Abstracts
The mechanism of interaction between Mycobacterium leprae and neural cells has not been elucidated so far. No satisfactory interpretation exists as to the bacterium tropism to the peripheral nervous system in particular. The present study is a review of the micro-physiology of the extracellular apparatus attached to Schwann cells, as well as on the description of morphological units probably involved in the process of the binding to the bacterial wall.
Carcinoma, squamous cell; Carcinoma, squamous cell; Incidence; Skin neoplasms
O mecanismo de interação entre o Mycobacterium leprae e as células neurais não está esclarecido até o momento. Não há interpretação satisfatória do tropismo da bactéria ao sistema nervoso periférico, em particular. O presente estudo é uma revisão da microfisiologia da estrutura do aparelho extracelular, ligado às células de Schwann, assim como a descrição das unidades morfológicas, provavelmente envolvidas no processo de ligação à parede celular da bactéria.
Carcinoma de células escamosas; Carcinoma de células escamosas; Incidência; Neoplasias cutâneas
REVIEW
Peripheral nervous system and grounds for the neural insult in leprosy
Jorge João ChachaI; Miriam N. SottoII; Lothar PetersIII; Silvia LourençoIV; Evandro A. RivittiV; Petr MelnikovVI
IPh.D., Discipline of Dermatology - Faculdade de Medicina da Universidade Federal de Mato Grosso do Sul (UFMS) Campo Grande (MS), Brazil
IIPh.D., Medical School, Universidade de São Paulo (USP) São Paulo (SP), Brazil
IIIMaster, Department of Pharmacy, Universidade Federal de Mato Grosso do Sul (UFMS) Campo Grande (MS), Brazil
IVPh.D., Faculdade de Medicina da Universidade de São Paulo (USP) São Paulo (SP), Brazil
VPh.D., Faculdade de Medicina da Universidade de São Paulo (USP) São Paulo (SP), Brazil
VIPh.D., Faculdade de Medicina da Universidade Federal de Mato Grosso do Sul (UFMS) Campo Grande (MS), Brazil
Mailing Address Mailing Address: Dr. Jorge João Chacha: Faculdade de Medicina FAMED Universidade de Federal de Mato Grosso do Sul Campo Grande Mato Grosso do Sul - Brasil Cx.postal: 549 79070 900 E-mail: ltf@nin.ufms.br
ABSTRACT
The mechanism of interaction between Mycobacterium leprae and neural cells has not been elucidated so far. No satisfactory interpretation exists as to the bacterium tropism to the peripheral nervous system in particular. The present study is a review of the micro-physiology of the extracellular apparatus attached to Schwann cells, as well as on the description of morphological units probably involved in the process of the binding to the bacterial wall.
Keywords: Antigens, CD29; Dystroglycans; Laminin; Peripheral nervous system; Schwann cells
INTRODUCTION
Leprosy is a chronic disease caused by Mycobacterium leprae with clinical polymorphic characteristics 1, relevant to the destruction of peripheral nerves, resulting in irreversible disabilities 2, causing suffering that goes beyond pain and embarrassment strictly related with the physical damage, with great social and psychological impact, justifying the advances in multidisciplinary approach of patients as the healthcare actions required to control the disease 3.
It is estimated today that there are 3,000,000 people with the disease in the world with permanent type 2 disability, and in Brazil, 55,000 have it, despite complete polychemotherapy (PCT) 4. It is known that 30% of the destruction of nerve fibers is required to produce clinical manifestations. The nervous aggression of leprosy may be defined into two ways: an initial one, in which there is absence of inflammatory cells, and a common one in which both paucibacillary and multibacillary forms occur, and late when the process become inflammatory, the later manifestations may developed auto-immunity 5,6 cytotoxicity 7,8 and presence of ROI (reactive oxygen intermediates)9 and NO (nitrous oxide) 10, fibroblasts 11, NGF-R (Nerve growth factor) 12, NgCAM (Neural Glial Cell Adhesion Molecule), interferon-gamma and TGF- beta (Transforming growth factor beta),13 TNF-alfa TNF-beta (Tumor necrosis factors), IL-6, IL-8, IL-12 and IL-10 (Interleukins)14,15 and matrix metalloproteinase 16.. The exact nature of the genetic components, such as HLA and non-HLA, MICA and MICB, NRA MP1 and others, especially the exact number of involved genes, their biological functions and the genetic variations of these genes responsible for the observed effects, are still completely unknown 17.
In the present paper, we focused on the role of these structures that are present in the peripheral nervous system and their relation with Mycobacterium leprae.
THE MAIN CHARACTERISTICS OF THE PERIPHERAL NERVOUS SYSTEM
The peripheral nervous system is comprised of nervous fiber formed by one or more axons involved by Schwann cells maintained by the endoneurum, plus amorphous material of extracellular matrix, capillaries, fibroblasts and mast cells, as well as epineurum and perineurum 18.
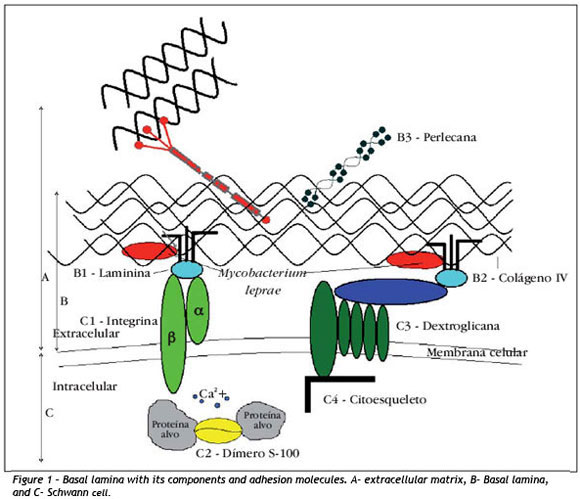
Considering that the perineural sheath of axons and Schwann cells are involved by a basal lamina, in figure 1 (line B) we can see the special organization of extracellular matrix (line A), comprising many molecules secreted by multiple cells, among which Schwann cells 19 (line C).
The basal lamina, through interactions with cellular membrane receptors, participates in cell metabolism, organization of proteins of plasma membrane, cell migration during embryogenesis and cell differentiation 19. Moreover, it influences axonal regeneration serving as a guide of nervous fibers in this process and it has structural and signaling functions 20. Laminin (Figure 1-B1), one of the components of basal lamina, is a large and flexible molecule formed by three long chains of polypeptides (γ, γ and γ) forming an asymmetrical cross, maintained together by disulfide bridges. To present, a total of 15 isoforms of laminins are known 21.
Basal lamina and laminins are important for the peripheral nervous system. Before birth, they support the correct development of the neural system, whereas after birth they guarantee the integrity and interaction with components of the extracellular matrix, in addition to programming myelin synthesis by Schwann cells 21,22.
In patients with muscle dystrophies and peripheral neuropathies, we can observe absence of one of the isoforms of laminin, laminin-2, causing discontinuation of basal lamina, demyelization and reduction of stimuli conduction. In the absence of laminin-2, Schwann cells become incapable of controlling these processes inside the axon, and the number of cells go down and proliferation gets slower 21,23.
Laminins in extracellular matrix binds to collagen IV, perlecan and entactins and cell surface receptors, especially integrins (Figure 1-C1). They are formed by two subunits γ (18) and γ (8), which work as heterodimeric transmembrane receptors. Subunits combine among each other, giving approximately 24 variations described to present 21,24.
Integrins work to maintain the morphological contacts between the extracellular matrix and the cytoskeleton to preserve the cell architecture and the balance of the internal medium through multiple signaling functions 21,25,26. They are located in sites named "areas of focal adhesion", where the intracytoplasm portion maintains contact with the cytoskeleton, providing a communication channel between the intracellular and the extracellular medium. They activate signaling paths that impact the cell behavior 27. Extracellularly, they act primarily as receptors of collagen, laminin and fibronectin whose ligants may bind to different types of integrins. At the same time, different binders activate specific signalization paths to the intracellular medium 26,28-30.
Group γ1 comprises 12 integrins, each one of them with different affinities for binding to components of extracellular matrix 22,24,31. In the nerve, unit γ1 of integrins can form dimers with many γ units, forming receptors to the many components of the extracellular matrix, including laminin present on the basal lamina 22,24.
Studies analyzing the expression of integrins in experimental autoimmune neuritis in rats and in Guillain Barré syndrome in humans evidenced failures in the expression of many integrins 32.
The abnormalities of their expressions, laminins and integrins, are of such importance that they can be used to characterize the progression and severity of cases of neuropathy in humans and in rats 32-34.
Among the other molecules expressed in the peripheral nervous system, there is protein S-100 (Figure 1-C2), which comprises21 different isoforms. The first visualization of protein S-100 in sensorial corpuscles was demonstrated by Iwanaga35 in 1982. Later, it was confirmed that isoforms S-100 a and S-100 β were the main forms present in the nervous tissue 36. It is a highly acid water-soluble dimer with molecular weight of 21kDa, which shows strong affinity for calcium 3737. It belongs to the family of modulating proteins expressed exclusively in vertebrates. It is present in glial cells, Schwann cells, melanocytes, Langherans cells, dermal dendrocytes, histiocytes, adipocytes, myoepithelial cells and some gland epithelial cells.
This protein participates in intra and extracellular regulating activities of multifunctional character. It inhibits phosphorilation and regulates the stability of the cytoskeleton by modifying its metabolism through enzymatic activities that include calcium homeostasis 38.
As to extracellular functions, there are influences on inflammatory cells, neurons, astrocytes, glial cells, as well as endothelial and epithelial cells 39. It has been shown in humans and guinea pigs that after compression or section of the nerve, expression of S 100 in corpuscles of Meissner and Paccini is irregular 40,41,42. The expression of protein S-100 has been used in studies involving leprosy, especially to better diagnose the disease, in special in undetermined and tuberculoid forms with negative bacilloscopy.
Fleury43 in 1987 detected abnormalities to protein S-100 in 8 cases (88.8%) out of 9 cases with non-specific histopathological granuloma without bacillus. Singh in 1994 44 studied granulomatous dermatoses and suggested the use of protein S-100 in diagnostic elucidation of leprosy.
Thomas in 199845 employed the method of protein S-100 also in granulomatous diseases, concluding that it was useful in the exclusion of leprosy precisely when nervous endings presented as normal.
Another important receptor of Schwann cell is dystroglycan (Figure 1-C3). It was originally isolated in skeletal muscles. It is subdivided into two polypeptides, a and β, in which the alpha part is related with extracellular environment and the beta part with the cytoskeleton (Figure 1-C4) of the cells 46.
Dystroglycan is a receptor of laminin-2. It maintains the adhesion of extracellular matrix of basal lamina with cell membrane of Schwann cells. There are evidences of the participation of dystroglycan in the myelinization of Schwann cells. Mice with absence of this molecule present abnormalities in myelinization and defects in nervous conduction 47. Its involvement is suggested in the pathogenesis of muscle dystrophies 48. By correlating Mycobacterium leprae and laminin 2, it is possible to assume that dystroglycan participates in the process of action of demyelinization and axonal depression of leprosy 49 with similar action mechanism of integrins, especially β1.50
INTERACTION OF MYCOBACTERIUM LEPRAE WITH PERIPHERAL NERVOUS SYSTEM
The affinity of Mycobacterium leprae by peripheral nerves is known since the first references by Danielssen and Boeck in 1848.51 Later, evidences were gathered towards having Schwann cells in the peripheral nerves as the target of the bacillus 52. Studies have demonstrated that the binding of Mycobacterium leprae to Schwann cells induces to demyelinization 56.
Schwann cells from the peripheral nervous system present 2 phenotypes, myelinized and non-myelinized, which present different response to Mycobacterium leprae.57 Even though it binds to both phenotypes of Schwann cells, the binds of non-myelinized cells are stronger. The cells in the second phenotype are the natural niche for the multiplication of bacteria, enabling the microorganism to be protected from immune responses of the host, providing an extremely favorable site for the proliferation and survival in the peripheral nervous system 58. There are evidences that the binding between Mycobacterium leprae and Schwann cells take place at the extracellular matrix basal lamina 59.
Therefore, in the case of leprosy, basal lamina does not represent a protective barrier against the entry of mycobacteria. To the contrary, it is through its structural components that occurs the invasion. There are "in vivo" and "in vitro" studies demonstrating that at molecular level the binding between Mycobacterium leprae and Schwann cells is made through laminin 60.
In fact, the cell wall of Mycobacterium leprae has an external electron-transparent layer which is mainly formed by fitiocerol dimerocerosic acid and glycolipids, especially phenolic glycolipid 1 (PGL-1). It is specific of this mycobacterium and contains a specific trisaccharide not found on the wall of any other mycobacteria 58,60. As shown, in studies with purified PGL-1, glycolipid is specifically binding to γ2 chain of laminin-2. In tissue culture, this binding is mediated by the specific trisaccharide that has been mentioned 61,62.
There is still another protein of molecular mass 21 kDa, associated with the mycobacteria wall named Mycobacterium leprae laminin binding protein (ML-LBP21), capable of binding to laminin-2. Differently from PGL-1, it is similar to proteins found in mycobacteria which are unable to invade Schwann cells, and do not seem to have the same importance 63.
CONCLUSION
The action mechanisms of Mycobacterium leprae are not sufficiently understood and totally accepted yet. The problem is its predilection for the peripheral nervous system, whereas other mycobacterium are morphologically similar and do not present this specific mechanism.
The integrity of peripheral nervous system fibers is maintained by adhesion or binding of basal lamina of Schwann cells. The aggression of Mycobacterium leprae on the extracellular matrix causes the disruption between the basal lamina and Schwann cells, causing abnormalities in physiological and neuronal functions, which are sometime irreversible.
The knowledge of molecular interactions between substances of bacterial cellular wall and the components of extracellular complex Schwann cells is getting more comprehensive. To present, the key known structures involved in the pathogenesis, such as laminins, integrins, proteins S-100, dystroglycans and others, will certainly enable early diagnosis and provide new therapeutic options.
REFERENCES
Conflict of interest: None
Financial funding: None
How to cite this article: Chacha JJ, Lourenço S, Rivitti E, Sotto M, Melnikov P, Peters L. Sistema nervoso periférico e pressupostos da agressão neural na hanseníase. An Bras Dermatol. 2009;84(5):495-500.
- 1. Sampaio SA, Rivitti, E. Dermatologia. 2. ed. São Paulo: Artes Médicas; 2001
- 2. Talhari S, Neves RG. Dermatologia tropical: hanseníase. 3. ed. Manaus: Instituto Superior de Estudos da Amazônia (Brasil); 1997
- 3. Martins BDL, Torres, FN, Oliveira, MLW. Impacto na qualidade de vida em pacientes com hanseníase: correlação do Dermatology Life Quality Index com diversas variáveis relacionadas à doença. An Bras Dermatol. 2008;83:39-43
- 4. Britton WJ, Lockwood DN. Leprosy. Lancet. 2004;363:1209-19
- 5. Dastur DK, Ramamohan Y, Shah JS. Ultrastructure of lepromatous nerves. Neural pathogenesis in leprosy. Int J Lepr Other Mycobact Dis. 1973;41:47-80
- 6. Job CK, Nayar A, Narayanan JS. Electronmicroscopic study of hypopigmented lesions in leprosy. A preliminary report. Br J Dermatol. 1972;87:200-12
- 7. Stanley JNA. Pathological changes in the sciatic nerves of mice with leprosy neuropathy an electromicroscopic study [Thesis]. Oxford: University of Oxford; 1988
- 8. Birdi TJ, Desai S, Antia NH. Suppression of lymph node lymphoproliferation to viable Mycobacterium leprae by peripheral blood-derived monocytes. Lepr Rev. 1996;67:338-42
- 9. Mistry NF, Shetty V, Shetty VP, Antia NH. The immunological role of the Schwann cells in leprosy in the peripheral nerve in leprosy and other neuropathies. Delhi: Oxford University Press; 1997. p. 215-30
- 10. Khanolkar-Young S, Snowdon D, Lockwood DN. Immunocytochemical localization of inducible nitric oxide synthase and transforming growth factor-beta (TGF-beta) in leprosy lesions. Clin Exp Immunol. 1998;113:438-42
- 11. Shetty VP, Antia NH. Myelination around multiple axons in the periferic nerve. Acta Neuropathol. 1980;50:147-51
- 12. Facer P, Mathur R, Pandya SS, Ladiwala U, Singhal BS, Anand P. Correlation of quantitative tests of nerve and target organ dysfunction with skin immunohistology in leprosy. Brain. 1998;121:2239-47
- 13. Singh RP. Immunoregulation of cytokines in infectious diseases (leprosy), future strategies. Nihon Hansenbyo Gakkai Zasshi. 1998;67:263-8
- 14. Sieling PA, Legaspi, A, Ochoa MT, Rea T, Modlin RL. Regulation of human T-cell homing receptor expression in cutaneous bacterial infection. Immunology. 2007;120:518-25
- 15. Mendonça VA, Costa RD, Melo GEBA, Antunes CM, Teixeira AL. Imunologia da hanseníase. An Bras Dermatol. 2008;83:343-50
- 16. Teles RM, Antunes SL, Jardim MR, Oliveira AL, Nery JA, Sales AM, et al Expressions of metalloproteinases (MMP-2, MMP-9 and TACE) and TNF-alpha in the nerves of leprosy patients. J Peripher Nerv Syst. 2007;12:195-204
- 17. Prevedello FC, Mira MT. Hanseníase: uma doença genética? An Bras Dermatol. 2007;82:451-9
- 18. Birdi TJ, Antia NH. Mechanisms involved in peripheral nerve damage in leprosy with special reference to insights obtained from in vitro studies and the experimental mouse model. Int J Lepr Other Mycobact Dis. 2003;71:345-54
- 19. Alberts B, Bray D, Lewis J, Raff M, Roberts K, Watson JD. Biologia molecular da célula. 3 ed. São Paulo: Artes Médicas; 1997
- 20. Stoll G, Müller HW. Nerve injury, axonal degeneration and neural regeneration: basic insights. Brain Pathol. 1999;9:313-35
- 21. Berti C, Nodari A, Wrabetz L, Feltri ML. Role of Integrins in Peripheral Nerves and Hereditary neuropathies. Neuromolecular Med. 2006;8:191-204
- 22. Bunge RP, Bunge MB. Interrelationchips between Schwann cell function and extracellular matrix production. Trends Neuroci. 1983;6:499-505
- 23. Feltri ML, Wrabetz L. Laminins and their receptors in Schwann cells and herediary neuropathies. J Periph Ner Sys. 2005;10:128-43
- 24. Previtalli SC, Feltri ML, Archelos JJ, Quattrini A, Wrabetz L, Hartung H. Role of integrins in peripheral nervous system. Progress in Neurobiology. 2001;64:35-49
- 25. Hynes RO. Integrines: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11-25
- 26. Hynes RO. The emergence of integrins: a personal and historical perspective. Matrix Biology. 2004;23:333-40
- 27. Giancotti FG, Ruoslathi E. Integrin signling. Science. 1999;285:1028-32
- 28. Heino J. The collagen receptor integrins have distinct ligand recognition and signaling functions. Matrix Biology. 2000;19:319-23
- 29. Hemler ME. Integrin associated proteins. Curr Opin Cell Biol. 1998;10:578-85
- 30. Darribère T, Skalski M, Cousin H, Gaultier A, Montmory C, Alfandary D. Integrins: Regulators of embryogenesis. Biol Cell. 2000;92:5-25
- 31. Brakebusch C, Fässler R. Beta-1 integrin function in vivo: adhesion, migration and more. Cancer and Metastasis Reviews. 2005;24:403-11
- 32. Previtali SC, Archelos JJ, Hartung HP. Expression of integrins in experimental autoimmune neuritis and Guillain Barré syndrome. Ann Neurol. 1998;44:611-21
- 33. Feltri ML, Porta DG, Previtali SC, Nodari A, Migliavacca B, Cassetti A, et al Conditional disruption of beta 1 integrin in Schwann cells impedes interactions with axons. J Cell Biol. 2002;156:199-209
- 34. Le Su, Xin Lv, JunYing Miao. Integrin, 4 in Neural Cells. NeuroMolecular Medicine. 2008; 4:316-21
- 35. Iwanaga T, Fujita T, Takahashi Y, Nakajima T. Meissners and Pacinian corpuscules as studies by immunohistochemistry for S-100 protein, neuron-specific enolase and neurofilament protein. Neurosci Lett. 1982;31:117-21
- 36. Haimoto, H, Hosoda S, Kato K. Differential distribution of immunoreactive S100-∙ and S100 , proteins in normal nonnervous human tissues. Lab Invest. 1987;57:489-98
- 37. Kligman D, Hilt DC. The S-100 protein family. Trends Biochem. Sci. 1988;13:437-43
- 38. Donato R. Intracellular and Extracellular roles of S100 proteins. Microsc Res Tech. 2003;60:540-51.
- 39. Marenholz I, Heizmann CW, Fritz G. S100 proteins in mouse and man: from evolution to function and pathology (incluiding an update of the nomeclature). Biochem Biophys Res Commun. 2004;322:1111-22
- 40. De Leon M, Van Eldik LJ, Shooter EM. Differential regulation of S100 , and mRNA coding for S100-like proteins (42A and 42C) during development and after lesion of rat sciatic nerve. J. Neurosci Res. 1991;29:155-62
- 41. Del Valle ME, Cabal A, Alvarez-Mendez JC, Calzada B, Haro JJ, Colier W, et al Effect of denervation on lamellar cells of Meissner-like sensory corpuscles of tehe rat. An immunohistochemical study. Cell Mol Biol. 1993;39:801-7
- 42. Gonzalez-Martinez T, Perez-Piñera P, Dias-Esnal B, Vega JA. S-100 proteins in the Human Peripheral Nervous System. Micros Res Tech. 2003;60:633-8
- 43. Fleury RN, Bacchi CE. S-100 protein and immunoperoxidase technique as an aid in the histopathology diagnoses of leprosy. Inst J Lep Others Mycobact Diseases. 1987;53:338-44
- 44. Singh N, Arora VK, Ramam M, Tickoo SK, Bhatia A. Na evaluation of the S-100 stain in the histological diagnosis of Tuberculoid leprosy and other granulomatous dermatosis. Int J Lepr. 1994;62:263-7
- 45. Thomas MM, Jacob M, Chandi SM, Soshamma G, Pulimood S, Jeyaseelan L, et al Role of S-100 staining in differentiating leprosy from other granulomatous diseases of the skin. Int J Lepr Other Mycobact Diseases. 1998;67:1-5
- 46. Ervasti JM, Campbell KP. A role for dystrophin-glycoprotein complex as transmembrane linker between laminin and actin. J Cell Biol. 1993;122: 809-23
- 47. Saito F, Masaki T, Kamakura K, Anderson LV, Fujita S, Fukuta-Ohi H, et al Characterization of the transmembrane molecular architecture of the dystroglycan complex in Schwann cells. J Biol Chem. 1999;274:8240-6
- 48. Campbell KP. Three muscular dystrophies: loss of cytoskeleton-extracellular matrix linkage. Cell. 1995;80:675-9
- 49. Henry MD, Campbell KP. Dystroglycan inside and out. Curr Opin Cell Biol. 1999;11:602-7
- 50. Feltri ML, Porta DG, Previtali SC, Nodari A, Migliavacca B, Cassetti A, et al Conditional disruption of beta 1 integrin in Schwann cells impedes interactions with axons. J Cell Biol. 2002;156:199-209
- 51. Danielssen DC, Boeck W. Traité de la Spedalskhed ou Elepnhatiasis des Grecs. Paris: J. B. Baillone; 1848
- 52. Lumsden CE. Leprosy and the Schwann cels in vitro and in vivo in Cochrane RD, Leprosy in Theory and Practice. Bristol John Wright & Sons Ltd. USA, 221-250, 1959.
- 53. Job CK. Nerve demage in leprosy. Int J Lepr. 1989;57:532-9
- 54. Mukherjee R, Antia NH. Host-parasite interelationship between M. leprae and Schwann cells in vitro. Int J Lepr. 1986;54:632-8
- 55. Scollard DM, McCormick G, Allen JL. Localization of Mycobacterium leprae to endothelial cells of epineural and perineural blood vesels and lymphatics. Am J Pathol. 1999;154:1611-20
- 56. Edwards GM, Willford FH, Liu XW, Hennighausen L, Djiane J, Streuli CH. Regulation on mammary differentiation by extracellular matrix involves protein-tyrosine phosphatases. J Biol Chem. 1988;273: 9495-9500
- 57. Leivo I, Engvall E. Merosin, a protein specific for basement membranes of Schwann cells, striated muscle, and trophoblasts, is expressed late in nerve and muscle development. Proc Natl Acad Sci USA. 1988;85:1544-8
- 58. Rambukkana A. Molecular Basis for the Peripheral Nerve Predilection of Mycobacterium Leprae. Curr Opin Microbiol. 2001;4:21-7
- 59. Ng V, Zanazzi G, Timpl R, Talts JF, Salzer JL, Brennan PJ, Rambukkana A. Role of the cell wall phenolic glycolipid- 1 in the peripheral nerve predilection of Mycobacterium leprae Cell . 2000;103:511-24
- 60. Rambukkana A, Salzer JL, Yurchenco PD, Tuomanen EI. Neural targeting of Mycobacterium leprae mediated by the G domain of the laminin-alpha2 chain. Cell. 1997;88:811- 21
- 61. Hunter SW, Brennan PJ. A novel phenolic glycolipid from Mycobacterium leprae possibly involved in immunogenicity and pathogenicity. J Bacteriol. 1981;147:728-35
- 62. Hunter SW, Fujiwara T, Brennan PJ. Structure and antigenicity of the major specific glycolipid antigen of Mycobacterium leprae J. Biol. Chem. 1982;257:15972-80
- 63. Shimoji Y, Ng V, Matsumura K, Fischetti V, Rambukkana A. A 21-Kda Surface Protein of Mycobacterium Leprae Binds Peripheral Nerve Laminin-2 and Mediates Schwann Cell Invasion. Proc Natl Acad Sci. 1999;96:9857-62
Publication Dates
-
Publication in this collection
07 Jan 2010 -
Date of issue
Oct 2009