Abstracts
BACKGROUND: Warts are epithelial proliferations in the skin and mucous membrane caused by various types of HPV. They can decrease spontaneously or increase in size and number according to the patient's immune status. The Propionium bacterium parvum is a strong immune stimulant and immune modulator and has important effects in the immune system and it is able to produce antibodies in the skin. OBJECTIVE: To show the efficacy of the Propionium bacterium parvum in saline solution in the treatment of skin warts. METHODS: A randomized double-blind study. Twenty patients with multiple warts were divided into two groups: one received 0,1ml intradermal injection of placebo solution in just one of the warts and the other received 0,1 ml of saline solution of Propionium bacterium parvum, one dose a month, for 3 to 5 months. RESULTS: Among the 20 patients who participated in the study, ten received the placebo and ten received the saline solution with Propionium bacterium parvum. In 9 patients treated with the Propionium bacterium parvum solution the warts disappeared without scars and in 1 patient it decreased in size. In 9 patients who received the placebo no change to the warts was observed and in 1 it decreased in size. CONCLUSIONS: The immune modulator and immune stimulant Propionium bacterium parvum produced antibodies in the skin which destroyed the warts without scars, with statistically significant results (P<0,001), and cured 90 % of the patients. We suggest the use of the immune stimulant in the treatment of warts.
Adjuvants, immunologic; Biological treatment; Immunologic factors; Immunotherapy; Therapeutics
FUNDAMENTOS: Verrugas são proliferações epiteliais na pele e mucosas causadas por diversos tipos de HPV. Elas podem involuir espontaneameme ou aumentar em número e tamanho de acordo com estado imunitário do paciente. O Propionium bacterium parvum é urn potente imunoestimulador e imunomodulador e tem efeitos importantes no sistema imune e é capaz de produzir anticorpos na pele. OBJETIVO: Mostrar a eficácia do Propionium bacterium parvum diluído em solução salina no tratamento de verrugas cutâneas. MÊTODOS: Estudo duplo-cego randomizado. Vinte pacientes com verrugas múltiplas foram divididos em dois grupos, um recebeu aplicação intradérmica do placebo em uma (1) única verruga e o outro da solução salina com Propionium bacterium parvum, uma dose por mês por 3 a 5 meses. RESULTADOS: Dos 20 pacientes do estudo, dez receberam placebo e 10 de solução salina com Propionium bacterium parvum. Dos pacientes tratados com Propionium bacterium parvum nove (9) foram curados e um teve diminuição das lesões. Do grupo do placebo nove (9) não apresentaram alterações e 1 (um) apresentou diminuição das lesões. CONCLUSÔES: O imunomodulador e imunoestimulador Propionium bacterium parvum produz anticorpos na pele que destroem as verrugas sem cicatrizes e mostrou uma significância de P<0,001, com cura de 90% dos pacientes submetidos à terapia. Sugerimos a utilização de imunoestimulante para o tratamento de verruga vulgar.
Adjuvantes imunológicos; Fatores imunológicos; Imunoterapia; Terapêutica; Tratamento biológico
INVESTIGATION
Treatment of common warts with the immune stimulant Propionium bacterium parvum* * Work performed in the Medicine course - Dermatology outpatient's clinic of the Fundação Universidade Regional de Blumenau (FURB) - Blumenau (SC), Brazil.
Tratamento das verrugas vulgares com o imunoestimulante Propionium bacterium parvum
Nilton Nasser
PhD in Dermatology - Full Professor at the Fundação Universidade Regional de Blumenau (FURB) - Blumenau (SC), Brazil
Mailing address Mailing address: Nilton Nasser Rua Curt Hering, 20 - Centro 8901030 Blumenau, SC E-mail: ninasser.bnu@terra.com.br
ABSTRACT
BACKGROUND: Warts are epithelial proliferations in the skin and mucous membrane caused by various types of HPV. They can decrease spontaneously or increase in size and number according to the patient's immune status. The Propionium bacterium parvum is a strong immune stimulant and immune modulator and has important effects in the immune system and it is able to produce antibodies in the skin.
OBJECTIVE: To show the efficacy of the Propionium bacterium parvum in saline solution in the treatment of skin warts.
METHODS: A randomized double-blind study. Twenty patients with multiple warts were divided into two groups: one received 0,1ml intradermal injection of placebo solution in just one of the warts and the other received 0,1 ml of saline solution of Propionium bacterium parvum, one dose a month, for 3 to 5 months.
RESULTS: Among the 20 patients who participated in the study, ten received the placebo and ten received the saline solution with Propionium bacterium parvum. In 9 patients treated with the Propionium bacterium parvum solution the warts disappeared without scars and in 1 patient it decreased in size. In 9 patients who received the placebo no change to the warts was observed and in 1 it decreased in size.
CONCLUSIONS: The immune modulator and immune stimulant Propionium bacterium parvum produced antibodies in the skin which destroyed the warts without scars, with statistically significant results (P<0,001), and cured 90 % of the patients. We suggest the use of the immune stimulant in the treatment of warts.
Keywords: Adjuvants, immunologic; Biological treatment; Immunologic factors; Immunotherapy; Therapeutics
RESUMO
FUNDAMENTOS: Verrugas são proliferações epiteliais na pele e mucosas causadas por diversos tipos de HPV. Elas podem involuir espontaneameme ou aumentar em número e tamanho de acordo com estado imunitário do paciente. O Propionium bacterium parvum é urn potente imunoestimulador e imunomodulador e tem efeitos importantes no sistema imune e é capaz de produzir anticorpos na pele.
OBJETIVO: Mostrar a eficácia do Propionium bacterium parvum diluído em solução salina no tratamento de verrugas cutâneas.
MÊTODOS: Estudo duplo-cego randomizado. Vinte pacientes com verrugas múltiplas foram divididos em dois grupos, um recebeu aplicação intradérmica do placebo em uma (1) única verruga e o outro da solução salina com Propionium bacterium parvum, uma dose por mês por 3 a 5 meses.
RESULTADOS: Dos 20 pacientes do estudo, dez receberam placebo e 10 de solução salina com Propionium bacterium parvum. Dos pacientes tratados com Propionium bacterium parvum nove (9) foram curados e um teve diminuição das lesões. Do grupo do placebo nove (9) não apresentaram alterações e 1 (um) apresentou diminuição das lesões.
CONCLUSÔES: O imunomodulador e imunoestimulador Propionium bacterium parvum produz anticorpos na pele que destroem as verrugas sem cicatrizes e mostrou uma significância de P<0,001, com cura de 90% dos pacientes submetidos à terapia. Sugerimos a utilização de imunoestimulante para o tratamento de verruga vulgar.
Palavras-chave: Adjuvantes imunológicos; Fatores imunológicos; Imunoterapia; Terapêutica; Tratamento biológico
INTRODUCTION
Warts are epithelial proliferations in the skin and mucosa caused by various types of human papilloma virus (HPV). Common warts are the most usual ones, characterized by papules or nodules with a hard surface, firm and hyperkeratotic consistency, found in any part of the skin, however they are mostly found on the dorsum of the hands and fingers. On the fingers they can be located in the nail bed and the periungual folds.1
They develop at any age and are more common in children and adolescents. The lesion is auto-inoculated and the incubation period varies from a few weeks to over a year.1 Contamination can be direct or indirect, mostly by exposure in swimming pools, sports precincts, beach and other places.
There are at least 70 types of HPV, but some of them tend to be more frequent in some areas of the body 2. A correlation between HPV serotype and infected zone might be possible; as such, palmoplantar lesions correspond to serotype 2, mucosal and mucocutaneous lesions to serotype 1, genital and perianal lesions to serotype 6 and periungual lesions to serotypes 2, 6, 10, 11.3-4
According to the immunological status the warts can regress spontaneously or increase in size and number. Spontaneous regression depends on the patient's anti HPV immunity.5
Forty-five percent (45%) of immune suppressed patients due to renal transplantation have warts, mainly common and plantar, less than five years after the transplant, a percentage that increases to more than 70% after this period.2
Even in the XXI century, common warts are still a challenge. Usually conservative therapy with topical products like keratolytics and caustic agents are the first option. If the topical agents fail, cryotherapy, curettage, electrocoagulation, liquid nitrogen, surgical excision or laser therapy can be considered.6-7
In some cases, due to pain and a great number of lesions, these treatments are impractical.1,3-5
However, all these treatments do not have completely satisfactory results. There is a high recurrence rate after their utilization as well as, in most cases, definitive scars and sequelae.7 As such, many of them cause lesions that are more severe than the warts themselves.3 Nevertheless, regardless of the treatment performed and the results obtained, it should not be forgotten that 30% of the warts disappear spontaneously within six months and the remaining ones disappear spontaneously within 3 years. It seems that the viral replication ceases and the warts fall.2
Immune modulators are substances that act on the immune system and have the ability to increase the host's resistance. Over the past few years they have been used in the treatment of many human illnesses, particularly from viral and neoplastic origin.8,9 Immune stimulants are substances that operate like the immune modulators.
Coryneobacterium parvum, also known as Propionibacterium acnes or Propionium bacterium parvum is a gram positive, pleomorphic, strictly anaerobic bacteria.8,9,10
It shows a potent stimulant effect in the reticuloendothelial system and because of that it has been used in recent years as an antibacterial and adjuvant immune stimulant to chemotherapy in numerous tumors.11,12,13 It can be parenterally or topically administered.10,13
Propionium bacterium parvum stimulates the activity of natural killer cells (NK), by releasing interferon and tumor necrosis factor.8, 9
Specific responses to the antigen prepared with dead Propionium bacterium parvum have been studied, including immediate and late hypersensitivity reactions, as well as erythematous reactions, which disappear 48 hours after the injection.13 This can be due to the inflammation generated by the complement activation by the antigen-antibody complex or by products found in the antigen preparation.13
Due to the dissatisfaction in relation to existing treatments and after studies showing that the Propionium bacterium parvum is a potent immune stimulant, our study shows that it can be used for that purpose, in a treatment which aims to eliminate common warts reaching only the virus, without destroying the surrounding tissues and, consequently, without leaving scars or sequelae.
METHODS Study
Type
This was a randomized double-blind clinical trial, with at least 20 volunteers. A double study is a study with human being where neither the patient nor the examiner knows what is being used as variable in any given moment (placebo or immune stimulant). A randomized study is an experimental study where the use of medications is tested, and the incidence of the disease is verified in the group which received the medication and in the one which received the placebo.
Ethics
The study was approved by the Ethics Committee of the Universidade Regional de Blumenau, according to the protocol number 025/06.
The patients with common warts were informed about the technical and scientific basis of the research project, through an informed consent form.
The information collected and the clinical observations from the lesions examination will be filed and in possession only of those involved with the project and the patients, if they want it. They will be available for technical and scientific purposes only.
Sample
28 volunteers with viral warts were studied, from both sexes, at the Dermatology Outpatients' Clinic of the Universidade Regional de Blumenau, who agreed with the intraepidermal test after reading the informed consent form. In case the patient with warts was younger than 18 years, the informed consent form was signed by the guardian.
Procedure
The protocol used for the patients with common warts consisted of:
Identification (sex, age and origin);
Lesion examination; number and location of the lesions;
Therapeutic assessment;
General questions in order to get inclusion and exclusion criteria into the research.
The visit included an initial consultation with anamnesis and examination of the lesion, clinical diagnosis and discussion of the case.
The patient could agree to take part in the study immediately or after some time or refuse participation.
Application of the medication and placebo
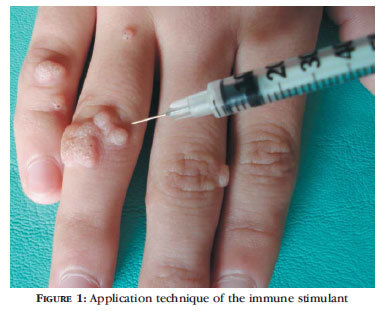
Intradermal application of 0,1 ml of the substance (drug or placebo) in only one wart, even if the patient had various lesions (Figure 1).
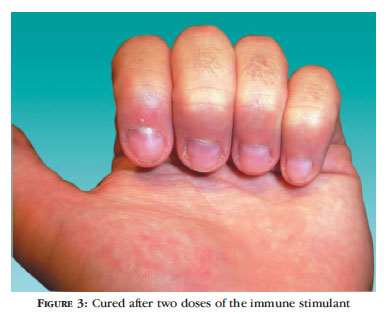
The patient would return to the dermatology outpatients' clinic of the Universidade Regional de Blumenau every 30-40 days so that subsequent applications could be done, in a total of 3 to 5 (if necessary) and for follow-up of the progress of the lesions, aiming at verifying the efficacy of the product (therapeutic assessment) (Figures 2, 3, 4 and 5).
A mild local reaction could develop, which was desired in order to demonstrate the antigen-antibody complex formation. All the applications were performed only by the physician responsible for the research, and were free of charge.
The patients who received the placebo substance were also treated, at the end of the study, with the proposed drug (Propionium bacterium parvum), at no cost.
Distribution of the patients
In half the patients the drug being studied was applied, and in the other half the placebo. This division was performed by order of agreement to participate in the project, that is, in the first patient who agreed the substance from the bottle with the blue label was applied, in the second the substance from the bottle with the orange label. From then on all odd patients (third, fifth, seventh,...) received the substance from the bottle with the blue label and all the even patients (forth, sixth, eighth,...) the substance from the bottle with the orange label.
Division of the substances into drug and placebo
The division of the substances into drug or placebo was done at the outpatients' clinic pharmacy of the Universidade Regional de Blumenau, by the pharmacist in charge, who also signed the informed consent form. The pharmacist in charge made the division of the substances by using colored labels on the bottles of the products to be used. Half the bottles received the blue label and the other the orange, and only the pharmacist in charge knew which of the two colors indicated the bottle with the drug (Propionium bacterium parvum) or with the placebo (saline solution).
Therefore, both the patients and the research physician did not know which one was the effective drug and which one the placebo.
The drugs used
The effective drug of Propionium bacterium parvum diluted in phenicated saline solution and buffered to pH 7,2 and the placebo, a phenicated saline solution buffered to pH 7,2 were prepared and provided free of charge by the laboratory RVP special manipulations, under the supervision of the pharmacist, Dr. Eliana Alves da Silva (CRF/SP - 19329).
The bottles were properly stored, at 10º C, according to manufacturing instructions.
Inclusion Criteria
Individuals with a clinical diagnosis of common wart, from both sexes, regardless of age or race, previously informed about the nature of the procedures and with formal approval (through the informed consent form).
Exclusion Criteria
Individuals with previous history of allergy of any kind, pregnant or lactating women, individuals with history of immune suppressive disease, using corticosteroids and/or other immune suppressive drugs.
Statistical Analysis
The Fisher's Exact Test was used to get the pvalue, considering the low number of patients studied (up to 20 frequencies).
RESULTS
There were 28 volunteers, and 20 completed the treatment and 8 gave up the participation in the research.
The location of the warts found in the patients who responded positively to the treatment were mostly (64,3%) in the hands (fingers and nail bed), and in the lower limbs (legs and feet) with 35,7%.
The results were reported to the pharmacist who then identified the substances used and informed that the blue bottles had the saline solution with Propionium bacterium parvum and the orange ones only contained the saline solution (placebo).
The results then considered can be found in table 1, and the statistical analysis by the Fisher's Exact Test showed a significance of P<0,001, which enables us to claim that the drug had a highly positive result when compared with the placebo.
The patients who had all their warts cured, with no scar, with the application in only one of them and fall of the remaining lesions, needed the following doses: one SC application of 0,5 ml = 2 patients, 2 applications (1 every 30 days) = 3 patients and 3 applications in 3 patients .
The patient who did not respond to the medication received the three scheduled doses by the protocol. The volunteer who received the placebo and whose wart decreased received three doses of it.
DISCUSSION
The usual treatment of common warts aims at destroying them by various highly aggressive methods which leave unavoidable sequelae like scars and deformations. There is also a high recurrence rate after these treatments and we should consider that 30% of the warts disappear spontaneously.
All these procedures seek to destroy not only the virus but also the tissue which they parasite.
Scientifically and in a logic manner we should look for alternative therapies which combat the HPV that causes the warts like vaccines, immune stimulants and other procedures which allow the human body's own immune defenses to destroy the virus, like the case of the genital HPV vaccine, a cervix cancer precursor.
The spontaneous regression and the "home remedies" which induce the patient to believe in the cure of the warts, show a natural increase of the antibodies which combat the virus and promote its elimination without causing sequelae. It seems that the virus replication ceases and the wart falls.2
We then decided to look for an immune stimulant capable of increasing the antibodies in the place where the warts develop. The stimulus to antibody formation at the place of the lesion allows their contact with the antigen (HPV virus) with consequent formation of specific antibodies which replicate and spread to the body, destroying all other lesions.
Propionium bacterium parvum was already used in order to increase the natural killer cells through the release of interferon and tumor necrosis factor.8, 9
Propionium bacterium parvum is produced by a French pharmaceutical laboratory as a stimulant vaccine and that is the reason we chose it for this research.
The double-blind randomized clinical study allows the patient´s psychological influence on the fall of the common warts to be ruled out and avoids misinterpretation by the researchers.
The results obtained with this experience enable us to indicate the immune stimulant therapy as an option in the treatment of common warts, having the advantage of not leaving scarring sequelae and immunizing indirectly to the type of virus found.
Received on 30.03.2011.
Approved by the Advisory Board and accepted for publication on 08.06.2011.
Financial Support: None.
Conflictc of Interests: None.
- 1. Sampaio SAP, Rivitti EA. Dermatologia Básica. 3 ed. São Paulo: Ed Artes Médicas; 2008. p. 565-7.
- 2. Verbov J. How to manage warts. Arch Dis Child. 1999;80:97-9.
- 3. Rodríguez C, Sánchez L, Minaya G, Vasquez J, Reymer D, Macher C. Tratamiento de lãs verrugas vulgares em niños com la hemolinfa del insecto Epicauta sp. Dermatol. Peru. 2000;10:94-96.
- 4. Beliaeva TL. [The population incidence of warts]. Vestn Dermatol Venerol. 1990;2: 55-8.
- 5. Silverberg NB, Lim JK, Paller AS, Mancini AJ. Square acid immunotherapy for warts in children. J Am Acad Dermatol. 2000;42:803- 8.
- 6. Siegfried EC. Warts on children: an approach to therapy. Pediatric Ann. 1996;25:79- 90.
- 7. Torrelo A. What`s new in treatment of viral warts in children. Pediatr Dermatol. 2000;19:191-9.
- 8. Santana CF, Asfora JJ, Lins LP, Lopes CAC, Santos ER. Efeitos imunoestimulantes do Corynebacterium parvum em pacientes portadores de neoplasias malígnas. Rev Inst Antib. 1979;19:137-41.
- 9. Megid J, Dias Junior JG; Aguiar DM, Nardi Júnior G, Silva WB, Ribeiro MG. Tratamento da papilomatose canina com Propionibacterium acnes. Arq Bras Med Vet Zootec. 2001;53:574-6.
- 10. Kalis C, Gumenscheimer M, Freudenberg N, Tchaptchet S, Fejer G, Heit A, et al. Requirement for TLR9 in the immunomodulatory activity of Propionibacterium acnes. J Immunolol. 2005;174:4295-300.
- 11. Sljivic VS, Watson SR. The adjuvant effect of corynebecterium parvum: T cell dependence of macrophage activation. J Exp Med. 1977;145:45-57.
- 12. Adlam C, Scott MT. Lymphoreticular stimulatory properties of Corynebacterium parvum and related bacteria. J Med Microbiol. 1973;6:261-74.
- 13. Cantrell JL, Wheat RW. Antitumor activity and lymphoreticular stimulation properties of fractions isolated from Corynebacterium parvum. Cancer Res. 1979;39:3554-63.
Publication Dates
-
Publication in this collection
10 Aug 2012 -
Date of issue
Aug 2012
History
-
Received
30 Mar 2011 -
Accepted
08 June 2011