Abstracts
Polycystic ovary syndrome (POS) is one of the most common endocrine abnormalities affecting women of reproductive age. It is a cause of significant social embarrassment and emotional distress. The pathogenesis of the disease is not yet fully understood, but it is thought to be a complex multigenic disorder, including abnormalities in the hypothalamic-pituitary axis, steroidogenesis, and insulin resistance. The main diagnostic findings of the syndrome are: hyperandrogenism, chronic anovulation and polycystic ovarian morphology seen on ultrasound. Hyperandrogenism is generally manifested as hirsutism, acne, seborrhea, androgenic alopecia and, in severe cases, signs of virilization. Treatment may improve the clinical manifestations of excess androgen production, normalize menses and ameliorate metabolic syndrome and cardiovascular complications. This article reviews the diagnosis, clinical manifestations, metabolic complications, and treatment of the syndrome. Early diagnosis and the consequent early treatment may prevent metabolic complications and emotional distress that negatively impact the patients' quality of life.
Acanthosis nigricans; Acne vulgaris; Alopecia; Hyperandrogenism; Polycystic ovary syndrome
A síndrome do ovário policístico (SOP) é uma das endocrinopatias mais freqüentes nas mulheres em idade reprodutiva. Caracteriza-se por morbidade elevada devido aos aspectos estéticos e por repercussões metabólicas importantes. Embora a sua patogênese permaneça incompletamente conhecida, acredita-se numa desordem multigênica complexa, incluindo anormalidades no eixo hipotálamohipofisário, esteroidogênese e resistência insulínica. Os achados principais para o diagnóstico são: hiperandrogenismo, anovulação crônica e ovários policísticos à ultrassonografia. As manifestações dermatológicas do hiperandrogenismo incluem: hirsutismo, acne, seborréia, alopecia e, em casos mais graves, sinais de virilização. Existe considerável heterogeneidade nos achados clínicos e também pode haver variação na mesma paciente com o passar do tempo. O tratamento visa reduzir as manifestações do hiperandrogenismo, restaurar os ciclos ovulatórios regulares e corrigir a síndrome metabólica. Este artigo apresenta revisão da fisiopatologia, diagnóstico e tratamento da síndrome do ovário policístico. Enfatiza-se a importância do diagnóstico e tratamento precoces no intuito de prevenir as complicações metabólicas e a repercussão emocional que afeta a qualidade de vida das pacientes.
Acantose nigricans; Acne vulgar; Alopecia; Hiperandrogenismo; Síndrome do ovário policístico
REVIEW
Polycystic ovary syndrome: a dermatologic approach*
Heloisa Helena Gonçalves de MouraI; Dailana Louvain Marinho CostaII; Ediléia BagatinIII; Celso Tavares SodréIV; Mônica Manela-AzulayV
ISpecialist in Clinical Medicine and Dermatology - Post-graduate student, Dermatology Service, Federal University of Rio de Janeiro (UFRJ) - Rio de Janeiro (RJ), Brazil
IISpecialist in Clinical Medicine with postgraduate training in dermatology, Federal University of Rio de Janeiro (UFRJ), Rio de Janeiro, Brazil
IIIPhD in Dermatology from the Federal University of São Paulo - Professor (PhD), Department of Dermatology, Federal University of São Paulo - São Paulo School of Medicine (Unifesp - EPM) - São Paulo, Brazil
IVSpecialist in Dermatology - Assistant Professor of Dermatology, Faculty of Medicine, Federal University of Rio de Janeiro (UFRJ); Assistant Professor of Dermatology, Santa Casa de Misericordia do Rio de Janeiro (SCMRJ); Assistant Professor of Dermatology, Souza Marques Technical-Educational Foundation - Rio de Janeiro (RJ), Brazil
VPhD in Dermatology, Federal University of Rio de Janeiro - Professor (PhD) of Dermatology, Faculty of Medicine, Federal University of Rio de Janeiro; Professor (PhD) of Dermatology, Souza Marques Technical-Educational Foundation - Rio de Janeiro (RJ), Brazil
Mailing address
ABSTRACT
Polycystic ovary syndrome (POS) is one of the most common endocrine abnormalities affecting women of reproductive age. It is a cause of significant social embarrassment and emotional distress. The pathogenesis of the disease is not yet fully understood, but it is thought to be a complex multigenic disorder, including abnormalities in the hypothalamic-pituitary axis, steroidogenesis, and insulin resistance. The main diagnostic findings of the syndrome are: hyperandrogenism, chronic anovulation and polycystic ovarian morphology seen on ultrasound. Hyperandrogenism is generally manifested as hirsutism, acne, seborrhea, androgenic alopecia and, in severe cases, signs of virilization. Treatment may improve the clinical manifestations of excess androgen production, normalize menses and ameliorate metabolic syndrome and cardiovascular complications. This article reviews the diagnosis, clinical manifestations, metabolic complications, and treatment of the syndrome. Early diagnosis and the consequent early treatment may prevent metabolic complications and emotional distress that negatively impact the patients' quality of life.
Keywords: Acanthosis nigricans; Acne vulgaris; Alopecia; Hyperandrogenism; Polycystic ovary syndrome
INTRODUCTION
Polycystic ovary syndrome (PCOS), originally described in the 1930s by Stein and Leventhal, is one of the most common endocrinopathies in women of reproductive age. 1 It is often characterized by hyperandrogenism, which may often be manifested as: hirsutism, acne, seborrhea, alopecia, menstrual irregularity, obesity and ovarian cysts. PCOS shows reproductive and metabolic complications that must be diagnosed and treated early due to the risk of infertility, endometrial cancer and plurimetabolic syndrome. Besides these complications, PCOS is associated with high morbidity due to aesthetic aspects that negatively affect women's self-esteem. Knowledge about the physiopathologic mechanisms of this syndrome is very important for an appropriate therapeutic approach.
CLASSIFICATION AND EPIDEMIOLOGY
The key findings for the diagnosis of PCOS are: hyperandrogenism, chronic anovulation and polycystic ovaries on ultrasound. However, other conditions may present with these manifestations, making differential diagnosis necessary.
Only a third of the patients have the classical form of the syndrome described by Stein and Leventhal, defined by the presence of amenorrhea, hirsutism and bilateral enlargement of the ovaries. Therefore, in 1990, the conference of the National Institutes of Health (NIH) suggested new diagnostic criteria represented by clinical and/or biochemical evidence of hyperandrogenism associated with oligoamenorrhea, with the exclusion of other causes of hyperandrogenism. In 2003, a new consensus was established by the European Society for Human Reproduction and Embryology and the American Society for Reproductive Medicine in Rotterdan - The Netherlands, which defined the syndrome as the presence of two or more manifestations that included, besides those defined by the NIH, the presence of polycystic ovaries on ultrasound.2 In 2006, the Androgen Excess Society gave emphasis to hyperandrogenism, suggesting that this would be a mandatory criterion for the diagnosis of the syndrome (Chart 1).3
PCOS affects women of childbearing age, with no predilection for race, but the signs and symptoms may differ in various ethnic groups. Prevalence varies from 4-10%, depending on the diagnostic criteria used.4, 5 By the Rotterdam criteria, the prevalence is five times higher than that defined by the criteria of the NIH. 6
PATHOGENESIS
The etiology of PCOS is not yet fully understood. It is believed that there is multigenic complex disorder, including abnormalities in the hypothalamic-pituitary axis, steroidogenesis and insulin resistance. 7
Abnormal steroidogenesis
Most authors consider that abnormal steroidogenesis, of ovarian or adrenal origin, is the primary disorder of PCOS. High concentrations of circulating testosterone and dehydroepiandrosterone (DHEA) occur in 60-80% and 20-25% of women with PCOS, respectively. 6, 8 There is also an increase in estradiol production by ovarian granulosa cells associated with excess androgen levels. (Chart 2). 9
Insulin resistance
Over the last decade, it has been observed that most women with PCOS have some degree of insulin resistance, even non-obese ones. Studies suggest the existence of genetic predisposition, which ends up manifesting as a result of lifestyle and obesity.
Hyperinsulinemia resulting from insulin resistance leads to an increase in the production of androgens and also in their biologically active portion. The common suggested mechanism for this is associated with changes in insulin receptors and in the enzyme that regulates the production of adrenal and ovarian androgens. 10 The systemic effects of hyperinsulinemia are described in chart 3.11, 12
Abnormalities in pituitary function:
An imbalance in the release of the luteinizing hormone (LH) and the follicle stimulating hormone (FSH) from the pituitary has been implicated in the pathogenesis of PCOS. In patients with PCOS, the LH / FSH ratio is altered and the secretion of LH is higher than that of FSH, resulting in increased androgen production by theca cells and in anovulatory cycles. 12, 13 However, recent studies have shown that changes in LH levels are a secondary and not primary event. 13
Thus, higher levels of circulating androstenedione are observed, with a consequent increase in the peripheral conversion of this hormone into testosterone. Excess androgen levels alter the regulation of female hormones, resulting in increased estrogen levels, menstrual irregularities and infertility. 6, 7, 12
CLINICAL MANIFESTATIONS
The clinical dermatologic manifestations of hyperandrogenism include: hirsutism, acne, seborrhea, alopecia, and in severe cases, signs of virilization. There is considerable heterogeneity in clinical findings, as there may be variation in the same patient over time. For instance, hyperandrogenism may not determine peripheral manifestations, as seen mainly in Asian women.
Hirsutism
Hirsutism is defined as excessive growth of terminal hair in androgen-dependent areas in women. It is one of the most commonly used clinical criteria for the diagnosis of excess androgen levels, being observed in 50-80% of the patients with hyperandrogenism. 14-16
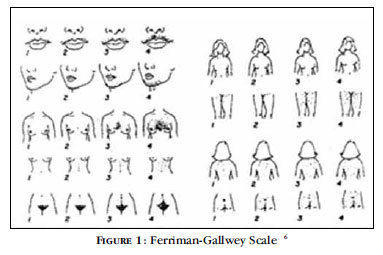
The Ferriman-Gallwey scale is used for the diagnosis of hirsutism. The disorder is considered present when the score is > 8. However, this score is examiner-dependent and does not consider the impact of hirsutism on the well being of the patient. 1518 There is no correlation of the score with androgen levels, since the response of the pilosebaceous unit to androgens varies considerably (Figure 1). 17, 19, 20
The formation of hair follicles occurs during fetal development and their concentration reflects ethnic differences.9 The speed of hair growth varies according to genetic differences in the activity of the enzyme 5α-reductase, which converts testosterone into dihydrotestosterone (DHT), which is the most potent metabolite. There are two isoenzymes of 5αreductase: type 1, present in the sebaceous glands and skin of the pubic region, and type 2, found in hair follicles, scalp and genital area. Differences in the activity of the two enzymes determine the variation of clinical presentations in women with hyperandrogenism. It is established that hirsute women have increased activity of 5α-reductase in the hair follicles. The activity of 5α-reductase is stimulated by hyperandrogenism, insulin-like growth factor (IGF) and by insulin itself. 21
Testosterone and DHT determine alterations in the hair and in its cycle. They irreversibly transform vellus hair into terminal hair, thicker and more pigmented, in androgen-sensitive areas (face, neck, chest and pubic region), after months or years of exposure.16, 17 The duration of the anagen phase is also related to levels of circulating androgens and varies in the different regions of the body. Androgens prolong the anagen phase in follicles of the body and shorten it in some areas of the scalp. 16
To differentiate hirsutism from hypertrichosis in women, it is necessary to determine the type of excess hair and its distribution. Hypertrichosis is excessive hair in the entire body; the growth of vellus hair in androgen-independent areas, such as forearms and calves, is also considered a manifestation of the disease. Excess of vellus hair alone does not reflect hyperandrogenism, as the former may be associated with hereditary factors, medication, metabolic disorders or physical irritation of the skin.17 In hirsutism there is an increase of terminal hair in androgen-dependent areas.
Acne
Acne is a pilosebaceous unit disorder with lesions on the face, neck, back and pectoral region.
The importance of androgens in the pathogenesis of acne is well documented. However, since in acne vulgaris androgen levels are usually normal, it is believed that local conversion is enhanced by increased receptor sensitivity to androgens in acne patients as compared with the normal population, perhaps representing the most important triggering factor of the disease . 15.22
Androgens not only enlarge the sebaceous glands and increase sebum production, but also cause abnormal desquamation of follicular epithelial cells. These factors determine the formation of comedones and, in combination with the colonization of the follicle by Propionibacterium acnes, result in inflammation and progressive development of papules, pustules, nodules, cysts and scars. 9
Several studies attempt to correlate the clinical presentation of acne with markers of hyperandrogenism. Although some authors have shown a correlation between acne and high levels of dehydroepiandrosterone sulfate (DHEA-S), DHT, androstenedione, testosterone and IGF, others did not corroborate this finding. 15, 20-22
Androgenetic Alopecia
Androgenetic alopecia in women is characterized by loss of hair in the central region of the scalp, with relevant psychosocial repercussions.
In the presence of androgen, high levels of 5alpha reductase, higher concentration of androgen receptors and lower levels of the enzyme cytochrome p450, the anagen phase is shortened, and the terminal follicles undergo miniaturization, turning into vellus hair. 16, 21 Changes may occur diffusely, but they are usually more evident in the frontal and parietal regions.
The diagnosis should exclude other causes of hair loss in women, such as telogen effluvium, alopecia areata, anagen loss syndrome and trichotillomania. 15
Most patients with androgenetic alopecia have normal endocrine function. Thus, physical examination and medical history are important in the search for other signs of hyperandrogenism. 23 Additional tests may be needed, such as hair density, hematological and biochemical evaluations, and histological examination of the scalp to demonstrate miniaturized hair.
Acanthosis nigricans
Acanthosis nigricans is characterized by brown and velvety hyperpigmentation of the skin with accentuation on skin folds. It is most commonly observed in the neck and intertriginous areas, such as armpits, groins and inframammary region. It is reported in 5% of the patients with PCOS. 9
Excessive binding of serum insulin to IGF-1 receptors in peripheral tissues determines the proliferation of keratinocytes and fibroblasts; hence, acanthosis nigricans is a cutaneous manifestation of hyperinsulinemia, and not just of obesity. Histologically there is papillomatosis, hyperkeratosis and acanthosis with minimal or no hyperpigmentation of the basal layer.
Despite being mostly associated with obesity, PCOS and diabetes, acanthosis nigricans may be associated with genetic diseases, drug reaction (nicotinic acid) and malignancies. 15
The presence of acanthosis nigricans suggests the performance of the glucose tolerance test. 24 When severe, extensive and progressive, it may be associated with malignancy, especially if mucous membranes are also involved. 15
Other Complications
The dysfunction of multiple hormone systems that characterize PCOS determines increased risk for many diseases. 8 It is therefore important to evaluate and monitor patients to reduce morbidity and clinical implications.
Glucose intolerance and diabetes are considered the most important disorders in women with PCOS.6, 9, 25 There is no consensus, but it is believed that PCOS is related to increased risk for cardiovascular disease.8, 26 However, clinical observations did not show greater morbidity and/or mortality in women with PCOS, despite the recognized association between insulin resistance and lipid profile changes. 8, 9, 12
Because PCOS is associated with irregular menstrual cycles, women may experience difficulty getting pregnant, endometrial hyperplasia and endometrial carcinoma.9, 12 These patients have a higher prevalence of abortion, ovarian and breast cancer, nonalcoholic steatohepatitis, obstructive sleep apnea and symptoms of depression. 8, 9, 27
Evaluation
The clinical diagnosis of PCOS is based on specific diagnostic criteria (Chart 4), but due to variable symptomatology, close attention to signs and symptoms is required. 6, 10
Clinical evaluation
The evaluation should include detailed menstrual history, information about the onset and duration of symptoms suggestive of hyperandrogenism and family history of PCOS and metabolic diseases 8-10,28. Abrupt changes in the menstrual pattern or symptoms of hyperandrogenism should alert us to other etiologies 28.
The most frequent clinical manifestations include menstrual irregularity or amenorrhea, which can be masked by the use of oral contraceptives (OC). However, some women with PCOS have regular ovulatory cycles.
Examination of the genital region can be accomplished using the Tanner Scale, which analyzes the development of primary and secondary sexual characteristics and identifies signs of virilization. Clitoromegaly and voice changes are rare findings suggestive of adrenal tumors 10.
Laboratory Evaluation
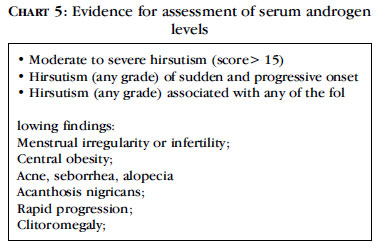
The main feature to confirm PCOS is the detection of excessive androgen levels through laboratory tests, although consensus allows only clinical evidence of hyperandrogenism recommendation of the Endocrine Society in 2008 for the evaluation of serum androgen levels in hirsute patients is listed below (Chart 5).
Among androgens, testosterone is the primary hormone in the evaluation of hirsutism and PCOS. However, most available methods are not very accurate, so their analysis should be cautious 17, 29.The most sensitive and precise methods are radioimmunoassays and tandem mass spectrometry.
Free testosterone is the most sensitive for the detection of hyperandrogenism 10, 29.This is because sex hormone-binding globulin (SHBG), which is the main determinant of the bioactive portion of plasma testosterone (free or bioavailable testosterone), is reduced in patients with hyperandrogenism. No tests are available for the direct measurement of this hormone. The best method is to calculate it by the product of total testosterone and SHBG function (e.g., free testosterone = total testosterone x percentage of free testosterone) 29, 30.These tests should be performed early in the morning, between the 4th-10th days of the menstrual cycle (women with regular cycles) or in women with amenorrhea. Patients with symptoms consistent of PCOS but without testosterone level changes must repeat the exam.
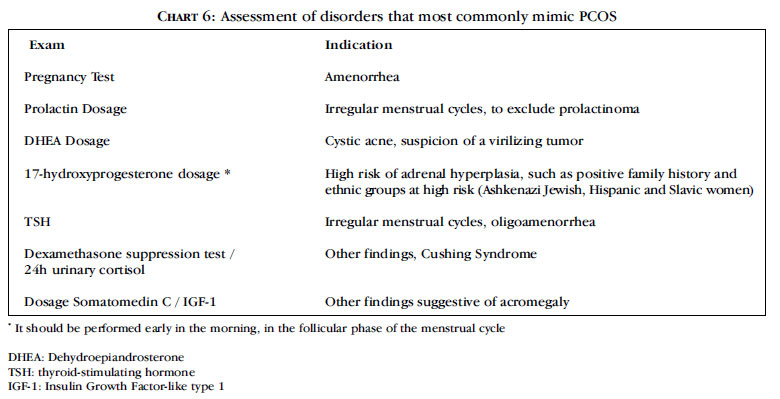
The dosage of other androgens in most cases is of little use. However, it is necessary to exclude other relatively common conditions that have specific treatment. The tests usually required and their main indications are listed in chart 6.
If the screening for disorders that most commonly mimic PCOS is negative, the association of high levels of testosterone and anovulatory symptoms meet the criteria for this syndrome. However, other rare disorders associated with androgen excess cannot be ruled out and an expert evaluation should be conducted to determine the source of androgen excess in these cases 17.
The LH / FSH ratio is no longer recommended for routine diagnosis of PCOS 2.This is because there are differences between the data supplied by various tests (radioimmunoassay with polyclonal antibodies, monoclonal antibody radioimmunoassay, immunometric assays), body mass index of the patient and day of the cycle in which the test is done. Moreover, the relationship of the LH/FSH ratio in the pathogenesis of PCOS has not been confirmed. However, FSH is important in the diagnosis of primary ovarian failure, when FSH levels are high in relation to LH and estrogen 31.
Among other laboratory tests, it is important to remember the metabolic changes that may be related to PCOS, since they are associated with high morbidity. The glucose tolerance test should be requested of obese women and those at risk for type 2 diabetes, such as family history 10. Periodic assessment of lipid profile is recommended, as well as liver function monitoring when there are risk factors for hepatic steatosis (nonalcoholic fatty liver disease)11, 32.
Radiological Assessment
Indication of pelvic ultrasonography is still controversial. The classical presentation of PCOS is characterized by ovaries with a polycystic aspect, with more than eight subcortical cysts smaller than 10 mm and/or increased ovarian volume (> 10ml) 2. The examination should be performed early in the follicular phase of the cycle (third to fifth day). In oligo / amenorrheic patients, the exam may be performed randomly or after 3-5 days of bleeding induced by progestogens. However, 20% of the patients did not reveal changes on ultrasound 6. Moreover, many healthy women or those with other disorders related to excess androgens may have polycystic ovaries to the examination without presenting the other findings of the syndrome 29.
TREATMENT
The treatment of PCOS seeks to reduce the signs of hyperandrogenism, restore regular ovulatory cycles and control features of the metabolic syndrome. The choice of treatment will depend on the severity of the symptoms and specific goals, always considering the possible long-term consequences.
Changes in lifestyle
It is the most effective and inexpensive approach, without adverse reactions. It consists in the practice of regular physical activity and in the adoption of a balanced diet. The loss of only 2-7% of weight improves almost all the parameters of PCOS by reducing androgen levels and improving ovarian function 10. These effects are related to the reduction in insulin levels and improvement of insulin resistance 33.
Hormonal Treatment
The estrogen-progesterone combination therapy remains the main option for the treatment of hyperandrogenism for women who do not wish to become pregnant. It can be effective for hirsutism, acne and androgenic alopecia, and to prevent endometrial hyperplasia and its complications 14.
Oral contraceptives (OC) are composed of ethinyl estradiol and a progestin. Estrogen suppresses the LH, decreasing androgen production by the ovaries and increasing the production of SHBG, reducing free testosterone 17, 33.
Most progestins are derivatives of testosterone and have androgenic activity (Chart 7).
Drospirenone (DRSP) and cyproterone acetate (CPA) are not structurally related to testosterone and act as androgen receptor antagonists 17. CPA also acts on androgen receptors, has little inhibitory effect on 5-·-reductase and reduces the secretion of androgen through anti-gonadotropin action 17, 34. It may be used as monotherapy in some cases.
Drospirenone is a spironolactone analogue with equivalent effect to a dose of 25mg. It acts as antimineralocorticoid, but has a weaker anti-androgen action 17.
The main OC used for the treatment of hirsutism contains CPA associated with estrogen. 33 Some studies confirm its efficacy, with a more rapid reduction in hair growth when compared with other anti-androgens. Similar efficacy has been observed with OC constituted by drospirenone 17.
The use of OC should be maintained until reproductive maturity, approximately 5 years after menarche or until the ideal weight. The normalization of menstrual cycles and the improvement of acne and hirsutism occur, in general, three months after the start of the treatment. Some studies have shown that suppression of serum androgens remains for at least 2 years after discontinuation of OC, although most patients experience worsening three months after the end of treatment 10.
Worsening of insulin resistance and increased levels of triglycerides and cholesterol associated with OC use have been reported 33. Other side effects include hypercoagulability, thrombogenic (mainly CPA) and vascular reactivity, demanding caution in patients with a history of vascular diseases such as migraine 10.
Anti-androgenic drugs block the binding of androgens to their tissue receptors or inhibit the 5αreductase enzyme. All anti-androgens should be prescribed in combination with oral contraceptives due to menstrual irregularities and risk of incomplete virilization of male embryo during pregnancy 35. In the treatment of hirsutism, they revert the transformation induced by androgens of vellus hair into terminal hair. Due to the long cycle of terminal hairs, the effect is only observed 9-12 months after the initiation of treatment 10.
Spironolactone, an aldosterone antagonist, has dose-dependent action, blocking the binding of 5alpha dihidrotestosterone to androgen receptors of the skin, increasing SHBG, reducing the activity of 5α-reductase and androgen production. It is effective in treating hirsutism and it is believed that it could also improve acne and alopecia. It is generally well tolerated, although hypotension, polyuria, nausea, headache, fatigue and menstrual irregularity have been reported 10, 17, 33. The therapeutic regimen for hirsutism is 25 to 100mg twice daily and for acne, 100mg once daily 15. The initial dose should be 25 mg / day and increased progressively over weeks 10. Hyperkalemia has been reported only in women with renal dysfunction or in those taking other potassiumsparing medication.
Flutamide, a potent nonsteroidal antiandrogen, blocks the action of androgens by competitive inhibition of receptors, while reducing androgen synthesis or increasing its inactivation 35. The recommended dose is 250 to 750mg/day, but lower doses (62.5 to 250 mg daily) also appear to be effective. It is very effective in the treatment of hirsutism, acne and alopecia, but it has a high cost and risk of liver toxicity that is dose dependent and, although rare, potentially fatal 17. The most common side effect is dry skin due to reduced production of sebum 33, 34. Its use is not authorized in Brazil.
Finasteride, a type 2 inhibitor of 5α-reductase, blocks the conversion of testosterone into DHT 15, 17. Despite the fact that the pilosebaceous unit has predominantly type 1 enzyme, studies show a beneficial effect in the treatment of hirsutism, as well as that of androgenic alopecia in women, with few side effects 10. However, it does not seem to be effective in treating acne. The dose used is 1 mg / day, although safety at higher doses has been described 15.
Dutasteride acts on both isoenzymes 5α-reductase and induces an even greater reduction in serum levels of DTH, with evidence of benefit in the treatment of female androgenic alopecia at a dose of 0.5 mg / day 23, 36.
Creams with anti-androgens have limited effectiveness. No benefit has been found with the use of 5% canrenone, the active metabolite of spironolactone. Studies have shown conflicting results with topical finasteride 17, 37.
Insulin sensitizers
These drugs reduce both hyperinsulinemia and hyperandrogenemia, in addition to their benefits on lipid profile, blood pressure and ovulation 33, 38, 39. The reduction in androgen levels occurs by direct influence on ovarian steroidogenesis through the decrease of insulin levels and increase of SHBG 15. Treatment of hirsutism with these drugs is still controversial, although studies have shown reduced growth and thickness of hair, and improvement of acne and acanthosis nigricans 15, 38, 40, 41.
Metformin has been suggested as a first-choice drug in the treatment of hirsutism in women with PCOS, metabolic and reproductive disorders 10, 15, 34. The initial dose is 500mg at dinner, which may be increased every week until a maximum dose of 2000mg/day, divided into two doses 10. The effect disappears 3 months after discontinuing the drug. The most frequent side effects are dose dependent, occurring nausea and diarrhea. Lactic acidosis is rare and insignificant when young subjects with PCOS are evaluated 10, 27.
These effects can also be obtained with pioglitazone and rosiglitazone, and they may be greater with the combination of oral contraceptive or flutamide 40, 42. However, the safety of treatment with these drugs still needs further evaluation. Patients should be informed of the chance of spontaneous ovulation.
Cosmetic treatment
Cosmetic procedures are important in the treatment of hirsutism, which may be sufficient to control mild to excessive hair growth or as an adjuvant in severe cases. Shaving, depilation or destruction of the dermal papilla by electrolysis or laser may be performed. Hair removal using wax is discouraged for patients with more severe cases of hirsutism. This technique can be effective, but there is a risk of skin irritation, folliculitis and ingrown hairs. Laser epilation is the most effective method and, despite the high cost, requires fewer sessions and the results are better.
Eflornithine hydrochloride is an irreversible inhibitor of ornithine decarboxylase, which was recently released for topical use in the treatment of facial hirsutism. This enzyme is modulated by androgens and regulates cell proliferation in the matrix of the follicle. Studies have shown significant reduction in the amount of hair, but the effect is rapidly reversed after discontinuation of the drug. Topical retinoids like calcipotriene and keratolytic drugs, such as ammonium lactate, can be useful in the treatment of acanthosis nigricans, as long as hyperinsulinemia is controlled 15 , 43.
Besides systemic treatment, alopecia can be controlled with topical minoxidil 2 or 5% and if necessary, with hair transplant 23, 44.
CONCLUSION
Polycystic ovary syndrome is a common endocrinopathy that affects women of reproductive age, which may cause metabolic disorders and psychosocial problems. Early diagnosis is essential for the prevention of complications and we should be attentive to the variety of clinical findings. Although its etiology has not yet been fully elucidated, knowledge and understanding of the abnormality in the hypothalamus-pituitary axis, steroidogenesis and insulin resistance are of utmost importance for early, effective and safe treatment.
REFERENCES
-
1Stein IF, Leventhal, M.L. Amenorrhea associated with bilateral polycystic ovaries. Am J Obstet Gynecol. 1935;29:10.
-
2Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and longterm health risks related to polycystic ovary syndrome (PCOS). Human Reproduction. 2004;19:41-7. Review.
-
3Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W, et al. Positions statement: criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: an Androgen Excess Society guideline. J Clin Endocrinol Metab. 2006;91:4237-45.
-
4Goodarzi MO, Azziz R. Diagnosis, epidemiology, and genetics of the polycystic ovary syndrome. Best Pract Res Clin Endocrinol Metab. 2006;20:193-205.
-
5Hart R, Hickey M, Franks S. Definitions, prevalence and symptoms of polycystic ovaries and polycystic ovary syndrome. Best Pract Res Clin Obstet Gynaecol. 2004;18:671-83.
-
6Buccola JM, Reynolds EE. Polycystic ovary syndrome: a review for primary providers. Prim Care. 2003;30:697-710.
-
7Yarak S, Bagatin E, Hassun KM, Talarico S, Parada MOA. Hyperandrogenism and skin: polycystic ovary syndrome and peripheral insulin resistance. An Bras Dermatol. 2005;80:16.
-
8Hoyt KL, Schmidt MC. Polycystic ovary (Stein-Leventhal) syndrome: etiology, complications, and treatment. Clin Lab Sci. 2004;17:155-63.
-
9Fraser IS, Kovacs G. Current recommendations for the diagnostic evaluation and follow-up of patients presenting with symptomatic polycystic ovary syndrome. Best Pract Res Clin Obstet Gynaecol. 2004;18:813-23.
-
10Harwood K, Vuguin P, DiMartino-Nardi J. Current approaches to the diagnosis and treatment of polycystic ovarian syndrome in youth. Horm Res. 2007;68:209-17.
-
11Ehrmann DA, Liljenquist DR, Kasza K, Azziz R, Legro RS, Ghazzi MN. Prevalence and predictors of the metabolic syndrome in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2006;91:48-53.
-
12Norman RJ, Dewailly D, Legro RS, Hickey TE. Polycystic ovary syndrome. Lancet. 2007;370:685-97.
-
13Doi SA. Neuroendocrine dysfunction in PCOS: a critique of recent reviews. Clin Med Res. 2008;6:47-53.
-
14Hatch R, Rosenfield RL, Kim MH, Tredway D. Hirsutism: implications, etiology, and management. Am J Obstet Gynecol. 1981;140:815-30.
-
15Lee AT, Zane LT. Dermatologic manifestations of polycystic ovary syndrome. Am J Clin Dermatol. 2007;8:201-19.
-
16Yildiz BO. Diagnosis of hyperandrogenism: clinical criteria. Best Pract Res Clin Endocrinol Metab. 2006;20:167-76.
-
17Martin KA, Chang RJ, Ehrmann DA, Ibanez L, Lobo RA, Rosenfield RL, et al. Evaluation and treatment of hirsutism in premenopausal women: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2008;93:1105-20.
-
18Yildiz BO, Bolour S, Woods K, Moore A, Azziz R. Visually scoring hirsutism. Hum Reprod Update. 2010;16:51-64.
-
19Lobo RA, Goebelsmann U, Horton R. Evidence for the importance of peripheral tissue events in the development of hirsutism in polycystic ovary syndrome. J Clin Endocrinol Metab.1983;57:393-7.
-
20Reingold SB, Rosenfield RL. The relationship of mild hirsutism or acne in women to androgens. Arch Dermatol. 1987;123:209-12.
-
21Archer JS, Chang RJ. Hirsutism and acne in polycystic ovary syndrome. Best Pract Res Clin Obstet Gynaecol. 2004;18:737-54.
-
22Ozdemir S, Ozdemir M, Gorkemli H, Kiyici A, Bodur S. Specific dermatologic features of the polycystic ovary syndrome and its association with biochemical markers of the metabolic syndrome and hyperandrogenism. Acta Obstet Gynecol Scand. 2010;89:199-204.
-
23Rogers NE, Avram MR. Medical treatments for male and female pattern hair loss. J Am Acad Dermatol. 2008;59:547-66; quiz 67-8.
-
24Araujo LM, Porto MV, Netto EM, Ursich MJ. Association of acanthosis nigricans with race and metabolic disturbances in obese women. Braz J Med Biol Res. 2002;35:59-64.
-
25Salley KE, Wickham EP, Cheang KI, Essah PA, Karjane NW, Nestler JE. Glucose intolerance in polycystic ovary syndrome--a position statement of the Androgen Excess Society. J Clin Endocrinol Metab. 2007;92:4546-56.
-
26Jovanovic VP, Carmina E, Lobo RA. Not all women diagnosed with PCOS share the same cardiovascular risk profiles. Fertil Steril. 2010;94:826-32. Epub 2009 Jun 6.
-
27Setji TL, Brown AJ. Comprehensive clinical management of polycystic ovary syndrome. Minerva Med. 2007;98:175-89.
-
28Setji TL, Brown AJ. Polycystic ovary syndrome: diagnosis and treatment. Am J Med. 2007;120:128-32.
-
29Rosenfield RL. What every physician should know about polycystic ovary syndrome. Dermatol Ther. 2008;21:354-61.
-
30Miller KK, Rosner W, Lee H, Hier J, Sesmilo G, Schoenfeld D, et al. Measurement of free testosterone in normal women and women with androgen deficiency: comparison of methods. J Clin Endocrinol Metab. 2004;89:525-33.
-
31Wallace AM, Sattar N. The changing role of the clinical laboratory in the investigation of polycystic ovarian syndrome. Clin Biochem Rev. 2007;28:79-92.
-
32Essah PA, Nestler JE. The metabolic syndrome in polycystic ovary syndrome. J Endocrinol Invest. 2006;29:270-80.
-
33Pelusi C, Pasquali R. Polycystic ovary syndrome in adolescents: pathophysiology and treatment implications. Treat Endocrinol. 2003;2:215-30.
-
34Moghetti P. Use of antiandrogens as therapy for women with polycystic ovary syndrome. Fertil Steril. 2006;86 Suppl 1:S30-1.
-
35Swiglo BA, Cosma M, Flynn DN, Kurtz DM, Labella ML, Mullan RJ, et al. Clinical review: Antiandrogens for the treatment of hirsutism: a systematic review and metaanalyses of randomized controlled trials. J Clin Endocrinol Metab. 2008;93:1153-60.
-
36Olszewska M, Rudnicka L. Effective treatment of female androgenic alopecia with dutasteride. J Drugs Dermatol. 2005;4:637-40.
-
37Lucas KJ. Finasteride cream in hirsutism. Endocr Pract. 2001;7:5-10.
-
38Lebinger TG. Metformin and polycystic ovary syndrome. Curr Opin Endocrinol Diabetes Obes. 2007;14:132-40.
-
39Teede HJ, Hutchison SK, Zoungas S. The management of insulin resistance in polycystic ovary syndrome. Trends Endocrinol Metab. 2007;18273-9. Epub 2007 Aug 16.
-
40Cosma M, Swiglo BA, Flynn DN, Kurtz DM, Labella ML, Mullan RJ, et al. Clinical review: Insulin sensitizers for the treatment of hirsutism: a systematic review and metaanalyses of randomized controlled trials. J Clin Endocrinol Metab. 2008;93:1135-42.
-
41Hutchison SK, Zoungas S, Teede HJ. Insulin levels, insulin resistance and the use of metformin in polycystic ovary syndrome. Med J Aust. 2007;186:268-9; author reply 71-2.
-
42Ibanez L, de Zegher F. Low-dose flutamide-metformin therapy for hyperinsulinemic hyperandrogenism in non-obese adolescents and women. Hum Reprod Update. 2006;12:243-52.
-
43Essah PA, Wickham EP 3rd, Nunley JR, Nestler JE. Dermatology of androgenrelated disorders. Clin Dermatol. 2006;24:289-98.
-
44Koulouri O, Conway GS. Management of hirsutism. BMJ. 2009 Mar 27;338:b847. doi: 10.1136/bmj.b847.
Publication Dates
-
Publication in this collection
21 Mar 2011 -
Date of issue
Feb 2011
History
-
Accepted
02 June 2010 -
Received
03 Dec 2009