Abstracts
Two tropical tree species viz. Alstonia venenata Br. and Alstonia neriifolia Don. (Apocynaceae) were investigated to detect size variation in different elements of the cambium and its derivative tissues. Although these two species were grown under identical climatic and edaphic conditions, fusiform initial dimensions and the elements derived from them were larger in A. venenata than in A. neriifolia. Ray initials are rectangular in A. venenata but isodiametric in A. neriifolia. An appreciable increase in length was observed in the phloem and xylem ray cells when compared to the mother cells. Maximum elongation was observed in xylem fibers during differentiation from the respective fusiform initials.
cambial fusiform initial length; cambial ray initials; sieve-tube elements; vessel elements; xylem fibers; Alstonia venenata; Alstonia neriifolia
Duas espécies de árvores tropicais (Alstonia venenata Br. e Alstonia neriifolia Don. - Apocynaceae) foram estudadas para detectar variação em tamanho de diversos elementos do cambio e seus tecidos derivados. Embora as condições de clima e edaficas destas duas espécies fossem identicas, as dimensões das iniciais fusiformes e os elementos derivados destas foram maiores em A. venenata do que em A. neriifolia. As iniciais radiais são retangulares em A. venenata porem são isodiamêtricas em A. neriifolia. Foi observado aumento substancial no comprimento das células do floema e xilema quando comparadas com as células mãe. Alongamento máximo foi observado nas fibras do xilema durante a diferenciação das respectivas iniciais fusiformes.
comprimento das iniciais funsiformes; iniciais radiais; elementos do tubo crivado; elementos de vaso; fibras do xilema; Alstonia venenata; Alstonia neriifolia
Size variation in the vascular cambium and its derivatives in two Alstonia species
Variação em tamanho do cambio vascular e seus tecidos derivados em duas espécies de Alstonia
Moin A. Khan1 1 Corresponding Author: moin_a_khan11@yahoo.co.in; b.siddiqui@rediffmail.com ; M. Badruzzaman Siddiqui1 1 Corresponding Author: moin_a_khan11@yahoo.co.in; b.siddiqui@rediffmail.com
Departament of Botany, Aligarh Muslim University, Aligarh-202002 (U.P.), India
ABSTRACT
Two tropical tree species viz. Alstonia venenata Br. and Alstonia neriifolia Don. (Apocynaceae) were investigated to detect size variation in different elements of the cambium and its derivative tissues. Although these two species were grown under identical climatic and edaphic conditions, fusiform initial dimensions and the elements derived from them were larger in A. venenata than in A. neriifolia. Ray initials are rectangular in A. venenata but isodiametric in A. neriifolia. An appreciable increase in length was observed in the phloem and xylem ray cells when compared to the mother cells. Maximum elongation was observed in xylem fibers during differentiation from the respective fusiform initials.
Key words: cambial fusiform initial length, cambial ray initials, sieve-tube elements, vessel elements, xylem fibers, Alstonia venenata, Alstonia neriifolia
RESUMO
Duas espécies de árvores tropicais (Alstonia venenata Br. e Alstonia neriifolia Don. - Apocynaceae) foram estudadas para detectar variação em tamanho de diversos elementos do cambio e seus tecidos derivados. Embora as condições de clima e edaficas destas duas espécies fossem identicas, as dimensões das iniciais fusiformes e os elementos derivados destas foram maiores em A. venenata do que em A. neriifolia. As iniciais radiais são retangulares em A. venenata porem são isodiamêtricas em A. neriifolia. Foi observado aumento substancial no comprimento das células do floema e xilema quando comparadas com as células mãe. Alongamento máximo foi observado nas fibras do xilema durante a diferenciação das respectivas iniciais fusiformes.
Palavras-chave: comprimento das iniciais funsiformes, iniciais radiais, elementos do tubo crivado, elementos de vaso, fibras do xilema, Alstonia venenata, Alstonia neriifolia
Introduction
Information on size variation of cambial initials and their derivatives is rather meager. The pioneering contribution in this field is by Chattaway (1936), Butterfield (1973) and Anand et al. (1978) who studied cambial initials and their derivatives in Ginkgo, several conifers and dicotyledons. Recently Iqbal (1990), Rao et al. (1996), Paliwal & Yadav (1999) and Paliwal et al. (2002) also observed the structural and size variation of different xylem elements in Leucaena leucocephala and Haldina cordifolia, respectively.
In three dimensional view, the cambium is a continuous cylindrical sheath about the xylem. There are two conceptually different views regarding the nature of cambium. One school of thought postulates a multiseriate zone distinguished in transections by radially narrow cells with thin walls in which all the cells are equally endowed with multiplication capacity. This view, proposed by Raatz (1892), has been strongly supported by Catesson (1964). The other school pleads for the uniseriate nature of cambium. There are two interpretations of this uniseriate concept based on terminological differences. According to one, there are single initial cells which in each radial file of cambial cells lie somewhere between the phloem and xylem mother cells and are responsible for the production of cambial derivatives on the outer and inner sides. This view is mainly advocated by Bannan (1955; 1968) and Newman (1956), and has been supported by ultra-structure studies of Mahmood (1968) and Murmanis (1970) pertaining to tangential wall characteristics. According to another group of workers (Wilson et al. 1966; Zimmermann & Brown 1971), the term cambium is applicable only to the initial cells, not the immediate derivatives. Following the former terminology, Butterfield (1975) defines cambium as a "multiseriate zone of periclinally dividing cells lying between the differentiating secondary xylem and phloem, with distinct initials capable of both periclinal and anticlinal divisions lying somewhere within each radial file of cells." The same terminology has been adopted for describing cambium in the present study.
The present investigation also aims to provide further information on size variations and relationship between cambial initials and their derivatives in two species of Alstonia namely A. venenata and A. neriifolia.
Materials and methods
Cambial samples along with the bark and some sap wood 1 inch square were collected from the main trunks, at chest height from the southern side of the tree using a chisel and hammer. Nine healthy 24-year-old trees of comparable size and vigour, each of Alstonia venenata Br. (6-8 m in height) and Alstonia neriifolia Don. (4-6 m in height) belonging to the family Apocynaceae and growing under natural climatic and edaphic conditions of the Western Ghats were selected for the purpose. The samples were collected at fortnightly intervals for a period of two consecutive calendar years (2004, 2005). Three samples were collected from three trees each month with a gap of ten days. The next sets of samples were collected from the same set of trees after at least three months. Care was taken to collect the sample at least 10 inches away from the wounded spot whenever the tree was used for the second or third time. All the samples were fixed on the spot in F.A.A. (Formalin-acetic-alcohol) and finally put in 70% ethanol after 5-7 days for preservation. Serial sections in transverse, tangential and radial longitudinal planes were obtained at a thickness of 6-8 µm on a Reichert's sliding microtome. Staining was done following the method of Foster (1934), Johansen (1940) and Cheadle et al. (1953).
Measurements of cambial initials and their derivatives (except for vessel elements and fibres) were carried out on transverse and tangential longitudinal sections with the help of an ocular micrometer scale under the specific magnification of a compound microscope. The dimensions of the fibres and vessel elements were taken after macerating the bark and wood separately following the method of Ghouse et al. 1974. An average of 500 measurements, macerated or sectioned, were taken on a random basis. The mean and range of cell dimensions were determined after pooling the readings obtained from different samples.
Results
Alstonia venenata Br. is a small, medium-sized evergreen tree with buttressed stem. The bark is yellow inside and exudes a milky juice when injured. Leaves were coriaceous, bright green and shiny above, 3-8 inches long tapering at the base into a short petiole. Flowers were greenish-white, arranged in compact, umbellately branched, pubescent cymes. It occurs in the Nilgiris and the evergreen forest of Western Ghats. The other species, Alstonia neriifolia Don., on the other hand, is widely distributed when compared to Alstonia venenata and occurs in East Nepal, Sikkim and Bhutan.
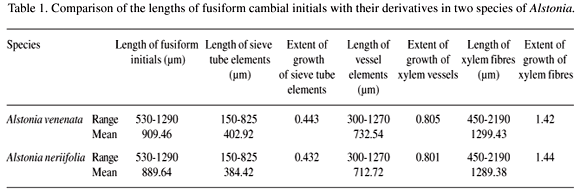
The transections show lenticels, periderm, secondary phloem, cambium and xylem from outside in. The cambium forms a multi-layered zone of 3-12 cells in transection (Figure 1A, B, D), whose component cells are arranged in non-storied or non-stratified manner when seen in tangential view (Figure 2). In both species fusiform initials are elongated, spindle shaped and have a prominent beaded appearance during the dormant period on their tangential walls (Figure 2B, C, F). The radial walls are slightly thicker than the tangential walls in both the active and dormant periods. These are uninucleate with usual cytoplasmic contents and length 909.46 µm in A. venenata and 889.64 µm in A. neriifolia while the widths are 32.49 µm and 34.89 µm, respectively (Table 1, 2). The ray initials are heterogenous, uni- to multi-seriate and consist of procumbent and roughly upright cells in A. venenata (Figures 2A, B, C) whereas these are multiseriate and heterogenous in A. neriifolia (Figures 2D, E, F).
Conducting phloem consists of sieve tube elements, companion cells, phloem fibres and phloem parenchyma in the axial system. The sieve tube elements possess simple and slightly oblique sieve plates (Figure 1C, F). At least one companion cell is associated with each sieve tube element (Figure 1E). Sieve tube elements vary in length; they are 402.92 µm in A. venenata and 384.42 µm in A. neriifolia and are nearly half the length of the respective mother cells in both trees under investigation (Table 1). Companion cells are almost half the width while these are 1/3 to 1/4 the length when compared to the length of fusiform initials. The phloem parenchyma strands are comprised of 6-8 vertically elongated cells. These are of two types - tanniniferous and crystalliferous. The phloem ray cells are heterogeneous and uni- to multi-seriate in A. venenata whereas similarly found in A. neriifolia (Figure 1C, F). The length of phloem ray cells experienced considerable elongation over the mother cells. The axial parenchyma strands also become twice as long in A. venenata but remain the same length in A. neriifolia when compared to fusiform initials.
The axial system of secondary xylem is made up of the usual 3 types of cells, vessel elements, xylem fibres and xylem parenchyma; the radial system consists of ray cells. Vessel elements are distributed in small multiples of 2-9 cells, thick walled, with pitted thickening (vestured intervessel pits) on the radial walls (Figure 3A, B, E, F). They bear slightly oblique simple perforation plates (Figure 3B, E). Vessel arrangement was observed to be diffuse both in A. venenata and A. neriifolia. The xylem fibres are elongated aseptate, thick walled, narrow lumened and with tapering apices. The axial parenchyma is fairly thin walled, aliform and confluent (Figure 3D). The parenchyma in the ray system is formed of heterogeneous, uni- and bi-seriate rays in A. neriifolia whereas these are uni- to multi-seriate in A. venenata (Figure 3C, E). The vessel elements are slightly shorter in both species: A. venenata (732.54 µm) and A. neriifolia (712.72 µm). These are 9-10 times wider when compared to the respective fusiform initials (Tables 1, 2). The length of xylem fibres are 1299.43 µm in A. venenata and 1289.38 µm in A. neriifolia (Table 1). The dimensions of ray parenchyma cells are certainly greater than the respective ray initials in both plants.
Discussion
In the present study, the term cambium/cambial zone applies to the entire region of tissue generation that includes the xylem and phloem mother cells in addition to the initiating layer. It is fully borne out from the data presented in the tables that all the derivatives of cambium undergo changes in their dimensions during and after differentiation. Also the plant exhibits successive active and dormant phases during a calendar year. This cambium behaviour is believed to be regulated by several internal and external factors which include heredity constitution, physiological phenomenon and environmental conditions of the habitat (Philipson et al. 1971).
The sieve tube elements were shortened during differentiation from the fusiform initials, probably due to the shift of oblique end walls of the latter to a more transverse position. Some of the sieve tube elements are nearly half the length of fusiform initials indicating that transverse or some other divisions occur in the sieve tube element mother cells. These results are parallel to those observed by Esau & Cheadle (1955) for numerous species of Ailanthus, Asimina, Buxus, Cercidiphyllum, Clethra, Hypericum and others, Evert, 1963 (Pyrus malus); Ghouse & Yunus, 1975 (Dalbergia sps.); Khan (1977, personal communication) (Psidium guajava); Iqbal & Ghouse, 1979 (Prosopis spicigera); Khan (1980, personal communication) (Callistemon citrinus, Eucalyptus maculata and Eugenia jambolana); as well as of Zahur (1959) for several dicotyledons. Companion cells are half the width and 1/3 to 1/4 the length when compared to their progenitors. The ray initials also experienced much elongation over their mother cells while no appreciable widening was observed in the respective progenitors in both trees investigated. Phloem and xylem parenchyma strands do not show any significant change and are more or less similar in size to their mother cells.
A comparison of the length of fusiform initials and vessel elements confirms the general affinity as proposed by Butterfield (1973) and Sharma et al. (1979). The slight decrease in length of vessel elements is due to the rearrangement of the end walls as the fusiform initials have long tapering apices. The natural vessel elements have transverse or slightly oblique end walls. The xylem fibres gain maximum elongation and become almost double the length of fusiform initials as a result of intrusive growth. These results are in agreement with those of Chattaway (1936), Ghouse & Siddiqui (1976), Ghouse & Hashmi (1978), Anand et al. (1981), Paliwal & Yadav (1999), and Paliwal et al. (2002). However, Siddiqui (1983, personal communication) has reported apical intrusive growth to the extent of 3.54 and 4.4 times over the size of mother initials in Ficus infectoria and F. religiosia. Khan (1984, personal communication) has reported that the xylem fibres undergo apical intrusive growth, 5.506.33 times over the size of their mother initials in Bombax melabaricum. Ajmal (1985, personal communication) has reported xylem fibres to exhibit 5.4 and 3.23 times length increase over fusiform initials in Ficus rumphii and Sterbulus asper. But Cheadle (1937) found xylem fibres to grow 15-40 times over the size of their mother initials, whereas Anand et al. (1978) reported xylem fibres in Dalbergia sisso to grow 8-9 times longer than their mother initials.
The present study shows an appreciable dimensional variation between the different elements of cambium and its derivative tissues although the two species are growing in same habitat. Fibres showed maximum elongation over the mother initials whereas the sieve-tube members and vessel elements showed a reduction in length with many times increase in width.
Received: March 13, 2006. Accepted: November 21, 2006
- Anand; Shashi, K.; Sajwan, V.S. & Paliwal, G.S. 1978. Size correlations among the cambium and its derivatives in Dalbergia sissoo Journal of Indian Botanical Society 57: 16-24.
- Anand; Shashi, K.; Singh, D.P.; Paliwal, S.P. & Paliwal, G.S. 1981. Size correlations among the cambial initials and its derivatives in Jacaranda mimosifolia D. Don. and Putranjiva roxburghii Wall. Indian Journal of Forestry 4: 179-185.
- Bannan, M.W. 1955. The vascular cambium and radial growth in Thuja occidentialis L. Canadian Journal of Botany 33: 113-138.
- Bannan, M.W. 1968. Anticlinal divisions and the organization of the conifer cambium. Botanical Gazette 129: 107-113.
- Butterfield, B.G. 1973. Variations in the size of fusiform initials and vessel members in Hoheria angustifalia Rauol. New Zealand Journal of Botany 11: 391-410.
- Butterfield, B.G. 1975. Terminology used for describing the cambium. IAWA Bulletin 1: 13-14.
- Catesson, A.M. 1964. Originie fonctionnement at variations cytologiques saisonnieres du cambium de 1" Acer pseudoplatanus L. (Aceraces) Annals of Science Nat. (Botany) 120: 229-498.
- Chattaway, M.M. 1936. The relation between fibre and cambial initial length in dicotyledonous woods. Tropical Woods 46: 16-20.
- Cheadle, V.I. 1937. Secondary growth by means of a thickening ring in certain monocotyledons. Botanical Gazette 98: 535-555.
- Cheadle, V.I.; Gifford Jr., E.M. & Esau, K. 1953. Staining combinations for phloem and contiguous tissues. Staining Technology 28: 49-53.
- Esau, K. & Cheadle, V.I. 1955. Significance of cell division differentiating secondary phloem. Acta Botanica Neerl. 1: 348-357.
- Evert, R.F. 1963. Ontogeny and structure of the secondary phloem in Pyrus malus American Journal of Botany 50: 8-37.
- Foster, A.S. 1934. The use of tannic acid and iron chloride for staining cell walls of meristematic tissues. Staining Technology 9: 91-92.
- Ghouse, A.K.M.; Yunus, M.; Farooqui, F. & Sabir, D. 1974. A simple maceration technique for the separation of sieve elements from the barks of woody plants. Current Science 43: 424-425.
- Ghouse, A.K.M. & Yunus, M. 1975. Intrusive growth in the phloem in Dalbergia Bulletin of Torrey Botanical Club 102: 14-17.
- Ghouse, A.K.M. & Siddiqui, F.A. 1976. Cell length variation in phloem fibres within the barks of some tropical fruit, Annona squamosa, Emblica officinalis, Feronia limonia and Grewia asiatica Phytomorphology 26: 109111.
- Ghouse, A.K.M. & Hashmi, S. 1978. Occurrence of intrusive growth in phloem fibres of some evergreen and deciduous Indian tropical trees. Journal of Indian Botanical Society 57: 366-368.
- Iqbal, M. & Ghouse, A.K.M. 1979. Anatomical changes in Prosopis spicigera with growing girth of stem. Phytomorphology 29: 204-211.
- Iqbal, M. 1990. The vascular cambium John Wiley & Sons Inc. New York. England, Research Studies Press Ltd. Tauton, Somerset.
- Johansen, D.A. 1940. Plant microtechnique New York, Mc. Graw Hill.
- Mahmood, A. 1968. Cell grouping and primary wall generations in the cambial zone, xylem and phloem in Pinus Australian Journal of Botany 16: 177-196.
- Murmanis, L. 1970. Locating the initials in the vascular cambium of Pinus strobus L. by electron microscopy. Wood Science & Technology 4: 1-14.
- Newman, I.V. 1956. Patterns in meristems of vascular plants. I. Cell partition in living apices and in the cambial zone in relation to the concepts of initial cells and apical cells. Phytomorphology 6: 1-19.
- Paliwal, S.P. & Yadav, A. 1999. Variations in the size of fusiform initials, xylem fibres and vessel elements along the axis and across in the stem of Leucaena leucocephala (Lam.) De. Wit. PCINL 29: 47-51.
- Paliwal, S.P.; Rajat; Usha; Yadav; Anita & Yadav, A. 2002. Size correlations among cambial initials and their derivatives in Haldina cordifolia (Roxb.). Ridsdale National Conference Palaco Botanical Society Lucknow, Nov. 50: 28-29.
- Philipson, W.R.; Word J.M. & Butterfield, B.G. 1971. The vascular cambium, its development and activity London, Chapmen & Hall.
- Rao, K.S.; Srinivas, T. & Rajput, K.S. 1996. Seasonal anatomy of vascular cambium in young branches of Bombax ceiba Brume. Acta Botanica Indica 24: 17-20.
- Raatz, W. 1892. Die Stabbildungen in Secundaren Holzkorper der Baume und die initial entheorine. Jahrbücher für Wissenschaftliche Botanik 23: 567-636.
- Sharma, D.D.; Sharma, H.K. & Paliwal, G.S. 1979. Size correlations among cambial initials and their derivatives in Polyalthia longiafolia Acta Society of Botany Poland 48: 93-98.
- Wilson, B.F.; Wodzicki, T.J. & Zahver, R. 1966. Differentiation of the cambial derivatives: proposed terminology. Forest Science 12: 438-440.
- Zahur, M.S. 1959. Comparative study of secondary phloem of 423 species of woody dicotyledons belonging to 85 families. Cornell University Agricultural Experimental Statement Memoir 358
- Zimmermann, M.H. & Brown, C.L. 1971. Trees: structure and function Berlin, Springer-Verlag.
Publication Dates
-
Publication in this collection
22 Nov 2007 -
Date of issue
Sept 2007
History
-
Accepted
21 Nov 2006 -
Received
13 Mar 2006