Abstracts
Four patients with Human Immunodeficiency Virus Infection in treatment with protease inhibitors for an average of 8 months and 3 weeks are reported. Fat accumulation in the cervical-dorsal region (buffalo hump) and moon face, similar to that of Cushing's Syndrome, are highlighted.
HIV; Lipodystrophy; Protease inhibitors
Apresentam-se quatro pacientes com infecção pelo vírus da imunodeficiência humana em tratamento com inibidores de proteases há oito meses e três semanas em média. Ressaltam-se o acúmulo de gordura na região dorsocervical e fáscies de lua cheia, semelhante à que ocorre na síndrome de Cushing.
HIV; Inibidores de proteases; Lipodistrofia
CASE REPORT
Redistribution of body fat induced by HIV protease inhibitors in patients with AIDS* * Work done at Department of Dermatology at the Medical School Hospital of the Universidade Federal de Juiz de Fora - Juiz de Fora (MG), Brazil. Conflict of interest: None
Cristina MansurI; Rosyane Rena de FreitasII; Felipe Torres RabêloIII; Leonora MansurIV; Fábio Torres RabêloV; Frank César Monteiro SantiagoV; Thaís de OliveiraV; Franceline Quintão AzevedoVI
IPhD at Universidade da Califórnia, San Francisco - California - EUA Professor at Universidade Federal de Juiz de Fora - Juiz de Fora (MG), Brazil
IIMedical student at Universidade Federal de Juiz de Fora - Juiz de Fora (MG), Brazil
IIIPhysician graduated at Universidade Federal de Juiz de Fora - Juiz de Fora (MG), Brazil
IVResident doctor in General surgery at University Hospital of the Universidade Federal de Juiz de Fora - Juiz de Fora (MG), Brazil
VMedical student at Universidade Federal de Juiz de Fora - Juiz de Fora (MG), Brazil
VIDermatologist
Mailing address Mailing address: Cristina Mansur Rua Olegário Maciel 297/801 - Centro 36036-000 - Juiz de Fora - MG - Brazil Tel.: +55 32 3215-4466 / Fax: +55 32 3217-4531 E-mail: cristina@mansur.com.br
ABSTRACT
Four patients with Human Immunodeficiency Virus Infection in treatment with protease inhibitors for an average of 8 months and 3 weeks are reported. Fat accumulation in the cervical-dorsal region (buffalo hump) and moon face, similar to that of Cushing's Syndrome, are highlighted.
Keywords: HIV; Lipodystrophy; Protease inhibitors
INTRODUCTION
Over the last decade, treatment of the Acquired Immunodefficiency Syndrome (AIDS), thanks to the use of protease inhibitors and introduction of Haart (highly active anti-retroviral therapy), was able to recover patients considered to be terminal, restoration of the immune system, decrease in the number of deaths, increase in lifespan and improvement in the quality of life of patients bearing AIDS/HIV (Human Immunodefficiency Virus).1
After two years of Haart introduction, a number of side effects became apparent. The first ones were fat accumulation in the abdominal region making it round (Pot belly) , enlargement of the dorsocervical region (Buffalo hump) and moon face, reminding Cushing's syndrome.2,3 Besides centripetal fat accumulation, progressive alterations of cellular subcutaneous tissue in extremities have been noted, producing a pseudo-athletic appearance with prominent muscles and vessels. Also observed were loss of peripheral cellular subcutaneous tissue (face, glutei, upper and lower limbs) and metabolic alterations, such as dislipidemia, insulin resistance, lactic acidosis, hypogonadism and osteoporosis. This alteration of body shape owing to a fat distribution abnormality in individuals affected by HIV/AIDS is known as the Fat Redistribution Syndrome (FRS).
CASE REPORTS
Case 1
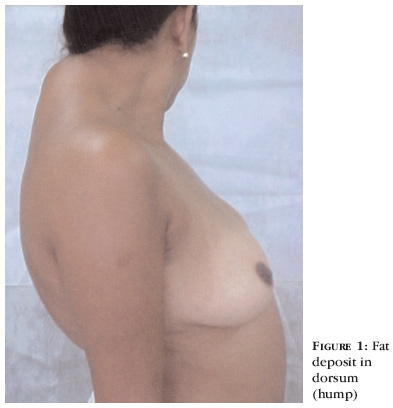
Thirty-five-year-old female patient, who had known to be an HIV bearer for eight years. She had used AZT (zidovudine), DDI (didanosine), D4T (estavudine) and 3TC (lamivudine). For one and a half year she had been using 3TC, AZT and nelfinavir (protease inhibitor) and from six months before had observed a centripetal fat accumulation, with enlargement of posterior cervical region (hump), lower portion of the face, malar and mandibular regions and intensive fat deposit in the dorsum (Figure 1).
Laboratorial tests: fasting blood glucose = 91mg/dl; total cholesterol = 216mg/dl; HDL = 38mg/dl e triglycerides = 152mg/dl.
Case 2
Forty-three-year-old male patient, knowingly HIV bearer for nine years. He had already used AZT, DDI and EFV (efavirenz). For two years he had been using 3TC, D4T and ritonavir (protease inhibitor), and since then he had been noticing progressive fat loss in the malar region.
Laboratorial tests: fasting blood glucose = 115mg/dl; total cholesterol = 322mg/dl; HDL = 34mg e triglycerides = 931mg/dl.
Case 3
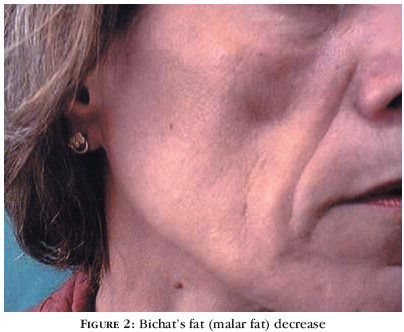
Forty-eight-year-old female patient (Figure 2), bearer of HIV for 10 years and 11 months. She had already used AZT, DDI, D4T and 3TC. For two years and four months she had been using D4T, 3TC and indinavir (protease inhibitor). After six months using the latter scheme, she observed fat loss in malar region and upper limbs (pseudo-athletic appearance).
Laboratorial tests: fasting blood glucose = 85mg/dl; total cholesterol total = 214mg/dl; HDL = 33mg/dl, e triglycerides = 221mg/dl.
Case 4
Forty-one-year-old male patient, infected by HIV for eight years and seven months. He had already used AZT, 3TC, D4T and DDI. Since January 2001, he had used AZT, 3TC, ritonavir and indinavir, and soon after beginning this scheme, noticed fat loss in the malar region.
Laboratorial tests: fasting blood glucose = 124mg/dl; total cholesterol = 318mg/dl; HDL = 41mg/dl e triglycerides = 169mg/dl.
In all four patients, assays of post-nocturnal plasmatic cortisol suppression with 1mg of dexametasone were performed, discarding Cushing's syndrome.
DISCUSSION
HIV infection can be responsible for triggering of a series of dermatological manifestations,4,5 and body fat redistribution is increasingly observed in these patients.6
The exact mechanism leading to the onset of lipodystrophy is not known yet. Among the mentioned, are: nucloside analogues reverse transcriptase inhibitors-induced mitochondrial toxicity; disregulation of TNF-alpha; protease inhibitor (PI)-related p450 citochrome inhibition; hypersteroidism (pseudo Cushing's syndrome); local effects of HIV on cortisol poduction and alterations of other steroidal hormones. None of those is able to explain alone all aspects of this syndrome, which is probably multifactorial.7
Decrease in face fat is typical and described; however, fat accumulation in the dorsocervical region (hump) and moon face, reminding classic Cushing's syndrome, are not so common.2,3
Lo and colaborators3 studied eight patients infected by HIV, with and average of 9.6 years of disease. Of these, four presented lipodystrophy between two and 18 months after beginning treatment with protease inhibitors. In the present patient set, average of onset was eight months and three weeks, corroborating data in the literature.2,3
Prevalence rate of lipodystrophy ranges from five to 83% in patients using PI, with an average of 50%, and it can even occur in HIV/AIDS patients who are not using PI.7
The syndrome has a larger incidence among females, and women present, proportionally, larger fat loss than men. Regional muscle loss was described in detail after Haart introduction.8
FRS, glucose intolerance, hyperlipidemia and mitochondrial toxicity are the major issues and may persist after suspension of treatment (Coasting phenomenon).8
With progression of lypodystrophy, several patients started presenting typical fascies, characteristic of FRS. This brought the AIDS stigma back, leading many patients to treatment interruption.6,7
It is necessary that specialists working with HIV/AIDS patients, and mainly dermatologists, identify these alterations and seek treatment options.
Abdominal treatment can be made with surgical removal by centripetal fat liposuccion. Fat absorption can be treated with growth hormone3 or aminoacid and vitamin supplements and supervised physical exercising, with satisfactory results. For the face, fat, collagen, hialuronic acid or metacrilate implantation can be used, with esthetical results and great improvement in patients' life quality.1
REFERENCES
Received on June 23, 2003.
Approved by the Consultive Council and accepted for publication on June 13, 2006.
- 1. Serra M. A Gordura corporal em pacientes com AIDS. Dermage News. 2001;6:5.
- 2. Miller KD, Jones E, Yanovski JA, Shankar R, Feuerstein I, Falloon J. Visceral abdominal-fat accumulation associated with use of indinavir. Lancet.1998;351:871-5.
- 3. Lo JC, Mulligan K, Tai VW, Algren H, Schambelan M. "Buffalo hump" in men with HIV-infection. Lancet. 1998;351:867-70.
- 4. Porro AM, Yoshioka MCN. Manifestações dermatológicas da infecção pelo HIV. An Bras Dermatol. 2000;75:665- 88.
- 5. Bonamigo RR, Nunes C, Arenzon S, Rietjens J. Dermatofibromas eruptivos múltiplos em paciente com infecção pelo HIV. An Bras Dermatol. 2002;77:337- 40.
- 6. Pujol RM, Domingo P, Xavier-Matias-Guiu, Sanbeat MA, Alomar A, Vasquez G. HIV-1 protease inhibitor associated partial lipodystrophy Clinicopathologic review of 14 cases. J Am Acad Dermatol. 2000;42:192-8.
- 7. Li HY, Silva ACCM, Santos S. Síndrome lipodistrófica e HIV/AIDS. J Bras Aids. 2002;3:23-35.
- 8. Herman JS, Easterbrook PJ. The metabolic toxicities of antiretroviral therapy. Int J STD AIDS. 2001;12:555-64.
Publication Dates
-
Publication in this collection
22 Dec 2006 -
Date of issue
Oct 2006
History
-
Received
23 June 2003 -
Accepted
13 June 2006