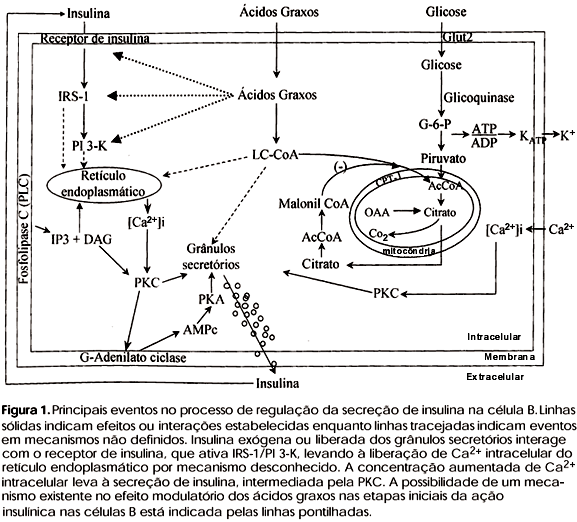
Insulin plays a central role in the regulation of glucose homeostasis and acts in a coordinated fashion on cellular events that regulate the metabolic and growth processes. The insulin receptor beta-subunit, which contains an intrinsic tyrosine kinase activity, undergoes tyrosyl autophosphorylation and is activated in response to insulin binding to the extracellular alpha-subunit. Subsequent steps in insulin signal transduction are mediated via the phosphorylation of specific intracellular proteins, including insulin receptor substrate-1 (IRS-1). In peptide motifs with the sequence Tyr-Met-x-Met (YMXM) or Tyr-x-x-Met (YXXM), tyrosine phosphorylated IRS-1 serves as a docking protein that interacts with signaling proteins containing SH2 or SH3 domains, such as the phosphatidylinositol 3-kinase (PI 3-kinase), thereby transmitting the signal downstream. The pancreatic B cell insulin receptor seems to mediate positive feedback for insulin secretion. Alterations in the molecular mechanisms of insulin signaling provide a potential link between insulin resistance and their impaired release, observed in non-insulin-dependent diabetes mellitus. Insulin resistance is also associated with elevated levels of free fatty acids (FFA) in the blood that may act directly on the exocytotic machinery to secrete insulin. The present review also describes the possible fatty acids and insulin signaling interactions on insulin exocytosis in pancreatic islets.
Insulin receptor substrates; Insulin action; Pancreatic beta cell; Free fatty acid; Insulin resistance