207.) in which all the distribution records were interpolated. We used two response variables to evaluate habitat use: number of sighting per grid and number of individuals per grid. Since sampling effort was uneven along the area we used the Encounter Rate index (ER), for number of sighting (1) and number of individuals (2) variables. Encounter rates were calculated as:
Where,
NS = Number of sightings in a given grid
SE = Survey effort, calculated as the linear boat trajectories, in kilometers, used to survey and follow dolphins
Where,
NI = Number of individuals in a given grid
SE = Survey effort, calculated as the linear boat trajectories, in kilometers, used to survey and follow dolphins
We analyzed group size and behavioral data using point-sampling methodology in which the recorded videos were separated by date and subsequently cut every 10 min, resulting in multiple clips (Mann 1999MANN J. 1999. Behavioral sampling methods for cetaceans: a review and critique. Mar Mamm Sci 15(1): 102-122.). From these 10min clips, behavior and group size were measured.
We used Monte-Carlo chi-square test with 5,000 simulations to investigate if the occurrence and residency of bottlenose dolphin differed significantly between seasons, to evaluate the most common behavioral state and to assess if group sizes varied between seasons and behavior. All analyses were done in R studio 1.0.44.
RESULTS
Our total effort comprised 99 boat trips corresponding to a total of 454.5h of observations including 46.4 hours of direct observations (10.1%), and 4,970 km surveyed. There were fifteen sightings (an overall of 104 GPS locations) of different groups of bottlenose dolphins throughout the year. Dolphins on Cabo Frio coast displayed dark-gray coloration and scars all over the body. No individual was sighted in May, September, October, and November (Table I).
Summary of common bottlenose dolphins occurrence, Tursiops truncatus truncatus in Cabo Frio coast, Rio de Janeiro, Brazil, showed in months for December 2010 to November 2012 and February to August 2014 period. Monthly Encounter Rate = Number of sightings in a given month/number of boat trips undertaken in the same given month.
Occurrence, residency patterns and habitat use
Dolphins occurrence did not vary significantly among seasons (Summer = 6, Fall = 4, Winter = 4, Spring = 1; Monte Carlo Chi-square = 3.4, p = 0.39).
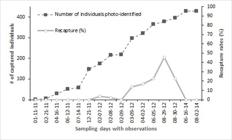
Based on the markings found on their dorsal fins, we identified 429 bottlenose dolphins. The frequency of daily recaptures ranged from 0 to 45.5% (x̄ = 8.0%, ± 13.1%) (Fig. 2).
Number of photo-identified common bottlenose dolphins, Tursiops truncatus truncatus, in Cabo Frio, RJ, Brazil and their respective recapture rates.
A total of 389 dolphins (90.7%) were seen once and classified as non-residents. The other 40 individuals (9.3%), sighted twice or more, were considered residents. The individual number of sightings ranged from 1 to 4, while the interval between the first and last sighting ranged from 1 to 416 days (x̄ = 106 ± 86). According to the measures used to define the degree of residency, 90.7% (N = 389) were not residents; 2.1% (N = 9) had a low degree of residency; 5.8% (N = 25) had a medium degree of residency; and only 1.4% (N = 6) had a high degree of residency (Table II).
Reference numbers used for each measurement according to each degree of residence for common bottlenose dolphins, Tursiops truncatus truncatus, in Cabo Frio, RJ, Brazil.
The number of individuals with a medium degree of residency predominated for each season. During the winter, only one individual (3.0%) presented low degree of residency while there was 16 individuals in summer (34.0%) (Table III). There was a statistical significant difference for the degree of fidelity between seasons (Chi-square = 12.1; p = 0.016).
Common bottlenose dolphins, Tursiops truncatus truncatus, sighted during each season according to their degree of residence (low, medium and high) in Cabo Frio, RJ, Brazil. No recaptured individual was seen during spring.
During the field work, we observed dolphins at depths that varied from 9.5m to 87 m, in distances of 0.3km to 13.9km from the coast. Our habitat use comparison of response variables showed slightly different results (Fig. 3 a, b). When using only sightings as response variables, our habitat use map indicated dolphins were more common off ~10km from Cabo Frio municipality between depths of 20-50m (mean encounter rate = 0.11 dolphins sightings/km surveyed) (Fig. 3a). When using number of individuals as response variable, our habitat use map indicated dolphins were most commonly found off Arraial do Cabo municipality, closer to Praia Grande beach and Cabo Frio island around depths of 30-70m (mean encounter rate = 4.9 individuals/km surveyed) (Fig. 3b).
Encounter rates of bottlenose dolphins Tursiops truncatus truncatus in Cabo Frio coast, Rio de Janeiro, Brazil within 1x1 km2 grid cells. Gray points indicate sightings of dolphins. Isobaths are shown as continuous black lines. Continuous blue line indicates Conservation Unit Reserva Extrativista Marinha do Arraial do Cabo and purple line Conservation Unit Area de Proteção Ambiental do Pau-Brasil. CFI = Cabo Frio island. Upper figure: Encounter rate calculated as number of sighted dolphin groups in a given grid/survey effort, calculated as the linear boat trajectories, in kilometers. Bottom figure: Encounter rate calculated as number of individuals in a given grid/survey effort, calculated as the linear boat trajectories, in kilometers.
Behavior, group size and composition
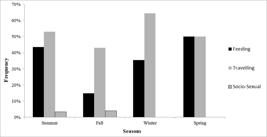
We observed T. truncatus truncatus on Cabo Frio coast more frequently travelling (117 min, 60.3%), followed by foraging (72 min, 37.1%) and Socio-sexual behavior (5.1 min, 2.6%) (Monte Carlo Chi-square = 98.0, p = 0.002). No resting behavior was observed. We observed dolphins feeding more frequently on Spring (Monte Carlo Chi-square = 16.1, p <0.001) and travelling on Winter (Monte Carlo Chi-square = 18.7, p <0.001), but Socio-sexual behavior did not vary between seasons (Monte Carlo Chi-square = 5.2, p = 0.17) (Fig. 4).
Frequency of Tursiops truncatus truncatus behaviors (Feeding, Travelling and Socio-sexual) among seasons in Cabo Frio coast, Rio de Janeiro, Southeastern Brazil.
Group sizes varied from three to 120 individuals (mean ± standard deviation = 40.4 ± 37.3 individuals), in which all these groups had at least one immature individual as a member, which accompanied adults in all activities. Larger groups were observed when travelling and foraging (Foraging = 36.3 ± 35.7, Travelling = 33.8 ± 30.5, Socio-sexual = 5.3 ± 0.5; Monte Carlo Chi-square = 23.6, p = 0.002). In only two occasions, dolphins displayed coordinated surface feeding behavior, in which it was possible to observe dolphins in wall formation – splitting into two subgroups and then joining in opposite directions (Bel’kovitch et al. 1991); in perpendicular feeding - dolphins splitting into two subgroups and then joining in perpendicular directions (Tardin et al. 2011TARDIN RH, ESPÉCIE MA, NERY MF, D’AZEREDO FT & SIMÃO SM. 2011. Coordinated feeding tactics of the Guiana dolphin, Sotalia guianensis (Cetacea: Delphinidae), in Ilha Grande Bay, Rio de Janeiro, Brazil. Zool 28(3): 291-296.); as well as in Kettle, when animals dove under a school of fish, forcing it to the surface, emerging from several directions (Bel’kovitch et al. 1991). On these occasions, group sizes were larger reaching approximately 70 and 120 individuals. On these feeding events, dolphins displayed intense vocal behavior with multiple individuals calling at the same time (I.S. Maciel, unpublished data).
DISCUSSION
Our study presents the first information about aspects on the ecology and behavior of T. truncatus truncatus on an upwelling area covered by two MPAs, in Cabo Frio coast, Southeastern Brazil. Despite the low sighting rate, we could quantify, for the first time, when dolphins occurred, how they behave, their residency patterns and their preferred areas.
Occurrence, residency patterns and habitat use
Despite dolphins did not occur differently among seasons, bottlenose dolphins occurred more frequently on January, February, June and August. The upwelling phenomenon, in which cold, deep, nutrient-rich waters mix with surface waters increasing local productivity occurs, especially, during January and February in this area (Carbonel 1998CARBONEL C. 1998. Modelling of upwelling in the coastal area of Cabo Frio (Rio de Janeiro – Brazil). Rev Bras Oceanogr 46(1): 1-17.).
The high rate of dolphins seen only once (90.7% of non-residents) suggest that the species home range is larger than the study area and, at a local scale, there is likely not enough food resources in the area to sustain a larger resident population. As pointed out in this study, feeding/foraging behaviors were often observed during the summer and winter, and recaptured individuals presented higher frequency during the summer, the upwelling period (Carbonel 1998CARBONEL C. 1998. Modelling of upwelling in the coastal area of Cabo Frio (Rio de Janeiro – Brazil). Rev Bras Oceanogr 46(1): 1-17., Coelho-Souza et al. 2012COELHO-SOUZA SA, LOPEZ MS, GUIMARÃES JRD, COUTINHO R & CANDELLA RN. 2012. Biophysical interactions in the Cabo Frio upwelling system, southeastern Brazil. Braz J Oceanogr 60(3): 353-365.). Movements of T. truncatus truncatus along the coast of Brazil are poorly understood. In Rio de Janeiro state, it was reported that no matches were found between individuals identified off the state’s coast and Cagarras archipelago, located only at 3.8km from the coast (Lodi & Tardin 2018LODI L & TARDIN RH. 2018. Site fidelity and residency of common bottlenose dolphins (Cetartiodactyla: Delphinidae) in a coastal insular habitat off southeastern Brazil. PanamJAS 13(1): 53-63.).
The residency pattern observed in our study is similar to those studies conducted in extensive study areas, such as oceanic islands (e.g. Dinis et al. 2016DINIS A, ALVES F, NICOLAU C, RIBEIRO C, KAUFMANN M, CAÑADAS A & FREITAS L. 2016. Bottlenose dolphin Tursiops truncatus group dynamics, site fidelity, residency and movement patterns in the Madeira Archipelago (North-East Atlantic). Afr J Mar Sci 38(2): 1-10. – Madeira Archipelago; Silva et al. 2008SILVA MA, PRIETO R, MAGALHÃES S, SEABRA MI, SANTOS RS & HAMMOND PS. 2008. Ranging patterns of bottlenose dolphins living in oceanic waters: implications for population structure. Mar Biol 156(2): 179-192. – Azores Archipelago) and open water regions (e.g. Oudejans et al. 2015OUDEJANS MG, VISSER F, ENGLUND A, ROGAN E & INGRAM SN. 2015. Evidence for distinct Coastal and Offshore communities of Bottlenose Dolphins in the North East Atlantic. PLoS ONE 10(4): e0122668. – northwest Ireland). In these areas, the probability of marking transient individuals is high, and a low degree of fidelity can indicate a large offshore population with animals displaying more extensive movement patterns, just passing on the coast (Silva et al. 2008SILVA MA, PRIETO R, MAGALHÃES S, SEABRA MI, SANTOS RS & HAMMOND PS. 2008. Ranging patterns of bottlenose dolphins living in oceanic waters: implications for population structure. Mar Biol 156(2): 179-192.). Extensive movement patterns are reported for bottlenose dolphin in others areas, for example, in the Southern California Bight (Defran & Weller 2006DEFRAN RH & WELLER DW. 1999. Occurrence, distribution, site fidelity, and school size of bottlenose dolphins (Tursiops truncatus) of San Diego, California. Mar Mamm. Sci 15(2): 366-380.) and UK and Ireland waters (Robinson et al. 2012ROBINSON KP ET AL. 2012. Discrete or not so discrete: Long distance movements by coastal bottlenose dolphins in UK and Irish waters. J Cet Res Manag 12(3): 365-371.).
Residency pattern studies in Brazil with T. truncatus truncatus are scarce (Milmann et al. 2016MILMANN LC, DANILEWICZ D, BAUMGARTEN J & OTT PH. 2016. Temporal-spatial distribution of an island-based offshore population of common bottlenose dolphins (Tursiops truncatus) in the equatorial Atlantic. Mar Mamm Sci 33(2): 496-519., Lodi & Tardin 2018LODI L & TARDIN RH. 2018. Site fidelity and residency of common bottlenose dolphins (Cetartiodactyla: Delphinidae) in a coastal insular habitat off southeastern Brazil. PanamJAS 13(1): 53-63.). However, for the T. truncatus gephyrus several studies report a high degree of residency in which dolphins present year-round occurrence, feeding and reproducing in these areas (e.g. Simões-Lopes & Fabian 1999, Flores & Fontoura 2006FLORES PAC & FONTOURA NF. 2006. Ecology of marine tucuxi, Sotalia guianensis, and bottlenose dolphin, Tursiops truncatus, in Baía Norte, Santa Catarina state, Southern Brazil. Lat Americ J Aquat Mamm 5(2): 105-115., Wedekin et al. 2008WEDEKIN LL, DAURA-JORGE FG, ROSSI-SANTOS MR & SIMÕES-LOPES PC. 2008. Notas sobre a distribuição, tamanho de grupo e comportamento do golfinho Tursiops truncatus (Cetacea: Delphinidae) na Ilha de Santa Catarina, sul do Brasil. Biota 8(4): 225-229., Fruet et al. 2011FRUET PF, SECCHI ER, DI TULLIO JC & KINAS PG. 2011. Abundance estimation of bottlenose dolphins, Tursiops truncatus (Cetacea, Delphinidae), inhabiting the Patos Lagoon estuary, southern Brazil: implications for conservation. RBZool 28(1): 23-30., Daura-Jorge et al. 2013bDAURA-JORGE FG, INGRAM SN & SIMÕES-LOPES PC. 2013b. Seasonal abundance and adult survival of bottlenose dolphins (Tursiops truncatus) in a community that cooperatively forages with fishermen in southern Brazil. Mar Mamm Sci 29(2): 293-311., Fruet et al. 2015FRUET PF, DAURA-JORGE FG, MÖLLER LM, GENOVES RC & SECCHI ER. 2015. Abundance and demography of bottlenose dolphins inhabiting a subtropical estuary in the Southwestern Atlantic Ocean. J Mammal 96(2): 332-343., Daura-Jorge et al. 2016DAURA-JORGE FG & SIMÕES-LOPES PC. 2016. Mark-recapture vs. line-transect abundance estimates of a coastal dolphin population: a case study of Tursiops truncatus from Laguna, southern Brazil. LAJAM 11(1-2): 133-143., Di Giacomo & Ott 2016DI GIACOMO AB & OTT PH. 2016. Long-term site fidelity and residency patterns of bottlenose dolphins (Tursiops truncatus) in the Tramandaí Estuary, southern Brazil. LAJAM 11(1-2): 155-161.)
Our habitat use analysis indicated common bottlenose dolphins used mainly the areas within the RESEX Arraial do Cabo. However, it is important to note that some high ER areas are located outside the limits of both MPAs. Dolphins habitat use in the area seems to be influenced by cold water with high chlorophyll concentration (Tardin et al. 2019TARDIN RH, CHUN Y, SIMÃO SM & ALVES MAS. 2019. Habitat use models of spatially auto-correlated data: a case study of the common bottlenose dolphin, Tursiops truncatus truncatus, in southeastern Brazil. Mar Biol Res 15(4-6): 305-316.), which is a proxy for the upwelling phenomenon (Carbonel 1998CARBONEL C. 1998. Modelling of upwelling in the coastal area of Cabo Frio (Rio de Janeiro – Brazil). Rev Bras Oceanogr 46(1): 1-17.).
Our habitat use comparison using two different response variables indicates the importance of choosing appropriate variables. As demonstrated in this paper, scientists must clearly bear in mind what they want to investigate, if it is sightings or density of individuals, since the maps of preferred areas may present differences. For managing purposes, such differences may be important in considering conservation priorities. For example, when using sightings as a response variable, most of common bottlenose dolphin occurrences were observed within the RESEX Arraial do Cabo, whereas when using number of individuals there were an increase in the areas used outside the MPAs. The existence of common bottlenose dolphin populations within and outside the limits of MPAs is reported for Moray Firth bay, Scotland (e.g. Wilson et al. 2004WILSON B, REID RJ, GRELLIER K, THOMPSON PM & HAMMOND PS. 2004. Considering the temporal when managing the spatial: a population range expansion impacts protected areas-based management for bottlenose dolphins. Anim Conserv 7(4): 331-338.), Pelagos Sanctuary, in the mediterranean sea (e.g. Gnone et al. 2011GNONE G ET AL. 2011. Distribution, abundance, and movements of the bottlenose dolphin (Tursiops truncatus) in the Pelagos Sanctuary MPA (north-west Mediterranean Sea). Aquat Conserv 21(4): 372-388.) and in Azores, Portugal (e.g. Silva et al. 2012SILVA MA, PRIETO R, MAGALHÃES S, SEABRA MI, MACHETE M & HAMMOND PS. 2012. Incorporating information on bottlenose dolphin distribution into marine protected area design. Aquat Conserv 22(1): 122-133.). Despite the existence of a MPA does not guarantee the protection of a species, it is an important management tool to protect a species habitat or at least part of it (Chape et al. 2005CHAPE S, HARRISON J, SPALDING M & LYSENKO I. 2005. Measuring the extent and effectiveness of protected areas as an indicator for meeting global biodiversity targets. Philos Trans R Soc Lond B Biol Sci 360(1454): 443-455., Hoyt 2011HOYT E. 2011. Marine protected areas for whales, dolphins and porpoises: A world handbook for cetacean habitat conservation and planning. Earthscan, London and New York, 1st ed., 448 p.). In Cabo Frio, specifically, despite the existence of two MPAs, the rapid human development in Cabo Frio may threaten this important area for bottlenose dolphin in terms of food resources and shelter from predators. Fishing and tourism activities are intense, especially during summer, and may alter dolphins’ behavior, as has been seen in other places (e.g. Doubtful Sound, New Zealand (Lusseau 2003LUSSEAU D. 2003. Effects of tour boats on the behavior of bottlenose dolphins: using Markov chains to model anthropogenic impacts. Conserv Biol 17(6): 1785-1793.); Zanzibar, Tanzania (Christiansen et al. 2010CHRISTIANSEN F, LUSSEAU D, STENSLAND E & BERGGREN P. 2010. Effects of tourist boats on the behaviour of Indo-Pacific bottlenose dolphins off the south coast of Zanzibar. Endanger Species Res 11(1): 91-99.); Archipelago of Bocas del Toro, Panamá (May-Collado et al. 2014MAY-COLLADO LJ, QUIÑONES-LEBRÓN SG, BARRAGÁN-BARRERA DC, PALACIOS JD & GAMBOA-POVEDA M. 2014. The dolphin watching industry of Bocas del Toro continues impacting the resident bottlenose dolphin population. Int Whal Comm SC/65b/WW06, 6 p.), Moray Firth, Scotland (Pirotta et al. 2015PIROTTA E, THOMPSON PM, CHENEY B, DONOVAN CR & LUSSEAU D. 2015. Estimating spatial, temporal and individual variability in dolphin cumulative exposure to boat traffic using spatially explicit capture–recapture methods. Anim Conserv 18(1): 20-31.). Although dolphin-watching tourism seems to be inconsistent in the region, a high number of tourism and fishing boats may affect their behavior. Thus, these activities may restrict the habitat use patterns of dolphins in Cabo Frio, where they should be able to avoid areas with high concentration of boats, which thus constrains their home range. More data are needed to test this hypothesis, but these considerations may help to investigate whether seasonal shifts of distribution are driven by anthropogenic activities in Cabo Frio coast.
Behavior, group size and composition
In most cases, we observed T. truncatus truncatus groups travelling through the area. When feeding/foraging, in most occasions, dolphins were searching for food closer to the bottom. Feeding was observed more frequently during summer and winter reflecting the opportunistic feeding behavior of common bottlenose dolphins that preys upon pelagic, demersal or benthic fish species (Wells & Scott 2008WELLS RS & SCOTT M. 2008. Common bottlenose dolphin. In: Perrin WF et al. (Eds), Encyclopedia of Marine Mammals. Academic Press, Chicago, 1352 p.). During summer, demersal species from the Engraulidae and Trichiuridae families are common at the region and during fall and winter the Brazilian sardine, Sardinella brasiliensis, a pelagic species, forms large schools (Paiva & Motta 2000PAIVA MP & MOTTA PCS. 2000. Cardumes da sardinha-verdadeira, Sardinella brasiliensis (Steindachner), em águas costeiras do estado do Rio de Janeiro, Brasil. RBZool 17(2): 339-346.). Species from both families are known to be part of bottlenose dolphin diet worldwide (e.g. Di Beneditto et al. 2001DI BENEDITTO APM, RAMOS RMA, SICILIANO S, DOS-SANTOS RA, BASTOS G & FAGUNDES-NETO E. 2001. Stomach contents of delphinids from Rio de Janeiro, southeastern Brazil. Aquat Mamm 2(2): 24-28., Bearzi 2005BEARZI M. 2005. Aspects of the ecology and behaviour of bottlenose dolphins (Tursiops truncatus) in Santa Monica Bay, California. J Cetacean Res Manage 7(1): 75-83., Carvalho & Rossi-Santos 2011CARVALHO MS & ROSSI-SANTOS MR. 2011. Sightings of the bottlenose dolphin (Tursiops truncatus) in Trindade island, South Atlantic Ocean. Mar Biodivers Rec 4(e15): 1-3., Bräger et al. 2016BRÄGER Z, GONZALVO J, AGAZZI S & BEARZI G. 2016. Identification of bottlenose dolphin (Tursiops truncatus) prey using fish scale analysis. Aquat Mamm 42(1): 63., Moura et al. 2016MOURA JF, TAVARES DC, SECCO HK & SICILIANO S. 2016. Bottlenose dolphins (Tursiops truncatus, Montagu 1821) in central-northern coast of Rio de Janeiro State, Brazil: stranding patterns and insights into feeding habits. Lat Americ J Aquat Mamm 11(1-2): 191-198.). In fact, cetaceans must forage constantly to meet their high energetic demands (Costa 2008COSTA D. 2008. Energetics. In: Perrin WF et al. (Eds), Encyclopedia of Marine Mammals. Academic Press, 2nd ed., Chicago, 1352 p.). In this situation, dolphins may be searching for food near the rocky coast, since some prey species that are associated with this habitat are included in the T. truncatus diet [e.g., Diplodus argenteus (Di Beneditto et al. 2001DI BENEDITTO APM, RAMOS RMA, SICILIANO S, DOS-SANTOS RA, BASTOS G & FAGUNDES-NETO E. 2001. Stomach contents of delphinids from Rio de Janeiro, southeastern Brazil. Aquat Mamm 2(2): 24-28.)]. The associated ichthyofauna of the study area is composed of both tropical and sub-tropical fishes, and most of these species are omnivorous (Ferreira et al. 2004FERREIRA CEL, FLOETER SR, GASPARINI JL, FERREIRA BP & JOYEUX JC. 2004. Trophic structure patterns of Brazilian reef fishes: a latitudinal comparison. J Biogeogr 31(7): 1093-1116.). The group composition we observed in Cabo Frio coast, indicates this area may play an important role for immature individuals to learn how to find and catch prey. Social learning is important and well documented for some cetacean species, in which immature individuals learn strategies from their mothers to find and capture food as well as to avoid potential hazards, such as predators and/or anthropogenic disturbances (Bender et al. 2008BENDER CE, HERZING DL & BJORKLUND DF. 2008. Evidence of teaching in Atlantic spotted dolphins by mother dolphins foraging in the presence of their calves. Anim Cogn 12(1): 43-53., Gibson & Mann 2008GIBSON QA & MANN J. 2008. The size, composition and function of wild bottlenose dolphin (Tursiops sp.) mother-calf groups in Shark Bay, Australia. Anim Behav 76(2): 389-405., Tardin et al. 2013TARDIN RH, SIMÃO SM & ALVES MAS. 2013. Distribution of Tursiops truncates in Southeastern Brazil: a modeling approach for summer sampling. Nat Conserv 11(1): 1-10.)The information reported about T. truncatus truncatus behavior and ecology may be used by MPAs managers to direct conservation efforts to specific areas and seasons.
Our results in the present paper indicate a transient population of T. truncatus truncatus using the area for foraging and travelling, in which all groups having at least one immature individual as a member. Most of these dolphins used the RESEX Arraial do Cabo, however high ER areas located outside the limits of both MPAs, indicating attention for protection of T. truncatus truncatus in the these areas, particularly in terms of anthropogenic pressures.
To increase our understanding of T. truncatus truncatus in the area and enhance its protection, we recommend the investigation of dolphins response to boat traffic, anthropogenic noise, and also biopsy sampling to identify individual gender and potential contaminant levels.
ACKNOWLEGMENTS
We thank Luciana D. Figueiredo, Liliane Lodi, Carine Gonçalves and Marco Aurelio B. Crespo for valuable field support. Also, we thank the contribution of two anonymous reviewers who significantly contributed to improve the paper. The authors gratefully acknowledge research grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico — CNPq (Grant # 479348/2010-3) and the Fundação Boticário de Proteção à Natureza (Grant #0997_20132). Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) granted scholarships to R.H. Tardin, (Process # E-26/100.866/2011), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for scholarship to I.S. Maciel and Conselho Nacional de Pesquisa e Desenvolvimento Grant to M.A.S. Alves (Process # 305798/2014-6 and #306579/2018-9). The study was conducted under permit Nº 26851-1.
REFERENCES
- BALLANCE LT. 1990. Residence patterns, group organization and surface association of bottlenose dolphins in Kino Bay, Gulf of California, Mexico. In: Leatherwood S & Reeves RR (Eds), The bottlenose dolphin,1st ed., Academic Press, San Diego, 653p.
- BARRETO AS. 2011. Golfinho nariz de garrafa. In: Rocha-Câmara CC et al. (Eds), Plano Nacional de Ação de Pequenos Cetáceos. Instituto Chico Mendes de Conservação da Biodiversidade, 1st ed., Brasília, 129 p.
- BEARZI M. 2005. Aspects of the ecology and behaviour of bottlenose dolphins (Tursiops truncatus) in Santa Monica Bay, California. J Cetacean Res Manage 7(1): 75-83.
- BEL’KOVICH VM, IVANOVA EE, EFREMENKOVA OVY, OZAROVITSKY LBK & HARITONOV SPK. 1991. Searching and hunting behavior in the bottlenose dolphin (Tursiops truncatus) in the Black Sea. In: Pryor K & Norris KS (Eds), Dolphin Societies: Discoveries and Puzzles. 1 st ed., University of California Press, California, p. 38-67.
- BENDER CE, HERZING DL & BJORKLUND DF. 2008. Evidence of teaching in Atlantic spotted dolphins by mother dolphins foraging in the presence of their calves. Anim Cogn 12(1): 43-53.
- BRÄGER Z, GONZALVO J, AGAZZI S & BEARZI G. 2016. Identification of bottlenose dolphin (Tursiops truncatus) prey using fish scale analysis. Aquat Mamm 42(1): 63.
- CARBONEL C. 1998. Modelling of upwelling in the coastal area of Cabo Frio (Rio de Janeiro – Brazil). Rev Bras Oceanogr 46(1): 1-17.
- CARVALHO MS & ROSSI-SANTOS MR. 2011. Sightings of the bottlenose dolphin (Tursiops truncatus) in Trindade island, South Atlantic Ocean. Mar Biodivers Rec 4(e15): 1-3.
- CHAPE S, HARRISON J, SPALDING M & LYSENKO I. 2005. Measuring the extent and effectiveness of protected areas as an indicator for meeting global biodiversity targets. Philos Trans R Soc Lond B Biol Sci 360(1454): 443-455.
- CHRISTIANSEN F, LUSSEAU D, STENSLAND E & BERGGREN P. 2010. Effects of tourist boats on the behaviour of Indo-Pacific bottlenose dolphins off the south coast of Zanzibar. Endanger Species Res 11(1): 91-99.
- COELHO-SOUZA SA, LOPEZ MS, GUIMARÃES JRD, COUTINHO R & CANDELLA RN. 2012. Biophysical interactions in the Cabo Frio upwelling system, southeastern Brazil. Braz J Oceanogr 60(3): 353-365.
- CONNOR RC, READ AJ & WRANGHAM R. 2000. Male reproductive strategies and social bonds. In: Mann J et al. (Eds), Cetacean societies: Field studies of dolphins and whales. The University of Chicago Press, 1st ed., Chicago, 448 p.
- COSTA APB, ROSEL PE, DAURA-JORGE FG & SIMÕES-LOPES PC. 2016. Offshore and coastal common bottlenose dolphins of the western South Atlantic face-to-face: What the skull and the spine can tell us. Mar Mamm Sci 32(4): 1433-1457.
- COSTA D. 2008. Energetics. In: Perrin WF et al. (Eds), Encyclopedia of Marine Mammals. Academic Press, 2nd ed., Chicago, 1352 p.
- DAURA-JORGE FG, CANTOR M, INGRAM SN, LUSSEAU D & SIMÕES-LOPES PC. 2013a. The structure of a bottlenose dolphin society is coupled to a unique foraging cooperation with artisanal fishermen. Biol Lett 8(1): 702-705.
- DAURA-JORGE FG, INGRAM SN & SIMÕES-LOPES PC. 2013b. Seasonal abundance and adult survival of bottlenose dolphins (Tursiops truncatus) in a community that cooperatively forages with fishermen in southern Brazil. Mar Mamm Sci 29(2): 293-311.
- DAURA-JORGE FG & SIMÕES-LOPES PC. 2016. Mark-recapture vs. line-transect abundance estimates of a coastal dolphin population: a case study of Tursiops truncatus from Laguna, southern Brazil. LAJAM 11(1-2): 133-143.
- DEFRAN RH & WELLER DW. 1999. Occurrence, distribution, site fidelity, and school size of bottlenose dolphins (Tursiops truncatus) of San Diego, California. Mar Mamm. Sci 15(2): 366-380.
- DE LEO FC & PIRES-VANIN AMS. 2006. Benthic megafauna communities under influence of the SACW (South Atlantic Central Water) intrusion onto the Brazilian Southeastern shelf: a comparison between an upwelling and a non-upwelling ecosystem. J Mar Syst 60(3-4): 268-284.
- DI BENEDITTO APM, RAMOS RMA, SICILIANO S, DOS-SANTOS RA, BASTOS G & FAGUNDES-NETO E. 2001. Stomach contents of delphinids from Rio de Janeiro, southeastern Brazil. Aquat Mamm 2(2): 24-28.
- DI GIACOMO AB & OTT PH. 2016. Long-term site fidelity and residency patterns of bottlenose dolphins (Tursiops truncatus) in the Tramandaí Estuary, southern Brazil. LAJAM 11(1-2): 155-161.
- DI TULLIO JC, FRUET PF & SECCHI ER. 2015. Identifying critical areas to reduce bycatch of coastal common bottlenose dolphins Tursiops truncatus in artisanal fisheries of the subtropical western South Atlantic. Endanger Species Res 29(1): 35-50.
- DI TULLIO JC, GANDRA TBR, ZERBINI A & SECCHI ER. 2016. Diversity and distribution patterns of cetaceans in the subtropical southwestern Atlantic outer continental shelf and slope. PLoS ONE: 11(5): e0155841.
- DINIS A, ALVES F, NICOLAU C, RIBEIRO C, KAUFMANN M, CAÑADAS A & FREITAS L. 2016. Bottlenose dolphin Tursiops truncatus group dynamics, site fidelity, residency and movement patterns in the Madeira Archipelago (North-East Atlantic). Afr J Mar Sci 38(2): 1-10.
- EDWARDS HH & SCHNELL GD. 2001. Status and ecology of Sotalia fluviatilis in the Cayos Miskito Reserve, Nicarágua. Mar Mamm Sci 17(3): 445-472.
- ESPÉCIE MA, TARDIN RHO & SIMÃO SM. 2010. Degrees of residence of Guiana dolphins (Sotalia guianensis) in Ilha Grande Bay, south-eastern Brazil: a preliminary assessment. J Mar Biolog Assoc UK 90(8): 1633-1639.
- FERREIRA CEL, FLOETER SR, GASPARINI JL, FERREIRA BP & JOYEUX JC. 2004. Trophic structure patterns of Brazilian reef fishes: a latitudinal comparison. J Biogeogr 31(7): 1093-1116.
- FLORES PAC & FONTOURA NF. 2006. Ecology of marine tucuxi, Sotalia guianensis, and bottlenose dolphin, Tursiops truncatus, in Baía Norte, Santa Catarina state, Southern Brazil. Lat Americ J Aquat Mamm 5(2): 105-115.
- FRUET PF, DALLA-ROSA L, GENOVES RC, VALIATI VH, DE-FREITAS TR & MÖLLER LM. 2017. Biopsy darting of common bottlenose dolphins (Tursiops truncatus) in southern Brazil: evaluating effectiveness, short-term responses and wound healing. Lat Americ J Aquat Mamm 11(1-2): 121-132.
- FRUET PF, DAURA-JORGE FG, MÖLLER LM, GENOVES RC & SECCHI ER. 2015. Abundance and demography of bottlenose dolphins inhabiting a subtropical estuary in the Southwestern Atlantic Ocean. J Mammal 96(2): 332-343.
- FRUET PF ET AL. 2014. Remarkably low genetic diversity and strong population structure in common bottlenose dolphins (Tursiops truncatus) from coastal waters of the Southwestern Atlantic Ocean. Conserv Genet 15(4): 879-895.
- FRUET PF, KINAS PG, SILVA KG, DI TULLIO JC, MONTEIRO DS, DALLA-ROSA L, ESTIMA SC & SECCHI ER. 2012. Temporal trends in mortality and effects of by-catch on common bottlenose dolphins, Tursiops truncatus, in southern Brazil. J Mar Biolog Assoc UK 92(8): 1865-1876.
- FRUET PF, SECCHI ER, DI TULLIO JC & KINAS PG. 2011. Abundance estimation of bottlenose dolphins, Tursiops truncatus (Cetacea, Delphinidae), inhabiting the Patos Lagoon estuary, southern Brazil: implications for conservation. RBZool 28(1): 23-30.
- GANDRA A. 2009. Comitê Gestor garantirá funcionamento de reserva marinha no estado do Rio de Janeiro. Disponível em https://arquivo.correiodobrasil.com.br/comite-gestor-garantira-funcionamento-de-reserva-marinha-no-rio/ Acessado em 25 de abril de 2019.
» https://arquivo.correiodobrasil.com.br/comite-gestor-garantira-funcionamento-de-reserva-marinha-no-rio/ - GIBSON QA & MANN J. 2008. The size, composition and function of wild bottlenose dolphin (Tursiops sp.) mother-calf groups in Shark Bay, Australia. Anim Behav 76(2): 389-405.
- GNONE G ET AL. 2011. Distribution, abundance, and movements of the bottlenose dolphin (Tursiops truncatus) in the Pelagos Sanctuary MPA (north-west Mediterranean Sea). Aquat Conserv 21(4): 372-388.
- HOFFMANN LS, TOLEDO FL & FREITAS TRO. 2008. Contribution to a behavioral data bank: association patterns and habitat use of a small group of coastal bottlenose dolphins Tursiops truncatus (Montagu, 1821) (Cetacea, Delphinidae) in southern Brazil. In: Braga ES (Ed), Oceanografia e mudanças globais, Instituto Oceanográfico da Universidade de São Paulo, 1st ed., São Paulo, p. 88-102.
- HOYT E. 2011. Marine protected areas for whales, dolphins and porpoises: A world handbook for cetacean habitat conservation and planning. Earthscan, London and New York, 1st ed., 448 p.
- ICMBIO - INSTITUTO CHICO MENDES DE CONSERVAÇÃO DA BIODIVERSIDADE. 2018. Livro Vermelho da Fauna Brasileira Ameaçada de Extinção: Brasília: ICMBio/MMA. Volume II – Mamíferos. 1st ed., ICMBio/MMA, Brasília, 625 p.
- KARCZMARSKI L, COCKCROFT VC & MCLACHLAN A. 2000. Habitat use and preferences of Indo-Pacific humpback dolphins Sousa chinensis in Algoa Bay, South Africa. Mar Mamm Sci 16(1): 65-79.
- KEIPER CA, AINLEY DG, ALLEN SG & HARVEY JT. 2005. Marine mammal occurrence and ocean climate off central California; 1986 to 1994 and 1997 to 1999. Mar Ecol Prog Ser 289(1): 285-306.
- LEHNER PN. 1996. Handbook of ethological methods, 2nd ed., Cambridge University Press, Cambridge, 403 p.
- LODI L 2016. Update on the current occurrence of Tursiops truncatus (Montagu, 1821) in Rio de Janeiro State. Lat Americ J Aquat Mamm 11(1-2): 220-226.
- LODI L, CANTOR M, DAURA-JORGE FG & MONTEIRO-NETO CA. 2014. A missing piece from a bigger puzzle: declining occurrence of a transient group of bottlenose dolphins off Southeastern Brazil. Mar Ecol 35(4): 516-527.
- LODI L, MAYERHOFER LC & MONTEIRO-NETO CA. 2009. Evaluation of the video-identification technique applied to bottlenose dolphins (Tursiops truncatus) in Cagarras Archipelago, Rio de Janeiro, Brazil. J Mar Biolog Assoc UK 89(5): 1077-1081.
- LODI L & TARDIN RH. 2018. Site fidelity and residency of common bottlenose dolphins (Cetartiodactyla: Delphinidae) in a coastal insular habitat off southeastern Brazil. PanamJAS 13(1): 53-63.
- LUSSEAU D. 2003. Effects of tour boats on the behavior of bottlenose dolphins: using Markov chains to model anthropogenic impacts. Conserv Biol 17(6): 1785-1793.
- MANN J. 1999. Behavioral sampling methods for cetaceans: a review and critique. Mar Mamm Sci 15(1): 102-122.
- MAY-COLLADO LJ, QUIÑONES-LEBRÓN SG, BARRAGÁN-BARRERA DC, PALACIOS JD & GAMBOA-POVEDA M. 2014. The dolphin watching industry of Bocas del Toro continues impacting the resident bottlenose dolphin population. Int Whal Comm SC/65b/WW06, 6 p.
- MILMANN LC, DANILEWICZ D, BAUMGARTEN J & OTT PH. 2016. Temporal-spatial distribution of an island-based offshore population of common bottlenose dolphins (Tursiops truncatus) in the equatorial Atlantic. Mar Mamm Sci 33(2): 496-519.
- MOURA JF, TAVARES DC, SECCO HK & SICILIANO S. 2016. Bottlenose dolphins (Tursiops truncatus, Montagu 1821) in central-northern coast of Rio de Janeiro State, Brazil: stranding patterns and insights into feeding habits. Lat Americ J Aquat Mamm 11(1-2): 191-198.
- OLIVEIRA LR, OTT PH, MORENO IB, TAVARES M, SICILIANO S & BONATTO SL. 2017. Effective population size of an offshore population of bottlenose dolphins, Tursiops truncatus, from the São Pedro and São Paulo Archipelago, Brazil. Lat Americ J Americ Mamm 11(1-2): 162-169.
- OUDEJANS MG, VISSER F, ENGLUND A, ROGAN E & INGRAM SN. 2015. Evidence for distinct Coastal and Offshore communities of Bottlenose Dolphins in the North East Atlantic. PLoS ONE 10(4): e0122668.
- PAIVA MP & MOTTA PCS. 2000. Cardumes da sardinha-verdadeira, Sardinella brasiliensis (Steindachner), em águas costeiras do estado do Rio de Janeiro, Brasil. RBZool 17(2): 339-346.
- PIROTTA E, THOMPSON PM, CHENEY B, DONOVAN CR & LUSSEAU D. 2015. Estimating spatial, temporal and individual variability in dolphin cumulative exposure to boat traffic using spatially explicit capture–recapture methods. Anim Conserv 18(1): 20-31.
- REDFERN JV ET AL. 2006. Techniques for cetacean–habitat modeling. Mar Ecol Prog Ser 310(1): 271-295.
- REIS AT ET AL. 2013. Origin of step-like and lobate seafloor features along the continental shelf off Rio de Janeiro State, Santos basin-Brazil. Geomorph 203(1): 25-45.
- ROBERTS JJ, BEST BD, DUNN DC, TREML EA & HAPLIN PN. 2010. Marine Geospatial Ecology Tools: An integrated framework for ecological geoprocessing with ArcGIS, Python, R, MATLAB, and C++. Environ Model Softw 25(10): 1197-1207.
- ROBINSON KP ET AL. 2012. Discrete or not so discrete: Long distance movements by coastal bottlenose dolphins in UK and Irish waters. J Cet Res Manag 12(3): 365-371.
- ROSSI-SANTOS M, WEDEKIN LL & SOUSA-LIMA RS. 2006. Distribution and habitat use of small cetaceans off Abrolhos Bank, eastern Brazil. Lat Americ J Aquat Mamm 5(1): 23-28.
- SHANE SH. 1990. Behavior and ecology of the bottlenose dolphin at Sanibel Island, Florida. In: Leatherwood S & Reeves RR (Eds), The bottlenose dolphin. Academic Press, San Diego, 1st ed., 653 p.
- SILVA MA, PRIETO R, MAGALHÃES S, CABECINHAS R, CRUZ A, GONÇALVES JM & SANTOS RS. 2003. Occurrence and distribution of cetaceans in the waters around the Azores (Portugal), Summer and Autumn 1999-2000. Aquat Mamm 29(1): 77-83.
- SILVA MA, PRIETO R, MAGALHÃES S, SEABRA MI, MACHETE M & HAMMOND PS. 2012. Incorporating information on bottlenose dolphin distribution into marine protected area design. Aquat Conserv 22(1): 122-133.
- SILVA MA, PRIETO R, MAGALHÃES S, SEABRA MI, SANTOS RS & HAMMOND PS. 2008. Ranging patterns of bottlenose dolphins living in oceanic waters: implications for population structure. Mar Biol 156(2): 179-192.
- SIMÕES-LOPES PC. 1988. Ocorrência de uma população de Sotalia fluviatilis (Gervais, 1853) (Cetácea, Delphinidae) no limite sul da sua distribuição, Santa Catarina. Biotemas 1(1): 57-62.
- SIMÕES-LOPES PC & FABIÁN ME. 1999. Residence patterns and site fidelity in bottlenose dolphins, Tursiops truncatus (Montagu) (Cetacea, Delphinidae) of Southern Brazil. RBZool 16(1): 1017-1024.
- SLOOTEN E 1994. Behaviour of Hector’s dolphin – classifying behaviour by sequence analysis. J Mammal 75(4): 956-964.
- TARDIN RH, CHUN Y, SIMÃO SM & ALVES MAS. 2019. Habitat use models of spatially auto-correlated data: a case study of the common bottlenose dolphin, Tursiops truncatus truncatus, in southeastern Brazil. Mar Biol Res 15(4-6): 305-316.
- TARDIN RH, ESPÉCIE MA, NERY MF, D’AZEREDO FT & SIMÃO SM. 2011. Coordinated feeding tactics of the Guiana dolphin, Sotalia guianensis (Cetacea: Delphinidae), in Ilha Grande Bay, Rio de Janeiro, Brazil. Zool 28(3): 291-296.
- TARDIN RH, SIMÃO SM & ALVES MAS. 2013. Distribution of Tursiops truncates in Southeastern Brazil: a modeling approach for summer sampling. Nat Conserv 11(1): 1-10.
- TORRES LG, READ AJ & HALPIN PN. 2008. Fine-scale habitat modeling of a top marine predator: do prey data improve predictive capacity. Ecol Appl 18(7): 1702-1717.
- WEDEKIN LL, DAURA-JORGE FG, ROSSI-SANTOS MR & SIMÕES-LOPES PC. 2008. Notas sobre a distribuição, tamanho de grupo e comportamento do golfinho Tursiops truncatus (Cetacea: Delphinidae) na Ilha de Santa Catarina, sul do Brasil. Biota 8(4): 225-229.
- WELLS RS. 1991. The role of long-term study in understanding the social structure of a bottlenose community. In: Pryor K & Norris KS (Eds), Dolphin societies: Discoveries and puzzles. University of California Press, 1st ed., Los Angeles, 405p .
- WELLS RS & SCOTT M. 2008. Common bottlenose dolphin. In: Perrin WF et al. (Eds), Encyclopedia of Marine Mammals. Academic Press, Chicago, 1352 p.
- WICKERT JC, VON-EYE SM, OLIVEIRA LR & MORENO IB. 2016. Revalidation of Tursiops gephyreus Lahille 1908 (Cetartiodactyla: Delphinidae) from the southwestern Atlantic Ocean. J Mammal 97(6): 1728-1737.
- WILSON B, REID RJ, GRELLIER K, THOMPSON PM & HAMMOND PS. 2004. Considering the temporal when managing the spatial: a population range expansion impacts protected areas-based management for bottlenose dolphins. Anim Conserv 7(4): 331-338.
Publication Dates
-
Publication in this collection
12 Aug 2020 -
Date of issue
2020
History
-
Received
15 Aug 2018 -
Accepted
1 Feb 2019