Abstracts
The variation in cloacal temperature, body weight loss and expression of the 70 kDa heat shock protein (Hsp70) in three naked neck broiler genotypes during heat stress were studied. Twelve birds of each genotype (Na/Na, Na/na and na/na) were reared to market weight (approximately 2.1kg) at thermoneutral temperature. Six birds from each group served as controls and the remaining six underwent gradual heat stress (from 28ºC to 36ºC). Cloacal temperature and body weight were measured before and after exposure to heat. Liver samples were collected and Hsp70 levels were quantified using western blotting with monoclonal anti-chicken Hsp70 antibody. Heterozygous (Na/na) birds had a significantly lower cloacal temperature variation and less body weight loss during heat stress than the other genotypes. There was no significant difference in the Hsp70 levels among the genotypes. Heterozygous birds (Na/na) appeared to have a slight advantage over the other genotypes during gradual heat stress, perhaps because of a heterotic effect.
Broiler chicken; naked neck genotype; Hsp70 expression; heat stress; cloacal temperature
Estudaram-se o efeito do estresse térmico sobre a temperatura cloacal, a perda de peso corporal e a expressão da proteína de estresse de 70 kDa (Hsp70) em três genótipos de galinhas de pescoço-pelado. Foram usadas 12 aves de cada genótipo (Na/Na, Na/na e na/na), com peso corporal médio de 2,100kg e criadas em temperatura termoneutra. Seis aves de cada grupo serviram como controle e as seis restantes foram submetidas a estresse térmico gradativo (28ºC - 36ºC). A temperatura cloacal e o peso das aves foram avaliados antes e depois do estresse. Amostras de fígado foram coletadas e os níveis de Hsp70 foram quantificados por "western blotting" com anticorpo monoclonal específico para Hsp70 de galinha. As aves heterozigotas (Na/na) tiveram variação de temperatura cloacal significativamente menor e menor perda de peso corporal durante o estresse térmico do que as dos outros genótipos. Não foi observada diferença significativa (P>0,05) para o aumento de Hsp70 no fígado. Aves heterozigotas parecem ter pequena vantagem sobre os outros genótipos durante estresse térmico gradativo, talvez devido ao efeito de heterose.
Frango; pescoço pelado; gene Na; proteína hsp70; estresse calórico; temperatura cloacal
Effect of heat exposure on the thermoregulatory responses of selected naked neck chickens
[Efeito da exposição ao calor na resposta termorregulatória de aves de pescoço pelado selecionadas]
C.M. Mazzi1, M.I.T. Ferro1, A.A.D. Coelho2, V.J.M. Savino2, M. Macari1, J.A. Ferro1* P.E.N. Givisiez1, P.F. Giachetto1, M.M. Silva1, N.J.L. Dionello3
1Faculdade de Ciências Agrárias e Veterinárias UNESP-Jaboticabal
14884-900 Jaboticabal, SP
2Departamento de Zootecnia, Escola Superior de Agricultura Luiz de Queiroz da USP - Piracicaba, SP
3Departamento de Zootecnia, Faculdade de Agronomia Eliseu Maciel, Universidade Federal de PelotasUFPel
Recebido para publicação em 28 de setembro de 2001.
*Autor para correspondência
E-mail: jesus@fcav.unesp.br
ABSTRACT
The variation in cloacal temperature, body weight loss and expression of the 70 kDa heat shock protein (Hsp70) in three naked neck broiler genotypes during heat stress were studied. Twelve birds of each genotype (Na/Na, Na/na and na/na) were reared to market weight (approximately 2.1kg) at thermoneutral temperature. Six birds from each group served as controls and the remaining six underwent gradual heat stress (from 28oC to 36oC). Cloacal temperature and body weight were measured before and after exposure to heat. Liver samples were collected and Hsp70 levels were quantified using western blotting with monoclonal anti-chicken Hsp70 antibody. Heterozygous (Na/na) birds had a significantly lower cloacal temperature variation and less body weight loss during heat stress than the other genotypes. There was no significant difference in the Hsp70 levels among the genotypes. Heterozygous birds (Na/na) appeared to have a slight advantage over the other genotypes during gradual heat stress, perhaps because of a heterotic effect.
Keywords: Broiler chicken, naked neck genotype, Hsp70 expression, heat stress, cloacal temperature.
RESUMO
Estudaram-se o efeito do estresse térmico sobre a temperatura cloacal, a perda de peso corporal e a expressão da proteína de estresse de 70 kDa (Hsp70) em três genótipos de galinhas de pescoço-pelado. Foram usadas 12 aves de cada genótipo (Na/Na, Na/na e na/na), com peso corporal médio de 2,100kg e criadas em temperatura termoneutra. Seis aves de cada grupo serviram como controle e as seis restantes foram submetidas a estresse térmico gradativo (28oC 36oC). A temperatura cloacal e o peso das aves foram avaliados antes e depois do estresse. Amostras de fígado foram coletadas e os níveis de Hsp70 foram quantificados por western blotting com anticorpo monoclonal específico para Hsp70 de galinha. As aves heterozigotas (Na/na) tiveram variação de temperatura cloacal significativamente menor e menor perda de peso corporal durante o estresse térmico do que as dos outros genótipos. Não foi observada diferença significativa (P>0,05) para o aumento de Hsp70 no fígado. Aves heterozigotas parecem ter pequena vantagem sobre os outros genótipos durante estresse térmico gradativo, talvez devido ao efeito de heterose.
Palavras-chave: Frango, pescoço pelado, gene Na, proteína hsp70, estresse calórico, temperatura cloacal
INTRODUCTION
Efforts to alleviate heat distress in commercial broiler chickens have focused on management procedures that include increased ventilation rates, pellet versus mash diets, changes in stocking densities, fogging, evaporative cooling and the inclusion of feed additives to improve the thermal resistance of the birds. A lack of heat tolerance in broilers is often measured by an increase in mortality or morbidity which results mainly from cardiovascular or renal failure, including acid-base imbalance and other metabolic dysfunctions. Many organisms have developed strategies to deal with adverse environmental changes that lead to a stress response. Part of the response to heat stress involves the activation of a specific set of conserved proteins known as heat shock proteins (Hsp) (Craig, 1985; Lindquist, 1986). Among the different heat shock proteins, Hsp70 has been the subject of intense study, because it is the most abundant translational product in stressed eukaryotic cells (Morimoto et al., 1986). Hsp70 has been suggested to serve as a molecular chaperone, which prevents the aggregation of denatured proteins and promotes their refolding (Beckman et al., 1990; Georgopoulos & Welch, 1993; Hendrick & Hart, 1993; Hightower et al., 1994). In addition, some studies have reported a correlation between the levels of synthesis of this protein with the acquisition of stress tolerance (Lee & Dewey, 1987; Laszlo, 1988; Parsell & Lindquist, 1994; Yahav et al., 1998). The molecular mechanisms involved in stress resistance and acclimatization in poultry are poorly understood. In view of the possible participation of Hsp70 in temperature regulation in stressed broilers, several studies have analyzed the expression of this protein in these birds, because exposure to a high ambient temperature directly affects their growth and development. Wang & Edens (1994) reported increased levels of this protein and its mRNA in different tissues from broilers exposed to high ambient temperatures. Gabriel et al. (1996) also reported a time-dependent increase in the levels of Hsp70 and its mRNA in the liver of broilers submitted to acute heat stress. More recently, Yahav et al. (1997) showed that conditioning chicks at 36ºC when they were five-day-old improved thermotolerance at 42 days, but that the levels of Hsp70 in heart and lung tissues were lower than in control birds, suggesting that improved thermotolerance decreased Hsp70 levels as a result of an improved capacity to control body temperature.
Naked skin seems to be advantageous during a thermal challenge because it markedly increases heat loss, and birds with reduced feather cover (bearing the gene for naked neck, Na) have better thermoregulatory and growth responses at high ambient temperatures (Cahaner et al., 1993; Eberhart & Washburn, 1993a; Decuypere et al., 1993). Recently, Yahav et al.(1998) studied the effect of different genotypes (Na/na, poor feathering and na/na, full feathering) and reported that there was no advantage of poor feathering under diurnal cyclic temperature conditions (12h at 15oC and 12h at 35oC).
This investigation was carried out aiming to compare the thermoregulatory responses of three genotypes of naked neck birds (Na/Na, Na/na and na/na) during heat stress and also to examine the involvement of Hsp70 in this process.
MATERIAL AND METHODS
Naked neck birds obtained from non-isogenic progeny selected for heavy body weight by crossing Na/na males with Na/na females (kindly supplied by ESALQ - USP) were used. Thirty-six chicks, consisting of 12 homozygous (Na/Na) naked neck, 12 heterozygous (Na/na) naked neck and 12 homozygous recessive (na/na) normal genotypes segregating from the same group of parents, were used in this experiment. Dominant homozygous (Na/Na) and heterozygous (Na/na) naked neck birds have 40% and 30% less feather cover, respectively, than normal recessive (na/na) birds, which are fully feathered (Touchburn et al., 1980). The difference between Na/Na and Na/na is based on a tuft of feathers on the ventral side of the neck (Scott & Crawford, 1977).
The birds were reared in cages (0.6m width x 0.5m height x 0.6m length) kept in a room in which the ambient temperature was adjusted according to their age (starting at 34oC during the first week and declining at an approximate rate of 1.8oC per week to 24oC at the age of six weeks) in order to grow the animals at thermoneutral temperature. Mash feed containing 13.39 kJ ME/kg and crude protein levels of 22% and 20% was supplied during the first (up to the 21st day) and second (from the 22nd day until the end of the experiment) phases of the study, respectively. The main ingredients of the feed were corn and soybean meal. Water and feed were supplied ad libitum.
The birds submitted to heat stress were removed from rearing temperature (24ºC) to 28oC for one hour and then the temperature was gradually increased to 36oC at a rate of 2oC/h. Cloacal temperature was recorded under rearing temperature conditions (24ºC) and hourly during heat stress using a cloacal thermistor probe (Yellow Springs Instruments, no. 702) connected to a telethermometer into the birds cloaca. Body weight was monitored at the beginning and at the end of the heat stress period. Food and water were not provided during heat stress.
For liver sampling and Hsp70 quantification six birds from each treatment were killed by cervical dislocation at 24oC (before heat stress) and at 36oC (after heat stress). Liver samples (0.5 g) were collected, immediately frozen in liquid nitrogen and stored at 70oC until assayed for Hsp70. These samples were homogenised using a 1:10 dilution in lysis buffer (20mM Tris-HCl, pH 7.5; 0,9% NaCl; 2mM b-mercaptoethanol) with an ultra-turrax homogeniser (Thomas Scientific, Swedesboro, NJ), at 20,000 rpm and ice-bath intervals of 30s. Lysates were centrifuged at 31,000 ×g for 30min at 4ºC and the protein concentration of the supernatant was then determined in triplicate (Hartree, 1972). Thirty micrograms of total protein were run on 9% polyacrylamide gels containing sodium dodecyl sulfate (SDS) according to Laemmli (1970), using a mini-Protean II apparatus (Bio-Rad) set at constant voltage (200 V). Before loading, the samples stored at 20oC were reboiled for 2min. An aliquot of a pool of liver lysates supernatants from six control birds (no heat stress) served as a reference sample in all gels. One hundred micrograms of Hsp70 (Sigma, H9776) was also included in all runs as a specific molecular weight marker. After fractionation on SDS-polyacrylamide gels, the proteins were transferred electrophoretically to polyvinylidene difluoride (PVDF) membranes using procedures adapted from Towbin et al. (1979) and Matsudaira (1987). The transfer was carried out for 30min at 4oC and at constant voltage (90 V) using a mini trans-blot cell (Bio-Rad). The membranes were stained with 0.05% Ponceau S in 1% acetic acid for 1min to assess the efficiency of the transfer. After several washes with deionised water, nonspecific interaction sites were blocked with 10ml of cold TBS buffer (10mM Tris-HCl, pH 8.0, 150mM NaCl) containing 5% dry skimmed milk and 0.02% Tween-20, for 1 h at room temperature on an orbital shaker (approximately 100rpm). The membranes were then incubated with 10 ml of monoclonal anti-Hsp70 antibody (H-5157, Sigma) diluted in 10ml of cold TBS-milk solution (1:1,000 dilution) containing 0.02% Tween-20 for 1 h at room temperature with shaking. Four washes (5min each) with 10ml of TBST (10mM Tris-HCl, pH 8.0, 150mM NaCl and 0.05% Tween-20) and a 10min wash with 10ml of cold TBS buffer were then performed. The membranes were subsequently incubated with 2ml of a secondary anti-mouse antibody conjugate to alkaline phosphatase (A-5153, Sigma) diluted in 10ml of cold TBS-milk (1:5,000 dilution) for 1 h at room temperature with constant shaking. After rinsing with cold TBST and TBS as described above, the color was developed for 2min with 66ml of nitro-blue tetrazolium chloride solution (50mg/ml in dimethylformamide) and 33ml of 5-bromo-4-chloro-3-indolyphosphate p-toluidine (50mg/ml in 70% dimethylformamide) in 10ml of AP buffer (100mM Tris-HCl, pH 9.5, 100mM NaCl and 5mMmgCl2). The reaction was blocked by adding 3% trichloroacetic acid. The membranes were washed with deionized water, dried at room temperature and protected from light. The color signal of the bands corresponding to Hsp70 was analyzed with a densitometer at 525nm (Shimadzu CS-9301) using the reflection mode and zigzag scanning. For the construction of the standard curve, Hsp70 (H-9776, Sigma) was resuspended in 30% glycerol (v/v) at a final concentration of 500ng/ml. Aliquots containing 25, 50, 100, 200, 300, 350, 400 and 450ng of protein were prepared and loaded onto SDS-polyacrylamide gels along with the reference sample (in triplicate). After electrophoresis, western blot analysis was performed as described. The bands were analyzed by densitometry and a standard curve for Hsp70 quantification was obtained by plotting the Hsp70 concentration against the ratio of the density of each concentration relative to that of the average value for the triplicate reference sample. The ratio between the test samples and the reference sample in each membrane was used to determine the quantity of Hsp70 in the supernatant. Data are expressed as ng Hsp70/mg total protein.
Cloacal temperatures (oC) and body weights (%) of the three genotypes before and after heat stress were compared by analysis of variance (ANOVA) in a completely random design. Hsp70 concentrations were analyzed using a 3 x 2 factorial arrangement (three genotypes and two ambient temperatures). Mean differences were determined by Tukeys test at the 5% level of significance (Steel & Torrie, 1980). The correlation between the increase in cloacal temperature (DT) and Hsp70 concentration was also examined.
RESULTS
The body weight of broiler chickens of the different genotypes was not significantly different between 44 and 46 days of age, immediately before heat stress. However, when chickens were submitted to a gradual heat stress (from 28ºC to 36ºC) there was a significant difference in body weight loss (expressed as percentage of initial body weight) among the genotypes (Tab. 1).
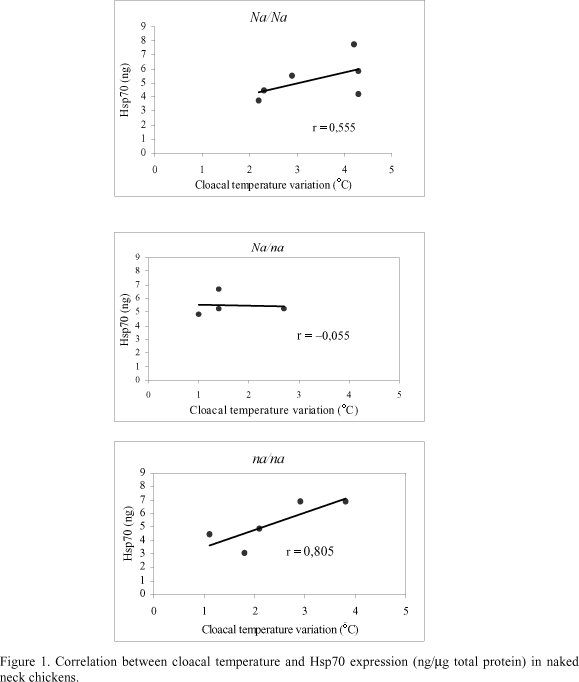
Cloacal temperature increased in all naked neck genotypes during gradual heat stress, although there was a significant lower increase (DT = 1.9ºC) in Na/na birds than in the other genotypes (DT = 3.7ºC for Na/Na; DT = 2.7ºC for na/na) (Tab. 2). There was also an increase in hepatic Hsp70 content, but there was no significant difference among the genotypes (Tab. 2). The correlation coefficient (r) between cloacal temperature increase and hepatic Hsp70 content revealed a positive effect between these variables for the homozygous birds (Na/Na and na/na), while for the heterozygous birds this effect was not observed (Fig. 1).
DISCUSSION
The findings of this study showed that heterozygous (Na/na) birds had better physiological performance during heat stress when compared with homozygous (Na/Na and na/na) broilers, since they showed lower body weight loss and less increase in cloacal temperature.
Based on productive parameters (feed conversion and body weight gain), Eberhart & Washburn (1993b), also reported that the Na gene was unable to confer resistance to chronic heat stress in a small body weight population of naked neck birds (Na/na), whereas for large body weight birds the Na gene produced resistance to chronic heat stress. These same authors studied the effect of the naked neck gene in birds reared at different environmental temperatures and exposed to acute heat stress (40.5oC) and found that the change in body temperature was higher for naked neck (Na/na) than for normally feathered birds (na/na) (Eberhart & Washburn, 1993a).
Our findings, using however less severe heat exposure (36oC), revealed that some thermoregulatory parameters such as body weight loss and cloacal temperature, were differentially affected, depending on the bird genotype. This suggests that body weight is not the only parameter affecting heat resistance since the birds had the same body weight during heat stress. Although the reduced feather covering produced by the naked neck gene (Na) has been associated with heat resistance (Merat, 1986; Webster & King, 1987), these birds did not show any great advantage during heat stress. Yahav et al. (1998) recently reported that the ability of naked neck chickens (Na/na) to thermoregulate at high environmental temperatures is only slightly better than that of normally feathered birds (na/na), and no genotype benefit was observed during exposure to temperature cycles (12 h at 35ºC and 12 h at 15ºC).
There was a positive tendency between the increase in cloacal temperature (Dt) and hepatic Hsp70 concentration (ng/ml total protein) in the dominant (Na/Na) and recessive (na/na) homozygous birds while in the heterozygous birds those was no such clear association. Gabriel et al. (1996) reported that hepatic Hsp70 synthesis in broiler chickens (Hubbard-Petersen strain) is heat-and time-dependent. Our investigation also showed that naked neck birds have an increase in hepatic Hsp70 expression during heat stress, exception to heterozygous (Na/na) birds.
In conclusion, the results of this experiment give evidence that the Na gene, in single dose, appear to confer a slightly advantage over the other genotypes during gradual heat stress, perhaps due to the heterotic effect, as suggested by Mathur & Horst (1989) and Horst & Mathur (1989).
ACKNOWLEDGMENTS
The authors thank Dr. Avigdor Cahaner and Dr. Eddy Decuypere for reading the manuscript and Fundação de Amparo à Pesquisa do Estado de São Paulo (Fapesp) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for financial support.
- BECKMANN, R.P.; MIZZEN, L.A.; WELCH, W.J. Interactions of Hsp70 with newly synthesized proteins: implications for protein folding and assembly. Science, v.248, p.850-854, 1990.
- CAHANER, A.; NADER, D.; GUTMAN, M. Effects of plumage reducing naked neck (Na) gene on the performance of fast growing broilers at normal and high ambient temperature. Poult. Sci., v.72, p.767-775, 1993.
- CRAIG, E. The heat shock response. Crit. Rev. Biochem., v.18, p.239-280, 1985.
- DECUYPERE, E.; BUYSE, J.; MERAT, P. et al. Growth, abdominal fat content, heat production and plasma hormone levels of naked neck and control broiler chickens. Anim. Prod, v.57, p.483-490, 1993.
- EBERHART, D.E.; WASHBURN, K.W. Assessing the effects of naked neck gene on chronic heat stress resistance in two genetic populations. Poult. Sci, v.72, p.1391-1399, 1993a.
- EBERHART, D.E.; WASHBURN, K.W. Variation in body temperature response of naked neck and normally feathered chickens to heat stress. Poult. Sci, v.72, p.1385-1390, 1993b.
- GABRIEL, J.E.; FERRO, J.A.; STEFANI, R.M.P. et al. Effect of acute heat stress on heat shock protein 70 messenger RNA and on heat shock protein expression in the liver of broilers. Br. Poult. Sci, v. 37, p.443-449, 1996.
- GEORGOPOULOS, C.; WELCH, W.J. Role of major heat shock proteins as molecular chaperones. Annu. Rev. Cell Biol, v.9, p.601-635, 1993.
- HENDRICK, J.P.; HART, F.U. Molecular chaperone functions of heat shock proteins. Annu. Rev. Biochem., v.62, p.349-384, 1993.
- HIGHTOWER, L.E.; SADIS, S.E.; TAKENAKA, I.M. Interactions of vertebrates hsc70 and hsp70 with unfolded proteins and peptides. In: MORIMOTO, R.I., TISSIÈRES, A.; GEORGOPOULOS, C. (Eds) The Biology of heat shock proteins and molecular chaperones New York: Cold Spring Harbor Laboratory, 1994. p.179-207.
- HARTREE, E.F. Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal. Biochem, v.48, p.422-427, 1972.
- HORST, P.; MATHUR, P.K. Position of local fowl for tropical oriented breeding activities. In: MÉRAT, P. (Ed.). Genotype x environment interactions in poultry production. Jouy-en-Josas: INRA, 1989. p.161-174.
- LAEMMLI, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, v. 226, p.112-115, 1970.
- LASZLO, A. The relationship of heat shock proteins, thermotolerance, and protein synthesis. Exp. Cell Res., v.178, p.401-414, 1988.
- LEE, Y.J.; DEWEY, W. Effect of cyclohexemide or puromycin on induction of thermotolerance by heat in Chinese hamster ovary cells: dose fractionation at 45.5oC. Cancer Res, v.47, p.5960-5966, 1987.
- LINDQUIST, S.L. The heat shock response. Annu. Rev. Biochem, v.55, p.1151-1191, 1986.
- MATHUR, P.K.; HORST, P. Temperature stress and tropical location as factors for genotype x environment interactions. In: MÉRAT, P. (Ed.). Genotype x environment interactions in poultry production. Jouy-en-Josas; INRA, 1989. p.83-96.
- MATSUDAIRA, P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J. Biol. Chem, v.262, p.10035-10038, 1987.
- MERAT, P. Potential usefulness of the Na (naked neck) gene in poultry production. World. Poult. Sci. J., v.42, p.124-142, 1986.
- MORIMOTO, R.; HUNT, S.Y.; BERG, K.L. et al. Organization, nucleotide sequences, and transcription of the chicken Hsp70 gene. J. Biol. Chem, v.261, p.12692-12699, 1986.
- PARSELL, D.A.; LINDQUIST, S. Heat shock proteins and stress tolerance. In: MORIMOTO, R.J.; TISSIČRES, A.; GEORGOPOULOS, S. (Eds.) The Biology of heat shock proteins and molecular chaperones New York: Cold Spring Harbor Laboratory, 1994. p. 457-494.
- SCOTT, T.; CRAWFORD, R.D. Feather number and distribution in the throat tuft of the naked neck chicks. Poult. Sci., v.56, p.1758 1977. (Abstract),
- STEEL, R.G.D.; TORRIE, J.H. Principles and procedures of statistics New York: McGraw Hill, 1980. p.633.
- TOUCHBURN, S.P.; GUILLAUME, J.; LECLERQ, B. et al. Lipid and energy metabolism in chicks affected by dwarfism (dw) and naked neck(Na). Poult. Sci, v.59, p.2189-2197, 1980.
- TOWBIN, H.; STAEHELIN, T.; GORDON, J. Electrophoretic transfer of proteins from polyacrylamide gels to nitro-cellulose sheets: procedure and some applications. Proc. Nat. Acad. Sci., v.76, p.4350-4354, 1979.
- YAHAV, S.; LUGER, D.; CAHANER, A. et al. Thermoregulation in naked neck chickens subjected to different ambient temperatures. Br. Poult. Sci, v.39, p.133-138, 1998.
- YAHAV, S.; SHAMAY, A.; HOREV, G. et al. Effect of acquisition of improved thermotolerance on the induction of heat shock proteins in broiler chickens. Poult. Sci., v.76, p.1428-1434, 1997.
- WANG, S.; EDENS, F.W. Hsp70 mRNA expression in heat-stressed chickens. Comp. Biochem. Physiol., v.107B, p.33-37, 1994.
- WEBSTER, M.D.; KING, J.R. Temperature and humidity dynamics of cutaneous and respiratory evaporation in pigeons, Columbia livia Comp. Biochem. Physiol., v.157, p.253-260, 1987.
Publication Dates
-
Publication in this collection
22 July 2002 -
Date of issue
Feb 2002
History
-
Received
28 Sept 2001