ABSTRACT
Soft tissue sarcomas (STS) comprise a heterogeneous group of malignancies derived from extra-skeletal mesenchymal tissues that may show similar histopathological changes. Histopathologic patterns suggestive of perivascular wall tumors (PWT) and peripheral nerve sheath tumors (PNST) have been described. This study investigated the histogenesis in a series of 71 cases of canine STS that showed morphological compatibility with what is described for PWT and PNST. Immunohistochemistry analysis were done to CD56, S100, SMA, Desmin, Von Willebrand Factor, NSE and GFAP. Twenty-one cases (29.6%) showed histopathologic features compatible with PWT, 23 cases (32.4%) with PNST and 27 cases (38.0%) shared both histopathological features. By immunohistochemistry, 59 (83.1%) cases showed positivity only for neural markers and 12 (16.9%) had simultaneous positivity for both neural and muscle markers. PNST was the most prevalent neoplasm and none of the cases were positive for muscle markers only. The histopathologic features were not useful to define the diagnosis of PWT, since most tumors were negative for muscle markers but positive for neural markers. Due to this immunoreactivity and the morphologic features, future studies may propose guidelines for the classification of these neoplasms.
Keywords:
dog; soft tissue sarcoma
RESUMO
Sarcoma de tecidos moles (STM) compreende um grupo heterogêneo de neoplasias malignas, derivadas de tecidos extraesqueléticos, que podem apresentar alterações histopatológicas similares. Os padrões histopatológicos sugestivos de tumor de parede perivascular (TPP) e de tumor de bainha de nervo periférico (TBNP) têm sido descritos. Este estudo investigou a histogênese de uma série de 71 STM caninos, que apresentavam compatibilidade morfológica com o que é descrito para TPP e TBNP. A análise imuno-histoquímica foi feita para CD56, S100, SMA, Desmina, Fator Von Willebrand, NSE e GFAP. Vinte e um casos (29,6%) apresentaram características histopatológicas compatíveis com TPP, 23 casos (32,4%) com TBNP e 27 casos (38,0%) apresentaram características histopatológicas de ambos. Na imuno-histoquímica, 59 (83,1%) casos apresentaram positividade somente para marcadores neurais e 12 (16,9%) tiveram positividade simultânea tanto para marcadores neurais como para marcadores musculares. TBNP foi a neoplasia mais prevalente e nenhum dos casos foi positivo para somente para marcadores musculares. As características histopatológicas não foram úteis para definir o diagnóstico de TPP, uma vez que a maioria foi negativa para marcadores musculares, mas positiva para marcadores neurais. Devido a essa imunorreatividade e às características morfológicas, pesquisas futuras poderão propor orientações para a classificação dessas neoplasias.
Palavras-chave:
cão; sarcoma de partes moles
INTRODUCTION
Soft tissue sarcomas (STS) comprise 15% of all subcutaneous tumors in dogs. They are considered a heterogeneous group of neoplasms, including peripheral nerve sheath tumors (PNST), hemangiopericytomas, liposarcomas, fibrosarcomas, myxosarcomas, perivascular wall tumors (PWT) and undifferentiated sarcomas. These tumors have histomorphological similarities and are composed of spindle cells arranged in interlacing / waving bundles and concentric whorls (Dennis et al., 2011DENNIS, M.M.; MCSPORRAN, K.D.; BACON, N.J. et al. Prognostic factors for cutaneous and subcutaneous soft tissue sarcomas in dogs. Vet. Pathol., v.48, p.73-84, 2011.).
Despite this similarity, specific histopathological findings have been described for PWT and PNST. Histologically, PWT have vascular growth patterns (staghorn, placentoid, pericapillary whorling and radiating bundles from media or adventitia tunica) and nonvascular growth patterns (interlacing or parallel bundles, solid areas, storiform arrangement, myxoid and Verocay - like Bodies) (Stefanello et al., 2011STEFANELLO, D.; AVALLONE, G.; FERRARI, R. et al. Canine cutaneous perivascular wall tumors at first presentation: clinical behavior and prognostic factors in 55 cases. J. Vet. Intern. Med., v.25. p.1398-1405, 2011.; Avallone et al., 2014AVALLONE, G.; BORACCHI, P.; STEFANELLO, D. et al. Canine perivascular wall tumors: high prognostic impact of site, depth, and completeness of margins. Vet. Pathol., v.51, p.713-721, 2014.). Peripheral nerve sheath tumors show neural patterns with fusiform cells arranged in solid and interlacing bundles, palisades and concentric whorls (Antoni A pattern), or the cells may be distributed in a fibrillar and mucinoid matrix, along with polygonal cells (Antoni B pattern) (Chijiwa et al., 2004CHIJIWA, K.; UCHIDA, K.; TATEYAMA, S. Immunohistochemical evaluation of canine peripheral nerve sheath tumors and other soft tissue sarcomas. Vet. Pathol., v.41, p.307-318, 2004.; Gross et al., 2005GROSS, T.L.; IHRKE, P.J.; WALDER, E.J. et al. Skin diseases of the dog and cat. clinical and histopathologic diagnosis. 2.ed. Iowa: Blackwell Publishing, 2005. 932p.PEREZ, J.; BAUTISTA, M.J.; ROLLON, E. et al. Immunohistochemical characterization of hemangiopericytomas and other spindle cell tumors in the dog. Vet. Pathol., v.33, p.391-397, 1996.; Gaitero et al., 2008GAITERO, L.; AÑOR, S.; FONDEVILA, D. et al. Canine cutaneous histopathological and immunohistochemical study. J. Comp. Pathol., v.139, p.16-23, 2008.).
However, the diagnosis of PWT and PNST is challenging in veterinary medicine, considering that many of these tumors share various immunostaining patterns, besides presenting histopathologic similarities (Williamson and Middleton, 1998WILLIAMSON, M.M.; MIDDLETON, D.J. Cutaneous soft tissue tumours in dogs: classification, differentiation and histogenesis. Vet. Dermatol., v.9, p.43-48, 1998.). Because of this and their common biological behavior and prognosis, some authors defend the idea of integrating them under the single term "spindle cell tumor of soft tissue" instead of classifying them according to their histogenesis (Williamson and Middleton, 1998). A recent study, however, pointed out differences in the biological behavior of PWT, which seem to represent a more benign form of the STS, with lower rates of recurrence and metastasis (Stefanello et al., 2011STEFANELLO, D.; AVALLONE, G.; FERRARI, R. et al. Canine cutaneous perivascular wall tumors at first presentation: clinical behavior and prognostic factors in 55 cases. J. Vet. Intern. Med., v.25. p.1398-1405, 2011.). Therefore, the precise definition of the cellular origin could be valuable as a prognostic factor for this specific group of STS, and could influence the therapeutic approach as well (Stefanello et al., 2011; Avallone et al., 2014AVALLONE, G.; BORACCHI, P.; STEFANELLO, D. et al. Canine perivascular wall tumors: high prognostic impact of site, depth, and completeness of margins. Vet. Pathol., v.51, p.713-721, 2014.). Thus, this study aimed to investigate the histogenesis of STS and compare it with previous morphological diagnoses for PWT and PNST.
MATERIAL AND METHODS
Microscopic slides from cases of the Pathology Service diagnosed as STS, with morphological findings compatible with PNST and PWT were reviewed. Soft tissue sarcomas with morphological findings suggestive of fibrosarcoma, liposarcoma, myxosarcoma, leiomyosarcoma and rhabdomyosarcoma were not included in the study.
Slides stained with H&E were reviewed under light microscopy for the classification of histologic type. The diagnosis of PWT was made when patterns of vascular growth such as staghorn, pericapillary whorls, placentoid, and whorls or bundles radiating from the middle tunica media and adventitia were seen alone or in combination (Avallone et al., 2007AVALLONE, G.; HELMBOLD, P.; CANIATTI, M. et al. The spectrum of canine cutaneous perivascular wall tumors: morphologic, phenotypic and clinical characterization. Vet. Pathol., v.44, p.607-620, 2007.). The classic neural pattern consisted of classical Antoni A (areas with thick spindle cell arrangements) and Antoni B (hypocellular areas with an abundant myxoid matrix), interwoven bundles, and whorls around collagen bundles (Dennis et al., 2011DENNIS, M.M.; MCSPORRAN, K.D.; BACON, N.J. et al. Prognostic factors for cutaneous and subcutaneous soft tissue sarcomas in dogs. Vet. Pathol., v.48, p.73-84, 2011.). The cases that shared both vascular and neural patterns were classified as mixed pattern.
For immunohistochemistry analysis, the paraffin blocks were cut into 3μm thick sections and placed on electrically charged plates (Starfrost®). The antibody marks, dilutions and clones were as follows: CD56 (Biocare Medical, Concord, CA, USA; BC56C04; 1:200); Polyclonal Rabbit Anti-S100 (Dako, Glostrup, Denmark; Policlonal; 1:1000); Monoclonal Mouse Anti-Human Smooth Muscle Actin (Dako, Glostrup, Denmark; A4; 1:1000); Monoclonal Mouse Anti-Human Desmin (Dako, Glostrup, Denmark; D33; 1:50); Polyclonal Rabbit Anti-Human Von Willebrand Factor (Dako, Glostrup, Denmark; Policlonal; 1:1000); Monoclonal Mouse Anti-Human Neuron-Specific Enolase (NSE) (Dako, Glostrup, Denmark; BBS/NC/VI-H14; 1:3000); Glial Fibrillary Acidic Protein Rabbit Polyclonal Antibody (Diagnostic BioSystems, Pleasanton, CA; Policlonal; 1:300).
After incubating at 54°C for 24h, tissue sections were deparaffinized in xylene, hydrated in ethanol and washed with deionized water. For antigen retrieval, citrate was used at pH 6.0 in a pressure cooker. The endogenous peroxidase blockage was performed with 3% hydrogen peroxide for 10min. After washing, the primary antibody and slides were kept overnight in a moist chamber at 4°C for 18h. After washing with Tris at pH 7.4, a peroxidase bound secondary antibody polymer system was used according to the manufacturer's recommendations (EnVision, DakoCytomation®). Visualization was accomplished with 3,3'-diaminobenzidine tetrachloride (DakoCytomation®) and counterstained with Harris hematoxylin. Internal positive controls (muscle tissue and endothelial cells) were used to smooth muscle actin, desmin, and Von Willebrand factor. For other antibodies, the positive control consisted of tumors known to be positive for that particular antibody. For all of the antibodies, negative controls were performed by omitting the primary antibodies.
The cases were considered positive when at least 10% of tumor cells showed immunostaining (Ramos-Vara and Miller., 2014). The sensitivity of the histopathologic pattern for the definitive diagnosis of STS were evaluated by the Chi square test. This study was approved by the Ethic Committee for Animal Use - CEUA/UFV - 95/2014.
RESULTS
Seventy-one cases of canine STS were reviewed; 21 cases (29.6%) showed histopathological findings compatible with PWT, and 23 cases (32.4%) were diagnosed as PNST (Figure 1A and 1B). Twenty-seven cases (38.0%) shared histopathological findings of both vascular and neural patterns (mixed pattern).
Canine PWT. A -Spindle cell in concentric whorl around a small vessel (H&E, X400); B. Canine PNST. Classical findings of Antoni A (a) and Antoni B (b) (H&E, X100).
None of the tumors were positive only for smooth muscle markers. All cases were negative for Von Willebrand factor (factor VIII). Most cases with a histopathologic diagnosis of PWT had immunostaining only for neural markers. The PWT pattern was not sensitive for the diagnosis of perivascular tumors (P< 0.01).
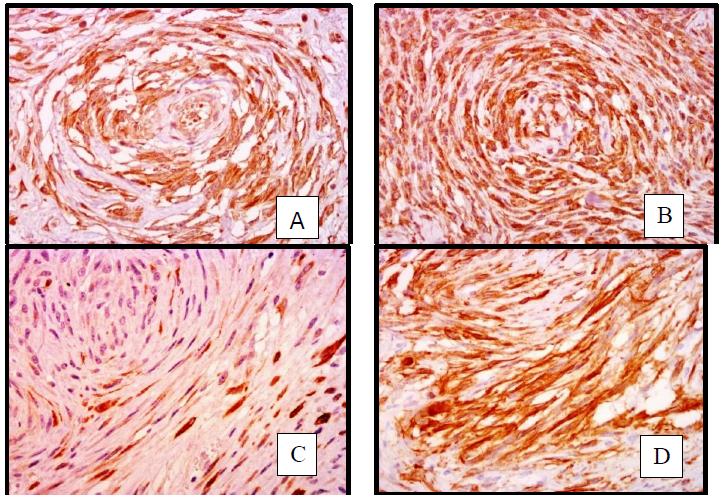
Out of the 71 cases, 59 (83.1%) were positive for neural markers only and therefore classified as PNST. The remaining 12 cases (16.9%) showed a mixed pattern of immunostaining, showing positivity for both neural and smooth muscle markers (desmin and smooth muscle actin) (Figure 2A-2D).
Canine STS. A- Positive immunostaining for GFAP (400x); B- Positive immunostaining for NSE (400x); C- Positive immunostaining for desmin (400x); D- Positive immunostaining for smooth muscle actin (400x).
Moreover, out of the 27 cases that shared histopathological findings of both PNST and PWT, only seven cases showed simultaneous immunostaining for neural and muscle markers (Table 1).
Positivity for neural markers showed variation between PNST. Simultaneous immunostaining for CD56, GFAP and NSE were the most prevalent combination. (Figure 3).
For tumors with mixed immunoreactivity, positivity for smooth muscle actin, CD56, GFAP and NSE was the most observed (Figure 4).
Mixed STS immunohistochemistry: positivity percentile for neural markers, desmin and actin smooth muscle (n= 12).
DISCUSSION
The term PWT, recently introduced in veterinary medicine, includes neoplasias derived from different cellular components of the vascular wall (excluding endothelial cells). This group includes hemangiopericytoma, myopericytoma, angioleiomyoma, angioleiomyosarcoma, adventitious tumor and angiofibroma (Avallone et al., 2007AVALLONE, G.; HELMBOLD, P.; CANIATTI, M. et al. The spectrum of canine cutaneous perivascular wall tumors: morphologic, phenotypic and clinical characterization. Vet. Pathol., v.44, p.607-620, 2007.). These tumors share some histopathologic features with PNST, making differentiating between them problematic.
In the present study, all cases were positive for neural markers, and it was not possible to identify tumors exclusively of perivascular origin, as in other investigations (Avallone et al., 2007AVALLONE, G.; HELMBOLD, P.; CANIATTI, M. et al. The spectrum of canine cutaneous perivascular wall tumors: morphologic, phenotypic and clinical characterization. Vet. Pathol., v.44, p.607-620, 2007.). Moreover, only a minority of cases with perivascular histopathologic patterns were positive for smooth muscle actin and / or desmin. These findings show that the histopathologic features have low specificity for the diagnosis of PWT. As most cases were negative for desmin and smooth muscle actin and were only positive for neural markers, PNST were the more prevalent diagnosis. Simultaneous positivity for muscle and neural markers, as seen in a smaller proportion of cases, supports the fact that some sarcomas may have cells of different lineages (Williamson and Middleton, 1998WILLIAMSON, M.M.; MIDDLETON, D.J. Cutaneous soft tissue tumours in dogs: classification, differentiation and histogenesis. Vet. Dermatol., v.9, p.43-48, 1998.). Similar to the results presented here, other authors also reported immunoreactivity for neural markers (S100, GFAP and NSE) and smooth muscle actin in the so-called hemangiopericytomas (Perez et al., 1996; Chijiwa et al., 2004CHIJIWA, K.; UCHIDA, K.; TATEYAMA, S. Immunohistochemical evaluation of canine peripheral nerve sheath tumors and other soft tissue sarcomas. Vet. Pathol., v.41, p.307-318, 2004.). This supports the statement that within canine STS there is a group of tumors with mixed histogenesis which should not be classified as purely hemangiopericytomas or as PWT.
In one study of 33 cases of canine STS with characteristics of PNST, immunostaining for GFAP was detected only in multinucleated cells, and the use of NSE marker was limited due to frequent nonspecific background staining (Gaitero et al., 2008GAITERO, L.; AÑOR, S.; FONDEVILA, D. et al. Canine cutaneous histopathological and immunohistochemical study. J. Comp. Pathol., v.139, p.16-23, 2008.). Herein, all 71 cases were positive for GFAP and NSE, with diffuse expression in most cases. Therefore, in the present study these markers proved useful for the diagnosis of canine STS.
CONCLUSION
In conclusion, in this series of canine STS, PNST were the most prevalent neoplasm. Pure perivascular tumors were not identified. The perivascular histopathological pattern was not useful to predict the tumor histogenesis. It was also clear that STS occur with mixed histogenesis. Due to this heterogeneity of the immunohistochemical results and poor specificity of histopathological patterns, criteria for the diagnosis of PNST and PWT are not currently clear. Undoubtedly, this is a limiting factor for comparison of different studies, including prognosis. A large consensus work may propose guidelines for the classification and standardization of these neoplasms to be accepted worldwide.
ACKNOWLEDGEMENTS
CAPES: Coordenação de Aperfeiçoamento de Pessoal de Nível Superior.
FUNARBE: Fundação Arthur Bernardes-UFV.
REFERENCES
- AVALLONE, G.; BORACCHI, P.; STEFANELLO, D. et al. Canine perivascular wall tumors: high prognostic impact of site, depth, and completeness of margins. Vet. Pathol., v.51, p.713-721, 2014.
- AVALLONE, G.; HELMBOLD, P.; CANIATTI, M. et al. The spectrum of canine cutaneous perivascular wall tumors: morphologic, phenotypic and clinical characterization. Vet. Pathol., v.44, p.607-620, 2007.
- CHIJIWA, K.; UCHIDA, K.; TATEYAMA, S. Immunohistochemical evaluation of canine peripheral nerve sheath tumors and other soft tissue sarcomas. Vet. Pathol., v.41, p.307-318, 2004.
- DENNIS, M.M.; MCSPORRAN, K.D.; BACON, N.J. et al. Prognostic factors for cutaneous and subcutaneous soft tissue sarcomas in dogs. Vet. Pathol., v.48, p.73-84, 2011.
- GAITERO, L.; AÑOR, S.; FONDEVILA, D. et al. Canine cutaneous histopathological and immunohistochemical study. J. Comp. Pathol., v.139, p.16-23, 2008.
- GROSS, T.L.; IHRKE, P.J.; WALDER, E.J. et al. Skin diseases of the dog and cat. clinical and histopathologic diagnosis. 2.ed. Iowa: Blackwell Publishing, 2005. 932p.PEREZ, J.; BAUTISTA, M.J.; ROLLON, E. et al. Immunohistochemical characterization of hemangiopericytomas and other spindle cell tumors in the dog. Vet. Pathol., v.33, p.391-397, 1996.
- RAMOS-VARA, J.A.; MILLER, M.A. When tissue antigens and antibodies get along: revisiting the technical aspects of immunohistochemistry - the red, brown, and blue technique. Vet. Pathol., v.5, p.42-87, 2014.
- STEFANELLO, D.; AVALLONE, G.; FERRARI, R. et al. Canine cutaneous perivascular wall tumors at first presentation: clinical behavior and prognostic factors in 55 cases. J. Vet. Intern. Med., v.25. p.1398-1405, 2011.
- WILLIAMSON, M.M.; MIDDLETON, D.J. Cutaneous soft tissue tumours in dogs: classification, differentiation and histogenesis. Vet. Dermatol., v.9, p.43-48, 1998.
Publication Dates
-
Publication in this collection
10 Oct 2019 -
Date of issue
Jul-Aug 2019
History
-
Received
01 May 2018 -
Accepted
19 Dec 2018