ABSTRACT
Heteranthery, the presence of different types of stamens in a flower, may reduce the conflict between pollinators and plants by ensuring the resource for the pollinator without drastically affecting the availability of viable male gametes for fertilization, according to the division-of-labor hypothesis. We investigated whether the poricidal anthers of Senna pendula, a buzz-pollinated heterantherous species, present morphological and physiological differences among pollen grains from the three sets of stamens. We compared quantity, ornamentation, size and fecundity of pollen from long, medium and short stamens. The short feeding stamens produced larger but fewer pollen grains than the long pollinating stamens, which produced smaller pollen grains but in higher quantity. The total pollen volume of pollinating and feeding stamens per flower, however, was the same. The medium stamen produced less-fertile small pollen grains and seems to play no specific role in bee feeding and pollination. Our results indicate differential allocation of pollen for pollinating and feeding stamens mediated by heteranthery. The differences in volume versus quantity of pollen grains fit the division-of-labor hypothesis well for heterantherous pollen-only flowers with poricidal anthers.
Keywords:
bees; buzz-pollination; division-of-labor; heteranthery; pollen economy; Senna pendula; stamen dimorphism
Introduction
Flowers that offer only pollen to pollinators face the dilemma to attract and reward their pollinators and at the same time to protect and guarantee pollen for sexual reproduction (Klinkhamer & Jong 1993Klinkhamer PG, Jong TJ. 1993. Attractiveness to pollinators: a plant's dilemma. Oikos 66: 180-184.; Westerkamp 1996Westerkamp C. 1996. Pollen in bee-flower relations, some considerations on melittophily. Plant Biology 109: 325-332.). To deal with this dilemma such pollen flowers have evolved several strategies to minimize pollen loss, among them heteranthery (Vogel 1978Vogel S. 1978. Evolution ary shifts from reward to deception in pollen flowers. In: Richard AJ. (ed.) The pollination of flowers by insects. Vol. 6. London/ New York, Linnean Society Symposium Series. p. 89-96.; Buchmann 1983Buchmann SL. 1983. Buzz pollination in angiosperms. In: Jones CE, Little RJ eds. Handbook of experimental pollination biology. New York, Van Nostrand Reinhold.).
Heterantherous flowers have stamens that differ in size, shape and/or position in a flower (Vallejo-Marín et al. 2009Vallejo-Marín M, Manson JS, Thomson JD, Barrett SC. 2009. Division of labour within flowers: heteranthery, a floral strategy to reconcile contrasting pollen fates. Journal of Evolutionary Biology 22: 828-839.). Several heterandrous species have a set of stamens with attractive coloration easily accessible to pollinators, while the other set usually has stamens of larger size, cryptic coloration and is located in a position that corresponds to the position of the stigma (Barrett 2010Barrett SC. 2010. Darwin's legacy: the forms, function and sexual diversity of flowers. Philosophical Transactions of the Royal Society of London B: Biological Sciences 365: 351-368.; Vallejo-Marín et al. 2014Vallejo-Marin M, Walker C, Friston-Reilly P, Solis-Montero L, Igic B. 2014. Recurrent modification of floral morphology in heterantherous Solanum reveals a parallel shift in reproductive strategy. Philosophical Transactions of the Royal Society of London 369: 20130256. doi: 10.1098/rstb.2013.0256
https://doi.org/10.1098/rstb.2013.0256...
). The stamen dimorphism of heteranthery is involved in a “division-of-labor” between stamens (Darwin 1862Darwin C. 1862. Letter to Asa Gray. http://www.darwinproject.ac.uk. 10 Nov. 2017.
http://www.darwinproject.ac.uk...
; Müller 1883Müller F. 1883. Two kinds of stamens with different functions in the same flower. Nature 27: 364-365.), one group contemplate the demand for food of pollinators (feeding stamens), and the other pollen export for pollination (pollinating stamens). Division-of-function has been empirically demonstrated for several heterantherous plant species, such as, Solanum rostratum Solanaceae (Vallejo-Marín et al. 2009Vallejo-Marín M, Manson JS, Thomson JD, Barrett SC. 2009. Division of labour within flowers: heteranthery, a floral strategy to reconcile contrasting pollen fates. Journal of Evolutionary Biology 22: 828-839.); Monochoria korsakowii Pontederiaceae (Tang & Huang 2007Tang LL, Huang SQ. 2007. Evidence for reductions in floral attractants with increased selfing rates in two heterandrous species. New Phytologist 175: 588-95.); Senna alata and S. bicapsularis (Luo et al. 2009Luo Z, Gu LL, Zhang DX. 2009. Intrafloral differentiation of stamens in heterantherous flowers. Journal of Systematics and Evolution 47: 43-56.); and S. reniformis Fabaceae (Mesquita-Neto et al. 2017Mesquita-Neto JN, Costa BKP, Schlindwein C. 2017. Heteranthery as a solution to the demands for pollen as food and for pollination-Legitimate flower visitors reject flowers without feeding anthers. Plant Biology 19: 942-950.). The division-of-labor between stamens may reduce the conflict between pollen-collecting bees and pollen flowers by ensuring the resource for pollinators without drastically affecting the availability of viable male gametes for fertilization (Vallejo-Marín et al. 2009Vallejo-Marín M, Manson JS, Thomson JD, Barrett SC. 2009. Division of labour within flowers: heteranthery, a floral strategy to reconcile contrasting pollen fates. Journal of Evolutionary Biology 22: 828-839.).
The presence of heteranthery in flowers is strongly correlated with poricidal anther dehiscence and absence of floral nectaries (Vallejo-Marín et al. 2010Vallejo-Marín M, Silva EM, Sargent RD, Barrett SC. 2010. Trait correlates and functional significance of heteranthery in flowering plants. New Phytologist 188: 418-425.). Flowers with both, heteranthery and poricidal anther dehiscence occur in at least 11 plant families, among them Fabaceae, Melastomataceae and Solanaceae (Vallejo-Marín et al. 2010Vallejo-Marín M, Silva EM, Sargent RD, Barrett SC. 2010. Trait correlates and functional significance of heteranthery in flowering plants. New Phytologist 188: 418-425.). The flowers with these characteristics are pollinated mainly by bees that extract pollen by vibrating anthers, a phenomenon known as buzz-pollination (Michener 1962Michener CD. 1962. An interesting method of pollen collecting by bees from flowers with tubular anthers. Revista de Biología Tropical 10: 167-175.; Vogel 1978Vogel S. 1978. Evolution ary shifts from reward to deception in pollen flowers. In: Richard AJ. (ed.) The pollination of flowers by insects. Vol. 6. London/ New York, Linnean Society Symposium Series. p. 89-96.; Buchmann 1983Buchmann SL. 1983. Buzz pollination in angiosperms. In: Jones CE, Little RJ eds. Handbook of experimental pollination biology. New York, Van Nostrand Reinhold.). The diversification of several clades of plants with poricidal anther dehiscence coincides with the appearance of buzz pollinating bees (Cardinal et al. 2018Cardinal S, Buchmann SL, Russell AL.2018. The evolution of floral sonication, a pollen foraging behavior used by bees (Anthophila). Evolution 72: 590-600.), which may be the result of a convergent evolution of both heteranthery and poricidal anther dehiscence (Buchmann 1983Buchmann SL. 1983. Buzz pollination in angiosperms. In: Jones CE, Little RJ eds. Handbook of experimental pollination biology. New York, Van Nostrand Reinhold.; Luca & Vallejo-Marín 2013Luca PA, Vallejo-Marín M. 2013. What's the ‘buzz’ about? The ecology and evolutionary significance of buzz-pollination. Current Opinion in Plant Biology 16: 429-435.; Russell et al. 2017Russell AL, Buchmann SL, Papaj DR. 2017. How a generalist bee achieves high efficiency of pollen collection on diverse floral resources. Behavioral Ecology 28: 991-1003.).
Flowers of the genus Senna have the typical floral traits associated with heteranthery: the flowers usually present poricidal anther dehiscence, enantiostyly and absence of floral nectar (Buchmann 1974Buchmann SL. 1974. Buzz pollination of Cassia quiedondilla (Leguminosae) by bees of the genera Centris and Melipona. Bulletin of the Southern California Academy of Sciences 73: 171-173.; Irwin & Barneby 1982Irwin HS, Barneby RC. 1982. The American Cassiinae: a synoptical revision of Leguminosae Tribe Cassieae subtribe Cassiinae in the New World. Memoires of the New York Botanical Garden 35: 1-1918.). Flowers of Senna show a considerable diversity of arrangements in floral symmetry and heterantherous types. Marazzi et al. (2007Marazzi B, Conti E, Endress PK. 2007. Diversity in anthers and stigmas in the buzz-pollinated genus Senna (Leguminosae, Cassiinae). International Journal of Plant Sciences 168: 371-391.) differentiate four types of stamens: (1) three uppermost stamens modified in staminodes (except Senna section Psilorhegma), (2) a set of four short-sized “feeding” middle stamens, (3) a set of three long-sized “pollinating” lowermost stamens, in which (4) the medium stamen may be reduced or even absent. The lowermost stamens show the greatest diversity in position, size and anther morphology in the genus Senna.
The division of anther function has already been reported for heterantherous representatives within this genus (Dulberger 1981Dulberger R. 1981. The floral biology of Cassia didymobotrya and C. auriculata (Caesalpiniaceae). American Journal of Botany 68: 1350-1360.; Marazzi et al. 2007Marazzi B, Conti E, Endress PK. 2007. Diversity in anthers and stigmas in the buzz-pollinated genus Senna (Leguminosae, Cassiinae). International Journal of Plant Sciences 168: 371-391.; Luo et al. 2009Luo Z, Gu LL, Zhang DX. 2009. Intrafloral differentiation of stamens in heterantherous flowers. Journal of Systematics and Evolution 47: 43-56.; Amorim et al. 2017Amorim T, Marazzi B, Soares AA, Forni-Martins ER, Muniz CR, Westerkamp C. 2017. Ricochet pollination in Senna (Fabaceae) − petals deflect pollen jets and promote division of labour among flower organs. Plant Biology 19: 951-962.; Mesquita-Neto et al. 2017Mesquita-Neto JN, Costa BKP, Schlindwein C. 2017. Heteranthery as a solution to the demands for pollen as food and for pollination-Legitimate flower visitors reject flowers without feeding anthers. Plant Biology 19: 942-950.). Heteranthery could accompany structural and qualitative differences in pollen (Lloyd 2000Lloyd DG. 2000. The selection of social actions in families: III. Reproductively disabled individuals and organs. Evolution ary Ecology Research 2: 29-40.), but only a few studies indicated such differences in pollen from pollinating and feeding stamens (Carvalho & Oliveira 2003Carvalho DA, Oliveira PE. 2003. Biologia reprodutiva e polinização de Senna sylvestris (Vell.) HS Irwin & Barneby (Leguminosae, Caesalpinioideae). Revista Brasileira de Botânica 26: 319-328.; Luo et al. 2009Luo Z, Gu LL, Zhang DX. 2009. Intrafloral differentiation of stamens in heterantherous flowers. Journal of Systematics and Evolution 47: 43-56.).
We studied Senna pendula a shrub widely distributed in South America and frequently cultivated as ornamental (Irwin & Barneby 1982Irwin HS, Barneby RC. 1982. The American Cassiinae: a synoptical revision of Leguminosae Tribe Cassieae subtribe Cassiinae in the New World. Memoires of the New York Botanical Garden 35: 1-1918.). The flowers of this species have three sets of stamens: two long, one medium-sized and four short. Based on the hypothesis that pollen grains might differ in morphology and function related to the division-of-labor of stamens, we asked: (1) Are there differences in pollen grain morphology (ornamentation, shape, volume) and pollen quantity (number of grains) from the three sets of stamens? (2) Are there differences in the fecundity of pollen from the three sets of stamens?
Materials and methods
Study area
The field study was conducted from March to July 2014, in a native population of Senna pendula (Humb. & Bonpl.ex Willd.) H.S.Irwin & Barneby in the nature reserve Serra do Curral (19º57'S 43º54'W, altitude 1200-1380 m), located in the metropolitan area of Belo Horizonte, Minas Gerais, Brazil. The climate is tropical, with mild winters and hot and rainy summers. Precipitation is concentrated in the rainy season from October to March with an annual average of 1,490 mm (Assis 2012Assis WL. 2012. Os climas naturais do município de Belo Horizonte - MG. Acta Geográfica 1: 115-135.). The vegetation is characteristic of the transition between Atlantic Forest and Cerrado.
Study species
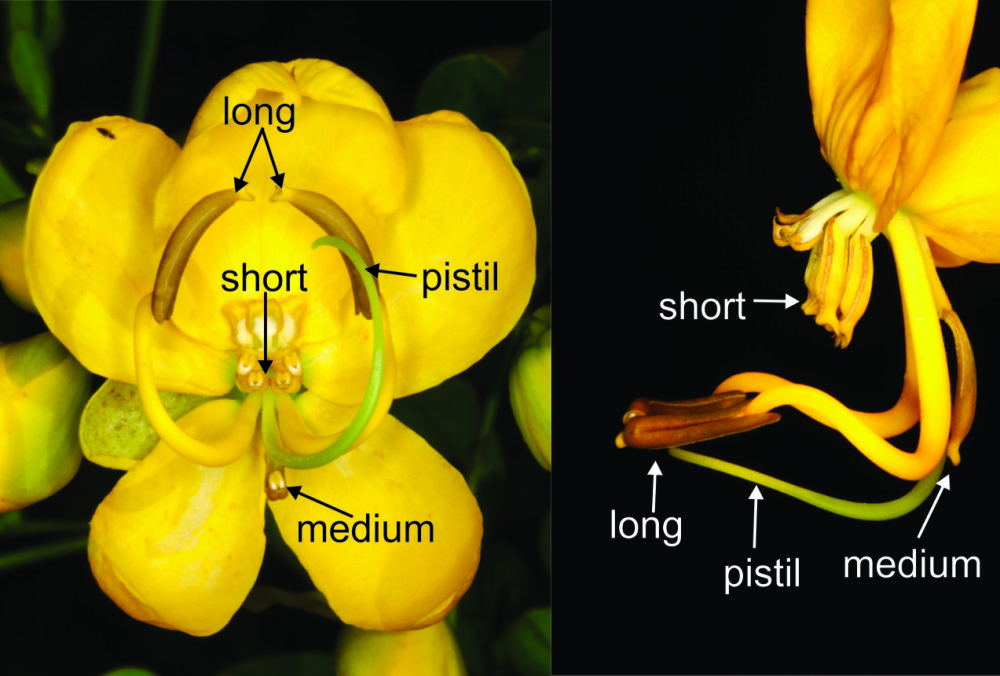
Senna pendula blooms from March to May, and fruits ripen from April to July. The shrub reaches a height of 2 m and has inflorescences with three to 12 flowers in racemes. The corolla has five golden-yellow non-concave petals and pistils oriented to the right or to the left, a condition known as enantiostyly. The androecium has two long lateral symmetric stamens (“pollinating stamens”), one centralized median-stamen, four short-central stamens (“feeding stamens”) and three staminodes (Fig. 1).
. Flower of Senna pendula, in front view (left side) and side view (right side). Arrows pointing the long, medium and short stamens and the pistil.
Pollen morphology, size and quantity
We counted the pollen grains for each set of stamens of S. pendula using twenty flower buds in pre-anthesis from ten individual plants (two buds from each plant), stored in 70 % ethanol. The anthers were macerated in Eppendorf® flasks and prepared according to Lloyd (1972Lloyd DG. 1972. Breeding Systems in Cotula L. Compositae, Anthemideae II. Monoecious Populations. New Phytologist 71: 1195-1202.). The pollen grains were counted in a Neubauer chamber (Maeda 1985Maeda JM. 1985. Manual para uso da câmara da Neubauer para contagem de pólen de espécies florestais. Universidade Federal do Rio de Janeiro, Rio de Janeiro.). The amount of pollen of the different stamen types was compared using analysis of variance (ANOVA) with general linear model procedure (GLM, Poisson distribution) (R Core Team 2017R Core Team. 2017. R: A language and environment for statistical computing. Vienna, R Foundation for Statistical Computing. https://www.R-project.org/
https://www.R-project.org/...
).
The pollen grains of the different types of anthers were stored in glacial acetic acid (N = 10 individuals, 10 flowers) to compare ornamentation and size of pollen grains from the three stamen types. We confected microscope slides with glycerinated gelatin, sealed with paraffin and analyzed the pollen grains in light microscope with 1000x magnification (N = 30 grains per type of stamen).
The pollen grain volumes were determined (μm3) from radial measurements taken of the polar and equatorial diameters of 50 non-acetolyzed pollen grains using a light microscope (1000x magnification). We adjusted the values obtained from the measures to a known geometric shape (oblate-spheroidal), whose volume was calculated by the following equation: v = (4/3) π a² b, where v is the volume (μm3), a is the radius of the polar diameter and b the radius of the equatorial diameter.
We multiplied the average grain volume by the pollen number of each type of stamen and calculated also the total volume of pollen grains for the three sets of stamens. We used ANOVA, with GLM procedure (Inverse Gaussian distribution), using the stats package in R version 3.3.3 (R Core Team 2017R Core Team. 2017. R: A language and environment for statistical computing. Vienna, R Foundation for Statistical Computing. https://www.R-project.org/
https://www.R-project.org/...
), to determine differences between volume and quantity of the pollen grains of the three groups of stamens. We calculated the pollen/ovule ratio for S. pendula, dividing the average number of ovules in a flower (N = 10 individuals, 20 flowers) by the average number of pollen grains per flower (N = 10 individuals, 10 flowers; Cruden 1977Cruden RW. 1977. Pollen‐ovule ratios: a conservative indicator of breeding systems in flowering plants. Evolution 31: 32-46.). We considered the number of samples (N) as the number of individuals of plants. When more than one flower per plant was used, we considered the average value of within-plant flowers as one sample.
Breeding system and fruit set
To determine the breeding system of S. pendula, controlled hand pollination was performed. Flower buds were bagged and flowers hand self-pollinated (N = four plants, 16 flowers) or maintained bagged (spontaneous self-pollination) (N = two plants, five flowers). We performed hand-cross pollination with pollen from long (N = four plants, 52 flowers), medium (N = four plants, 61 flowers) and short stamens (N = four plants, 51 flowers) to verify fertility of pollen from the three types of stamens. Pollen donors were at least 100 meters distant from pollen receiving plants. Hand cross- and hand self-pollination treatments were conducted in new open flowers in the morning. Moreover, non-bagged marked flowers were maintained accessible to flower visitors. We compared fruit set (number of fruits/number of flowers) and seed set (number of seeds/fruit) of the different treatments. The statistical significance the fruit and seed set were determined by the chi-square test and ANOVA (GLM procedure, Poisson distribution), respectively. The GLM-ANOVA was performed in stats package in R version 3.3.3 (R Core Team 2017R Core Team. 2017. R: A language and environment for statistical computing. Vienna, R Foundation for Statistical Computing. https://www.R-project.org/
https://www.R-project.org/...
). Sample number (N) was the number of plant individuals.
Floral visitors
To evaluate the behavior of floral visitors, observations were performed on different individuals between 8h00min and 17h00min. After floral visits, the insects were collected, mounted, labeled, identified and included in the entomological collection of Universidade Federal de Minas Gerais. We noted which species of bees vibrate flowers to collect pollen and touch the stigmas.
Results
Pollen morphology, ornamentation and quantity in the different anther types
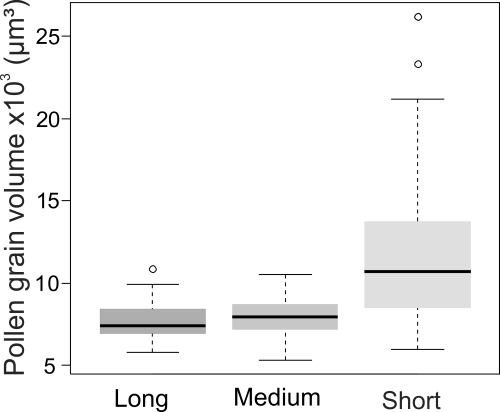
The tricolporate, oblate-spheroidal pollen grains of S. pendula exhibited no differences in the microreticulate ornamentation at 1000x magnification under the light microscopy. However, short-stamens produced pollen grains with larger volume (mean ± SD: 11,810.1 ± 4,549.2 µm³), about 37% larger than pollen grains from long (7,644.2 ± 1,014.0 µm³) and medium-stamens (7,926.6 ± 1,120.9 µm³) (Fig. 2; GLM, F = 34.7, p ≤ 0.001, Inverse Gaussian distribution).
Median and percentiles (25-75 %) of the volume of a single pollen grain (μm3) from long, medium and short stamens of Senna pendula (N = 25). Statistical analysis: GLM (F = 34.7, p ≤ 0.001, Inverse Gaussian distribution).
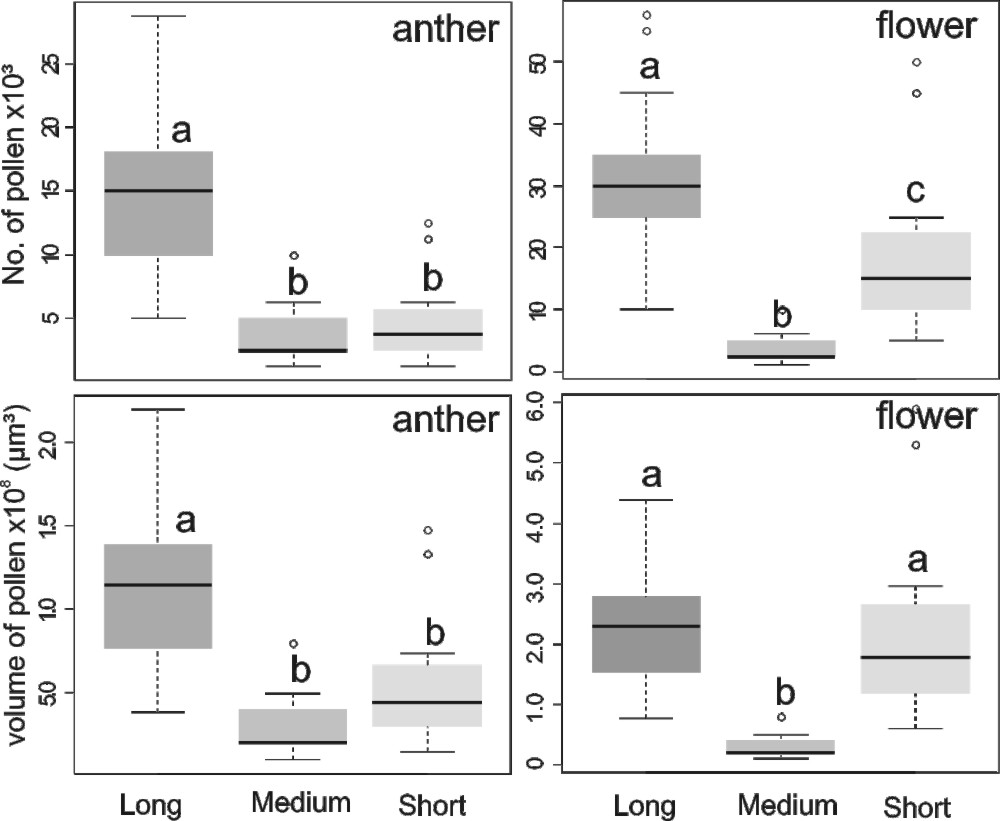
Single long stamens have more pollen grains (mean ± SD: 15,062.5 ± 56,657.4) than the medium (3,812.5 ± 2,515.5) and short stamens (4,812.5 ± 3,225.9) (GLM, F = 49.9, p ≤ 0.001, Poisson distribution; Fig. 3). Considering the total set of stamens in a flower, on average, 56 % of the total number of pollen grains of a flower are allocated to long, 7.5 % to medium and 36.5 % to short stamens (GLM, F = 75.4, p ≤ 0.001, Poisson distribution).
Median and percentiles (25-75 %) of number of pollen grains (upper) and volume of pollen (bottom) in long, medium and short stamen (left side “stamen”) and by stamen groups within flowers (right side “flower”) in Senna pendula. Number of pollen grains (top) and volume of pollen (bottom). Different letters represent significant differences between the stamens groups indicated by GLM: number of pollen per anther (F = 38.1, p ≤ 0.001) and per flower (F = 31.4, p ≤ 0.001), Poisson distribution; volume of pollen per flower (F = 23.2, p ≤ 0.001) and per anther (F = 3.1, p ≤ 0.05), Inverse Gaussian distribution; Nplants = 10 (20 flowers).
However, relating the number of grains per stamens to the respective volume of the pollen grains (Fig. 2, GLM, F = 34.7, p ≤ 0.001, Inverse Gaussian distribution), the set of long and short stamens have no difference in pollen volume per flower (Fig. 3, GLM, p = 0.93).
Breeding system and fruit set and fecundity of pollen from different stamens
Hand self-pollination experiments set no fruits. Fruit set using pollen from long and short stamens after hand cross-pollination was similar, both were 25 % (X 2 = 0.02, p = 0.62) and higher than the 8 % seed set sired by pollen from medium size stamens (long versus medium: X 2 = 4.68, p ≤ 0.05; short versus medium: X 2 = 4.89, p ≤ 0.05; Tab. 1). However, seed set in the mature fruits after hand cross-pollination with pollen from the three stamen types did not differ (Tab. 1). A flower produced, on average, 53,187.5 ± 19,258.9 pollen grains (Nplants = 10) and 103.8 ± 7.97 ovules (Nplants = 10), a pollen/ovule ratio of 512.4:1.
Breeding system of Senna pendula. Fruit and seed set in flowers previously bagged in the bud stage: spontaneous self-pollination (flowers maintained bagged), hand self-pollination (pollination with pollen of the same flower), hand cross-pollination (pollination with pollen of other plant individuals), and open (non-bagged control) treatments. Hand cross-pollination treatments were conducted with pollen from long, medium or short stamens. Number (percent) of fruits set and mean (± SD); (Nplants) represents the number of plant individuals tested per treatment. Values with the different superscript letters indicate significant differences between fruit (p ≤ 0.05; Mantel-Haenszel Chi-square) and seed sets (GLM, F = 0.69, p = 0.51, Poisson distribution).
Floral visitors
The floral visitors to S. pendula were mainly bees of the tribes Bombini, Centridini, Xylocopini, Exomalopsini and Meliponini. The large bees of Xylocopa (Neoxylocopa) frontalis, Xylocopa (Neoxylocopa) grisescens, Bombus (Fervidobombus) pauloensis, Centris (Trachina) fuscata, and Centris (Melacentris) collaris collected pollen by anther vibration. The females of these species hovered at close distance in front of the flowers before they decided to visit a flower or not. Their flower visits were uniformly in a specific position with both long lateral stamens positioned right and left-handed to the bee’s dorsal surface. While the ventral surface faced the short stamens, the dorsal surface contacted the stigma. Vibrations were applied to the flower in this position removing pollen simultaneously from all stamens. During the flower visits, the dorsum (mesoscutum, scutellum and metanotum) of the bees touched the stigma.
Females of Melipona bicolor, Exomalopsis collaris, and Pseudaugochlora graminea landed in the center of the flower, positioned the abdomen close to the anther opening and individually vibrated short, medium and long stamens. These bees contacted the stigma only occasionally.
Flower visits of workers of Paratrigona subnuda did not vibrate the flowers or the anthers, and collected pollen inserting their mouthparts into the anther pores of the different stamen types. Workers of this bee species spent long periods in a single flower removing pollen grains from their mouthparts with the fore legs and transferring them to the corbicula.
Discussion
Flowers of S. pendula show differential allocation of pollen grains to the long and short stamens. Short feeding stamens contain fewer but larger pollen grains, while the long pollination stamens have more but much smaller grains. These differences in volume versus quantity of pollen grains fit the division-of-labor hypothesis among stamens, as suggested by Müller (1881Müller H. 1881. Two kinds of stamens with different functions in the same flower. Nature 24: 307-308.) and Darwin (1862Darwin C. 1862. Letter to Asa Gray. http://www.darwinproject.ac.uk. 10 Nov. 2017.
http://www.darwinproject.ac.uk...
).
The meaning of feeding and pollinating anthers for large bees
The division-of-labor hypothesis predicts that the long stamens contain pollen destined for pollination and the short ones pollen for bee feeding (Darwin 1862Darwin C. 1862. Letter to Asa Gray. http://www.darwinproject.ac.uk. 10 Nov. 2017.
http://www.darwinproject.ac.uk...
; Müller 1881Müller H. 1881. Two kinds of stamens with different functions in the same flower. Nature 24: 307-308.). In our studies, large carpenter bees (Xylocopa), bumblebees (Bombus) and oil-collecting bees (Centris) were the effective pollinators, which vibrated all anthers simultaneously in a single position defined by the complex morphology of the zygomorphic to slightly asymmetric flowers of S. pendula (Westerkamp 2004Westerkamp C. 2004. Ricochet pollination in cassias - and how bees explain enantiostyly preliminary communication. In: Freitas BM, Pereira JOP. (eds.) Solitary bees, conservation, rearing and management for pollination. Fortaleza, Universidade Federal do Ceará. p. 225-230. ). The dorsum of the large bees receives the ejected pollen from the long stamens during the applied vibration; this part is a “safe site” for pollen deposition, because this region gets into contact with the stigma and the bees rarely remove these pollen loads (Westerkamp 2004Westerkamp C. 2004. Ricochet pollination in cassias - and how bees explain enantiostyly preliminary communication. In: Freitas BM, Pereira JOP. (eds.) Solitary bees, conservation, rearing and management for pollination. Fortaleza, Universidade Federal do Ceará. p. 225-230. ; Westerkamp & Classen-Bockhoff 2007Westerkamp C, Classen-Bockhoff R. 2007. Bilabiate flowers: the ultimate response to bees? Annals of Botany 100: 361-374; Amorim et al. 2017Amorim T, Marazzi B, Soares AA, Forni-Martins ER, Muniz CR, Westerkamp C. 2017. Ricochet pollination in Senna (Fabaceae) − petals deflect pollen jets and promote division of labour among flower organs. Plant Biology 19: 951-962.). Differently, pollen from the four short central feeding stamens is ejected onto the large bee’s mesepisternum, a region from which it is frequently removed and transferred to the scopa on the hind legs. Moreover, the large bees only associate the feeding stamens with presence of food and fail to visit flowers whose short (but not medium and long stamens) are removed as demonstrated in Senna reniformis (Mesquita-Neto et al. 2017Mesquita-Neto JN, Costa BKP, Schlindwein C. 2017. Heteranthery as a solution to the demands for pollen as food and for pollination-Legitimate flower visitors reject flowers without feeding anthers. Plant Biology 19: 942-950.), which has the same flower morphology as S. pendula. The deposition of pollen in different body parts of the large and buzzing-bees, mediated by heteranthery, is characteristic for division-of-labor of stamens in heterantherous flowers (Vallejo-Marín et al. 2009Vallejo-Marín M, Manson JS, Thomson JD, Barrett SC. 2009. Division of labour within flowers: heteranthery, a floral strategy to reconcile contrasting pollen fates. Journal of Evolutionary Biology 22: 828-839.; Tong & Huang 2018Tong ZY, Huang SQ. 2018. Safe sites of pollen placement: a conflict of interest between plants and bees? Oecologia 186: 163-171.).
Senna pendula belongs to clade VII (sensuMarazzi et al. 2007Marazzi B, Conti E, Endress PK. 2007. Diversity in anthers and stigmas in the buzz-pollinated genus Senna (Leguminosae, Cassiinae). International Journal of Plant Sciences 168: 371-391.), in which most of the representatives have monosymmetric flowers that possess upper and lower petals with similar shapes or have lower petals slightly longer and thinner than the upper ones (Marazzi & Endress 2008Marazzi B, Endress PK. 2008. Patterns and development of floral asymmetry in Senna (Leguminosae, Cassiinae). American Journal of Botany 95: 22-40.). In the latter, the pollen jet of the long stamens is directed to an elastic concave petal then it is again deflected from a second upper petal until the pollen grains are finally deposited on the bee’s dorsum. In this pollination mechanism, denominated ricochet (Westerkamp 2004Westerkamp C. 2004. Ricochet pollination in cassias - and how bees explain enantiostyly preliminary communication. In: Freitas BM, Pereira JOP. (eds.) Solitary bees, conservation, rearing and management for pollination. Fortaleza, Universidade Federal do Ceará. p. 225-230. ; Amorim et al. 2017Amorim T, Marazzi B, Soares AA, Forni-Martins ER, Muniz CR, Westerkamp C. 2017. Ricochet pollination in Senna (Fabaceae) − petals deflect pollen jets and promote division of labour among flower organs. Plant Biology 19: 951-962.), two petals are involved in the deflection of the pollen jet and the two long stamens are directed ventrally to one of the concave petals. The ricochet mechanism associated with the strongly concave petals and the deflected pollen grains from the pollination stamens occurs in numerous species of Senna and in different clades (Marazzi et al. 2007Marazzi B, Conti E, Endress PK. 2007. Diversity in anthers and stigmas in the buzz-pollinated genus Senna (Leguminosae, Cassiinae). International Journal of Plant Sciences 168: 371-391.; Amorim et al. 2017Amorim T, Marazzi B, Soares AA, Forni-Martins ER, Muniz CR, Westerkamp C. 2017. Ricochet pollination in Senna (Fabaceae) − petals deflect pollen jets and promote division of labour among flower organs. Plant Biology 19: 951-962.). In S. pendula, the five petals are elliptical to oval, possess only weak concavity in the upper petals and do not interfere the direction of the pollen jet from the long stamens, which are here positioned dorsally and almost symmetrically left and right-hand of a large buzzing bee. Pollen in S. pendula, thus is ejected directly onto the bee’s dorsum from the long stamens during the flower visit, similar to S. reniformis (Mesquita-Neto et al. 2017Mesquita-Neto JN, Costa BKP, Schlindwein C. 2017. Heteranthery as a solution to the demands for pollen as food and for pollination-Legitimate flower visitors reject flowers without feeding anthers. Plant Biology 19: 942-950.), without deflection by any petal (sensuWesterkamp 2004Westerkamp C. 2004. Ricochet pollination in cassias - and how bees explain enantiostyly preliminary communication. In: Freitas BM, Pereira JOP. (eds.) Solitary bees, conservation, rearing and management for pollination. Fortaleza, Universidade Federal do Ceará. p. 225-230. ; Amorim et al. 2017Amorim T, Marazzi B, Soares AA, Forni-Martins ER, Muniz CR, Westerkamp C. 2017. Ricochet pollination in Senna (Fabaceae) − petals deflect pollen jets and promote division of labour among flower organs. Plant Biology 19: 951-962.). Because the long pollination stamens have the same relative position to the bee’s body in floral morphs with left and right-sided pistil in S. pendula, enantiostyly in this species has not the role to promote cross-pollination (seeGottsberger & Silberbauer-Gottsberger 1988Gottsberger G, Silberbauer-Gottsberger I. 1988. Evolution of flower structures and pollination in Neotropical Cassiinae (Caesalpiniaceae) species. Phyton 28: 293-320.; Mesquita-Neto et al. 2017Mesquita-Neto JN, Costa BKP, Schlindwein C. 2017. Heteranthery as a solution to the demands for pollen as food and for pollination-Legitimate flower visitors reject flowers without feeding anthers. Plant Biology 19: 942-950.), and the stigma of right and left-sided pistils might indiscriminately contact pollen grains from both pollination stamens on the dorsal surface of large bees.
Poricidal dehiscence of anthers and rigorous self-incompatibility makes S. pendula dependent of large buzz-pollinating bees that move between conspecific plants to produce fruits. Fruit and seed production after hand cross-pollination with pollen from pollination and feeding stamens reached similar levels in S. pendula. Several studies have demonstrated differential fertility among heteromorphic stamens (Fægri & Pijl 1979Faegri K, Pijl K. 1979. The principles of pollination ecology. Oxford, Cambridge Pergamon Press.; Sarala et al. 1999Sarala, BS, Lokesha R, Vasudeva R. 1999. Anther dimorphism, differential anther dehiscence, pollen viability and pollination success in Caesalpinia pulcherrimma L. (Fabaceae). Current Science 76: 1490-1494.; Luo et al. 2009Luo Z, Gu LL, Zhang DX. 2009. Intrafloral differentiation of stamens in heterantherous flowers. Journal of Systematics and Evolution 47: 43-56.; Mesquita-Neto et al. 2017Mesquita-Neto JN, Costa BKP, Schlindwein C. 2017. Heteranthery as a solution to the demands for pollen as food and for pollination-Legitimate flower visitors reject flowers without feeding anthers. Plant Biology 19: 942-950.), while others found similar rates of fructification between pollen from pollination stamens and feeding anthers (Tang & Huang 2007Tang LL, Huang SQ. 2007. Evidence for reductions in floral attractants with increased selfing rates in two heterandrous species. New Phytologist 175: 588-95.; Gross & Kukuk 2001Gross CL, Kukuk PF. 2001. Foraging strategies of Amegilla anomala at the flowers of Melastoma affine - no evidence for separate feeding and pollinating anthers. Acta Horticulturae 561: 171-178.). Thus, the differential fecundity of pollen from different types of stamens is not necessarily associated with heteranthery.
Different pollen allocation to feeding and pollinating stamens economizes gametes
The pollen/ovule ratio was lower than that predicted by Cruden (1977Cruden RW. 1977. Pollen‐ovule ratios: a conservative indicator of breeding systems in flowering plants. Evolution 31: 32-46.) for obligatory xenogamic plants. It is expected that flowers that offer only pollen as a reward for pollinators produce more pollen than flowers that offer nectar or multiple rewards, because pollen in the former is also used to feed the bees (Vogel 1978Vogel S. 1978. Evolution ary shifts from reward to deception in pollen flowers. In: Richard AJ. (ed.) The pollination of flowers by insects. Vol. 6. London/ New York, Linnean Society Symposium Series. p. 89-96.; Cruden 2000Cruden RW. 2000. Pollen grains: why so many? Plant Systematics and Evolution 222: 143-165.). In this context, lower pollen production than expected might be an evolutionary response due to a high efficiency in pollination (Cruden & Jensen 1979Cruden RW, Jensen KG. 1979. Viscin threads, pollination efficiency and low pollen-ovule ratios. American Journal of Botany 66: 875-879.) associated with pollen economy mediated by heteranthery (also see Ferreira & Araújo 2016Ferreira QIX, Araújo FPD. 2016. Pollen economy enhanced by heteranthery in Desmocelis villosa (Melastomataceae). Rodriguésia 67: 347-355.).
Heterantherous flowers that provide a high quantity of its pollen to pollinators may reduce available male gametes for fertilization (Vogel 1978Vogel S. 1978. Evolution ary shifts from reward to deception in pollen flowers. In: Richard AJ. (ed.) The pollination of flowers by insects. Vol. 6. London/ New York, Linnean Society Symposium Series. p. 89-96.; Buchmann 1983Buchmann SL. 1983. Buzz pollination in angiosperms. In: Jones CE, Little RJ eds. Handbook of experimental pollination biology. New York, Van Nostrand Reinhold.; Vallejo-Marín et al. 2010Vallejo-Marín M, Silva EM, Sargent RD, Barrett SC. 2010. Trait correlates and functional significance of heteranthery in flowering plants. New Phytologist 188: 418-425.). Senna pendula seems to have another way to mediate the dilemma: about 40 % of the total pollen number in the flower is available to pollinators in the feeding stamens. High proportions of pollen for feeding are also found in other species of Senna (S. reniformis, Mesquita-Neto et al. 2017Mesquita-Neto JN, Costa BKP, Schlindwein C. 2017. Heteranthery as a solution to the demands for pollen as food and for pollination-Legitimate flower visitors reject flowers without feeding anthers. Plant Biology 19: 942-950.; S. bicapsularis; S. alata, Luo et al. 2009Luo Z, Gu LL, Zhang DX. 2009. Intrafloral differentiation of stamens in heterantherous flowers. Journal of Systematics and Evolution 47: 43-56.). Papaj et al. (2017Papaj DR, Buchmann SL, Russell AL. 2017. Division of labor of anthers in heterantherous plants: flexibility of bee pollen collection behavior may serve to keep plants honest. Arthropod-Plant Interaction 11: 307-315.) showed that storage of a minimum quantity of pollen in the feeding stamens is necessary to prevent bees to also collect pollen from the pollination stamens. Therefore, it seems to be necessary that pollination by large bees requires that the flowers allocate significant amounts of pollen to the feeding stamens. The total pollen grain volume of the four short feeding stamens is equivalent to the total pollen volume of the two long pollination anthers. However, the pollination anthers bear 50 % more pollen grains than feeding anthers. For bees, the volume of pollen grains represents better its nutritional value than the number of grains (Silveira 1991Silveira FA. 1991. Influence of pollen grain volume on the estimation of the relative importance of its source to bees. Apidologie 22: 495-502.). While a greater food volume may satisfy pollen-collecting bees, a high number of viable pollen grains is fundamental for pollen allocated to the reproduction of the plant. Probably there is an advantage for the plant to produce fewer but larger pollen grains for bee consumption. Several studies attribute the pollen number/size relationship as trade-off by the way that the reduction in the number of pollen grains should be associated with an increase in the size of pollen grains (eg. Charnov 1982Charnov EL. 1982. The theory of sex allocation.Vol. 18. Princeton, Princeton University Press.; Vonhof & Harder 1995Vonhof MJ, Harder LD. 1995. Size-number trade-offs and pollen production by Papilionaceous legumes. American Journal of Botany 82: 230-238.).
A negative correlation between pollen grain size and pollen protein content was found in buzz-pollinated plants by the way that smaller grains tend to have higher protein richness (Roulston et al. 2000Roulston TH, Cane JH, Buchmann SL. 2000. What governs protein content of pollen: pollinator preferences, pollen-pistil interactions, or phylogeny? Ecological Monographs 70: 617-643.). If we assume that the volume of a pollen grain is a reliable negative indicator of the nutrient content, and the bees have to collect a minimum quantity of pollen to feed their larvae, the found differences here in pollen volume and pollen grain numbers seem to be well explained: S. pendula offers larger grains but with less essential nutrients in the short feeding stamens and save protein-rich pollen in long pollinating anther. To our knowledge, there is still no information on differences of the nutritional content of pollen grains from feeding and pollination stamens in heterantherous buzz-pollinated species. We hypothesize that pollen grains from pollinating anthers contain more high-value nutrients (P, N, proteins) than the possibly more energy-rich pollen grains from the feeding stamens in species with poricidal anthers. Measuring pollen quality in these species may help to understand the role of specialized feeding and resource allocation.
The role of medium-sized stamens and staminodes
In S. pendula, the small pollen grains of medium-sized stamens had much lower viability than the larger ones of the feeding stamens. The medium stamen seems to play no role in division-of-labor, and the decrease of pollen fertility indicates loss of the reproductive function, alike the morphologically similar S. reniformis (Mesquita-Neto et al. 2017Mesquita-Neto JN, Costa BKP, Schlindwein C. 2017. Heteranthery as a solution to the demands for pollen as food and for pollination-Legitimate flower visitors reject flowers without feeding anthers. Plant Biology 19: 942-950.). In some species and among several clades within the genus Senna, the medium stamen can be strongly reduced, sterile or absent (Irwin & Barneby 1982Irwin HS, Barneby RC. 1982. The American Cassiinae: a synoptical revision of Leguminosae Tribe Cassieae subtribe Cassiinae in the New World. Memoires of the New York Botanical Garden 35: 1-1918.; Marazzi et al. 2006Marazzi B, Endress PK, Queiroz LP, Conti E. 2006. Phylogenetic relationships within Senna (Leguminosae, Cassiinae) based on three chloroplast DNA regions: patterns in the evolution of floral symmetry and extrafloral nectaries. American Journal of Botany 93: 288-303.; 2007Marazzi B, Conti E, Endress PK. 2007. Diversity in anthers and stigmas in the buzz-pollinated genus Senna (Leguminosae, Cassiinae). International Journal of Plant Sciences 168: 371-391.). We interpret the lower fertility of pollen from the medium stamen in S. pendula as related to an evolutionary process of stamen reduction as demonstrated in other species of Senna clade VII.
The staminodes, like the central short stamens, might possess a signaling function. In S. pendula they show bright white reflectance to the human eye, similar to the region surrounding the apical open pores of the anthers (Fig. 1A). This visual contrast, possibly enhanced by uv-absorbance, might amplify the signaling function of short stamens, indicating the presence of food for effective pollinators. This could be verified experimentally by removing staminodes and with the use of spectrophotometry.
We conclude that pollen from short feeding stamens and long pollinating stamens in S. pendula exhibit different quantity and size but similar fecundity. Whereas pollen from short feeding stamens have considerably larger size than pollen from long pollination stamens, those of the latter are smaller and more numerous, which is advantageous for pollination. The indicative of differential allocation of pollen grains for pollination and feeding of pollinators can be considered as manifestation of the division-of-labor function in pollen rewarding flowers with heteromorphic stamens.
Acknowledgements
We thank Fundação de Parques Municipais de Belo Horizonte (FPM) for the license to work in the Nature Reserve “Serra do Curral” and anonymous reviewers for constructive comments, which improved the manuscript. This study was supported by Fundação de Amparo à Pesquisa do Estado de Minas Gerais (APQ-01707-14). Moreover, we thank Coordenação de Aperfeiçoamento de Pessoal de Nível Superior for individual research fellowships to BKPC and JNM, and Conselho Nacional de Desenvolvimento Científıco e Tecnológico to BKPC and CS.
References
- Amorim T, Marazzi B, Soares AA, Forni-Martins ER, Muniz CR, Westerkamp C. 2017. Ricochet pollination in Senna (Fabaceae) − petals deflect pollen jets and promote division of labour among flower organs. Plant Biology 19: 951-962.
- Assis WL. 2012. Os climas naturais do município de Belo Horizonte - MG. Acta Geográfica 1: 115-135.
- Barrett SC. 2010. Darwin's legacy: the forms, function and sexual diversity of flowers. Philosophical Transactions of the Royal Society of London B: Biological Sciences 365: 351-368.
- Buchmann SL. 1974. Buzz pollination of Cassia quiedondilla (Leguminosae) by bees of the genera Centris and Melipona Bulletin of the Southern California Academy of Sciences 73: 171-173.
- Buchmann SL. 1983. Buzz pollination in angiosperms. In: Jones CE, Little RJ eds. Handbook of experimental pollination biology. New York, Van Nostrand Reinhold.
- Cardinal S, Buchmann SL, Russell AL.2018. The evolution of floral sonication, a pollen foraging behavior used by bees (Anthophila). Evolution 72: 590-600.
- Carvalho DA, Oliveira PE. 2003. Biologia reprodutiva e polinização de Senna sylvestris (Vell.) HS Irwin & Barneby (Leguminosae, Caesalpinioideae). Revista Brasileira de Botânica 26: 319-328.
- Charnov EL. 1982. The theory of sex allocation.Vol. 18. Princeton, Princeton University Press.
- Cruden RW, Jensen KG. 1979. Viscin threads, pollination efficiency and low pollen-ovule ratios. American Journal of Botany 66: 875-879.
- Cruden RW. 1977. Pollen‐ovule ratios: a conservative indicator of breeding systems in flowering plants. Evolution 31: 32-46.
- Cruden RW. 2000. Pollen grains: why so many? Plant Systematics and Evolution 222: 143-165.
- Darwin C. 1862. Letter to Asa Gray. http://www.darwinproject.ac.uk 10 Nov. 2017.
» http://www.darwinproject.ac.uk - Dulberger R. 1981. The floral biology of Cassia didymobotrya and C. auriculata (Caesalpiniaceae). American Journal of Botany 68: 1350-1360.
- Faegri K, Pijl K. 1979. The principles of pollination ecology. Oxford, Cambridge Pergamon Press.
- Ferreira QIX, Araújo FPD. 2016. Pollen economy enhanced by heteranthery in Desmocelis villosa (Melastomataceae). Rodriguésia 67: 347-355.
- Gottsberger G, Silberbauer-Gottsberger I. 1988. Evolution of flower structures and pollination in Neotropical Cassiinae (Caesalpiniaceae) species. Phyton 28: 293-320.
- Gross CL, Kukuk PF. 2001. Foraging strategies of Amegilla anomala at the flowers of Melastoma affine - no evidence for separate feeding and pollinating anthers. Acta Horticulturae 561: 171-178.
- Irwin HS, Barneby RC. 1982. The American Cassiinae: a synoptical revision of Leguminosae Tribe Cassieae subtribe Cassiinae in the New World. Memoires of the New York Botanical Garden 35: 1-1918.
- Klinkhamer PG, Jong TJ. 1993. Attractiveness to pollinators: a plant's dilemma. Oikos 66: 180-184.
- Lloyd DG. 1972. Breeding Systems in Cotula L. Compositae, Anthemideae II. Monoecious Populations. New Phytologist 71: 1195-1202.
- Lloyd DG. 2000. The selection of social actions in families: III. Reproductively disabled individuals and organs. Evolution ary Ecology Research 2: 29-40.
- Luca PA, Vallejo-Marín M. 2013. What's the ‘buzz’ about? The ecology and evolutionary significance of buzz-pollination. Current Opinion in Plant Biology 16: 429-435.
- Luo Z, Gu LL, Zhang DX. 2009. Intrafloral differentiation of stamens in heterantherous flowers. Journal of Systematics and Evolution 47: 43-56.
- Maeda JM. 1985. Manual para uso da câmara da Neubauer para contagem de pólen de espécies florestais. Universidade Federal do Rio de Janeiro, Rio de Janeiro.
- Marazzi B, Conti E, Endress PK. 2007. Diversity in anthers and stigmas in the buzz-pollinated genus Senna (Leguminosae, Cassiinae). International Journal of Plant Sciences 168: 371-391.
- Marazzi B, Endress PK. 2008. Patterns and development of floral asymmetry in Senna (Leguminosae, Cassiinae). American Journal of Botany 95: 22-40.
- Marazzi B, Endress PK, Queiroz LP, Conti E. 2006. Phylogenetic relationships within Senna (Leguminosae, Cassiinae) based on three chloroplast DNA regions: patterns in the evolution of floral symmetry and extrafloral nectaries. American Journal of Botany 93: 288-303.
- Mesquita-Neto JN, Costa BKP, Schlindwein C. 2017. Heteranthery as a solution to the demands for pollen as food and for pollination-Legitimate flower visitors reject flowers without feeding anthers. Plant Biology 19: 942-950.
- Michener CD. 1962. An interesting method of pollen collecting by bees from flowers with tubular anthers. Revista de Biología Tropical 10: 167-175.
- Müller F. 1883. Two kinds of stamens with different functions in the same flower. Nature 27: 364-365.
- Müller H. 1881. Two kinds of stamens with different functions in the same flower. Nature 24: 307-308.
- Papaj DR, Buchmann SL, Russell AL. 2017. Division of labor of anthers in heterantherous plants: flexibility of bee pollen collection behavior may serve to keep plants honest. Arthropod-Plant Interaction 11: 307-315.
- R Core Team. 2017. R: A language and environment for statistical computing. Vienna, R Foundation for Statistical Computing. https://www.R-project.org/
» https://www.R-project.org/ - Roulston TH, Cane JH, Buchmann SL. 2000. What governs protein content of pollen: pollinator preferences, pollen-pistil interactions, or phylogeny? Ecological Monographs 70: 617-643.
- Russell AL, Buchmann SL, Papaj DR. 2017. How a generalist bee achieves high efficiency of pollen collection on diverse floral resources. Behavioral Ecology 28: 991-1003.
- Sarala, BS, Lokesha R, Vasudeva R. 1999. Anther dimorphism, differential anther dehiscence, pollen viability and pollination success in Caesalpinia pulcherrimma L. (Fabaceae). Current Science 76: 1490-1494.
- Silveira FA. 1991. Influence of pollen grain volume on the estimation of the relative importance of its source to bees. Apidologie 22: 495-502.
- Tang LL, Huang SQ. 2007. Evidence for reductions in floral attractants with increased selfing rates in two heterandrous species. New Phytologist 175: 588-95.
- Tong ZY, Huang SQ. 2018. Safe sites of pollen placement: a conflict of interest between plants and bees? Oecologia 186: 163-171.
- Vallejo-Marín M, Manson JS, Thomson JD, Barrett SC. 2009. Division of labour within flowers: heteranthery, a floral strategy to reconcile contrasting pollen fates. Journal of Evolutionary Biology 22: 828-839.
- Vallejo-Marín M, Silva EM, Sargent RD, Barrett SC. 2010. Trait correlates and functional significance of heteranthery in flowering plants. New Phytologist 188: 418-425.
- Vallejo-Marin M, Walker C, Friston-Reilly P, Solis-Montero L, Igic B. 2014. Recurrent modification of floral morphology in heterantherous Solanum reveals a parallel shift in reproductive strategy. Philosophical Transactions of the Royal Society of London 369: 20130256. doi: 10.1098/rstb.2013.0256
» https://doi.org/10.1098/rstb.2013.0256 - Vogel S. 1978. Evolution ary shifts from reward to deception in pollen flowers. In: Richard AJ. (ed.) The pollination of flowers by insects. Vol. 6. London/ New York, Linnean Society Symposium Series. p. 89-96.
- Vonhof MJ, Harder LD. 1995. Size-number trade-offs and pollen production by Papilionaceous legumes. American Journal of Botany 82: 230-238.
- Westerkamp C. 1996. Pollen in bee-flower relations, some considerations on melittophily. Plant Biology 109: 325-332.
- Westerkamp C. 2004. Ricochet pollination in cassias - and how bees explain enantiostyly preliminary communication. In: Freitas BM, Pereira JOP. (eds.) Solitary bees, conservation, rearing and management for pollination. Fortaleza, Universidade Federal do Ceará. p. 225-230.
- Westerkamp C, Classen-Bockhoff R. 2007. Bilabiate flowers: the ultimate response to bees? Annals of Botany 100: 361-374
Publication Dates
-
Publication in this collection
30 July 2018 -
Date of issue
Jul-Sep 2018
History
-
Received
05 Apr 2018 -
Accepted
22 June 2018