Abstracts
BACKGROUND: The sebaceous glands are susceptible to the effects of androgens. A benign proliferation of these hormones, i.e. hyperplasia, occurs with age. OBJECTIVES: This was a pilot study to demonstrate whether any correlation exists between circulating androgen levels and an increase in the incidence of sebaceous hyperplasia. METHODS: Sixteen female patients with a diagnosis of sebaceous hyperplasia were compared to a control group of females of a similar age without the disease. Blood tests were performed on participants of both groups to measure circulating androgen levels (free and total testosterone and androstenedione levels). Results were tabulated for statistical analysis. RESULTS: These data showed no statistically significant differences in circulating androgen levels between the patients with sebaceous hyperplasia and the control group. CONCLUSION: These data suggest that no significant changes occur in circulating androgen levels [free and total testosterone, androstenedione, dehydroepiandrosterone (DHEA) and DHEA sulfate] in patients with sebaceous hyperplasia.
Hormones; Hormones, hormone substitutes, and hormone antagonists; Hyperplasia; Sebaceous glands
FUNDAMENTOS: As glândulas sebáceas são suscetíveis à ação dos hormônios androgênios e apresentam proliferação benigna com a idade, ou seja, hiperplasia. OBJETIVOS: Estudo piloto para verificar se há correlação entre a taxa de hormônios masculinos circulantes e o aumento da incidência da hiperplasia das glândulas sebáceas. MÉTODOS: 16 pacientes do sexo feminino, com diagnóstico de hiperplasia sebácea cutânea, foram comparados a um grupo-controle de mesmo gênero e idades semelhantes, sem a doença. Ambos os grupos foram submetidos a testes de dosagem sanguínea para avaliação das taxas de hormônios androgênios circulantes (testosterona livre e total, androstenediona). Os resultados foram tabulados e analisados estatisticamente. RESULTADOS: Os dados demonstraram não haver mudanças nos níveis de hormônios masculinos circulantes dos pacientes com hiperplasia sebácea cutânea, quando comparados ao grupo-controle. CONCLUSÃO: Os dados sugerem que não há alterações estatisticamente significantes nas taxas dos hormônios circulantes (testosterona livre e total, androstenediona, deidroepiandrosterona, sulfato de deidroepiandrosterona) dos pacientes com hiperplasia sebácea cutânea.
Glândulas sebáceas; Hiperplasia; Hormônios; Hormônios, substitutos de hormônios e antagonistas de hormônios
INVESTIGATION
Sebaceous hyperplasia: a pilot study to correlate this skin disease with circulating androgen levels*
Sandra TagliolattoI; Mauricio M. A. AlchorneII; Mauro EnokiharaIII
IMaster's degree in Dermatology awarded by the Federal University of São Paulo (UNIFESP), São Paulo, Brazil
IIFull Professor of Dermatology, Federal University of São Paulo (UNIFESP), São Paulo, Brazil
IIIPhD in Dermatology awarded by the Federal University of São Paulo (UNIFESP), São Paulo, Brazil. Professor, Department of Dermatology, Federal University of São Paulo (UNIFESP), São Paulo, Brazil
Mailing address
ABSTRACT
BACKGROUND: The sebaceous glands are susceptible to the effects of androgens. A benign proliferation of these hormones, i.e. hyperplasia, occurs with age.
OBJECTIVES: This was a pilot study to demonstrate whether any correlation exists between circulating androgen levels and an increase in the incidence of sebaceous hyperplasia.
METHODS: Sixteen female patients with a diagnosis of sebaceous hyperplasia were compared to a control group of females of a similar age without the disease. Blood tests were performed on participants of both groups to measure circulating androgen levels (free and total testosterone and androstenedione levels). Results were tabulated for statistical analysis.
RESULTS: These data showed no statistically significant differences in circulating androgen levels between the patients with sebaceous hyperplasia and the control group.
CONCLUSION: These data suggest that no significant changes occur in circulating androgen levels [free and total testosterone, androstenedione, dehydroepiandrosterone (DHEA) and DHEA sulfate] in patients with sebaceous hyperplasia.
Keywords: Hormones; Hormones, hormone substitutes, and hormone antagonists; Hyperplasia; Sebaceous glands
INTRODUCTION
The sebaceous glands are an important site of androgen activity. 1 They are susceptible to regulation by the sex hormones and undergo changes in accordance with alterations in hormone levels that occur over an individualís lifetime. Their size may increase with age, although their number remains approximately the same. 2
The development and function of the sebaceous glands in the fetal-neonatal period are regulated by maternal androgens and the synthesis of endogenous steroids, leading to an increase in sebaceous secretion shortly after birth. A further peak occurs at around 9 years of age, with these levels being maintained up to approximately 17 years of age when adult levels are reached. In women, sebaceous production tends to decrease following menopause, whereas no major changes are found in men up to the eighth decade of life. 1,3
Sebaceous hyperplasia may be considered a benign proliferation of the sebaceous glands.4 Studies conducted in animal models or in vitro have shown the effect of androgens in triggering this condition. 5-7
To the best of our knowledge, no studies have been conducted in vivo to correlate the appearance of sebaceous hyperplasia lesions in the skin of patients with no other related comorbidities and serum androgen levels.
Ultraviolet radiation and the use of cyclosporine have been described as co-factors responsible for triggering sebaceous hyperplasia, and studies of familial cases have shown an autosomal dominant inheritance with incomplete penetrance. 3,8
Clinically, sebaceous hyperplasia lesions affect the face of elderly, white individuals, presenting as raised, tumorous, rounded, salient but superficial lesions of 2-5 mm in diameter that are yellowish or flesh-colored. They are principally located in the sebaceous areas: on the frontal region and, less commonly, on the malar, nasal and temporal regions, as well as on the neck and chest. 4,8 Although the condition is referred to as ìsenile sebaceous hyperplasiaî, it may also occur in young people. 9-11
Diagnosis is generally clinical and can be confirmed by histopathology. Dermoscopy and confocal microscopy may be useful in the clinical evaluation of the patient, principally to differentiate in vivo between sebaceous hyperplasia and basal cell carcinoma, its principal differential diagnosis. 4, 12-14
Tissue evaluation in cases of sebaceous hyperplasia, comparing the size of the different segments of the pilosebaceous unit with sebaceous hyperplasia, shows a clear increase in the diameter of the sebaceous acini, as well as a greater number of immature sebocytes and dilatation of the follicular infundibulum. 15
At immunohistochemistry, cytokeratin-14 (CK14) expression was found in the keratinocytes of the infundibulum, the isthmus and the sebaceous duct, and in both mature and immature sebocytes; CK-1 expression was found in the infundibular keratinocytes, CK-17 expression in the keratinocytes of the sebaceous duct and an increase in the Ki-67 cell proliferation marker (MIB-1) in the immature sebocytes. Expression of cytokeratins CK-18 and CK-19 was not detected. 15
Numerous forms of treatment have been described for sebaceous hyperplasia. These include systemic isotretinoin, photodynamic therapy with methyl aminolevulinate or aminolevulinic acid, oral administration of a 5-lipoxygenase inhibitor (ZileutonÆ), which acts by directly decreasing sebaceous production, the use of lasers such as the laser diode, pulsed dye laser (PDL) and erbium. Other procedures that have also been used include excision, electrocauterization, cryotherapy, shaving, curettage and cauterization with chemical agents used topically, although there is a risk of depigmentation or scarring with these latter options. 16-25
Many laboratory studies have shown the effect of androgens on the sebaceous glands. A review of the literature on several research models confirmed the effect of androgens in triggering the activity of the sebaceous glands and indicated which of these hormones may be implicated in triggering these glands.
The association between sebaceous hyperplasia and androgens was confirmed in a study conducted in rats in which this condition was induced by increasing testosterone levels. 6
Additionally, genetically mutant rats were used in a study model of sebaceous hyperplasia conducted to evaluate whether increased sebum secretion and sebaceous hyperplasia are androgen-dependent. The effects of inhibitors of steroid 5-alpha-reductase type I and type II and an androgen receptor blocker on the regression of hyperplasia were evaluated. The three agents were administered to rats; however, the control group and the castrated rats received only the vehicle. The androgen receptor blocker induced regression equivalent to that found in the castrated rats, thus confirming its efficacy in suppressing sebaceous gland development, while only a moderate reduction was achieved in the gland with the type I and type II steroid inhibitors. 7 It should be emphasized that no statistically significant changes were found in serum testosterone or dihydrotestosterone (DHT) levels in the rats treated with the drugs. 7
Another study was conducted to evaluate the effect of DHT in sebaceous production. This study was carried out in adult men with inherited 5-alpha-reductase 2 deficiency (pseudohermaphroditism), therefore with a consequent decrease in dihydrotestosterone. The reduction in DHT was shown to have no effect on the development or function of the sebaceous glands, since sebaceous production was found to be normal in these subjects. 2
A study was conducted in oophorectomized female rats to evaluate the effect of another androgen, dehydroepiandrosterone (DHEA), on the skin sebaceous glands. A DHEA precursor was administered to a group of oophorectomized rats, while other groups of female rats that had also been oophorectomized were concomitantly treated with DHEA alone or in combination with antiandrogens or antiestrogens with the objective of determining the estrogenic or androgenic effects of DHEA. The appearance of sebaceous hyperplasia was noted in the oophorectomized rats treated with DHEA; however, in the rats using the antiandrogen, this change induced by DHEA in the sebaceous gland was inhibited, while no effect was obtained with the antiestrogen. These data show the exclusively androgenic effect of DHEA in stimulating the glands. 26
In the field of research involving the sebaceous glands, studying the human sebocyte culture model is considered fundamental in clarifying the activity of the sebaceous gland, since these glands are exclusive to each species. The culture model is able to show that hormonal control of the sebaceous gland consists of a complex mechanism, in which not only androgens have a stimulating effect on the activity of the sebocytes in vitro but also other hormones such as insulin, thyroid hormone and hydrocortisone. 27
MATERIAL AND METHODS
This was a single-center, prospective, observational, pilot clinical trial, approved by the internal review board of the Federal University of São Paulo (UNIFESP), in which 16 female volunteers over 18 years of age with facial sebaceous hyperplasia were selected either from the dermatology clinic of the same institute or from another collaborating service during the second semester of 2006. These patients had to be able to read, understand and sign a copy of the informed consent form approved for this study and were provided with a copy of the form. Women in use of any medication that could interfere with the results of hormone measurement and women with any known hormonal changes at the time of enrollment or who had any comorbidity that could interfere with the evaluation and clinical inference of the patient were excluded from the study.
In each patient, a biopsy was performed on a lesion clinically diagnosed as sebaceous hyperplasia and the material was sent for histopathological confirmation of the diagnosis of the disease under evaluation. Next, the patients were referred for blood sampling to measure the hormones that constitute the androgen cascade: dehydroepiandrosterone (DHEA), total testosterone (TT), free testosterone (FT), androstenedione and dehydroepiandrosterone sulfate (DHEA-S). 28
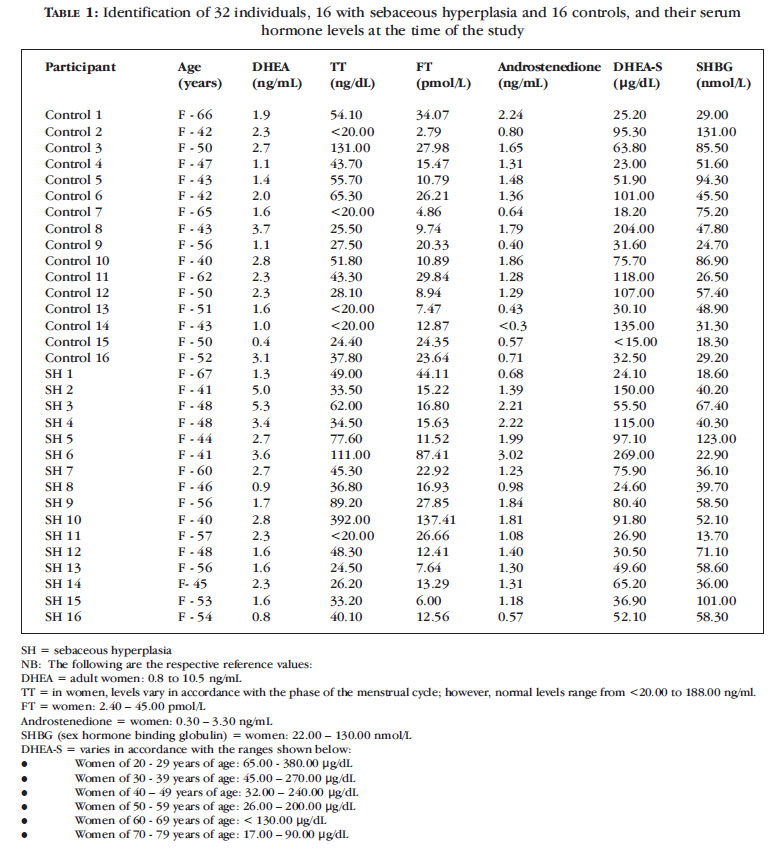
In parallel, 16 volunteers without any sebaceous hyperplasia lesions and of a similar age to the women in the study group were paired, one to one, with a patient from the study group (Table 1).
These volunteers were selected at random at the same sites at which the women in the study group were recruited while attending routine consultations. This group of women also answered a questionnaire on comorbidities and medication. Except for the condition under evaluation, all other inclusion and exclusion criteria were applied and all the women were referred to the same laboratory for hormone measurement.
All the data from both groups were recorded and analyzed statistically. Due to the small sample size and the low incidence of abnormality found in the two groups, Fisherís exact test was used to compare the groups with respect to normal hormone profiles. The non-parametric Mann-Whitney (or Wilcoxon) test was used to compare the median hormone levels in the two groups, since the distribution of hormone levels was not close to normal (Gaussian).
RESULTS
Sixteen female patients with sebaceous hyperplasia lesions were analyzed, together with their respective controls of the same gender and a similar age. Mean age was 53.5 years (range 40-67 years). No changes were found in the DHEA levels of the 16 patients in the study group; however, a 50-year old woman in the control group had low levels of this hormone, 0.4 ng/ml, compared to the normal range of 0.8 to 10.5 ng/ml.
With respect to total testosterone, an increased level of 392.00 ng/dl was found in a 40-year old patient with sebaceous hyperplasia (normal range: < 20.00 to 118.00 ng/dl). In the control group, there was also a case of a higher level compared to the same reference limits: 131.00 ng/dl. Two patients in the study group had increased free testosterone levels: a 41-year old patient with a measurement of 87.41 pmol/l and a 40-year old patient with a measurement of 137.41 pmol/l (normal range: 2.40 ñ 45.0 pmol/l). No changes were found in any patients in the control group with respect to this hormone.
No changes in androstenedione were found in any of the patients in the study group and only one case with a decreased level was found in the control group: a 43-year old patient with a measurement of < 0.30 ng/ml compared to the normal range of 0.30 ñ 3.30 ng/ml.
Three cases were found in the study group in which DHEA-S measurements were outside the normal range according to age (32.00 to 240.00 ºg/dl). A 41-year old patient had an increased level of 269.10 ºg/dl and two other patients had decreased levels (a 46-year old patient with a measurement of 24.60 ºg/dl and a 48-year old patient with a measurement of 30.50 ºg/dl). In the control group, two decreased values were found: a 50-year old woman with a DHEA-S level below 15.00 ºg/dl (normal range: 26.00-200.00 ºg/dl) and a 47-year old patient with a measurement of 23.00 ºg/dl (normal range: 32.00-240.00 ºg/dl).
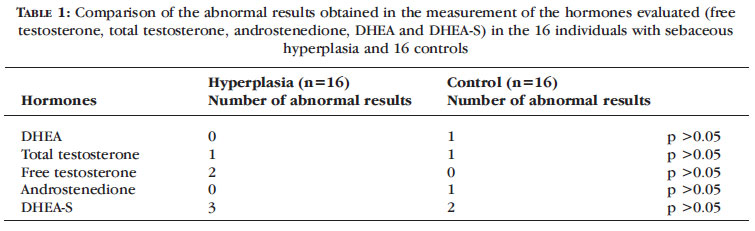
These results are summarized in Tables 1 and 2.
DISCUSSION
The sebaceous glands are susceptible to the effect of androgens, with a benign proliferation of these glands, i.e. hyperplasia, occurring with age. Therefore, the onset of sebaceous hyperplasia with advancing age may be explained by slower cell renovation in the glands secondary to a decline in androgen levels. 1-4 Nevertheless, laboratory studies have reported a relationship between the appearance of sebaceous hyperplasia lesions and androgenic stimuli. 2,5,7,26
The effect of other factors such as genetic alterations found in familial cases of sebaceous hyperplasia, chronic exposure to solar radiation or factors related to immunosuppression such as the use of cyclosporins has yet to be fully clarified in the etiopathogenesis of this sebaceous gland condition. 3,8,29,30
Hyperplasia is classified as a benign tumor. Since it affects exposed areas such as the face, it is a frequent cause of complaint in clinical dermatology; however, for the dermatologist, the principal concern is to differentiate between this condition and basal cell carcinoma. 4,8,13,14
In order to study sebaceous hyperplasia, the etiopathogenesis of which is yet to be established, the present protocol was based on studies correlating the appearance of sebaceous hyperplasia lesions with the effect of androgens. 2,5-7,26 In the present study, serum androgen levels were evaluated in female patients with sebaceous hyperplasia in order to analyze whether changes occur in the levels of these hormones in patients with this skin disease.
A literature review revealed various studies on the effect of androgens in stimulating the sebaceous gland, principally the effect of DHEA; however, most consisted of in vitro studies conducted using human sebocyte culture, studies involving animal models or evaluations in humans with known hormonal changes. To the best of our knowledge, no studies had yet been conducted using serum androgen measurements in individuals with sebaceous hyperplasia lesions and with no known hormonal changes. 2,5-7,26
The studies identified in this review showed the effect of androgens on the sebaceous glands. The effects of androgens in triggering hyperplasia of the sebaceous glands were reported in a study using an animal model in which an androgen-stimulating substance was administered. Some investigators have shown that blocking the androgenic effect leads to consequent suppression of the growth of the sebaceous glands. 6,7
Conducted to identify which hormones were involved in inducing sebaceous hyperplasia, some studies were able to confirm that reducing DHT has no effect on the development of the sebaceous gland or its function in sebaceous production; however, they confirmed the androgenic effect of DHEA in triggering sebaceous hyperplasia. 2,7,26
The present study evaluated a group of women with sebaceous hyperplasia and another group without the disease, none of whom had any previously known hormonal changes or were in use of exogenous hormones. The two groups, composed of women of a similar age, were compared and no association was found between the hormonal conditions diagnosed during the study [changes in serum androgen levels (DHEA, DHEA-S, TT, FT, androstenedione)] and the onset of sebaceous hyperplasia lesions in this sample. Thus far, despite the fact that the appropriate statistical tests for the sample size were applied, no statistically significant differences were found in the measurements of any of the androgens studied between the patients with sebaceous hyperplasia and the control group (Table 2).
Therefore, although a strong effect of androgens in stimulating the sebaceous gland has been shown in various laboratory studies, when the hormone levels of patients with sebaceous hyperplasia lesions were evaluated, no statistically significant differences were found in comparison to a control group of women without the disease.
The results of this pilot study suggest that skin lesions may occur in sebaceous hyperplasia due to: 1) increased sensitivity of hormone receptors in the sebaceous glands; or 2) a delayed consequence of exposure of the sebocytes to the normal effect (both quantitatively and qualitatively) of circulating androgens throughout the individualís lifetime; or 3) individual or genetic predisposition (not hormonedependent). Moreover, it suggests that hormone dependency only exists in experimental models. Nevertheless, further studies should be conducted with larger sample sizes to correlate clinical and laboratory findings, both hormonal and biomolecular, in order to fully clarify these findings.
CONCLUSION
Although the physiopathogenesis of sebaceous hyperplasia is associated with hormonal effects, in the present sample in which women with sebaceous hyperplasia were compared with women of a similar age without the condition, no association was found between serum hormone levels and the appearance of sebaceous hyperplasia lesions. Further studies with larger sample sizes should be conducted to correlate clinical and laboratory findings (hormonal and biomolecular) in order to reach a final conclusion on this skin disorder.
REFERENCES
- 1. Zouboulis CC. Acne and sebaceous gland function. Clin.Dermatol. 2004;22:360-6.
- 2. Imperato-McGinley J, Gautier T, Cai LQ, Yee B, Epstein J, Pochi P. The androgen control of sebum production. Studies of subjects with dihydrotestosterone deficiency and complete androgen insensitivity. J Clin Endocrinol. Metab. 1993;76:524-8.
- 3. Zouboulis CC, Boschnakow A. Chronological ageing and photoageing of the human sebaceous gland. Clin.Exp.Dermatol. 2001;26:600-7.
- 4. Marques A, Rocha GL. Hiperplasia sebacea senil. An Bras Dermatol. 1969;44:191-7.
- 5. Ito M, Motoyoshi K, Suzuki M, Sato Y. Sebaceous gland hyperplasia on rabbit pinna induced by tetradecane. J Invest Dermatol. 1985;85:249-54.
- 6. Sandbank M, Abramovici A, Wolf R, Ben DE. Sebaceous gland hyperplasia following topical application of citral. An ultrastructural study. Am.J Dermatopathol. 1988;10:415-8.
- 7. Ye F, Imamura K, Imanishi N, Rhodes L, Uno H. Effects of topical antiandrogen and 5-alpha-reductase inhibitors on sebaceous glands in male fuzzy rats. Skin Pharmacol. 1997;10:288-97.
- 8. Weisshaar E, Schramm M, Gollnick H. Familial nevoid sebaceous gland hyperplasia affecting three generations of a family. Eur J Dermatol. 1999;9:621-3.
- 9. Bhawan J, Calhoun J. Premature sebaceous gland hyperplasia. J.Am.Acad.Dermatol. 1983;8:136.
- 10. De Villez RL, Roberts LC. Premature sebaceous gland hyperplasia. J Am Acad Dermatol. 1982;6:933-35.
- 11. Dupre A, Bonafe JL, Lamon P. Functional familial sebaceous hyperplasia of the face and premature sebaceous gland hyperplasia: a new and unique entity. J Am Acad Dermatol. 1983;9:768-9.
- 12. Zaballos P, Ara M, Puig S, Malvehy J. Dermoscopy of sebaceous hyperplasia. Arch Dermatol. 2005;141:808.
- 13. Prioleau PG, Santa Cruz DJ. Sebaceous gland neoplasia. J Cutan Pathol. 1984;11:396-414.
- 14. Propperova I, Langley RG. Reflectance-mode confocal microscopy for the diagnosis of sebaceous hyperplasia in vivo. Arch Dermatol. 2007;143:134.
- 15. Schirren CG, Jansen T, Lindner A, Kind P, Plewig G. Diffuse sebaceous gland hyperplasia. A case report and an immunohistochemical study with cytokeratins. Am J Dermatopathol. 1996;18:296-301.
- 16. Grimalt R, Ferrando J, Mascaro JM. Premature familial sebaceous hyperplasia: successful response to oral isotretinoin in three patients. J Am Acad Dermatol. 1997;37:996-8.
- 17. Perrett CM, McGregor J, Barlow RJ, Karran P, Proby C, Harwood CA. Topical photodynamic therapy with methyl aminolevulinate to treat sebaceous hyperplasia in an organ transplant recipient. Arch Dermatol. 2006;142:781-82.
- 18. Alster TS, Tanzi EL. Photodynamic therapy with topical aminolevulinic acid and pulsed dye laser irradiation for sebaceous hyperplasia. J.Drugs Dermatol. 2003;2:501-4.
- 19. Zouboulis C, Saborowski A, Boschnakow A. Zileuton, an oral 5-lipoxygenase inhibitor, directly reduces sebum production. Dermatology. 2005;210:36-8.
- 20. No D, McClaren M, Chotzen V, Kilmer SL. Sebaceous hyperplasia treated with a 1450-nm diode laser. Dermatol Surg. 2004;30:382-4.
- 21. González S, White WM, Rajadhyaksha M, Anderson RR, González E. Confocal imaging of sebaceous gland hyperplasia in vivo to assess efficacy and mechanism of pulsed dye laser treatment. Lasers Surg.Med. 1999;25:8-12.
- 22. Riedel F, Bergler W, Baker-Schreyer A, Stein E, Hörmann K. [Controlled cosmetic dermal ablation in the facial region with the erbium:YAG laser]. HNO 1999;47:101-06.
- 23. Bader RS, Scarborough DA. Surgical pearl: intralesional electrodesiccation of sebaceous hyperplasia. J Am Acad Dermatol. 2000;42:127-8.
- 24. Rosian R, Goslen JB, Brodell RT. The treatment of benign sebaceous hyperplasia with the topical application of bichloracetic acid. J.Dermatol.Surg.Oncol. 1991;17:876-9.
- 25. Wheeland RG, Wiley MD. Q-tip cryosurgery for the treatment of senile sebaceous hyperplasia. J Dermatol Surg Oncol. 1987;13:729-30.
- 26. Sourla A, Richard V, Labrie F, Labrie C. Exclusive androgenic effect of dehydroepiandrosterone in sebaceous glands of rat skin. J.Endocrinol. 2000;166:455-62.
- 27. Zouboulis CC, Xia L, Akamatsu H, Seltmann H, Fritsch M, Hornemann S, et al. The human sebocyte culture model provides new insights into development and management of seborrhoea and acne. Dermatology. 1998;196:21-31.
- 28. Yarak S, Bagatin E, Hassun KM, Parada MOAB, Talarico Filho S. Hiperandrogenismo e pele: síndrome do ovário policístico e resistência periférica à insulina. An Bras Dermatol. 2005;80:395-410.
- 29. Kumar P, Marks R. Sebaceous gland hyperplasia and senile comedones: a prevalence study in elderly hospitalized patients. Br J Dermatol. 1987;117:231-6.
- 30. Pang SM, Chau YP. Cyclosporin-induced sebaceous hyperplasia in renal transplant patients. Ann Acad Med Singapore. 2005;34:391-3.
Publication Dates
-
Publication in this collection
01 Dec 2011 -
Date of issue
Oct 2011
History
-
Accepted
09 July 2011 -
Received
20 May 2010