Abstract
Background
In spite of proven effectiveness of implantable cardioverter defibrillators (ICDs), shock therapy delivered by the device may result in increased levels of anxiety and depression, leading to deleterious effects on quality of life.
Objective
To carry out the translation, cross-cultural adaptation and validation of the Florida Shock Anxiety Scale (FSAS) scale into Brazilian Portuguese.
Methods
In this psychometric study, construct validity was performed by exploratory (EFA) and confirmatory (CFA) factor analyses, and by item response theory (IRT). The adjustment indexes of the CFA were: Robust Mean-Scaled Chi Square/df NNFI, CFI (Comparative Fit Index), GFI (Goodness Fit Index), AGFI (Adjusted Goodness Fit Index), RMSEA (Root Mean Square Error of Approximation) and RMSR (Root Mean Square of Residuals). Reliability was evaluated through Cronbach’s Alpha, McDonald’s Omega and Greatest Lower Bound (GLB). The analyses were carried out with the programs SPSS 23 and Factor 10.8.01. A 5 percent significance level was used.
Results
The final Portuguese version of the FSAS was administered to 151 ICD patients, with a mean age of 55.7 ± 14.1 years, and predominantly male. The parallel analysis indicated that the FSAS is unidimensional, with an explained variance of 64.4%. The correlations ranged from 0.31 to 0.77, factor loadings from 0.67 to 0.86, and communalities from 0.46 to 0.74. The adjustment indexes of the CFA were above the quality threshold. Satisfactory reliability evidence was provided by the FSAS.
Conclusions
The FSAS-Br showed consistent validity and reliability evidence. Therefore, it can be used in ICD patients in Brazil. (Arq Bras Cardiol. 2020; 114(5):764-772)
Implantable defibrillator; Shock therapies; Arrhythmias; Anxiety; Psychometric
Resumo
Fundamento
A despeito da comprovada efetividade do cardioversor-desfibrilador implantável (CDI), as terapias de choque deflagradas pelo dispositivo podem causar níveis elevados de ansiedade e depressão, provocando efeitos deletérios na qualidade de vida.
Objetivo
Realizar a tradução, adaptação transcultural e validação do instrumento Florida Shock Anxiety Scale (FSAS) para a língua portuguesa falada no Brasil.
Métodos
Nesse estudo psicométrico, a validade de construto foi realizada pela análise fatorial exploratória (AFE) e confirmatória (AFC) e pela Teoria de Resposta ao Item. Os índices de ajustamento da AFC foram: Robust Mean-Scaled Chi Square/df NNFI, CFI (Comparative Fit Index), GFI (Goodness Fit Index), AGFI (Adjusted Goodness Fit Index), RMSEA (Root Mean Square Error of Approximation) e RMSR (Root Mean Square of Residuals). A confiabilidade foi verificada pelo Alfa de Cronbach, Ômega de McDonald e Greatest Lower Bound. As análises foram realizadas no SPSS 23.0 e Factor 10.8.01, com nível de significância de 5%.
Resultados
A versão final em português do FSAS foi administrada em 151 portadores de CDI, com idade média de 55,7 ± 14,1 anos e predomínio do sexo masculino. A análise paralela indicou que o FSAS é unidimensional, com variância explicada de 64,4%. As correlações variaram de 0,31 a 0,77; as cargas fatoriais de 0,67 a 0,86 e as comunalidades de 0,46 a 0,74. Os índices de ajustamento da AFC estabeleceram-se acima dos limites de qualidade. Encontramos evidências satisfatórias de confiabilidade da escala FSAS.
Conclusão
O instrumento FSAS-Br apresentou evidências consistentes de validade e confiabilidade, podendo, portanto, ser utilizado em portadores de CDI do Brasil. (Arq Bras Cardiol. 2020; 114(5):764-772)
Desfibrilador implantável; Terapias de choque; Arritmias; Ansiedade; Qualidade de vida; Validade; Confiabilidade; Psicometria
Introduction
Nowadays, there are no doubts regarding the role of the implantable cardioverter defibrillator (ICD) for prevention of sudden cardiac death, especially among patients with ventricular dysfunction and arrhythmogenic genetic diseases.11. Al-Khatib SM, Friedman P, Ellenbogen KA. Defibrillators: Selecting the Right Device for the Right Patient. Circulation. 2016;134(18):1390-404.
2. Colquitt JL, Mendes D, Clegg AJ, Harris P, Cooper K, Picot J, Bryant J. Implantable cardioverter defibrillators for the treatment of arrhythmias and cardiac resynchronization therapy for the treatment of heart failure: systematic review and economic evaluation. Health Technol Assess. 2014;18(56):1-560.-33. Winkle RA. Evolution of the implantable cardioverter-defibrillator: from bullets to BBs. J Am Coll Cardiol 2012;60(23):2399-401. Due to its proven efficacy in identifying and correcting potentially lethal ventricular tachyarrhythmias, the number of ICD implantations has increased significantly worldwide, and more than 250,000 procedures are performed every year.44. Camm AJ, Nisam S. European utilization of the implantable defibrillator: has 10 years changed the “enigma”? Europace 2010;12:1063-9.
The primary purpose of ICD is to correct potentially fatal ventricular arrhythmias by delivering low- or high-energy therapy. Low-energy therapy, known as antitachycardia pacing or antitachycardia pacing (ATP), is a painless method. High-energy therapy delivers shocks of up to 40 J which, in spite of causing major discomfort, usually occur after the patient has lost consciousness, since they are applied about 15 seconds after the initiation of ventricular fibrillation or fast ventricular tachyarrhythmia. In undesirable situations, such as arrhythmias resistant to overstimulation, or in electrical storm, high-energy discharges can occur in awake patients.33. Winkle RA. Evolution of the implantable cardioverter-defibrillator: from bullets to BBs. J Am Coll Cardiol 2012;60(23):2399-401.,55. Wilkoff BL, Fauchier L, Stiles MK, Morillo CA, Al-Khatib SM, Almendral J, et al. 2015 HRS/EHRA/APHRS/ SOLAECE Expert consensus statement on optimal implantable cardioverter-defibrillator programming and testing. Heart Rhythm. 2016;13(2):e50–e86.,66. Daubert JP, Zareba W, Cannom DS, McNitt S, Rosero SZ, Wang P, et al. Inappropriate implantable cardioverter-defibrillator shocks in MADIT II: frequency, mechanisms, predictors, and survival impact. J Am Coll Cardiol. 2008;51(14):1357-65.
It is estimated that the chances of ICD patients will need appropriate electric shocks for primary prevention of sudden cardiac death varies between 2 and 15% per year.55. Wilkoff BL, Fauchier L, Stiles MK, Morillo CA, Al-Khatib SM, Almendral J, et al. 2015 HRS/EHRA/APHRS/ SOLAECE Expert consensus statement on optimal implantable cardioverter-defibrillator programming and testing. Heart Rhythm. 2016;13(2):e50–e86.
6. Daubert JP, Zareba W, Cannom DS, McNitt S, Rosero SZ, Wang P, et al. Inappropriate implantable cardioverter-defibrillator shocks in MADIT II: frequency, mechanisms, predictors, and survival impact. J Am Coll Cardiol. 2008;51(14):1357-65.
7. Sood N, Ruwald AC, Solomon S, Daubert JP, McNitt S, Polonsky B, et al. Association between myocardial substrate, implantable cardioverter defibrillator shocks and mortality in MADIT-CRT. Eur Heart J. 2014;35(2):106-15.-88. Desai H, Aronow WS, Ahn C, Gandhi K, Hussain S, Lai HM, et al. Risk factors for appropriate cardioverter-defibrillator shocks, inappropriate cardioverter-defibrillator shocks, and time to mortality in 549 patients with heart failure. Am J Cardiol. 2010;105(9):1336-8. On the other hand, when the ICD is used for secondary prevention, the incidence of shock therapies may vary between 35 and 53%, within the first year after implantation.55. Wilkoff BL, Fauchier L, Stiles MK, Morillo CA, Al-Khatib SM, Almendral J, et al. 2015 HRS/EHRA/APHRS/ SOLAECE Expert consensus statement on optimal implantable cardioverter-defibrillator programming and testing. Heart Rhythm. 2016;13(2):e50–e86.
6. Daubert JP, Zareba W, Cannom DS, McNitt S, Rosero SZ, Wang P, et al. Inappropriate implantable cardioverter-defibrillator shocks in MADIT II: frequency, mechanisms, predictors, and survival impact. J Am Coll Cardiol. 2008;51(14):1357-65.
7. Sood N, Ruwald AC, Solomon S, Daubert JP, McNitt S, Polonsky B, et al. Association between myocardial substrate, implantable cardioverter defibrillator shocks and mortality in MADIT-CRT. Eur Heart J. 2014;35(2):106-15.-88. Desai H, Aronow WS, Ahn C, Gandhi K, Hussain S, Lai HM, et al. Risk factors for appropriate cardioverter-defibrillator shocks, inappropriate cardioverter-defibrillator shocks, and time to mortality in 549 patients with heart failure. Am J Cardiol. 2010;105(9):1336-8. Despite the high level of technological sophistication of ICDs, unfortunately, there is the risk that the patient may receive inappropriate shock deliveries as a result of erroneous discrimination between supraventricular and ventricular tachyarrhythmias. On these occasions, the sensation reported is a painful and distressing experience.99. Ahmad M, Bloomstein L, Roelke M, Bernstein AD, Parsonnet V. Patients’ attitudes towards implanted defibrillator shocks. Pacing Clin Electrophysiol 2000; 23(6):934–8.
10. Costa R, Silva KR, Mendonça RC, Nishioka SA, Siqueira SF, Tamaki WT, et al. Incidence of shock and quality of life in young patients with implantable cardioverter-defibrillator. Arq Bras Cardiol. 2007;88(3):258-64.
11. Pedersen SS, Sears SF, Burg MM, Van Den Broek KC. Does ICD indication affect quality of life and levels of distress? Pacing Clin Electrophysiol. 2009; 32(2):153-6.
12. Sears SF, Conti JB. Quality of life and psychological functioning of ICD patients. Heart 2002; 87(5): 488–93.
13. Carroll SL, Strachan PH, de Laat S, Schwartz L, Arthur HM. Patients’ decision making to accept or decline an implantable cardioverter defibrillator for primary prevention of sudden cardiac death. Health Expect 2013;16(1):69-79-1414. da Silva KR, Costa R, Rodrigues CG, Schasechter A, Nobre MC, Passman R, et al. Quality of life in patients with implantable cardioverter-defibrillator: systematic review of randomized controlled trials. Eur J Cardiovasc Nurs. 2018;17(3):196-206.
ICD patients live with the expectation that, at any moment, the device will deliver shock therapies to interrupt ventricular arrhythmias resulting from their heart disease. Thus, although they recognize the benefits of the treatment, some patients may present with anxiety, depression, mood disorders, post-traumatic stress disorder, as well as fear that the device will not operate in crucial situations.99. Ahmad M, Bloomstein L, Roelke M, Bernstein AD, Parsonnet V. Patients’ attitudes towards implanted defibrillator shocks. Pacing Clin Electrophysiol 2000; 23(6):934–8.
10. Costa R, Silva KR, Mendonça RC, Nishioka SA, Siqueira SF, Tamaki WT, et al. Incidence of shock and quality of life in young patients with implantable cardioverter-defibrillator. Arq Bras Cardiol. 2007;88(3):258-64.
11. Pedersen SS, Sears SF, Burg MM, Van Den Broek KC. Does ICD indication affect quality of life and levels of distress? Pacing Clin Electrophysiol. 2009; 32(2):153-6.
12. Sears SF, Conti JB. Quality of life and psychological functioning of ICD patients. Heart 2002; 87(5): 488–93.
13. Carroll SL, Strachan PH, de Laat S, Schwartz L, Arthur HM. Patients’ decision making to accept or decline an implantable cardioverter defibrillator for primary prevention of sudden cardiac death. Health Expect 2013;16(1):69-79-1414. da Silva KR, Costa R, Rodrigues CG, Schasechter A, Nobre MC, Passman R, et al. Quality of life in patients with implantable cardioverter-defibrillator: systematic review of randomized controlled trials. Eur J Cardiovasc Nurs. 2018;17(3):196-206. On the other hand, ICD implantation has been reported to provide the patient with a great sense of safety, considering the device’s capacity to interrupt unexpected episodes of potentially fatal ventricular arrhythmias.1010. Costa R, Silva KR, Mendonça RC, Nishioka SA, Siqueira SF, Tamaki WT, et al. Incidence of shock and quality of life in young patients with implantable cardioverter-defibrillator. Arq Bras Cardiol. 2007;88(3):258-64.
11. Pedersen SS, Sears SF, Burg MM, Van Den Broek KC. Does ICD indication affect quality of life and levels of distress? Pacing Clin Electrophysiol. 2009; 32(2):153-6.
12. Sears SF, Conti JB. Quality of life and psychological functioning of ICD patients. Heart 2002; 87(5): 488–93.
13. Carroll SL, Strachan PH, de Laat S, Schwartz L, Arthur HM. Patients’ decision making to accept or decline an implantable cardioverter defibrillator for primary prevention of sudden cardiac death. Health Expect 2013;16(1):69-79-1414. da Silva KR, Costa R, Rodrigues CG, Schasechter A, Nobre MC, Passman R, et al. Quality of life in patients with implantable cardioverter-defibrillator: systematic review of randomized controlled trials. Eur J Cardiovasc Nurs. 2018;17(3):196-206.
In face of the concern about the deleterious effects of ICD on psychosocial adaptation, an scale was specifically developed to assess the level of anxiety related to the presence of ICD and to the shocks delivered by the device, for use both in clinical practice and in the context of scientific research.1515. Kuhl EA, Dixit NK, Walker RL, Conti JB, Sears SF. Measurement of patient fears about implantable cardioverter defibrillator shock: an initial evaluation of the Florida Shock Anxiety Scale. Pacing Clin Electrophysiol. 2006;29(6):614-8.,1616. Ford J, Finch JF, Woodrow LK, Cutitta KE, Shea J, Fischer A, et al. The Florida Shock Anxiety Scale (FSAS) for patients with implantable cardioverter defibrillators: testing factor structure, reliability, and validity of a previously established measure. Pacing Clin Electrophysiol. 2012;35(9):1146-53. The Florida Shock Anxiety Scale (FSAS) quickly achieved wide international acceptance, and has been translated and validated in several countries (Netherlands,1717. Versteeg H, Starrenburg A, Denollet J, Palen Jv, Sears SF, Pedersen SS. Monitoring device acceptance in implantable cardioverter defibrillator patients using the Florida Patient Acceptance Survey. Pacing Clin Electrophysiol. 2012;35(3):283-93. Denmark,1818. Pedersen SS, Spindler H, Johansen JB, Mortensen PT, Sears SF. Correlates of patient acceptance of the cardioverter defibrillator: cross-validation of the Florida Patient Acceptance Survey in Danish patients. Pacing Clin Electrophysiol. 2008;31(9):1168-77Poland,1919. Kochańska A, Zarzycka B, Świątecka G, Majkowicz M, Kozłowski D, Raczak G.Quality of life in patients with an implantable cardioverter-defibrillator – the significance of clinical factors. Arch Med Sci 2008; 4(4):409-16. China,2020. Chair SY, Lee CK, Choi KC, Sears SF. Quality of life outcomes in chinese patients with implantable cardioverter defibrillators. Pacing Clin Electrophysiol. 2011;34(7):858-67 Norway,2121. Morken IM, Isaksen K, Karlsen B, Norekvål TM, Bru E, Larsen AI. Shock anxiety among implantable cardioverter defibrillator recipients with recent tachyarrhythmia. Pacing Clin Electrophysiol. 2012;35(11):1369-76 Turkey2222. Oz Alkan H, Enç N. Validity and reliability of the Florida Patient Acceptance Survey and Florida Shock Anxiety Scale in Turkish patients with implantable cardioverter defibrillation. Int J Med Res Health Sci 2017,6(10):21-32.), with consistent results.
Objectives
The purpose of the present study was to assess the psychometric properties of the Brazilian version of the FSAS for ICD patients.
Methods
Study design
This study was conducted in a high-complexity cardiology hospital and it was approved by that hospital’s Committee of Ethics in Research. All subjects signed a free and informed consent form.
Study location and ethical aspects
This was a psychometric study of cross-cultural adaptation and validation of the FSAS.
The Florida Shock Anxiety Scale (FSAS)
The FSAS was developed in 2006 in the United States to provide a quantitative measure of ICD shock-related anxiety. The instrument consists of 10 items, with five response options (“not at all”,”rarely”,”some of the time”,”most of the time”,”all the time”), corresponding to a 5-point Likert scale.1515. Kuhl EA, Dixit NK, Walker RL, Conti JB, Sears SF. Measurement of patient fears about implantable cardioverter defibrillator shock: an initial evaluation of the Florida Shock Anxiety Scale. Pacing Clin Electrophysiol. 2006;29(6):614-8.,1616. Ford J, Finch JF, Woodrow LK, Cutitta KE, Shea J, Fischer A, et al. The Florida Shock Anxiety Scale (FSAS) for patients with implantable cardioverter defibrillators: testing factor structure, reliability, and validity of a previously established measure. Pacing Clin Electrophysiol. 2012;35(9):1146-53.
Questions are related to patients’ fear or anxiety caused by the expectation that the device may deliver shock therapies and to the behavioral changes (not engaging in physical exercise or in sexual activity, or not getting angry or upset, for instance) to avoid the occurrence of ICD therapies.
The FSAS total score is determined by the sum of all items, with a maximum score of 50 points. The higher the score, the higher the anxiety level. The items receiving three points or more should be considered the most critical aspects.1717. Versteeg H, Starrenburg A, Denollet J, Palen Jv, Sears SF, Pedersen SS. Monitoring device acceptance in implantable cardioverter defibrillator patients using the Florida Patient Acceptance Survey. Pacing Clin Electrophysiol. 2012;35(3):283-93.,1818. Pedersen SS, Spindler H, Johansen JB, Mortensen PT, Sears SF. Correlates of patient acceptance of the cardioverter defibrillator: cross-validation of the Florida Patient Acceptance Survey in Danish patients. Pacing Clin Electrophysiol. 2008;31(9):1168-77The instrument can be self-administered or administered by interview.
Stage 1 – Translation and cross-cultural adaptation of the instrument
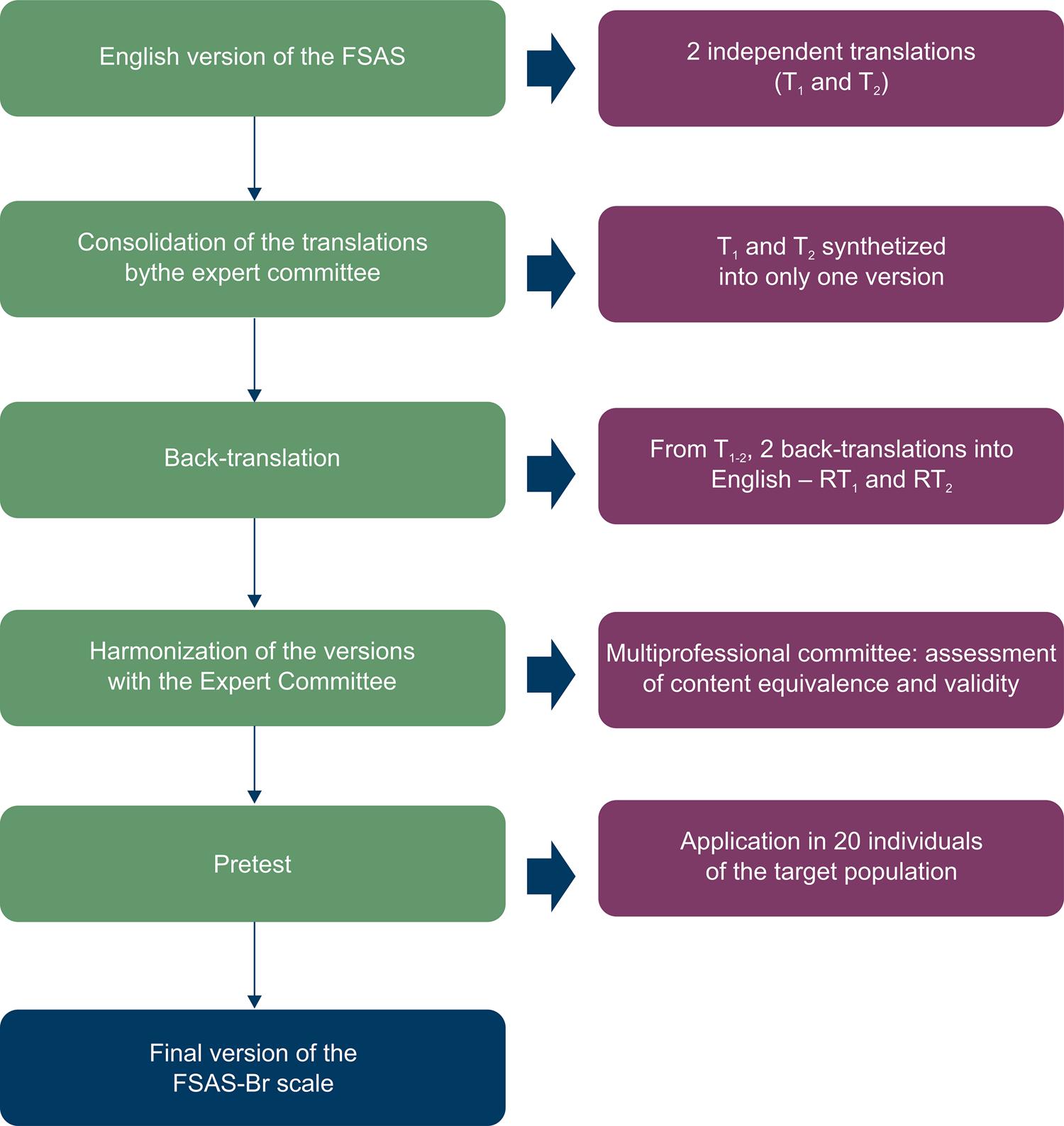
The cross-cultural adaptation process of the FSAS followed international guidelines and included five stages: (1) Translation by two independent translators; (2) Synthesis of the translations; (3) Back-translation; (4) Harmonization of the translations by the expert committee; (5) Pretest with the target population; (6) Pretest review and final translation.2323. Wild D, Grove A, Martin M. Principles of good practice for the translation and cultural adaptation process for patient-reported outcomes (PRO) measures: report of the ISPOR task force for translating adaptation. Value Health> 2005;8(2):94-104.
24. Beaton D, Bombardier C, Guillemin F, Ferraz MB. Guidelines for the process of cross-cultural adaptation of self-report measures. Spine (Phila Pa 1976). 2000;25(24):3186-91.-2525. Sousa VD, Rojjanasrirat WJ. Translation, adaptation and validation of instruments or scales for use in cross-cultural health care research: a clear and user-friendly guideline. Eval Clin Pract. 2011;17(2):268-74.The expert committee was composed by professionals of the area of artificial cardiac stimulation and by nurses.
The translation of the original instrument into Portuguese was performed by two independent Brazilian translators, proficient both in the Portuguese and English languages. In this stage, the translations produced by the two independent translators (T1 and T2) were reconciled into one version (T1-2), after discussion with the expert committee. Working from the final version and blind to the original version, two bilingual teachers carried out the back-translations (RT1 and RT2). The aim of this stage was to measure the semantic and idiomatic consistency of the translations produced in the first stage. Finally, a new meeting was held with the expert committee to review all the cross-cultural adaptation process and undertake the harmonization across versions, thus obtaining the pre-final version of the instrument (Figure 1).
The pretest version was administered in a convenience sample of 20 ICD patients, aged between 18 and 80 years, All patients were recruited during an outpatient cardiovascular clinic appointment, and were at least 6 months postimplant. The pretest was conducted to identify and correct possible translation problems. Following self-completion of the instrument, a clarifying interview was held to verify the existence of irrelevant or hardly understandable items, as well as to measure the understanding of each item of the instrument. It was established that the translation would be reviewed or reformulated if less than 80% of the participants were able to understand the items.
Stage 2 – construct validation of the FSAS
The final Portuguese version of the FSAS was administered to a convenience sample of 151 participants with the following characteristics: (1) adults, aged between 18 and 80 years, of both sexes and with any education level; (2) ICD implanted for more than 6 months; (3) capable of understanding and answering the questionnaire used in the study; (4) having agreed to participate in the study by signing the informed consent form. We did not include in the study patients presenting at least one of the following situations: (1) indication for cardiac transplantation; (2) ongoing pregnancy; (3) malignant neoplasia.
Patients were selected consecutively, during outpatient care or by visits to the inpatient unit of our institution. Individuals who met the eligibility criteria were invited to answer the FSAS questionnaire. At the same time, demographic, clinical and ICD data were collected by using electronic case report forms developed in REDCap2626. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) - A metadata-driven methodology and workflow process for providing translational research informatics support, J Biomed Inform. 2009;42(2):377-81. (Research Electronic Data Capture) hosted at the hospital’s server.
Sample size
Sample size determination for psychometric studies is usually calculated based on the number of items of the instrument. Some studies have demonstrated that a ratio of 20:1 or greater, that is, 20 participants per item would be ideal. However, ratios of 10:1 are sufficient to allow for adequate analysis. Thus, a minimum sample number of 150 patients was established.2525. Sousa VD, Rojjanasrirat WJ. Translation, adaptation and validation of instruments or scales for use in cross-cultural health care research: a clear and user-friendly guideline. Eval Clin Pract. 2011;17(2):268-74.
Statistical Analysis
Descriptive analysis
Detailed descriptive analysis was performed, using measures of central tendency (mean, standard deviation, median, trimmed average, confidence intervals and interquartile interval). The Kolmogorov-Smirnov (KS) test was used to test the normality of each item in the questionnaire, whereas the Mardia test was employed to assess multivariate normality.
All analyses were performed using SPSS 23 statistical package software and Factor 10.8.01, adopting a level of significance of 5%.
Construct validity and dimensionality
In this study, we conducted an exploratory factor analysis (EFA) and a confirmation factor analysis (CFA) to verify the dimensionality of the FSAS in its Portuguese version.
The dimensionality testing was performed using Robust Parallel Analysis (RPA) through the Optimal implementation of Parallel Analysis (PA) with minimum rank factor analysis (MRFA), which minimizes the common variance of residuals.2727. Timmerman ME, Lorenzo-Seva U. Dimensionality assessment of ordered polytomous items with parallel analysis. Psychol Methods. 2011;16(2):209-20.,2828. Lorenzo-Seva, U. How to report the percentage of explained common variance in exploratory factor analysis. Tarragona (Italia):Universita Technical Report. Department of Psychology, Universitat Rovira i Virgili, Tarragona (Italia): Universita Rovira-Virgili; 2013, (Technical Report).The robustness of the test was determined from the association of a bootstrap with sample extrapolation to 5,000. Factor extraction was done initially with Robust Unweighted Least Squares (RULS), which reduces the matrix of residuals.2929. Briggs NE, MacCallum RC. Recovery of Weak Common Factors by Maximum Likelihood and Ordinary Least Squares Estimation. Multivariate Behav Res. 2003;38(1):25-56.
Item Response Theory
Item discrimination index was used (a), which measures the association strength between the item and the latent variable, and whose interpretation is similar to factor loading in the exploratory factor analysis.
Quality parameters of the translated and adapted versions of the FSAS
To adequate the items and the models, the following criteria were taken into account: the explained variance of the model (60 to 70%), factor loading values (> 0.50), communalities (> 0.40) and item discrimination, and collinearity and multicollinearity problems (factor loads ranging from 0.80 to 0.85).
Indices of adjustment obtained in the Confirmatory Factor Analysis
The model adjustment indices and their respective expected values were: Robust Mean-Scaled Chi Square/df NNFI (Non-Normed Fit Index > 0.93), CFI (Comparative Fit Index > 0.94), GFI (Goodness Fit Index > 0.95), AGFI (Adjusted Goodness Fit Index > 0.93), RMSEA (Root Mean Square Error of Approximation < 0.07) and RMSR (Root Mean Square of Residuals < 0.08).2929. Briggs NE, MacCallum RC. Recovery of Weak Common Factors by Maximum Likelihood and Ordinary Least Squares Estimation. Multivariate Behav Res. 2003;38(1):25-56.
30. Matt C. Howard. A Review of Exploratory Factor Analysis Decisions and Overview of Current Practices: What We Are Doing and How Can We Improve? Int J Hum Comput Interact. 2016;32(1):51-62.-3131. Kahn JH. Factor analysis in counseling psychology research, training, and practice: principles, advances, and applications. Couns Psychol. 2006;34(5):684-718.
Reliability
Three indicators were adopted to assess the reliability of the Brazilian version of the FSAS questionnaire: Coefficient Alpha (“Cronbach’s Alpha”), Omega and the Greatest Lower Bound (GLB).
Results
The final version of the FSAS
The stages of the translation and cross-cultural adaptation resulted in similar versions of the FSAS instrument. The synthesis of the translations was quite concise and combined the most coherent elements of each translation. The back-translations confirmed the good quality of the translations and the synthesis process carried out in the initial stages.
A total of 20 ICD patients, with a mean of age 55.6 ± 6.8 years, took part in the pretest. Of these, 50% were female, 50% were white and 30% had studied up to High School. All participants reported that the items were relevant, easy to understand and that the response options were clear. No modifications in the instrument were required. Table 1 shows the instrument items in its English and Portuguese versions.
Psychometric properties of the FSAS
Population composition
In this stage of the study, 151 ICD patients, with a mean of 55.7±14.1 years (range, 19- 80 years), were included. There was a male sex predominance, which corresponded to 64% of the cases. Most patients were white (85.4%) and 49% had attended Middle School (Table 2).
Among the cardiac diseases, there was a predominance of Chagas disease, which was present in 30.5% of the cases, followed by ischemic cardiomyopathy in 25.2%. Brugada syndrome and congenital long-QT syndrome (LQTS) were identified in 4.6 and 3.3% of patients, respectively.
Baseline assessment showed that most patients were in the New York Heart Association (NYHA) functional classes I (37.1%) and II (47.7%). Left ventricular function was determined by bidimensional transthoracic echocardiography and ranged from 18 to 77%, with a median of 35%.
Only 29.1% of the patients did not present any associated comorbidities. Dyslipidemia and arterial hypertension were the most frequent comorbidities, being present in 51.4% and 49.5% of patients, respectively. Atrial fibrillation was present in 27.1% of the individuals studied (Table 2).
As expected, 80.1% of the indications for ICD were r secondary prophylaxis of sudden cardiac death. In Brazil, due to lack of resources, ICD implantation is still underused for primary prophylaxis of sudden cardiac death.
Descriptive analysis of the FSAS items
Through descriptive analysis of the instrument items, it was possible to identify that normality of distribution was violated, indicating, therefore, the need for polychoric correlation, instead of Pearson’s correlation coefficient.
The means of the instrument items ranged from 1.5 to 2.9. The FSAS average score was 22.8 ± 11.1, with a median of 20 points and variation of 10 to 50 points. There was no impact of extreme values on the mean (Table 3).
Construct validity and dimensionality of the FSAS
The values obtained from the Kaiser-Meyer-Olkin index (KMO= 0.88), the Bartlett’s sphericity test (X2= 565.5, df= 45; p<0.001) and the matrix determinant (0.0206 (p<0.0001)) revealed a significant correlation between the items, which confirmed the adequacy of the EFA.
The parallel analysis indicated the existence of only one dimension for the instrument. Moreover, this item set can explain 64.4% of latent variable (above the values recommended in the literature).2929. Briggs NE, MacCallum RC. Recovery of Weak Common Factors by Maximum Likelihood and Ordinary Least Squares Estimation. Multivariate Behav Res. 2003;38(1):25-56.
30. Matt C. Howard. A Review of Exploratory Factor Analysis Decisions and Overview of Current Practices: What We Are Doing and How Can We Improve? Int J Hum Comput Interact. 2016;32(1):51-62.-3131. Kahn JH. Factor analysis in counseling psychology research, training, and practice: principles, advances, and applications. Couns Psychol. 2006;34(5):684-718. The eigenvalue criteria also indicated only one dimension, with a an eigenvalue of of 6.08. The fact that the instrument was unidimensional waived requirements for methods of matrix factor rotation. Unidimensionality indicated the use of the normal-ogive graded response IRT model, which is more adequate for a unidimensional polytomous model.3131. Kahn JH. Factor analysis in counseling psychology research, training, and practice: principles, advances, and applications. Couns Psychol. 2006;34(5):684-718.
Table 4 presents the factor loads, which ranged from 0.67 to 0.86, representing excellent levels of adherence of the items to the latent variable, greater than the minimum criterion of 0.50, with no evidence of multicollinearity. The unidimensionality ruled out the possibility of cross-loading. Communalities varied between 0.46 and 0.74, with all the items above the threshold of 0.40. For item discrimination (a), the values ranged from 0.91 to 1.71, also indicating good adherence to the latent variable and corroborating the data obtained from factor loading.
The CFA revealed good adjustment to the unidimensional model, with values similar to those recommended by the literature: Robust Mean and Variance-Adjusted Chi Square X2/ df (35) = 40.40; p < 0.243; NNFI= 0.997; CFI= 0,997; GFI= 0.986; AGFI= 0.982. The residual indicators were at good levels (RMSEA= 0.032; RMSR= 0.077), showing little difference between the original matrix and the matrix generated from factor loadings.3131. Kahn JH. Factor analysis in counseling psychology research, training, and practice: principles, advances, and applications. Couns Psychol. 2006;34(5):684-718.
Reliability of the FSAS-Br
Satisfactory reliability evidence was provided by the FSAS-Br scale, with a Cronbach’s alpha coefficient of 0.92, a McDonald’s Omega coefficient of 0.92 and GLB of 0.98.
Discussion
In the present study, we described the translation and cross-cultural adaptation process of a brief scale designed to provide a quantitative measure of ICD shock-related anxiety, folllowing international methodological standards.2323. Wild D, Grove A, Martin M. Principles of good practice for the translation and cultural adaptation process for patient-reported outcomes (PRO) measures: report of the ISPOR task force for translating adaptation. Value Health> 2005;8(2):94-104.
24. Beaton D, Bombardier C, Guillemin F, Ferraz MB. Guidelines for the process of cross-cultural adaptation of self-report measures. Spine (Phila Pa 1976). 2000;25(24):3186-91.-2525. Sousa VD, Rojjanasrirat WJ. Translation, adaptation and validation of instruments or scales for use in cross-cultural health care research: a clear and user-friendly guideline. Eval Clin Pract. 2011;17(2):268-74. The final translation of the FSAS into Brazilian Portuguese (FSAS-Br) presented conceptual, semantic, cultural and measurement equivalences compared to the original items in English.1515. Kuhl EA, Dixit NK, Walker RL, Conti JB, Sears SF. Measurement of patient fears about implantable cardioverter defibrillator shock: an initial evaluation of the Florida Shock Anxiety Scale. Pacing Clin Electrophysiol. 2006;29(6):614-8.,1616. Ford J, Finch JF, Woodrow LK, Cutitta KE, Shea J, Fischer A, et al. The Florida Shock Anxiety Scale (FSAS) for patients with implantable cardioverter defibrillators: testing factor structure, reliability, and validity of a previously established measure. Pacing Clin Electrophysiol. 2012;35(9):1146-53.
Efforts were made to include patients with different sociodemographic profiles and various types of underlying heart diseases to ensure heterogeneous representation, aiming at providing the best calibration of the items. Thus, patients with different ICD types (ventricular, atrioventricular or associated with cardiac resynchronization therapy) were included, as well as patients with indications for primary or secondary prophylaxis of sudden cardiac death. Notwithstanding, the most common kinds of heart disease among these patients’ profiles have also been contemplated, with expressive prevalence of Chagas Disease, ischemic and hypertrophic cardiomyopathy.
In the international scenario, the FSAS scale has been widely used in different scenarios, since it presents good sensitivity to identify the level of ICD shock-related anxiety and requires reduced time for completion.1515. Kuhl EA, Dixit NK, Walker RL, Conti JB, Sears SF. Measurement of patient fears about implantable cardioverter defibrillator shock: an initial evaluation of the Florida Shock Anxiety Scale. Pacing Clin Electrophysiol. 2006;29(6):614-8.
16. Ford J, Finch JF, Woodrow LK, Cutitta KE, Shea J, Fischer A, et al. The Florida Shock Anxiety Scale (FSAS) for patients with implantable cardioverter defibrillators: testing factor structure, reliability, and validity of a previously established measure. Pacing Clin Electrophysiol. 2012;35(9):1146-53.
17. Versteeg H, Starrenburg A, Denollet J, Palen Jv, Sears SF, Pedersen SS. Monitoring device acceptance in implantable cardioverter defibrillator patients using the Florida Patient Acceptance Survey. Pacing Clin Electrophysiol. 2012;35(3):283-93.
18. Pedersen SS, Spindler H, Johansen JB, Mortensen PT, Sears SF. Correlates of patient acceptance of the cardioverter defibrillator: cross-validation of the Florida Patient Acceptance Survey in Danish patients. Pacing Clin Electrophysiol. 2008;31(9):1168-77
19. Kochańska A, Zarzycka B, Świątecka G, Majkowicz M, Kozłowski D, Raczak G.Quality of life in patients with an implantable cardioverter-defibrillator – the significance of clinical factors. Arch Med Sci 2008; 4(4):409-16.
20. Chair SY, Lee CK, Choi KC, Sears SF. Quality of life outcomes in chinese patients with implantable cardioverter defibrillators. Pacing Clin Electrophysiol. 2011;34(7):858-67
21. Morken IM, Isaksen K, Karlsen B, Norekvål TM, Bru E, Larsen AI. Shock anxiety among implantable cardioverter defibrillator recipients with recent tachyarrhythmia. Pacing Clin Electrophysiol. 2012;35(11):1369-76-2222. Oz Alkan H, Enç N. Validity and reliability of the Florida Patient Acceptance Survey and Florida Shock Anxiety Scale in Turkish patients with implantable cardioverter defibrillation. Int J Med Res Health Sci 2017,6(10):21-32. Thus, it is important to highlight that the FSAS was not designed to assess relevant aspects of adaptation to the device and its real impact on quality of life, which makes it necessary to use other instruments to complement the assessment of these patients. In this sense, the authors who had created the FSAS developed another instrument, the Florida Patient Acceptance Survey (FPAS),3232. Burns JL, Serber ER, Keim S, Sears SF. Measuring patient acceptance of implantable cardiac device therapy: initial psychometric investigation of the Florida Patient Acceptance Survey. J Cardiovasc Electrophysiol. 2005;16(4):384-90. which aims at assessing the psychosocial adjustment of ICD patients. The results of the cross-cultural adaptation and validation process of the FPAS into Portuguese will be published in due course.
Evidence of validity of an instrument has been recommended by the scientific community as a way to check whether the instrument actually and accurately measures the latent variable of interest. In addition, it is important to analyze whether the instrument factor structure is adequately represented by its dimensionality, that is, the number of dimensions that make up the instrument of assessment.2727. Timmerman ME, Lorenzo-Seva U. Dimensionality assessment of ordered polytomous items with parallel analysis. Psychol Methods. 2011;16(2):209-20.
28. Lorenzo-Seva, U. How to report the percentage of explained common variance in exploratory factor analysis. Tarragona (Italia):Universita Technical Report. Department of Psychology, Universitat Rovira i Virgili, Tarragona (Italia): Universita Rovira-Virgili; 2013, (Technical Report).
29. Briggs NE, MacCallum RC. Recovery of Weak Common Factors by Maximum Likelihood and Ordinary Least Squares Estimation. Multivariate Behav Res. 2003;38(1):25-56.
30. Matt C. Howard. A Review of Exploratory Factor Analysis Decisions and Overview of Current Practices: What We Are Doing and How Can We Improve? Int J Hum Comput Interact. 2016;32(1):51-62.-3131. Kahn JH. Factor analysis in counseling psychology research, training, and practice: principles, advances, and applications. Couns Psychol. 2006;34(5):684-718. In the original publication of the FSAS, the authors claim that the instrument was bidimensional, presenting two dimensions: Consequence (composed of 7 items) and Trigger (composed of 3 items).1515. Kuhl EA, Dixit NK, Walker RL, Conti JB, Sears SF. Measurement of patient fears about implantable cardioverter defibrillator shock: an initial evaluation of the Florida Shock Anxiety Scale. Pacing Clin Electrophysiol. 2006;29(6):614-8.This model was not reproducible to the Brazilian version, because all analyses performed in this study supported the FSAS-Br scale unidimensionality. Revisiting the study by Kuhl et at.,1515. Kuhl EA, Dixit NK, Walker RL, Conti JB, Sears SF. Measurement of patient fears about implantable cardioverter defibrillator shock: an initial evaluation of the Florida Shock Anxiety Scale. Pacing Clin Electrophysiol. 2006;29(6):614-8. it is important to highlight that the sample was constituted by only 72 participants, which may have had an impact on the results of the psychometric analyses.
Afterwards, the psychometric properties of the FSAS were evaluated, with a sample of 443 participants.1616. Ford J, Finch JF, Woodrow LK, Cutitta KE, Shea J, Fischer A, et al. The Florida Shock Anxiety Scale (FSAS) for patients with implantable cardioverter defibrillators: testing factor structure, reliability, and validity of a previously established measure. Pacing Clin Electrophysiol. 2012;35(9):1146-53. The CFA showed that the two previously identified dimensions were highly related to a second-order factor (“Shock anxiety”). In other words, the two dimensions identified previously could have been better explained by their association to a common factor, namely the “shock-related anxiety” dimension. Due to these results, the authors recommended that the total scale score may be more clinically useful, instead of subdividing it into the two dimensios described before. These results corroborate the factor structure identified in our study.
Reliability assessment of the FSAS-Br scale revealed the accuracy of the Brazilian version, which was confirmed by adequate values of Cronbach’s alpha, McDonald’s Omega and GLB. The adoption of these three indications aimed to increase the accuracy of interpretation, since the Cronbach’s coefficient alpha is affected by the nature of data distribution and by sample size. Besides, its values may be increased by extensive scales, parallel and/or redundant elements or limited coverage of the construct under analysis, decreasing the reliability of the measurement.3333. Panayides P. Coefficient alpha: interpret with caution. Eur J Psychol. 2013;9(4):687-96.
In general, the results observed in the present study showed that the instrument is reliable and valid for application in Brazil, meeting the quality requirements for patient-reported outcome measurements.
Study limitations
Although the population studied is larger than the samples of several other studies which have used the FSAS, further studies with more robust samples are crucial for the consolidation of its validity and for attesting its stability in the various possible scenarios and profiles of ICD patients.
Further studies, evaluating the association of the FSAS-Br scores with the occurrence of ICD shock therapies and other clinic parameters will be useful to identify factors which may be associated with increased anxiety levels and, therefore, allow for the establishment of specific and personalized interventions for these patients.
Conclusions
The FSAS-Br instrument presented consistent validity and reliability evidence and, therefore, its use can be recommended for the ICD population in Brazil, both in clinical practice and in scientific research.
Referências
-
1Al-Khatib SM, Friedman P, Ellenbogen KA. Defibrillators: Selecting the Right Device for the Right Patient. Circulation. 2016;134(18):1390-404.
-
2Colquitt JL, Mendes D, Clegg AJ, Harris P, Cooper K, Picot J, Bryant J. Implantable cardioverter defibrillators for the treatment of arrhythmias and cardiac resynchronization therapy for the treatment of heart failure: systematic review and economic evaluation. Health Technol Assess. 2014;18(56):1-560.
-
3Winkle RA. Evolution of the implantable cardioverter-defibrillator: from bullets to BBs. J Am Coll Cardiol 2012;60(23):2399-401.
-
4Camm AJ, Nisam S. European utilization of the implantable defibrillator: has 10 years changed the “enigma”? Europace 2010;12:1063-9.
-
5Wilkoff BL, Fauchier L, Stiles MK, Morillo CA, Al-Khatib SM, Almendral J, et al. 2015 HRS/EHRA/APHRS/ SOLAECE Expert consensus statement on optimal implantable cardioverter-defibrillator programming and testing. Heart Rhythm. 2016;13(2):e50–e86.
-
6Daubert JP, Zareba W, Cannom DS, McNitt S, Rosero SZ, Wang P, et al. Inappropriate implantable cardioverter-defibrillator shocks in MADIT II: frequency, mechanisms, predictors, and survival impact. J Am Coll Cardiol. 2008;51(14):1357-65.
-
7Sood N, Ruwald AC, Solomon S, Daubert JP, McNitt S, Polonsky B, et al. Association between myocardial substrate, implantable cardioverter defibrillator shocks and mortality in MADIT-CRT. Eur Heart J. 2014;35(2):106-15.
-
8Desai H, Aronow WS, Ahn C, Gandhi K, Hussain S, Lai HM, et al. Risk factors for appropriate cardioverter-defibrillator shocks, inappropriate cardioverter-defibrillator shocks, and time to mortality in 549 patients with heart failure. Am J Cardiol. 2010;105(9):1336-8.
-
9Ahmad M, Bloomstein L, Roelke M, Bernstein AD, Parsonnet V. Patients’ attitudes towards implanted defibrillator shocks. Pacing Clin Electrophysiol 2000; 23(6):934–8.
-
10Costa R, Silva KR, Mendonça RC, Nishioka SA, Siqueira SF, Tamaki WT, et al. Incidence of shock and quality of life in young patients with implantable cardioverter-defibrillator. Arq Bras Cardiol. 2007;88(3):258-64.
-
11Pedersen SS, Sears SF, Burg MM, Van Den Broek KC. Does ICD indication affect quality of life and levels of distress? Pacing Clin Electrophysiol. 2009; 32(2):153-6.
-
12Sears SF, Conti JB. Quality of life and psychological functioning of ICD patients. Heart 2002; 87(5): 488–93.
-
13Carroll SL, Strachan PH, de Laat S, Schwartz L, Arthur HM. Patients’ decision making to accept or decline an implantable cardioverter defibrillator for primary prevention of sudden cardiac death. Health Expect 2013;16(1):69-79
-
14da Silva KR, Costa R, Rodrigues CG, Schasechter A, Nobre MC, Passman R, et al. Quality of life in patients with implantable cardioverter-defibrillator: systematic review of randomized controlled trials. Eur J Cardiovasc Nurs. 2018;17(3):196-206.
-
15Kuhl EA, Dixit NK, Walker RL, Conti JB, Sears SF. Measurement of patient fears about implantable cardioverter defibrillator shock: an initial evaluation of the Florida Shock Anxiety Scale. Pacing Clin Electrophysiol. 2006;29(6):614-8.
-
16Ford J, Finch JF, Woodrow LK, Cutitta KE, Shea J, Fischer A, et al. The Florida Shock Anxiety Scale (FSAS) for patients with implantable cardioverter defibrillators: testing factor structure, reliability, and validity of a previously established measure. Pacing Clin Electrophysiol. 2012;35(9):1146-53.
-
17Versteeg H, Starrenburg A, Denollet J, Palen Jv, Sears SF, Pedersen SS. Monitoring device acceptance in implantable cardioverter defibrillator patients using the Florida Patient Acceptance Survey. Pacing Clin Electrophysiol. 2012;35(3):283-93.
-
18Pedersen SS, Spindler H, Johansen JB, Mortensen PT, Sears SF. Correlates of patient acceptance of the cardioverter defibrillator: cross-validation of the Florida Patient Acceptance Survey in Danish patients. Pacing Clin Electrophysiol. 2008;31(9):1168-77
-
19Kochańska A, Zarzycka B, Świątecka G, Majkowicz M, Kozłowski D, Raczak G.Quality of life in patients with an implantable cardioverter-defibrillator – the significance of clinical factors. Arch Med Sci 2008; 4(4):409-16.
-
20Chair SY, Lee CK, Choi KC, Sears SF. Quality of life outcomes in chinese patients with implantable cardioverter defibrillators. Pacing Clin Electrophysiol. 2011;34(7):858-67
-
21Morken IM, Isaksen K, Karlsen B, Norekvål TM, Bru E, Larsen AI. Shock anxiety among implantable cardioverter defibrillator recipients with recent tachyarrhythmia. Pacing Clin Electrophysiol. 2012;35(11):1369-76
-
22Oz Alkan H, Enç N. Validity and reliability of the Florida Patient Acceptance Survey and Florida Shock Anxiety Scale in Turkish patients with implantable cardioverter defibrillation. Int J Med Res Health Sci 2017,6(10):21-32.
-
23Wild D, Grove A, Martin M. Principles of good practice for the translation and cultural adaptation process for patient-reported outcomes (PRO) measures: report of the ISPOR task force for translating adaptation. Value Health> 2005;8(2):94-104.
-
24Beaton D, Bombardier C, Guillemin F, Ferraz MB. Guidelines for the process of cross-cultural adaptation of self-report measures. Spine (Phila Pa 1976). 2000;25(24):3186-91.
-
25Sousa VD, Rojjanasrirat WJ. Translation, adaptation and validation of instruments or scales for use in cross-cultural health care research: a clear and user-friendly guideline. Eval Clin Pract. 2011;17(2):268-74.
-
26Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) - A metadata-driven methodology and workflow process for providing translational research informatics support, J Biomed Inform. 2009;42(2):377-81.
-
27Timmerman ME, Lorenzo-Seva U. Dimensionality assessment of ordered polytomous items with parallel analysis. Psychol Methods. 2011;16(2):209-20.
-
28Lorenzo-Seva, U. How to report the percentage of explained common variance in exploratory factor analysis. Tarragona (Italia):Universita Technical Report. Department of Psychology, Universitat Rovira i Virgili, Tarragona (Italia): Universita Rovira-Virgili; 2013, (Technical Report).
-
29Briggs NE, MacCallum RC. Recovery of Weak Common Factors by Maximum Likelihood and Ordinary Least Squares Estimation. Multivariate Behav Res. 2003;38(1):25-56.
-
30Matt C. Howard. A Review of Exploratory Factor Analysis Decisions and Overview of Current Practices: What We Are Doing and How Can We Improve? Int J Hum Comput Interact. 2016;32(1):51-62.
-
31Kahn JH. Factor analysis in counseling psychology research, training, and practice: principles, advances, and applications. Couns Psychol. 2006;34(5):684-718.
-
32Burns JL, Serber ER, Keim S, Sears SF. Measuring patient acceptance of implantable cardiac device therapy: initial psychometric investigation of the Florida Patient Acceptance Survey. J Cardiovasc Electrophysiol. 2005;16(4):384-90.
-
33Panayides P. Coefficient alpha: interpret with caution. Eur J Psychol. 2013;9(4):687-96.
-
Study AssociationThis study is not associated with any thesis or dissertation.
-
Ethics approval and consent to participateThis study was approved by the Ethics Committee of the CAPPesq under the protocol number CAAE:54522516.2.000.0068. All the procedures in this study were in accordance with the 1975 Helsinki Declaration, updated in 2013. Informed consent was obtained from all participants included in the study.
-
Sources of FundingThis study was funded by FAPESP. (Grant # 2016/02104-6).
Publication Dates
-
Publication in this collection
01 June 2020 -
Date of issue
May 2020
History
-
Received
19 Dec 2018 -
Reviewed
04 June 2019 -
Accepted
23 June 2019