Abstracts
The present work investigated the occupation and the correlation of the shrimp abundance in relation to environmental variables in different habitats (mangroves, salt marshes and rocky outcrops) in an Amazon estuary. The collections were made in August and November 2009, at low syzygy tide on Areuá Beach, situated in the Extractive Reserve of Mãe Grande de Curuçá, Pará, Brazil totaling 20 pools. In each environment, we recorded the physical-chemical factors (pH, salinity, and temperature) and measured the area (m2) and volume (m3) of every pool through bathymetry. The average pH, salinity, temperature, area and volume of tide pools were 8.75 (± 0.8 standard deviation), 35.45 (± 3), 29.49 °C (± 2.32), 27.41 m2 (± 41.18), and 5.19 m3(± 8.01), respectively. We caught a total of 4,871 shrimps, distributed in three families and four species: Farfantepenaeus subtilis (98.36%) (marine) followed byAlpheus pontederiae (0.76%) (estuarine), Macrobrachium surinamicum(0.45%) and Macrobrachium amazonicum(0.43%) predominantly freshwater. The species F. subtilis and A. pontederiae occurred in the three habitats, whereas M. surinamicum occurred in salt marsh and rocky outcrop and M. amazonicum only in marisma. Temperature and pH were the most important environmental descriptors that significantly affected the density and biomass of shrimps.
habitat; density; biomass; Decapoda
O presente trabalho investigou a ocupação e a correlação da abundância de camarões em relação às variáveis ambientais nos diferentes habitats (manguezal, marisma e afloramento rochoso) em um estuário amazônico. As coletas foram realizadas em agosto e novembro de 2009, na maré baixa de sizígia na praia do Areuá, situada na RESEX Mãe Grande de Curuçá, Pará, totalizando 20 poças. Em cada ambiente foram registrados os fatores físicoquímicos (pH, salinidade e temperatura) e mensuradas a área (m2) e o volume (m3) de cada poça através da técnica de batimetria. A média do pH, salinidade, temperatura, área e volume das poças-de-maré foram 8,75 (± 0,8 desvio padrão) 35,45 (± 3), 29,49 °C (± 2,32), 27,41 m2 (± 41,18) e 5,19 m3 (± 8,01), respectivamente. Foi capturado um total de 4.871 indivíduos, distribuídos em três famílias e quatro espécies:Farfantepenaeus subtilis (marinha) a mais frequente (98,36%), seguida de Alpheus pontederiae (0,76%) (estuarina),Macrobrachium surinamicum (0,45%) eMacrobrachium amazonicum (0,43%) predominantemente dulcícolas. As espécies F. subtilise A. pontederiae ocorreram nos três habitats, enquanto queM. surinamicum ocorreu no afloramento rochoso e marisma eM. amazonicum somente no marisma. O pH e a temperatura foram os descritores ambientais mais importantes que afetaram significativamente a densidade e a biomassa dos camarões.
hábitat; densidade; biomassa; Decapoda
INTRODUCTION
Estuaries are places to which many species migrate for reproduction or for developing stages of their life cycle and where many others species are residents. Some marine shrimps, specially Penaeidae family, are examples of temporary estuarine species. During the juvenile phase (post-larvae), they make use of marine currents and occupy these food-rich areas to ensure growth. Marine shrimpsFarfantepenaeus subtilis, Litopenaeus schmittiand X. kroyeri spend about four months in estuarine environments (Martinelli 2005Martinelli JM. 2005. Estrutura populacional dos camarões Penaeidae no estuário do rio Caeté, litoral Norte do Brasil. Tese (Doutorado em Ciências Biológicas) – Instituto de Ciências Biológicas, Universidade Federal do Pará, Belém, p. 174. (Unpublished).) and then return to the sea. Sexual maturity is usually complete in the sixth month, when the individuals are ready for mating and complete their life cycle (Vinatea 2004Vinatea AL. 2004. Fundamentos de Aquicultura. Ed. UFSC - Florianópolis, v. 1, p. 349.). Some species of freshwater shrimps (Macrobrachium) also need to spend part of their life cycle in estuaries, since the larvae develop in brackish water (salinity from 12 to 16) until they reach the juvenile stage, when they initiate the migration to fresh water, where they become adults, sexually mature and ready for mating (SEBRAE/ES 2005). An example of this is Macrobrachium amazonicum (Heller 1862), a species with great plasticity whose populations widely inhabit Amazon estuaries and can also be exclusively a freshwater species.
Intertidal zones are highly variable environments due to the action of tides and nycthemeral and seasonal variations, where inhabitants are subject to temperature and salinity variations, desiccation and hypoxia conditions (Horn et al. 1999Horn MH, Martin KLM and Chotkowski MA. 1999. Intertidal Fishes: Life in two worlds. Academic Press, San Diego, p. 399.). In this environment, tide pools can be formed during low tides, which according to Zander et al. (1999)Zander CD, Nieder J and Martin KL. 1999. Vertical Distribution Patterns. In: Horn MH, Martin KL and Chotkowski MA (Eds), Intertidal Fishes- Life In Two Worlds. Academic Press, San Diego, p. 26-53. are still waters dammed in depressions and cavities without direct communication with the sea. Survival conditions become critical in this environment due to increased temperature and salinity (Nybbaken 1997). Tide pools can determine local differences about the diversity of species (Araújo and Feitosa 2003Araújo ME and Feitosa CV. 2003. Análise de agrupamento da ictiofauna recifal do Brasil com base em dados secundários: Uma avaliação crítica. Trop Ocean 31(2): 171-192.), because they are environments with a complex biodiversity, which are internally subject to intense variations of physical-chemical conditions and biological interactions (Metaxas and Scheibling 1993Metaxas A and Scheibling RE. 1993. Community structure and organization of tide pools. Mar Ecol Prog Ser 98: 187-198.).
The coastline of the State of Pará is surrounded by islands, bays and estuaries with a wide range of mangroves that correspond to 20% of Brazilian mangroves (Herz 1991Herz R. 1991. Manguezais do Brasil. Universidade de São Paulo. São Paulo. p. 54.). Most studies on decapod crustaceans from the coast of Pará refer to subtidal areas (e.g., Corrêa and Martinelli 2009Corrêa AB and Martinelli JM. 2009. Composição da População do camarão-rosa Farfantepenaeus subtilis (Pérez-Farfante, 1936) no estuário do Rio Curuçá, Pará, Brasil. Rev Cient UFPA 7(1): 1-19.,Nevis et al. 2009Nevis AB, Martinelli JM, Carvalho ASS and Nahum VJI. 2009. Abundance and spatial-temporal distribution of the family Portunidae (Crustacea, Decapoda) in the Curuçá estuary on the northern coast of Brazil. J Aquat Sci Tech 13(1): 71-79., Bentes et al. 2011Bentes BS, Martinelli JM, Souza LS, Cavalcante DV, Almeida MC and Isaac VJ. 2011. Spatial distribution of the Amazon river prawn Macrobrachium amazonicum (Heller, 1862) (Decapoda, Caridea, Palaemonidae) in two perennial creeks of an estuary on the northern coast of Brazil (Guajará Bay, Belém, Pará).Braz J Biol 71(4): 925-935., Oliveira et al. 2012Oliveira DB, Silva DC and Martinelli JM. 2012. Density of larval and adult forms of the burrowing crustaceans Lepidophthalmus siriboia (Callianassidae) and Upogebia vasquezi (Upogebiidae) in an Amazon estuary, northern Brazil. J Mar Biol Assoc UK 92(2): 295-303., Silva and Martinelli-Lemos 2012Silva DC and Martinelli-Lemos JM. 2012. Species composition and abundance of the benthic community of Axiidea and Gebiidea (Crustacea: Decapoda) in the Marapanim Bay, Amazon estuary, northern Brazil. Zoologia (Curitiba) 29(2): 144-158., Cavalcante et al. 2012Cavalcante DV, Silva B and Martinelli-Lemos JM. 2012. Biodiversity of decapod crustaceans in the estuarine floodplain around the city of Belém (Pará) in Brazilian Amazonia. Zoologia (Curitiba) 29(3): 203-209.). Most published works related to intertidal areas of the coast of Pará were focused on ichthyofauna (e.g., Giarrizzo and Krumme 2007Giarrizzo T and Krumme U. 2007. Spatial differences and seasonal cyclicity in the intertidal fish fauna from four mangrove creeks in a salinity zone of the Curuçá estuary, north Brazil. Bull Mar Sci 80(3): 739-754., 2009Giarrizzo T and Krumme U. 2009. Temporal patterns in the occurrence of selected tropical fishes in mangrove creeks: implications for the fisheries management in North Brazil. Braz Archiv Biol Technol 52(3): 679-688.) and benthos (e.g., Rosa Filho et al. 2006).
Taking into consideration that tide pools are environments that can be formed in different locations with large variations of physical-chemical conditions, the present work aims to verify the occupation and the correlation of shrimp abundance in relation to environmental variables in different habitats (mangroves, salt marshes and rocky outcrops) found on Areuá Beach, Guarás Island, situated in the Extractive Reserve of Mãe Grande de Curuçá, State of Pará (coast of Brazilian Amazon) in order to identify the importance of the area for the species.
MATERIALS AND METHODS
Area of Study
The study was carried out on the coast of Curuçá situated in the Extractive Reserve of Mãe Grande de Curuçá, Areuá Beach (Geographic coordinates: 35°00′ 30.43″ S, 47°51′07.97″ W), Guarás Island, northeast of the State of Pará, Brazilian equatorial region (Figure 1).
The temperature in this region is relatively constant, averaging 27 °C with a range of 6 °C. It has abundant rainfall exceeding 2,000 mm per year, with the rainiest season from December to June and the less rainy season from July to November (SEPOF-PA 2011). The Curuçá estuary has a perimeter, length and area of 133 km, 21 km and 200 km2, respectively (Giarrizzo and Krumme 2007Giarrizzo T and Krumme U. 2007. Spatial differences and seasonal cyclicity in the intertidal fish fauna from four mangrove creeks in a salinity zone of the Curuçá estuary, north Brazil. Bull Mar Sci 80(3): 739-754.). The estuary suffers a strong marine influence and is dominated by semi-diurnal macrotides, ranging from 2 to 4 m tall. This tidal influence reaches 12 to 16 km in land (Mácola and El-Robrini 2004Mácola G and El-Robrini M. 2004. Ilha dos Guarás (Mariteua) - Município de Curuçá (NE do Pará): Aspectos Físicos, Meteorológicos e Oceanográficos. Relatório Final: Cartografia, Hidrografia e Digitalização - CHD and Grupo de Estudos Marinhos e Costeiros – GEMC, p. 35.).
The vegetation of the area is predominantly composed of mangrove forest and the prevailing species are: Rhizophora manglefollowed by Avicennia germinans, in addition to some spots of salt marshes (Spartina spp.). Geologically, the island has Barreiras Group (Tertiary), Post-Barreiras and recent (Quaternary) sediments (Companhia Docas do Pará 2004).
Sampling
Samples were collected in August and November 2009, at low syzygy tide during the day. The collections were made in tide pools from three distinct habitats: rocky outcrops, salt marshes and mangroves (Figure 2), totaling 20 pools: eight in rocky outcrops, six in salt marshes and six in mangroves. Firstly, we recorded physical-chemical factors (pH, salinity and water temperature) in each habitat. The pH was determined using tapes (ColorpHast) with 0 to 14 marks; salinity was obtained in the laboratory with an optical refractometer (Atago); and the temperature was measured with a mercury thermometer (max 50 °C) at intervals of 30 minutes.
Location of the three sampled habitats: rocky outcrop, salt marsh and mangrove on Guarás Island, Areuá Beach, Municipality of Curuçá, Brazilian Amazon.
The area and volume of tide pools were determined using two three-meter rulers marked every 20 cm. The rulers were placed on the edges of the tide pools for designing the “Cartesian plane” (X and Y), where in the Y axis we measured the depth each 20 cm. Data were entered in the Surfer@ 8.0 program (Golden Software Inc 2002), obtaining the maximum and minimum values of the area, volume and a 3D image of the pool. The shrimps were caught manually with a hand net (150 mm long; 101.6 mm opening; and 3 mm mesh) and packed in 70% alcohol.
We counted and identified the shrimps to the lowest taxonomic level according to Holthuis (1952)Holthuis LB. 1952. A general revision of the Palaemonidae (Crustacea, Decapoda, Natantia) of the Americas. II. The subfamily Palaemoninae. Allan Hancock Foundation Publications 12: 1-396.,Pérez-Farfante (1978)Pérez-Farfante I. 1978. Shrimps and prawns. In: FISHER W (Ed), FAO species identification sheets for fishery purposes, Western Central Atlantic (Fishery Area 31). FAO, Rome, v. 6, p. 40.,Christoffersen (1984)Christoffersen ML. 1984. The western atlantic snapping shrimps related to Alpheus heterochaelis Say (Crustacean, Caridea) with the description of a new species. Papéis Avulsos Zool 35(19): 189-208. andMelo (2003)Melo GAS. 2003. Manual de identificação dos Crustacea Decapoda de água doce do Brasil. 1a ed., São Paulo: Edições Loyola, p. 430. at the laboratory. The weight of each individual was assessed with a precision scale accurate to 0.01 g (Marte). Subsequently, we measured the total length (TL) and the length of the cephalothorax (CL) with a precision digital caliper accurate to 1 mm (Vonder). Ecological descriptors of shrimps registered were: number of individuals, species and families; density (ind/m3); and biomass (g/m3).
The species accumulation curve, also known as collector's curve, was generated through the program PRIMER® version 5.0 (Clarke and Warwick 1994Clarke KR and Warwick RM. 1994. Change in Marine Communities: an approach to statistical analysis and interpretation. Natural Environmental Research Council, Plymouth, UK, p. 144.) in order to verify the sufficiency of sampling, relating the number of species obtained to the increase in the collection effort. The nonparametric estimators of richness used were: Sobs (species observed); Chao1 (number of rare species); Chao2 (data of presence/absence taking into account the distribution of species among samples); Jacknife1 (species that only occurred in one sample); Jackknife2 (number of species occurring in only one sample and the number of species that occurred in exactly two samples); and Bootstrap (data based on the ratio of stands containing each of the species).
To test whether there was significant difference among the medians of the abiotic factors of different habitats (rocky outcrops, salt marshes and mangroves) we performed a non-parametric Kruskal-Wallis test with a significance level of 95% since the assumptions of normality and homoscedasticity were not met, even underwent transformations. An analysis of Spearman linear correlation was performed to verify the correlation of abiotic factors (pH, salinity, water temperature, area, and volume of tide pools) as independent variables, with the density and biomass of shrimps caught, regarded as dependent variables. Both analyses were carried out using the program Statistica® version 7.0 (Statsoft 2004Statsoft Inc. 2004. Statistica: data analysis software system, version 7. Disponível em: <http://www.statsoft.com>. Acesso em: 15 jul. 2012.
http://www.statsoft.com...
).
RESULTS
Abiotic Factors
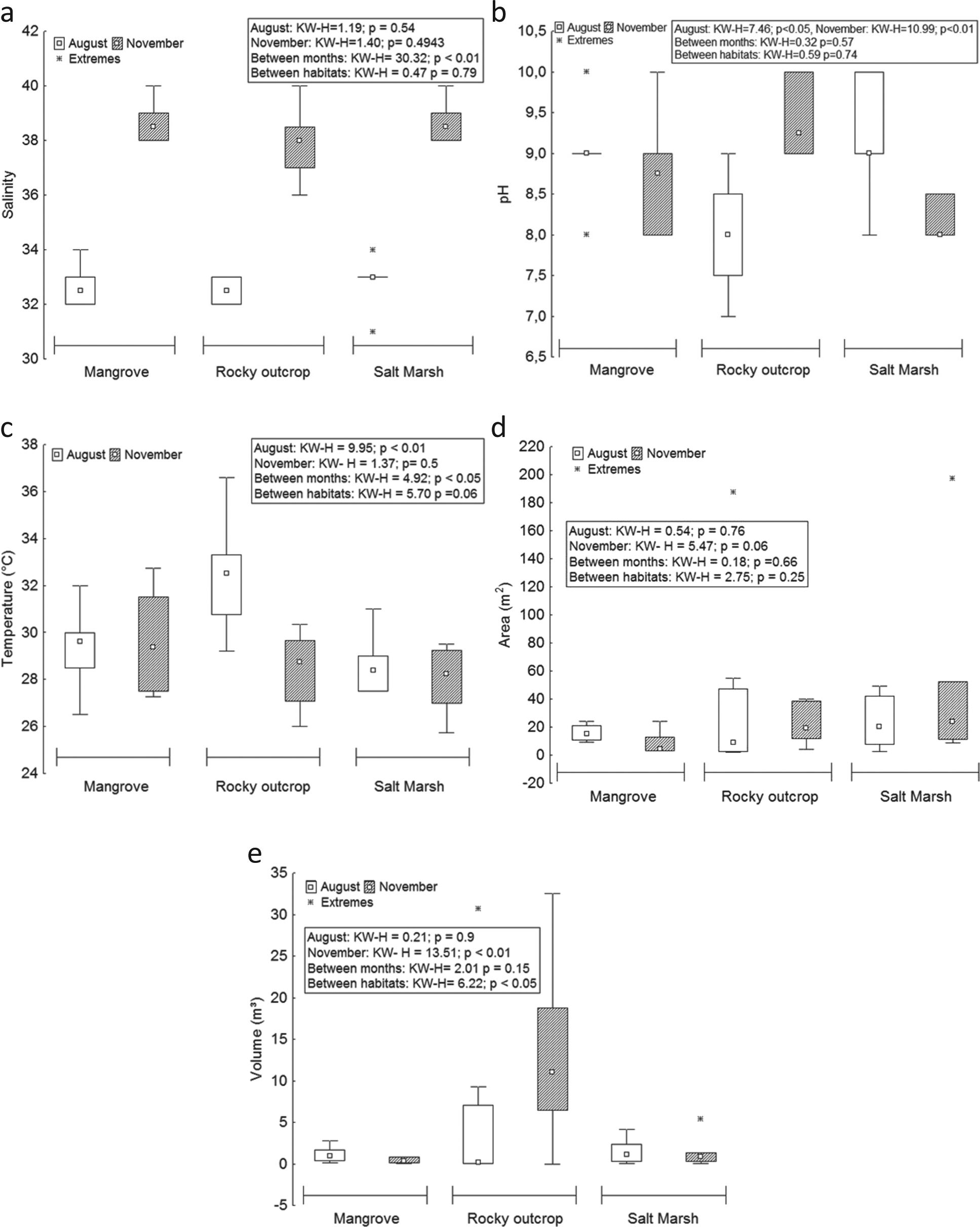
The median of salinity was 35, with a maximum of 40 in the three habitats in November and a minimum of 31 in the salt marsh in August (Table I). The habitat of salt marsh had the lowest salinity, possibly due to the physical and morphometric characteristics of pools in this environment, which are more extensive and wooded, which reduces the incidence of sunlight, diminishing water evaporation and consequently increasing salinity. There were highly significant differences in the median of this factor among months (H = 30.32; p<0.01) (Figure 3a).
Variation of abiotic factors (temperature, pH, salinity, area and volume) of tide pools in three different habitats of Areuá Beach, Guarás Island, Curuçá, Pará, Brazilian Amazon. A = salinity; B= pH; C = temperature; D = area; and E = volume.
Descriptive statistic of abiotic factors of tide pools on Guarás Island, Curuçá, State of Pará (Me = Mean; Md = Median; Min = Minimum; Max = Maximum; ST = Standard Deviation; CI = Confidence Interval; and SE = Standard Error).
Regarding the pH, the median was 9, with a maximum of 10 in August and November in the three habitats (mangrove, salt marsh and rocky outcrop) and a minimum of 7 in the rocky outcrop in August (Table I). There was significant difference of pH in August (H = 7.466; p<0.05) and November (H = 10.99; p<0.01) (Figure 3b).
The median of temperature was 29.23 °C, the maximum value was registered in August in the rocky outcrop (36.6 °C) and the minimum was 25.75 °C in the salt marsh in November (Table I). The values show significant difference of this factor in August (H = 9.95; p<0.01) and among habitats (H = 4.92; p<0.05) (Figure 3c).
The median of the areas measured of tide pools was 12.82 m2, with a maximum of 197.2 m2 in the pool of salt marsh in November and a minimum of 1.91 m2 in the rocky outcrop in August (Table I). There was no significant difference in the median of this factor (Figure 3d). The median of the volume of tide pools was 1.08 m3, of which the largest volume was found in the pool of the rocky outcrop (32.5 m3) and the lowest in the pool of the mangrove (0.02 m3); both values were recorded in November. There was significant difference between the median of the volumes of pools among habitats (H = 6.22; p<0.05) and measured in November (H = 13.51; p<0.01) (Figure 3e).
Carcino-Group
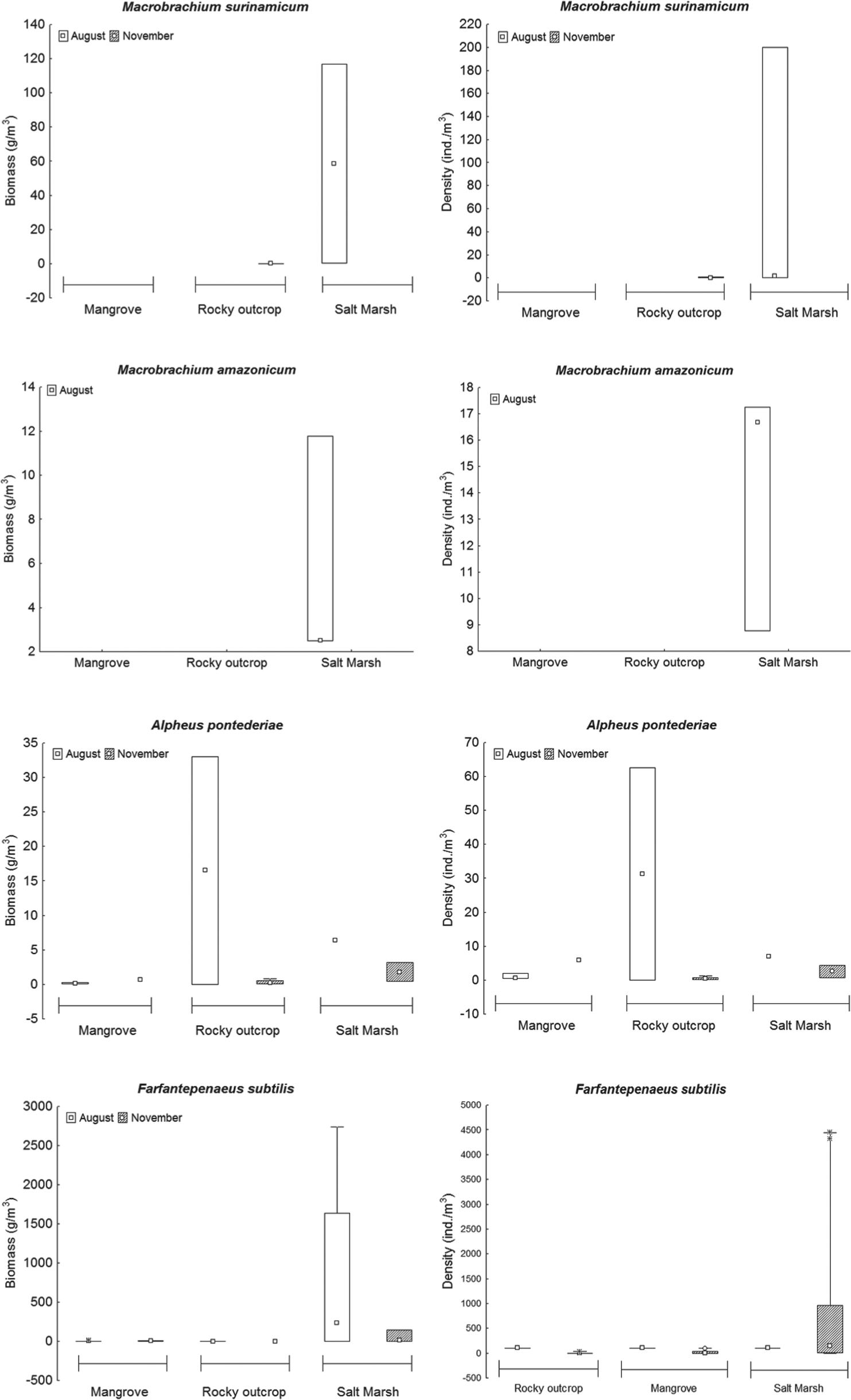
We collected a total of 4,871 shrimps, distributed in three families and four species (Figure 4 and Table II), withFarfantepenaeus subtilis (Pérez-Farfante 1967) representing 98.36% of the total collection, followed byAlpheus pontederiae (Rochebrune 1883), 0.76%; Macrobrachium surinamicum (Holthuis 1948), 0.45%; and Macrobrachium amazonicum(Heller 1862), 0.43%. Palaemonidae was the most specious family and Penaeidae and Alpheidae occurred in all habitats. The species F. subtilis and A. pontederiae occurred in the three habitats, while M. surinamicum occurred in the rocky outcrop and the salt marsh and M. amazonicum only in the salt marsh. All species occurred in the two months with exception of M. amazonicum, which only occurred in August.
Abundance in three habitats on Areuá Beach, Guarás Island, Curuçá, State of Pará, Brazilian Amazon.A = biomass (g/m3); and B = density (ind/m3).
Composition of shrimp species on Areuá Beach, Guarás Island, Curuçá, State of Pará, Brazilian Amazon.
Regarding the species collected, two were predominantly freshwater species (M. amazonicum and M. surinamicum), one estuarine (A. pontederiae) and the other marine (F. subtilis). The lowest frequency was obtained in the rocky outcrop (0.78%) and the largest in the salt marsh (96.16%).
The median biomass of shrimps was 0.52 g/m3. The habitat with the largest and smallest biomass was the salt marsh, with 2,752.86 g/m3 and 0.14 g/m3, respectively. There was significant difference in the median of the biomass of shrimps in August (H = 11.91; p<0.01) and November (H = 8.70; p<0.05) and among habitats (H = 15.34; p<0.01) (Figure 4 a). With regards to density (ind/m3), the habitats had a median of 3 ind/m3, where the largest density (4,533.33 ind/m3) and the lowest density (1.44 ind/m3) both occurred in the salt marsh. Density differed among months (H = 11.04; p<0.01) and among habitats (H = 15.11; p<0.01) (Figure 4b), being higher in the salt marsh in August.
Maximum density (4,443.37 ind/m3) and biomass (2,734.75 g/m3) of F. subtilis occurred in the salt marsh (Figures 5h and g, respectively). M. amazonicum had the lowest density (17.24 ind/m3) in the salt marsh (Figure 5d) and F. subtilis had the lowest biomass (0.14 g/m3) in the salt marsh (Figure 5g).
Biomass (g/m3) and density (ind/m3) of species in relation to the habitats on Areuá Beach, Guarás Island, Curuçá, State of Pará, Brazilian Amazon.
The average CL, TL and total weight (TW) was 7.84 ± 2.57 mm, 33.54 ± 10.84 mm, and 0.34 ± 0.36 g, respectively (Tables III and IV). The species with the largest and smallest CL and TW was F. subtilis (Tables III and IV). The largest and smallest TL corresponded to M. amazonicum andA. pontederiae, respectively (Table IV). Shrimps with the smallest CL (2.66 mm) and TL (10 mm) were collected in the mangroves. Bigger and heavier shrimps (CL: 18.03 mm; TL: 77.1 mm; TW: 3.08 g) occurred in the pools of the salt marshes.
Descriptive statistic of weight (g) of shrimps from Guarás Island, Curuçá, State of Pará, Brazilian Amazon. CI = Confidence Interval.
Descriptive statistic of size (Cephalothorax length - CL and Total length - TL) of shrimps from Guarás Island, Curuçá, State of Pará, Brazilian Amazon. (Me = Mean; Md = Median; Min= Minimum; Max = Maximum; SD = Standard deviation; CI = Confidence Interval; and SE = Standard error).
The estimates of richness showed an interval of 4 for Jacknife1 and 2 and Chao1 and 2 to 4.04 for Bootstrap; indicating that we sampled between 99 to 100% of the species available to the collection method. Most estimators reached asymptote (Figure 6).
Species accumulation curve for estimated values of richness (Sobs, Chao1, Chao2, Jacknife1, Jacknife2, and Bootstrap) in tide pools of Areuá Beach, Guarás Island, Curuçá, State of Pará, Brazilian Amazon.
Relationship between Environmental Variables and the Carcino-Group
The environmental variables did not show significant correlation with density, nor with the biomass of shrimp species of mangrove pools (Table V). Among the abiotic factors studied (temperature, salinity, pH, area and volume of tide pools), the pH showed significant negative correlation with the density and biomass ofF. subtilis and positive correlation with the density ofA. pontederiae in the rocky outcrop (Table VI) and the density ofM. amazonicum in the salt marsh (Table VII).
Spearman's correlation coefficient among abiotic factors (independent variables), density and biomass (dependent variables) of shrimp species from mangrove pools on Areuá Beach, Guarás Island, Curuçá, State of Pará, Brazilian Amazon.
Spearman's correlation coefficient among abiotic factors (independent variables), density and biomass (dependent variables) of shrimp species from rocky outcrop pools on Areuá Beach, Guarás Island, Curuçá, State of Pará, Brazilian Amazon. Significant correlations highlighted in bold type.
Spearman's correlation coefficient among abiotic factors (independent variables), density and biomass (dependent variables) of shrimp species from salt marsh pools on Areuá Beach, Guarás Island, Curuçá, State of Pará, Brazilian Amazon. Significant correlations highlighted in bold type.
The temperature correlated significantly and negatively with the density and biomass of M. surinamicum and positively with the biomass of F. subtilis in the pools of the rocky outcrop (Table VI). Salinity was the only physical-chemical factor that did not correlate significantly with the abundance of shrimp species (Tables VI and VII).
DISCUSSION
High temperatures in tide pools can be explained by high exposure to the sun during the period of low tides (Macieira 2008Macieira RM. 2008. Estrutura de Comunidade e Distribuição espacial dos peixes das Poças de maré em um recife do Atlântico sudoeste, Brasil. Dissertação (Mestrado em Ciências Biológicas) – Centro de Ciências Humanas e Naturais, Universidade Federal do Espírito Santo, p. 67. (Unpublished)., Machado 2013Machado FS. 2013. Ictiofauna de poças de maré ao longo do litoral do Brasil: uma análise latitudinal. Dissertação (Mestrado em Ecologia Aquática e Pesca) – Instituto de Ciências Biológicas, Universidade Federal do Pará, Belém, p. 97. (Unpublished).). In the rocky outcrops, the highest temperatures recorded can be explained by the lesser extent of these pools and less vegetation cover over them, since there are no shrubs protecting them from solar rays. However, the pools of the salt marshes with similar characteristics had the lowest temperature values.
The occurrence of predominantly freshwater species (M. amazonicum and M. surinamicum), estuarine (A. pontederiae) and marine (F. subtilis) in the salt marsh (96.16%) reflects the extreme ecological importance of this habitat, raising questions for future management plans. This result is consistent with other studies that found high numbers of crustaceans in salt marshes, such as works by Kneib and Wagner (1994)Kneib RT and Wagner SL. 1994. Nekton use of vegetated marsh habitat at different stages of tidal inundation. Mar Ecol Prog Ser 106: 227-238., which studied nektons in the Duplin River on the west side of Sapelo Island, Georgia, United States; Rozas and Minello (1998)Rozas LPA and Minello TJ. 1998. Nekton use of saltmarsh, seagrass and nonvegetated habitats in a South Texas (USA) estuary. Bull Mar Sci 63: 481-501. who through a comparative study found that in an estuary in the south of Texas, United States, the total density of crustaceans was greater in vegetated habitats (salt marshes and marine angiosperms) than in non-vegetated habitats (shallow waters, <1 m depth); and Minello and Zimmerman (1992)Minello TJ and Zimmerman RJ. 1992. Utilization of natural and transplanted Texas salt marshes by fish and decapod crustaceans. Mar Ecol Prog Ser 90: 273-285. who investigated the use of transplanted and natural salt marshes habitats by fish and crustaceans on the coast of Texas, United States, suggesting that density is correlated with the density of prey.
Farfantepenaeus subtilis represents a high ecological contribution to the aquatic food web, in addition to being one of the major resources for industrial fishing in the northern Brazilian coast, where its capture is performed in one of the most important shrimp fishing grounds of the world, which extends from Tutóia, in the State of Maranhão, to the border of Brazil and French Guiana (Isaac et al. 1992Isaac VJ, Dias Neto J and Damasceno FG. 1992. Biologia, dinâmica de populações e administração pesqueira do camarão rosa Penaeus subtilis da Região Norte do Brasil. Série estudos de Pesca, Coleção Meio Ambiente Brasília, p. 187.). The northern limit of distribution of this species is represented by Antilles (Central America), with continuous occurrances in Cabo Frio, Rio de Janeiro (South America) as well. The predominance and the high density ofF. subtilis in salt marshes prove that they are important areas for recruitment of juveniles, corroborated by the presence of small sized shrimps.
The predominantly freshwater species M. amazonicum is the main commercially exploited shrimp in estuaries and continental waters of the states of Pará and Amapá, by artisanal fisheries, where there is a significant market (Odinetz-Collart 1987Odinetz-Collart O. 1987. La pêche crevettiêre de Macrobrachium amazonicum (Palaemonidae) dans le Bas-Tocantins, après la fermeture du barrage de Tucuruí (Brésil). Rev Hydrobiol Trop 20(2): 134-144., Bentes et al. 2011Bentes BS, Martinelli JM, Souza LS, Cavalcante DV, Almeida MC and Isaac VJ. 2011. Spatial distribution of the Amazon river prawn Macrobrachium amazonicum (Heller, 1862) (Decapoda, Caridea, Palaemonidae) in two perennial creeks of an estuary on the northern coast of Brazil (Guajará Bay, Belém, Pará).Braz J Biol 71(4): 925-935.). According to Brito and Furtado Júnior (2002), the Municipality of Curuçá in northeastern Pará, represents one of the most important marine and estuarine shrimp landing sites, where the average annual production was of approximately 30 tons between the years of 1997 and 2000.
Temperature and pH were the factors that most influenced the variation in the density and biomass of shrimps in tide pools of Areuá Beach, northeastern Pará, in the Brazilian equatorial region. The large temperature variation can lead these organisms to osmotic stress conditions being decisive in their occupation, in spite of the fact that crustaceans and estuarine organisms are eurythermal (Garcia and Le Reste 1987). However, this result differs from that obtained by Masunari et al. (1998)Masunari S, Oliveira E and Kowalczuk VGL. 1998. Crustacea Decapoda da praia rochosa da Ilha do Farol, Matinhos, Paraná. I. Distribuição temporal de densidade das populações. Rev Bras Zool 15(1): 219-239. who described that the temporal density variation of most Decapoda species of an intertidal area on Farol Island, coast of Paraná (Brazilian subtropical region) did not show any correlation with temperature variation. However, this comparison should be interpreted with caution, since the environment of tide pools suffers greater amplitude of physical-chemical factors variation than intertidal zones.
Salinity was the only physical-chemical factor which was not significantly correlated with the abundance of shrimp species; however, according toTeixeira and Sá (1998)Teixeira RL and Sá HS. 1998. Abundância de macrocrustáceos decápodas nas áreas rasas do complexo lagunar Mundaú/Manguaba. Revta Bras Biol 58(3): 393-404., salinity appears to be the most important factor that acts as a regulator of the distribution and abundance of macrocrustaceans in estuarine complexes, even with highly significant difference in the median of this factor among months.
Most shrimps were captured in the juvenile phase, suggesting that tide pools are used as nurseries, refuge areas and shelters for shrimp species inhabiting the estuarine region, a fact seldom in scientific literature. Tide pools are environments that offer favorable resources for shrimps' development, being crucial for the maintenance and survival of this group.
The authors are especially grateful to the Fundação Amazônia Paraense de Amparo à Pesquisa (FAPESPA 137/2008 Universal Project) for the financial resources for field collections, which was a project coordinated by Tommaso Giarrizzo; to the colleagues who helped in the field collections, especially Rory Sena; to Alexandre Oliveira de Almeida from the State University of Santa Cruz for the identification of Alpheus pontederiae; and to Eduardo Gentile for the English version of this article, supported by the Vice-rectory of Research, Federal University of Pará (PROPESP/UFPA) and the Fundação de Amparo ao Desenvolvimento da Pesquisa (FADESP). All experiments conducted in this study complied with current applicable state and federal laws (IBAMA/MMA, Process No. 16346-3).
REFERENCES
- Araújo ME and Feitosa CV. 2003. Análise de agrupamento da ictiofauna recifal do Brasil com base em dados secundários: Uma avaliação crítica. Trop Ocean 31(2): 171-192.
- Bentes BS, Martinelli JM, Souza LS, Cavalcante DV, Almeida MC and Isaac VJ. 2011. Spatial distribution of the Amazon river prawn Macrobrachium amazonicum (Heller, 1862) (Decapoda, Caridea, Palaemonidae) in two perennial creeks of an estuary on the northern coast of Brazil (Guajará Bay, Belém, Pará).Braz J Biol 71(4): 925-935.
- Brito CSF and Furtado Júnior I. 2002. Boletim estatístico da pesca marítima e estuarina do Brasil/1997-2000. CEPNOR/IBAMA, Belém-Pará, p. 56.
- Cavalcante DV, Silva B and Martinelli-Lemos JM. 2012. Biodiversity of decapod crustaceans in the estuarine floodplain around the city of Belém (Pará) in Brazilian Amazonia. Zoologia (Curitiba) 29(3): 203-209.
- Christoffersen ML. 1984. The western atlantic snapping shrimps related to Alpheus heterochaelis Say (Crustacean, Caridea) with the description of a new species. Papéis Avulsos Zool 35(19): 189-208.
- Clarke KR and Warwick RM. 1994. Change in Marine Communities: an approach to statistical analysis and interpretation. Natural Environmental Research Council, Plymouth, UK, p. 144.
- Companhia Docas do Pará. 2004. Ilha dos Guarás (Mariteua) município de Curuçá (NE do Pará): Aspectos físicos, meteorológicos e oceanográficos. Relatório final. Belém, p. 4. Disponível em: http://www2.cdp.com.br/Skin/Imagens/espardate_estudo_figuras.pdf. Acesso em: 28 mai. 2012.
» http://www2.cdp.com.br/Skin/Imagens/espardate_estudo_figuras.pdf - Corrêa AB and Martinelli JM. 2009. Composição da População do camarão-rosa Farfantepenaeus subtilis (Pérez-Farfante, 1936) no estuário do Rio Curuçá, Pará, Brasil. Rev Cient UFPA 7(1): 1-19.
- Garcia S and Le Reste L. 1987. Ciclos vitales, dinámica, explotación y ordenación de las poblacionaes de camarones penaeídeos costeros. Roma: FAO Documento Técnico de Pesca, n. 203, p. 180.
- Giarrizzo T and Krumme U. 2007. Spatial differences and seasonal cyclicity in the intertidal fish fauna from four mangrove creeks in a salinity zone of the Curuçá estuary, north Brazil. Bull Mar Sci 80(3): 739-754.
- Giarrizzo T and Krumme U. 2009. Temporal patterns in the occurrence of selected tropical fishes in mangrove creeks: implications for the fisheries management in North Brazil. Braz Archiv Biol Technol 52(3): 679-688.
- Golden Software Inc. 2002. Surfer Version 8.0 Contouring, gridding, and surface mapping package for scientists and engineers <http://www.statsoft.com>. Acesso em: 15 jul. 2012.
» http://www.statsoft.com - Herz R. 1991. Manguezais do Brasil. Universidade de São Paulo. São Paulo. p. 54.
- Holthuis LB. 1952. A general revision of the Palaemonidae (Crustacea, Decapoda, Natantia) of the Americas. II. The subfamily Palaemoninae. Allan Hancock Foundation Publications 12: 1-396.
- Horn MH, Martin KLM and Chotkowski MA. 1999. Intertidal Fishes: Life in two worlds. Academic Press, San Diego, p. 399.
- Isaac VJ, Dias Neto J and Damasceno FG. 1992. Biologia, dinâmica de populações e administração pesqueira do camarão rosa Penaeus subtilis da Região Norte do Brasil. Série estudos de Pesca, Coleção Meio Ambiente Brasília, p. 187.
- Kneib RT and Wagner SL. 1994. Nekton use of vegetated marsh habitat at different stages of tidal inundation. Mar Ecol Prog Ser 106: 227-238.
- Machado FS. 2013. Ictiofauna de poças de maré ao longo do litoral do Brasil: uma análise latitudinal. Dissertação (Mestrado em Ecologia Aquática e Pesca) – Instituto de Ciências Biológicas, Universidade Federal do Pará, Belém, p. 97. (Unpublished).
- Macieira RM. 2008. Estrutura de Comunidade e Distribuição espacial dos peixes das Poças de maré em um recife do Atlântico sudoeste, Brasil. Dissertação (Mestrado em Ciências Biológicas) – Centro de Ciências Humanas e Naturais, Universidade Federal do Espírito Santo, p. 67. (Unpublished).
- Mácola G and El-Robrini M. 2004. Ilha dos Guarás (Mariteua) - Município de Curuçá (NE do Pará): Aspectos Físicos, Meteorológicos e Oceanográficos. Relatório Final: Cartografia, Hidrografia e Digitalização - CHD and Grupo de Estudos Marinhos e Costeiros – GEMC, p. 35.
- Martinelli JM. 2005. Estrutura populacional dos camarões Penaeidae no estuário do rio Caeté, litoral Norte do Brasil. Tese (Doutorado em Ciências Biológicas) – Instituto de Ciências Biológicas, Universidade Federal do Pará, Belém, p. 174. (Unpublished).
- Masunari S, Oliveira E and Kowalczuk VGL. 1998. Crustacea Decapoda da praia rochosa da Ilha do Farol, Matinhos, Paraná. I. Distribuição temporal de densidade das populações. Rev Bras Zool 15(1): 219-239.
- Melo GAS. 2003. Manual de identificação dos Crustacea Decapoda de água doce do Brasil. 1a ed., São Paulo: Edições Loyola, p. 430.
- Metaxas A and Scheibling RE. 1993. Community structure and organization of tide pools. Mar Ecol Prog Ser 98: 187-198.
- Minello TJ and Zimmerman RJ. 1992. Utilization of natural and transplanted Texas salt marshes by fish and decapod crustaceans. Mar Ecol Prog Ser 90: 273-285.
- Nevis AB, Martinelli JM, Carvalho ASS and Nahum VJI. 2009. Abundance and spatial-temporal distribution of the family Portunidae (Crustacea, Decapoda) in the Curuçá estuary on the northern coast of Brazil. J Aquat Sci Tech 13(1): 71-79.
- Nybakken JW. 1997. Marine Biology: An Ecological Approach. 4th ed., Benjamin Cummings, Menlo Park, California, p. 481.
- Odinetz-Collart O. 1987. La pêche crevettiêre de Macrobrachium amazonicum (Palaemonidae) dans le Bas-Tocantins, après la fermeture du barrage de Tucuruí (Brésil). Rev Hydrobiol Trop 20(2): 134-144.
- Oliveira DB, Silva DC and Martinelli JM. 2012. Density of larval and adult forms of the burrowing crustaceans Lepidophthalmus siriboia (Callianassidae) and Upogebia vasquezi (Upogebiidae) in an Amazon estuary, northern Brazil. J Mar Biol Assoc UK 92(2): 295-303.
- Pérez-Farfante I. 1978. Shrimps and prawns. In: FISHER W (Ed), FAO species identification sheets for fishery purposes, Western Central Atlantic (Fishery Area 31). FAO, Rome, v. 6, p. 40.
- Rosa Filho JS, Busmana DV, Viana AP, Gregório AM and Oliveira DM. 2006. Macrofauna bentônica de zonas entre-marés não vegetadas do estuário do Rio Caeté, Bragança, Pará. Bol Mus Para Emilio Goeldi Cienc Nat 1(3): 85-96.
- Rozas LPA and Minello TJ. 1998. Nekton use of saltmarsh, seagrass and nonvegetated habitats in a South Texas (USA) estuary. Bull Mar Sci 63: 481-501.
- SEBRAE/ES - Serviço de Apoio às Micro e Pequenas Empresas do Espírito Santo. 2005. “Tecnologia de criação do Camarão da Malásia (Macrobrachium rosenbergii).” Manual de Carcinicultura de água doce, Centro de Tecnologia em Aquicultura e Meio Ambiente - CTA, Vitória, p. 12.
- SEPOF - Secretaria de Estado de Planejamento Orçamento e Finanças. 2011. Estatística Municipal. Belém, p. 44. Disponível em: http://iah.iec.pa.gov.br/iah/fulltext/georeferenciamento/curuca.pdf. Acesso em: 15 jul. 2012.
» http://iah.iec.pa.gov.br/iah/fulltext/georeferenciamento/curuca.pdf - Silva DC and Martinelli-Lemos JM. 2012. Species composition and abundance of the benthic community of Axiidea and Gebiidea (Crustacea: Decapoda) in the Marapanim Bay, Amazon estuary, northern Brazil. Zoologia (Curitiba) 29(2): 144-158.
- Statsoft Inc. 2004. Statistica: data analysis software system, version 7. Disponível em: <http://www.statsoft.com>. Acesso em: 15 jul. 2012.
» http://www.statsoft.com - Teixeira RL and Sá HS. 1998. Abundância de macrocrustáceos decápodas nas áreas rasas do complexo lagunar Mundaú/Manguaba. Revta Bras Biol 58(3): 393-404.
- Vinatea AL. 2004. Fundamentos de Aquicultura. Ed. UFSC - Florianópolis, v. 1, p. 349.
- Zander CD, Nieder J and Martin KL. 1999. Vertical Distribution Patterns. In: Horn MH, Martin KL and Chotkowski MA (Eds), Intertidal Fishes- Life In Two Worlds. Academic Press, San Diego, p. 26-53.
Publication Dates
-
Publication in this collection
Mar 2014
History
-
Received
20 Dec 2012 -
Accepted
12 June 2013