Abstracts
Some organisms disperse energy, associated with the transportation of resource, which is not necessarily food. Stingless bees of Central Amazonia (Melipona flavolineata and M. lateralis) collect clay in banks along streams for nest building. The moisture of the clay varies along the bank, and bees collect clay from specific location, indicating that there is some sort of preference regarding their selection. This study aims at identifying: if larger bees carry more clay; if there is a preference for moisture of substrates; and if bees are less efficient accumulating and transporting clay when it is wet. In order to do so, I measured the size of the bees and of the pellets of clay found in the corbicula. I set up a field experiment to test substrate preferences. The amount of clay transported, increased exponentially in accordance to the size of the bee, and the preferred substrate was the driest clay. The amount and the efficiency of removal of clay were not affected by the moisture of the substrate. Despite the wet clay being denser, it does not reduce the efficiency of exploitation of the resource, but suggests that bees spend more energy to carry the same quantity of wet clay, which may be the underlying mechanism explaining their preference for removing drier clay.
corbicula; Melipona ; substrate preference; removal efficiency; nest building
Alguns organismos têm custos energéticos associados com o transporte de recursos, que não necessariamente são alimentos. Algumas abelhas sem ferrão da Amazônia Central (Melipona flavolineata e M. lateralis) coletam argila na margem de córregos para a construção do ninho. A umidade da argila varia ao longo do barranco e as abelhas coletam argila de pontos específicos, o que sugere que há preferência. Aqui testo se abelhas maiores transportam mais argila; se existe preferência pela umidade do substrato; e se abelhas são menos eficientes na coleta e transporte de argila com elevada umidade. Para isso, eu medi o tamanho das abelhas e das agregações de argila na corbícula. Eu realizei um experimento de campo para testar a preferência de umidade do substrato. A quantidade de argila transportada aumentou exponencialmente com o tamanho da abelha e o substrato preferido foi o de menor umidade. A quantidade de argila removida e a eficiência de remoção não foram influenciadas pela umidade do substrato. A argila com maior umidade é mais densa, o que sugere que as abelhas gastam mais energia para transportar a mesma quantidade de argila quando ela está mais úmida. Esse pode ser o mecanismo para explicar a preferência de remoção pela argila com menor umidade.
corbícula; Melipona ; preferência de substrato; eficiência de remoção; construção do ninho
INTRODUCTION
Individuals that maximize energetic liquid gain during foraging should be favored by natural selection (Smith 2006Smith JM. 2006. Optimization theory in evolution. In: SOBBER E (Ed), Conceptual issues in evolutionary biology. London: Bradford Books, p. 99-130.). Thus, an optimum foraging theory was proposed, that postulates that there is probably a balance that minimizes energetic loss associated with the search and exploration of resources, and maximize the energy return provided by this resource (Charnov 1976Charnov EL. 1976. Optimal foraging: The marginal value theorem. Theor Popul Biol 9: 129-136., Stephens and Krebs 1986Stephens DW and Krebs JR. 1986. Foraging theory. Princeton University Press, 247 p.).
For species that carry the resource prior to consuming it, the energetic costs associated with the transportation of the load were also considered. Birds that transport food to their babies in the nest (Kacelnick 1984, Jones 1987Jones G. 1987. Parental foraging ecology and feeding behavior during nestling rearing in the swallow. Ardea 75: 169-174.), bees that carry pollen to the colony (Schmid-Hempel 1986Schmid-Hempel P. 1986. Do honeybees get tired? The effect of load weight on patch departure. Anim Behav 34: 1243-1250.), hunting-wasps that store prey in their nests (Araújo and Gonzaga 2007Araújo MS and Gonzaga MO. 2007. Individual specialization in the hunting wasp Trypoxylon (Trypargilum) albonigrum (Hymenoptera, Vrabronidae). Behav Ecol Sociobiol 61: 1855-1863.) and leaf-cutting ants that carry leaf fragments (Burd 2000Burd M. 2000. Foraging behaviour of Atta cephalotes (leaf-cutting ants): an examination of two predictions for load selection. Anim Behav 60: 781-788.) are all examples of organisms that have energetic costs associated to the transportation of resources. In these cases the weight and the distance of the resource from the nest, the energy necessary for its transportation, the number of trips required for its transportation, and their specific experience and strength determine the load size that an individual is capable of carrying (Charnov 1976Charnov EL. 1976. Optimal foraging: The marginal value theorem. Theor Popul Biol 9: 129-136., Krebs and Davies 1993Krebs JR and Davies NB. 1993. An introduction to behavioral ecology. Oxford: Blackwell Publishing, 432 p.). The optimum load size would be one that maximizes energy gain of individuals and minimizes the costs associated with transportation (Charnov and Orians 1973Charnov EL and Orians GH. 1973. Optimal foraging: some theoretical explorations. Seattle: University of Washington, 160 p.).
Habitat characteristics and quality of resources may influence the optimum load (Robakiewicz and Daigle 2004Robakiewicz P and Daigle W. 2004. Patch quality and foraging time in the crab spider Misumenops asperatus Hentz (Araneae: Thomisidae). Northeastern Nat 11: 23-32.). For instance, in environments where quality resources are plentiful, individuals tend to decrease the load carried and increase the number of trips (Krebs and Davies 1993Krebs JR and Davies NB. 1993. An introduction to behavioral ecology. Oxford: Blackwell Publishing, 432 p.). Additionally, regardless of the characteristics of the resource and habitat, the individual's body size can also be determinant in their ability to exploit resources (Mittelbach 1981Mittelbach GG. 1981. Foraging efficiency and body size: a study of optimal diet and habitat use by Buegills. Ecology 62: 1370-1386., Ramalho et al. 1994Ramalho M, Giannini TC, Malagodi-Braga KS and Imperatriz-Fonseca VL. 1994. Pollen harvest by stingless bee foragers (Hymenoptera, Apidae, Meliponinae). Grana 33: 239-244.). Size variation of worker, within-colony, is well documented for the Apidae group, including the stingless bees Meliponinae (Lacerda et al. 1991Lacerda LM, Zucchi R and Zucoloto FS. 1991. Colony condition and bionomic alterations in Geotrigona inusitata (Apidae, Meliponinae). Acta Biol 20: 109-123.). For most organisms, the largest individuals are generally stronger and more experienced, and could therefore indicate a greater capacity for carrying and manipulating resources (Schoener 1971Schoener TW. 1971. Theory of feeding strategies. Annu Rev Ecol Syst 2: 369-404., Polis 1984Polis G. 1984. Age structure component of niche width and intraspecific resource partitioning: can age groups function as ecological species? Am Nat 123: 541-564.). However, this pattern is not so evident for stingless bees (Ramalho et al. 1994Ramalho M, Giannini TC, Malagodi-Braga KS and Imperatriz-Fonseca VL. 1994. Pollen harvest by stingless bee foragers (Hymenoptera, Apidae, Meliponinae). Grana 33: 239-244., 1998Ramalho M, Imperatriz-Fonseca VL and Giannini TC. 1998. Within-colony size variation of foragers and pollen load capacity in the stingless bee Melipona quadrifasciata anthidioides Lepeletier (Apidae, Hymenoptera). Apidologie 19: 221-228.).
The optimum load size theory was created in order to provide a scientific standard model regarding the transport of alimentary items by animals (Charnov 1976Charnov EL. 1976. Optimal foraging: The marginal value theorem. Theor Popul Biol 9: 129-136., Krebs and Davies 1993Krebs JR and Davies NB. 1993. An introduction to behavioral ecology. Oxford: Blackwell Publishing, 432 p.). However, organisms do not carry food resources only. As an example, some species of stingless bees can search for and remove clay from specific regions associated with streams, to build nests (Cane 1991Cane JH. 1991. Soils of Ground-Nesting bees (Hymenoptera: Apoidea): Texture, moisture, cell depth and climate. J Kansas Entomol Soc 64: 406-413.). Individuals remove small bits of clay with their jaws and place it on the corbicula, which is a part of the tibia on the hind legs (Carvalho-Zilse et al. 2007Carvalho-Zilse G, Porto EL, Silva CGN and Pinto MFC. 2007. Atividades de vôo de operárias de Melipona seminigra (Hymenoptera: Apidae) em um sistema agroflorestal da Amazônia. Bioscience 23: 94-99.). The fact that bees ignore other sources of clay which are found just a few meters away, indicates that there are some characteristic of the substrate which are determinant to the bees upon making their selection (Potts and Willmer 1997Potts SG and Willmer PG. 1997. Abiotic and biotic factors influencing nest-site selection by Halictus rubicundus, a ground nesting halictine bee. Ecol Entomol 22: 319-328.). The amount of water in the clay varies along the edge of the stream, presenting more humid regions near the stream and drier clay at higher elevations. The wet clay has more water between sand granules, and therefore is denser. Drier clay, on the other hand is more difficult to aggregate in the corbicula due to the decreased consistency of lower humidity clay. Therefore, the amount of water on the substrate may influence the bees selection and removal efficiency of clay.
In this paper I set out to evaluate the following hypotheses: I) larger individuals carry larger amounts of clay, II) bees select clay with intermediate moisture conditions, III) bees have lower exploitation efficiency of wet clay substrates. If the hypotheses are true, I expect that: a) the anteroposterior distance of bees has a positive relationship with the size of the clay pellets in the corbicula. With regards to hypothesis II, I expect that: b) the clay from the specific location where the bees are making their removal (natural humidity conditions) will be the preferred clay, as opposed to clays which are drier and wetter. Regarding hypothesis III, I expect that c) bees have a lower rate of collection, when collecting clay from wet substrates, and d) bees will carry less clay when the humidity of the substrate is higher.
MATERIALS AND METHODS
Study Site
I sampled a patch of clay bank beside a stream located on the Esteio Farm, which belongs to the Área de Relevante Interesse Ecológico (Area of Relevant Ecological Interest) of the Projeto Dinâmica Biológica de Fragmentos Florestais (PDBFF) in Central Amazonia, Brazil (02° 25′ S, 59° 45′ O). I observed two species of stingless bees, Melipona flavolineata Friese, 1900 and Melipona lateralis Erichson, 1848, that were removing clay specifically from this site. Both exhibited the same behavior regarding the collection of clay, adding clay granules to the corbicula. Bees initiated the removal of clay by 6:00 AM and remained active for 10 h.
Body Size vs. Load Size
To test whether the size of the aggregation of clay transported increased in accordanace to the size of individual bees, I used a camera SONY HX1 to film the bees removing clay. I filmed the bees from 6:00 AM to 8:30 AM and from 10:00 AM to 12:30 PM. for two days in August, 2011. During filming, I placed graduated scales (5 mm) scattered along the collecting areas of clay, in order to always have a scale near the focal bee that was being filmed removing clay. A snapshot frame of each individual bee just before it left the clay site to head toward the nest was made from the images obtained during filming. In these images, I measured the linear anteroposterior length of the individual bee (from the vertex of the bee's head to the distal end of the abdomen), and the width and length of pellets of clay in one of the corbicula, using the program ImageTool. I calibrated the units measured using the graduated scales in the background of the frames. I calculated the area where the clay was removed, using the formula for the area of the ellipse. I correlated the total length of the bees and the square root of the pellet area on the corbicula. I used the square root of the area of the pellet to remove the effect of any exponential relationship caused by the difference in the number of dimensions between the anteroposterior length (linear) and the area of the clay (bidimensional). To test this relationship I performed a Pearson correlation with the data transformed into logarithm.
Preference of Substrate
To test if the bees preferred the clay moisture of the specific site in which they were active I performed a field experiment. First I prevented access to this specific part of the clay bank with a plastic cover. Then I manipulated the humidity of the clay, creating three treatments: control clay (clay removed from the bank through bees activities), dry clay (control clay oven-dried for 10 minutes at 60°C, simulating the higher sites of the clay bank), and wet clay (with humidity similar to the clay near the stream and after the afternoon rains). I compiled six sampling blocks, and in each block I used two Petri dishes (diameter = 9 cm) with each treatment of clay humidity, totalizing six dishes/ block. I randomized the order of dishes in each experiment block. I counted the number of bees collecting clay from each of the dishes every 2 min for 10 min in each sampling block. I waited half an hour prior to the start of the counting of bees in each block in order to allow time for the bees to get used to the new arrangement of substrates. I calculated the average number of individuals in each treatment for each block, and tested if the logarithm of the average abundance differed between the different types of treatments of substrate humidity, using an ANOVA for block design.
Quality of the Substrate vs. Efficiency of Resource Exploitation
To test whether bees have lower removal rates of clay when the substrate is wet, I filmed individual bees removing clay in the bank without modifications (n = 21) and after precipitation (n = 17). In the footages, I selected two snapshot frames of the same individual at different times collecting clay (separated by at least 30 s). I considered the time interval between frames as the collecting time of clay. In each snapshot frame, I measured the linear anteroposterior length of the focal individual at different moments (separated by at least 30 s). I calculated the difference in the measurement of the area of the pellets in these two frames for each focal individual. Then I estimated the clay collection rate by dividing the amount of accumulated clay (difference in area measurement of pellets between frames) by the time it took to collect the clay. I transformed the data into logarithms and tested if the average clay collection rate differed between the control clay and the wet clay using Student t-test
To test whether the final size of clay pellets on the corbicula was smaller when the clay was wet, I used the snapshot frames recorded when the individuals were leaving the clay bank. In these frames, I measured the anteroposterior linear length and the size of the clay aggregation on the corbicula for each individual bee (n = 20 for each treatment). I expected that larger individuals would transport more clay; the size of clay aggregation on the corbicula should be dependent of the individual size of the bee. Thus, I eliminated this effect by getting the residual of the regression between individual size and final size of the pellets of clay, and transformed them into logarithm. Then, I tested if the average values of this residue differed between control and wet clay using Student t-test.
RESULTS
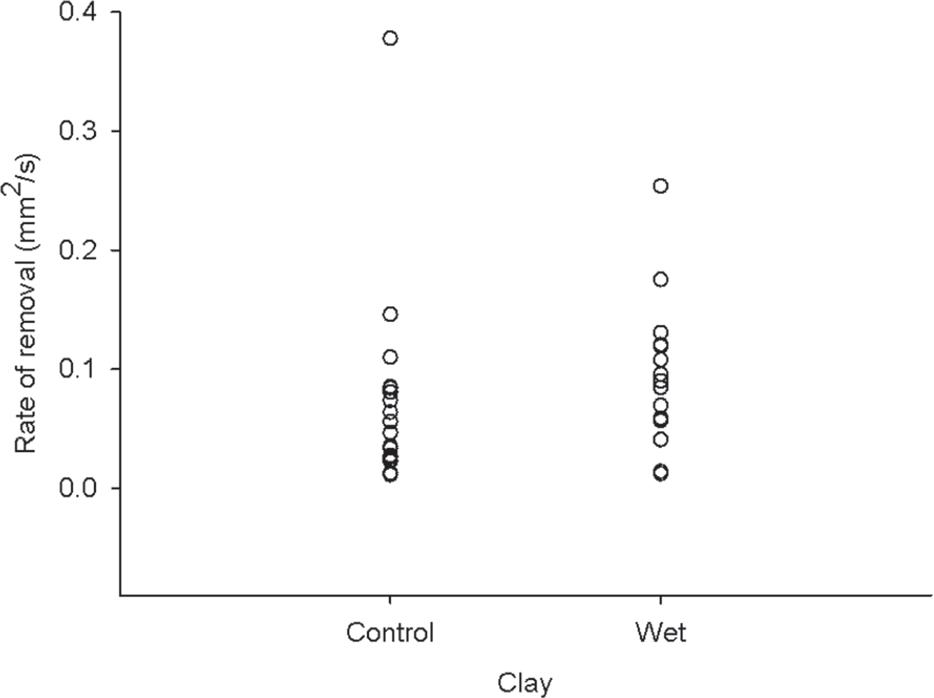
Larger bees carried more clay (F(1,38)=58,43, r =0.61, p<0.001), and this relationship was exponential (Figure 1). In the presence of three treatments associated with the humidity of the substrate, the average abundance of bees was 44% higher for the treatment of dry clay when compared with control clay, and 94% higher than in wet clay (F(2,10)=27,8, p<0.001, Figure 2). With regards to the removal of clay, bees removed on average 0.008 mm2 (SD = 0.07) of clay per second and transported on average 6.5 mm2 (SD = 4.56) of clay. The removal rate (t = 1.0, df = 36, p = 0.31, Figure 3) and the amount of clay removed by individual bees (t <0.01, df = 38, p = 1, Figure 4) were not lower in wet clay when compared to the control group.
Relationship between the size of the bees and the square root of the area of clay removed in a stream clay bank in Esteio Farm, Central Amazonia. White circles = Melipona flavolineata; Black circles = Melipona lateralis.
Average number of bees collecting clay in each treatment of clay humidity in the Esteio Farm, Central Amazonia. The vertical bars represent the standard deviation.
Clay removal rate by Melipona bees according to the relative level of clay moisture in a bank of a stream in Central Amazonia.
Residual of the regression between log-transformed values of individual bee size and area of clay removed in two categories of substrate moisture of a stream bank in Esteio Farm, Central Amazonia.
DISCUSSION
The positive relationship between the anteroposterior length of the bees and area of clay accumulated in the corbicula indicates that the optimum load transported depends on the size of the individual. Contrary to expectations, bees prefer drier substrates to collect clay. This preference cannot be explained by increased operating efficiency of the resource, since the rate of removal and the area of removed clay in the corbicula were similar between the control and moistened clay.
The exponential relationship between size and quantity of transported clay indicates that larger individuals carry disproportionately higher amounts of clay. This contradicts the linear relationship between the amount of load and body mass found for bees carrying pollen (Schmid-Hempel 1986Schmid-Hempel P. 1986. Do honeybees get tired? The effect of load weight on patch departure. Anim Behav 34: 1243-1250.). Therefore, it is possible that large individuals contribute more to the nest building or nest repairing than small ones in each individual event of clay transportation. Another possibility is that bees of different sizes belong to different colonies. In this scenario, colonies with larger bees tend to build or repair nest in a faster way. This finding opens the possibility to test if small bees should make a disproportionately greater number of trips to take the same amount of clay as the larger bees.
The preference for dry substrate can be associated with facilities to collect lighter granules of clay with a little water. As they used clay which was not totally dehydrated, the water present in the treatment of the dry clay could still be sufficient to maintain it aggregated in the bees' corbicula. However, since the bees preferred the clay with lower humidity, collecting clay on the observed stream bank under natural conditions would be sub-optimal when compared to the use of dry clay offered in the experiments. This may indicate that there is a great lack of resources available within the area where individuals are collecting clay. On the other hand the mechanism involved in selecting the amount of water in the substrate within a small spatial and temporal scale may be associated with individual cognitive skills. Bees have a system of decision making in which the accuracy of the choice depends on the time of resource assessment (Chittka et al. 2003Chittka L, Dyer AG, Bock F and Dornhaus A. 2003. Bees trade off foraging speed for accuracy. Nature 424: 388.). In general, this assessment and learning process is quick (Real 1991Real L. 1991. Animal choice behavior and the evolution of cognitive architecture. Science 253: 980-986.). Thus it is possible that the bees assess the quality of the clay available and select the one with less water before starting the collection of the granules.
The efficiency of resource exploitation did not explain the bees preference for substrates with less water, once they carried the same amount of clay and had similar rates of removal of clay in both the control and the wet treatments. This scenario contradicts what is expected by the theory of optimal size of load, which predicts that the efficiency of exploitation is a potential mechanism to indicate the preference for certain resources (Krebs and Davies 1993Krebs JR and Davies NB. 1993. An introduction to behavioral ecology. Oxford: Blackwell Publishing, 432 p.).
However wet clay is denser, since it retains water between the granules, which means that the same amount of wet clay is heavier than the dry clay. In this regard, despite Melipona spp. bees carrying the same amount of clay independent of the amount of water in the substrate, individuals potentially expend more energy transporting wet clay due to the greater weight. This energetic divergence to carry the same amount of clay in different moisture scenarios may be the underlying mechanism to explain the observed preference for drier clay.
In summary, individual bee size act strongly on resource load size, and the preference for drier clay treatment may be associated to energetic constraints. These findings open the possibility for the study of patterns of load size and preferences to other resources exploited and carried by stingless bees, such as pollen, propolis, resin and wax (Barth 2004Barth OM. 2004. Melissopalynology in Brazil: a review of pollen analysis of honeys, propolis and pollen loads of bees. Sci Agri 61: 342-350.).
This is article #635 of the Technical Series of Biological Dynamics of Forest Fragments Project (PDBFF - INPA/STRI) and part of the activities developed in the Curso de Campo Ecologia da Floresta Amazônica (EFA 2011). I am grateful to all organizers – especially José Luis Camargo – and to the friends I made during EFA, particularly Paulo Peixoto, Laura Leal, Manoela Borges and Karla Campião. They contributed on sampling designs, data collection and manuscript review. I am equally grateful to Gislene Zilse and Felipe Verussa for the aid in identifying bees.
REFERENCES
- Araújo MS and Gonzaga MO. 2007. Individual specialization in the hunting wasp Trypoxylon (Trypargilum) albonigrum (Hymenoptera, Vrabronidae). Behav Ecol Sociobiol 61: 1855-1863.
- Barth OM. 2004. Melissopalynology in Brazil: a review of pollen analysis of honeys, propolis and pollen loads of bees. Sci Agri 61: 342-350.
- Burd M. 2000. Foraging behaviour of Atta cephalotes (leaf-cutting ants): an examination of two predictions for load selection. Anim Behav 60: 781-788.
- Cane JH. 1991. Soils of Ground-Nesting bees (Hymenoptera: Apoidea): Texture, moisture, cell depth and climate. J Kansas Entomol Soc 64: 406-413.
- Carvalho-Zilse G, Porto EL, Silva CGN and Pinto MFC. 2007. Atividades de vôo de operárias de Melipona seminigra (Hymenoptera: Apidae) em um sistema agroflorestal da Amazônia. Bioscience 23: 94-99.
- Charnov EL. 1976. Optimal foraging: The marginal value theorem. Theor Popul Biol 9: 129-136.
- Charnov EL and Orians GH. 1973. Optimal foraging: some theoretical explorations. Seattle: University of Washington, 160 p.
- Chittka L, Dyer AG, Bock F and Dornhaus A. 2003. Bees trade off foraging speed for accuracy. Nature 424: 388.
- Jones G. 1987. Parental foraging ecology and feeding behavior during nestling rearing in the swallow. Ardea 75: 169-174.
- Kacelnik A. 1984. Central place foraging in Starlings (Sturnus vulgaris). I. Patch residence time. J Anim Ecol 53: 283-299.
- Krebs JR and Davies NB. 1993. An introduction to behavioral ecology. Oxford: Blackwell Publishing, 432 p.
- Lacerda LM, Zucchi R and Zucoloto FS. 1991. Colony condition and bionomic alterations in Geotrigona inusitata (Apidae, Meliponinae). Acta Biol 20: 109-123.
- Mittelbach GG. 1981. Foraging efficiency and body size: a study of optimal diet and habitat use by Buegills. Ecology 62: 1370-1386.
- Polis G. 1984. Age structure component of niche width and intraspecific resource partitioning: can age groups function as ecological species? Am Nat 123: 541-564.
- Potts SG and Willmer PG. 1997. Abiotic and biotic factors influencing nest-site selection by Halictus rubicundus, a ground nesting halictine bee. Ecol Entomol 22: 319-328.
- Ramalho M, Giannini TC, Malagodi-Braga KS and Imperatriz-Fonseca VL. 1994. Pollen harvest by stingless bee foragers (Hymenoptera, Apidae, Meliponinae). Grana 33: 239-244.
- Ramalho M, Imperatriz-Fonseca VL and Giannini TC. 1998. Within-colony size variation of foragers and pollen load capacity in the stingless bee Melipona quadrifasciata anthidioides Lepeletier (Apidae, Hymenoptera). Apidologie 19: 221-228.
- Real L. 1991. Animal choice behavior and the evolution of cognitive architecture. Science 253: 980-986.
- Robakiewicz P and Daigle W. 2004. Patch quality and foraging time in the crab spider Misumenops asperatus Hentz (Araneae: Thomisidae). Northeastern Nat 11: 23-32.
- Schmid-Hempel P. 1986. Do honeybees get tired? The effect of load weight on patch departure. Anim Behav 34: 1243-1250.
- Schoener TW. 1971. Theory of feeding strategies. Annu Rev Ecol Syst 2: 369-404.
- Smith JM. 2006. Optimization theory in evolution. In: SOBBER E (Ed), Conceptual issues in evolutionary biology. London: Bradford Books, p. 99-130.
- Stephens DW and Krebs JR. 1986. Foraging theory. Princeton University Press, 247 p.
Publication Dates
-
Publication in this collection
Sept 2014
History
-
Received
25 May 2013 -
Accepted
14 Oct 2013