Abstracts
The high pressure liquid chromatography (HPLC) was used for the identification of isoniazid (isonicotinic acid hydrazide) in the milk of cattle treated with a dose of 25 mg/kg/day in alternated days. The effect of milk pasteurization on the isoniazid residue concentration was also studied. The drug excretion presented a cyclic variation, with higher levels in the first day after administration (aa), a mean of 1104.48µg/l, and a decrease two days aa, with a mean of 104.12µg/l. Four days after the last administration of the drug it was not possible to identify residues of isoniazid in the milk of treated animals. Body weight and milk yield influenced the amount of the excreted drug, and pasteurization decreased (mean 47.07%) the concentration of isoniazid residue in milk.
Cattle; tuberculosis; isoniazid; milk; HPLC
Cromatografia líqüida de alta eficiência (HPLC) foi utilizada na identificação de isoniazida (hidrazida do ácido isonicotínico) no leite de bovinos tratados com a dose de 25 mg/kg/dia em dias alternados. Também foi verificado o efeito da pasteurização sobre a concentração residual de isoniazida no leite. A excreção da droga no leite apresentou variação cíclica com picos no primeiro dia após a administração da droga, média de 1104,48µg/l, e queda dois dias após, média de 104,12µg/l. Quatro dias após a última administração da droga não foi mais possível a identificação de resíduos de isoniazida no leite. Observou-se influência do peso corporal e da produção de leite sobre a quantidade da droga excretada. A pasteurização diminuiu, em média 47,07%, a concentração de resíduo de isoniazida no leite.
Bovino; tuberculose; isoniazida; leite; HPLC
[Identificação de resíduos de isoniazida no leite por HPLC]
R.M.H. Leite1, R.C. Leite2,J.A. Lima3, C.B. Fóscolo2 , P.M.P.C. Mota3, F.C.F. Lobato2, A.P. Lage2**Autor para correspondência
1Empresa Estadual de Pesquisa Agropecuária da Paraíba S.A.- EMEPA - PB
2Escola de Veterinária da UFMG
Caixa Postal 567
30123-970 - Belo Horizonte, MG
3Laboratório Regional de Apoio Animal - LARA - Pedro Leopoldo, MG
Recebido para publicação, após modificações, em 19 de agosto de 2000.
E-mail: alage@vet.ufmg.br
ABSTRACT
The high pressure liquid chromatography (HPLC) was used for the identification of isoniazid (isonicotinic acid hydrazide) in the milk of cattle treated with a dose of 25 mg/kg/day in alternated days. The effect of milk pasteurization on the isoniazid residue concentration was also studied. The drug excretion presented a cyclic variation, with higher levels in the first day after administration (aa), a mean of 1104.48µg/l, and a decrease two days aa, with a mean of 104.12µg/l. Four days after the last administration of the drug it was not possible to identify residues of isoniazid in the milk of treated animals. Body weight and milk yield influenced the amount of the excreted drug, and pasteurization decreased (mean 47.07%) the concentration of isoniazid residue in milk.
Keywords: Cattle, tuberculosis, isoniazid, milk, HPLC
RESUMO
Cromatografia líqüida de alta eficiência (HPLC) foi utilizada na identificação de isoniazida (hidrazida do ácido isonicotínico) no leite de bovinos tratados com a dose de 25 mg/kg/dia em dias alternados. Também foi verificado o efeito da pasteurização sobre a concentração residual de isoniazida no leite. A excreção da droga no leite apresentou variação cíclica com picos no primeiro dia após a administração da droga, média de 1104,48µg/l, e queda dois dias após, média de 104,12µg/l. Quatro dias após a última administração da droga não foi mais possível a identificação de resíduos de isoniazida no leite. Observou-se influência do peso corporal e da produção de leite sobre a quantidade da droga excretada. A pasteurização diminuiu, em média 47,07%, a concentração de resíduo de isoniazida no leite.
Palavras-chave: Bovino, tuberculose, isoniazida, leite, HPLC.
INTRODUCTION
Human tuberculosis is the main cause of death due to a single infectious agent among adults in the world. It is estimated that the incidence and mortality rates of tuberculosis in the 1990s reached 88 million and 30 million people respectively, with the majority of cases occurring in developing countries. Mycobacterium tuberculosis is the most common cause of human tuberculosis, but a proportion of cases is due to M. bovis (Cosivi et al., 1998).
Bovine tuberculosis could become a serious public health threat for people belonging to risk groups, especially those infected by HIV, particularly in countries in which M. bovis infection is present in animals and conditions are favorable to zoonotic transmission (Cosivi et al., 1998).
Although the majority of developed countries continue to use testing and culling for bovine tuberculosis control, it has not yet been eradicated in some of these countries, for example in the United States. An alternative approach to these classical programs would be the treatment of infected animals by isoniazid (isonicotinic acid hydrazid-IHN) (Langenegger et al., 1991).
In Brazil, intermitent treatment protocols using IHN showed a 100% efficiency rate in controlling bovine tuberculosis in infected herds (Langenegger et al., 1991). Due to these studies, the use of treatment with IHN in the control of bovine tuberculosis has become widespread in some regions without the adequate follow-up. However animal health legislation does not consider treatment in the control of bovine tuberculosis (Brasil, 1934).
A series of analytical methods, including spectrophotometry, spectrofluorometry and gas chromatography were described for the identification of IHN and some of its metabolites in biological fluids of human patients submitted to treatment with this drug. However, there is only scarce information concerning the specific identification of IHN in milk (Defilippi et al., 1994).
Only three procedures are currently used for quantitative determination of IHN in milk. The first two, which were developed by Ruffo (1964), are based on biological and spectrophotometric measurements and have low sensitivity: 100 and 50 mg/l, respectively. The third is based on the high pressure liquid chromatography (HPLC) (Defilippi et al., 1994; Defilippi et al., 1995), being able to detect up to 0.1 mg/l of IHN.
The objective of the present study was to determine the amount of isoniazid excreted in milk of cattle treated with IHN and to verify the effect of milk pasteurization on the drug residue concentration.
MATERIALS AND METHODS
Six Jersey/Zebu crossbred cows free from bovine tuberculosis in the third stage of lactation and weighing from 330 to 424kg were used in the experiment. Milk yield was recorded daily.
Isoniazid (Interchange. China)was administered orally, 25mg/kg/day, on Mondays, Wednesdays and Fridays for two weeks for a total of six applications. A suspension of the dose was prepared in 50ml of water and then given orally to the animals with a syringe. The doses and the interval of drug administration were adopted as proposed for bovine tuberculosis treatment, which is done with 80 doses during a six months period (Langenegger et al., 1991).
Five mililiters of milk from each animal, and from a pooled sample of milk of all animals, before and after pasteurization, were collected three days (day -3) prior to IHN administration and up to six days after the last application of the drug (day 17). All samples were processed within four hours of collection. Samples collected on days 5, 6, 12 and 13 were frozen at 20° C for two days before being analyzed.
After taking a 5ml sample, the pooled milk was submited to rapid pasteurization at 72 to 75° C for 16 seconds in a hot plate pasteurizer (Mec-Brasil. Pompéia, São Paulo, Brazil), according to Kessler (1981).
Alkaline phosphatase and peroxidase tests were used for the quality control of the pasteurization. Properly pasteurized milk should present a negative reaction to the alkaline phosphatase test but positive reaction to the peroxidase test (Brasil, 1981).
Deproteinization of 2ml milk samples were done by addition of 20% (w/w) aqueous trichloroacetic acid solution, mixed for 30 seconds, left at room temperature for 10 minutes and then centrifuged at 5500 ´ g for 15 minutes. The supernatants (600ml) were filtered through a 0.45mm nitrocellulose membrane and then derivatized using a methanolic solution of cinnamaldehyde 1% w/v, vortex mixed for a few seconds, left at room temperature for five minutes and injected into the HPLC system (Defilippi et at., 1994).
The identification of isoniazid residues in milk was performed on a TSP chromatograph (Thermo Separation Products Inc.,California, U.S.A.), using a 5mm LiChrosphere 100 RP18 column (250mm ´ 4mm ID) (Merck, Germany). All experimental analyses were carried out isocratically at a flow-rate of 1ml/min, UV detector operating at 330nm and 100ml volume injection of prepared milk samples (Defilippi et al., 1995).
Isoniazid standard solution was obtained diluting the pure drug in IHN-free milk produced by non-treated cattle to the required concentration. The results obtained with IHN free milk standards were similar to those obtained from a standard solution prepared using water.
For the comparisons of the residue concentrations of IHN in milk the Student t test was used. The relationship between weight and milk production and IHN residue concentrations excreted was evaluated by the Student-Newman-Keuls (SNK) test. The effect of pasteurization on IHN residue concentration on milk was evaluated by the Tukey test (P< 0.05; Snedecor & Cochran, 1980). Regression analysis was used to study the effect of pasteurization on the concentration of IHN residues in milk (Snedecor & Cochran, 1980).
RESULTS
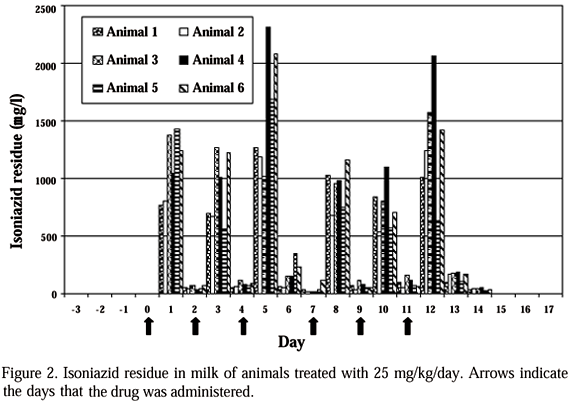
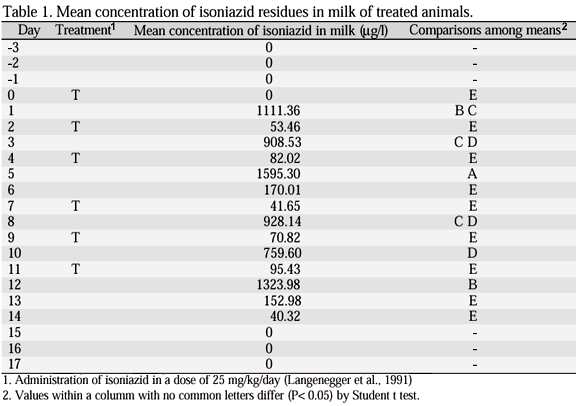
Chromatograms of milk samples from animal 1, before administration of the drug on day 3 of the experiment and after one day (24h) of the first IHN dose (day 1) are shown on Fig. 1. A peak of IHN in 4.429 minutes of retention was observed on day 1, between the trichloroacetic acid and cinnamaldehyde peaks (Fig.1B), which was absent in the milk of animal 1 before drug administration (day 3) (Fig.1A). Negative profiles were observed in samples from all animals on days -3, -2, -1 and 0, demonstrating the absence of IHN in milk before drug administration (Tab. 1, Fig. 2).
The mean daily concentration of isoniazid residues in milk samples collected during the experiment, as well as the comparisons between the means, are shown in Tab. 1.
A significant (P<0.05) fluctuation on the concentration of IHN residues in milk associated with the time after drug administration was observed. An increase in drug concentration in milk was observed one day (24h) after IHN administration, mean 1104.48 mg/l, decreasing to 104.12 mg/l, two days (48h) after administration (Tab.1, Fig.2).
The period of IHN excretion in milk after the end of the drug administration, along with the variation on drug excretion during the treatment for the drug administration protocol used, presented the same pattern for all the animals in the experiment (Fig. 2). IHN residues were no more detectable in milk of treated animals on day 15, four days after the last IHN administration (Tab.1, Fig. 2).
The mean daily milk yield during the 21-day-period of the experiment was 39.42kg of milk/day. Tab. 2 presents body weight of the experimental animals, total milk yield from day 0 to day 15 of the experiment, and milk yield per body weight of all animals. A cumulative IHN index was calculated multiplying the body weight, milk yield and the total amount of residual IHN in milk. The analysis of the cumulative IHN index showed a significant difference among drug excretion in animals as a function of body weight and milk yield (Tab. 2).
The concentration of IHN residues in the pooled milk before and after pasteurization is shown in Tab. 3. All milk samples presented a negative reaction to the alkaline phosphatase test and a positive reaction to the peroxidase test after pasteurization, demonstrating that samples were adequately pasteurized (Brasil, 1981). There was a significant reduction in the concentration of IHN residues in pooled milk after pasteurization (t = 2.25, P = 0.01 and DF = 8), with a mean reduction of 47.07% (range 10.8 to 100%) (Tab. 3). Regression analysis of the reduction of IHN in milk after pasteurization resulted in the equation Y=26.20+0.49*X, with r2 = 0.76 and P = 0.001.
DISCUSSION
The identification of IHN residues in bovine milk by HPLC proved to be a practical, rapid and sensitive technique which is adequate for routine detection of this drug in milk.
Despite the differences in the dose and administration route used, these results could be compared with those from Leschiera et al. (1993) and Defilippi et al. (1995), which found that IHN is rapidly eliminated from the milk and blood of cattle, presenting maximum levels 5 to 7 hours after its administration and that it can still be identified up to 4 to 5 days after the last administration of the drug. Leschiera et al. (1993) found a mean concentration of IHN in milk of 575 mg/l, one day after administration of 10 mg/kg/day of IHN in ration, which is half of the one observed in the present study using 2.5 times that daily IHN dose.
The excretion of IHN in milk follows a cyclic pattern with a maximum level one day after administration and a reduction two days after administration. No significant difference among IHN excretion between the first and the second day after drug administration was observed. The only exceptions observed were found on days 5 and 12 of the experiment (Tab. 1, Fig. 2). It is worth mentioning that the samples taken on days 5 and 12 were frozen at 20°C and analyzed two days after collection. Leschiera et al. (1993) also observed variations from 13.3% to 11.3% in the concentration of IHN residues in milk analyzed freshly produced and milk frozen at 20°C for six months, indicating that freezing could interfere with IHN detection in milk by HPLC.
According to Kleeberg et al. (1961), the higher the milk production the smaller the residual IHN concentration in milk. Analysis of the cumulative IHN index confirms the association between IHN residues in milk and milk yield. However it also shows that IHN residues in milk are influenced by body weight. Animal 4, showing the lowest milk yield (94.8 kg), and the heaviest body weight (435 kg), received the highest IHN dose. This resulted in a higher concentration of the drug in the milk characterized by a higher IHN residue excreted. A reverse relation was observed in animal 2 which had the lowest body weight (330 kg) and produced the highest amount of milk (107.9 kg). This animal received the smallest dose of IHN which resulted in the lowest concentration of the drug in the milk.
Pasteurization reduced the concentration of IHN residue, but did not completely eliminate the drug present in milk, as suggested by Kleeberg et al. (1961). These authors suggest that many different procedures such as pasteurization, heating and storage resulted in the total elimination of IHN from milk. However, all determinations were done by a spectrophotometric technique which has a detection limit of 107.0 mg/l (Kleeberg et al., 1961). The HPLC technique used in this study presents a much higher sensitivity (0.1 mg/l) according to Defilippi et al. (1994). The lowest concentration of IHN detected in milk in this experiment was 19.85 mg/l.
Kleeberg et al. (1961) verified that daily administration of IHN in cattle at doses of 10 mg/kg/day and 20 mg/kg/day resulted in 240 mg/l and 400 mg/l IHN residue in milk, respectively. In the present study, the average IHN residue concentration in the milk of animals treated with 25 mg/kg/day, was 523.82 mg/l with a range of 1595.30 to 40.32 mg/l (Tab. 1). This suggests that the milk of animals receiving IHN should not be used for human consumption even after pasteurization, because this procedure can not completely eliminate this drug.
ACKNOWLEDGMENT
We are indebted to Prof. Iran Borges for helping with the statistical analyses and Raul Lara Resende de Carneiro and Valdelaine Etelvina Miranda de Araújo for technical assistance. This study was supported by PRPq - UFMG, FAPEMIG and FEP-MVZ Coordenação Preventiva. R.M.H. Leite, R.C. Leite, F.C.F. Lobato and A.P. Lage are recipients of fellowships from Conselho Nacional de Desenvolvimento Científico e Tecnológico CNPq.
- BRASIL. Decreto Nº 24.548 - de 03 de julho de 1934. Regulamento do Serviço de Defesa Sanitária Animal. cap. VI, art. 63, p. 9.
- BRASIL. Ministério da Agricultura. Métodos analíticos oficiais para controle de produtos de origem animal e seus ingredientes II. Métodos físicos e químicos. Brasilia: LANARA, 1981. p.irreg.
- COSIVI, O., GRANGE, J.M., DABORN, C.J. et al. Zoonotic tuberculosis due to Mycobacterium bovis in developing countries. Emerg. Infect. Dis, v.4, p.59-70, 1998.
- DEFILIPPI, A., PIANCONE, G., TARTAGLINO, L. et al. High-performance liquid chromatography with UV detection and diode-array UV confirmation of isonicotinic acid hydrazide in cattle. J. Chromatogr, B656, p.466-471, 1994.
- DEFILIPPI, A., PIANCONE, G., LAIA, R.C. et al. An HPLC screening method for the detection of isonicotinic acid hydrazide in cattle milk. Chromatographia, v.40, p.170-174, 1995.
- KESSLER, H.G. Food engineering and dairy technology. Germany: Verlag A. Kessler, 1981. 654p.
- KLEEBERG, H.H., GERICKE, J.J., WEYLAND, H. The excretion and stability of isoniazid in cows milk. J.S. Afr. Vet. Med. Assoc, v.32, p.77-83. 1961.
- LANGENEGGER, J., LEITE, G.O., OLIVEIRA, Jr., J. Tratamento intermitente da tuberculose bovina com isoniazida. Pesq. Vet. Bras., v.11, p.55-59, 1991.
- LESCHIERA, M., DEFILIPPI, A., LAIA, R.C. et al. Pesquisa de hidrazida do ácido isonicotínico no leite de bovinos tratados ilegalmente. Sel. Vet, v.34, p.679-687, 1993.
- RUFFO, G. Excretion of isoniazid in the milk of cows. Atti. Soc. Ital. Sci., v.17, p.756-757, 1964.
- SNEDECOR, G.W., COCHRAN, W.G. Statistical methods, Iowa, Ed. The Iowa State University Press, 1980. 507p.
Publication Dates
-
Publication in this collection
08 Feb 2001 -
Date of issue
Dec 2000
History
-
Received
19 Aug 2000