Abstract
PURPOSE:
To investigate gender differences in the evolution of the inflammatory process in rats subjected to brain death (BD).
METHODS:
Adult Wistar rats were divided into three groups: female; ovariectomized female; and male rats. BD was induced using intracranial balloon inflation and confirmed by maximal pupil dilatation, apnea, absence of reflex, and drop of mean arterial pressure. Six hours after BD, histological evaluation was performed in lungs, heart, liver and kidneys, and levels of inflammatory proteins, estrogen, progesterone, and corticosterone were determined in plasma.
RESULTS:
In the lungs, females presented more leukocyte infiltration compared to males (p<0.01). Ovariectomized female rat lungs were more hemorrhagic compared to other groups (p<0.001). In the heart, females had higher leukocyte infiltration and tissue edema compared to males (p<0.05). In the liver and kidneys, there were no differences among groups. In female group estradiol and progesterone were sharply reduced 6 hours after BD (p<0.001) to values observed in ovariectomized females and males. Corticosterone levels were similar.
CONCLUSIONS:
Sex hormones influence the development of inflammation and the status of organs. The increased inflammation in lungs and heart of female rats might be associated with the acute reduction in female hormones triggered by BD.
Brain Death; Sex Characteristics; Estradiol; Inflammation; Rats
Introduction
Brain death (BD) is a relevant event that causes pathological changes on all the organs11. Simas R, Kogiso DH, Correia Cde J, Silva LF, Silva IA, Cruz JW, Sannomiya P, Moreira LF. Influence of brain death and associated trauma on solid organ histological characteristics. Acta Cir Bras. 2012 Jul;27(7):465-70. PMID: 22760831.
2. Smith M. Physiologic changes during brain stem death--lessons for management of the organ donor. J Heart Lung Transplant. 2004 Sep;23(9 Suppl):S217-22. PMID: 15381167.-33. Simas R, Sannomiya P, Cruz JW, Correia Cde J, Zanoni FL, Kase M, Menegat L, Silva IA, Moreira LF. Paradoxical effects of brain death and associated trauma on rat mesenteric microcirculation: an intravital microscopic study. Clinics (Sao Paulo). 2012 Jan; 67(1):69-75. PMID: 22249483.. When used for transplantation marginal organs can influence the survival of the receptor44. Salim A, Martin M, Brown C, Belzberg H, Rhee P, Demetriades D. Complications of brain death: frequency and impact on organ retrieval. Am Surg. 2006 May;72(5):377-81. PMID: 16719188.. As brain dead patients are the most common source of transplanted organs, the comprehension of BD alterations is useful to show the professionals involved in the process of capture and transplantation of organs how to treat the brain dead patient increasing, therefore, the percentage of organs suitable for transplantation.
Many issues are considered in donor organ evaluation55. Chaney J, Suzuki Y, Cantu E, van Berkel V. Lung donor selection criteria. J Thorac Dis. 2014 Aug;6(8):1032-8. PMID: 25132970., such as donor and recipient ages, comorbidities, size matching, ABO compatibilities, serology and a minimal ischemic time. However, donor and recipient genders are not directly matched. There are few reports about this feature and many of them have limited sample sizes66. Fessart D, Dromer C, Thumerel M, Jougon J, Delom F. Influence of gender donor-recipient combinations on survival after human lung transplantation. Transplant Proc. 2011 Dec;43(10):3899-902. PMID: 22172868.,77. Roberts DH, Wain JC, Chang Y, Ginns LC. Donor-recipient gender mismatch in lung transplantation: impact on obliterative bronchiolitis and survival. J Heart Lung Transplant. 2004 Nov;23(11):1252-9. PMID: 15539123.. Some studies confirm donor gender as a factor that influences significantly the outcome of organ transplantation88. Sato M, Gutierrez C, Kaneda H, Liu M, Waddell TK, Keshavjee S. The effect of gender combinations on outcome in human lung transplantation: the International Society of Heart and Lung Transplantation Registry experience. J Heart Lung Transplant. 2006 Jun;25(6):634-7. PMID: 16730568.,99. Zeier M, Döhler B, Opelz G, Ritz E. The effect of donor gender on graft survival. J Am Soc Nephrol. 2002 Oct;13(10):2570-6. PMID: 12239247. and the survival after transplantation of lung, heart or kidney is clearly shorter when a male recipient receives a female organ88. Sato M, Gutierrez C, Kaneda H, Liu M, Waddell TK, Keshavjee S. The effect of gender combinations on outcome in human lung transplantation: the International Society of Heart and Lung Transplantation Registry experience. J Heart Lung Transplant. 2006 Jun;25(6):634-7. PMID: 16730568.,1010. Prendergast TW, Furukawa S, Beyer AJ, Browne BJ, Eisen HJ, Jeevanandam V. The role of gender in heart transplantation. Ann Thorac Surg. 1998 Jan;65(1):88-94. PMID: 9456101.,1111. Vereerstraeten P, Wissing M, De Pauw L, Abramowicz D, Kinnaert P. Male recipients of kidneys from female donors are at increased risk of graft loss from both rejection and technical failure. Clin Transplant. 1999 Apr;13(2):181-6. PMID: 10202615.. That sex hormones influence the development of chronic renal allograft rejection was provided by the observation that a male rat kidney benefits from the absence of testosterone, while estradiol ameliorates a female rat kidney function1212. Müller V, Szabó A, Viklicky O, Gaul I, Pörtl S, Philipp T, Heemann UW. Sex hormones and gender-related differences: their influence on chronic renal allograft rejection. Kidney Int. 1999 May;55(5):2011-20. PMID: 10231466.. Female sex hormones play an important role in the preservation of functionality of various organs and systems. As female rats present a better response to shock, trauma and sepsis when compared to male rats, several studies state that female hormones (estradiol, mainly) have a protective action modulating the generation of inflammatory mediators1313. Breithaupt-Faloppa AC, Fantozzi ET, de Assis Ramos MM, Vitoretti LB, Couto GK, Lino-dos-Santos-Franco A, Rossoni LV, Oliveira-Filho RM, Vargaftig BB, Tavares-de-Lima W. Protective effect of estradiol on acute lung inflammation induced by an intestinal ischemic insult is dependent on nitric oxide. Shock. 2013 Sep;40(3):203-9. PMID: 23846411.
14. Oberholzer A, Keel M, Zellweger R, Steckholzer U, Trentz O, Ertel W. Incidence of septic complications and multiple organ failure in severely injured patients is sex specific. J Trauma. 2000 May;48(5):932-7. PMID: 10823539.-1515. Xu J, Xiang Q, Lin G, Fu X, Zhou K, Jiang P, Zheng S, Wang T. Estrogen improved metabolic syndrome through down-regulation of VEGF and HIF-1alpha to inhibit hypoxia of periaortic and intra-abdominal fat in ovariectomized female rats. Mol Biol Rep. 2012 Aug;39(8):8177-85. PMID: 22570111.. It has been shown that estrogen is also a protective factor to infections, reason why women would be more resistant than men1414. Oberholzer A, Keel M, Zellweger R, Steckholzer U, Trentz O, Ertel W. Incidence of septic complications and multiple organ failure in severely injured patients is sex specific. J Trauma. 2000 May;48(5):932-7. PMID: 10823539.. Furthermore, it has been demonstrated the presence of estradiol receptors (subtypes α and β) on lymphocytes, neutrophils, macrophages, dendritic cells and NK lymphocytes, suggesting that estrogens may play a role in immunocompetence regulation1616. Cutolo M, Sulli A, Seriolo B, Accardo S, Masi AT. Estrogens, the immune response and autoimmunity. Clin Exp Rheumatol. 1995 Mar-Apr;13(2):217-26. PMID: 7656468..
Pituitary failure is associated with hormonal changes after BD22. Smith M. Physiologic changes during brain stem death--lessons for management of the organ donor. J Heart Lung Transplant. 2004 Sep;23(9 Suppl):S217-22. PMID: 15381167., but there is not a clear explanation for the sex hormones influence on organ transplantation. Considered the immunomodulatory role of female sex hormones, this study aimed to investigate the influence of gender on the course of the inflammatory process in the lungs, heart, liver and kidneys, in a rat model of BD.
Methods
The experimental protocols were approved by the Animal Subject Committee, Instituto do Coração (InCor), Universidade de São Paulo (USP), School of Medicine.
In this study, 15 female and five male Wistar rats (200-300 g) were divided into three groups: female rats (n=10); ovariectomized female rats (n=5); and male rats (n=5). Animals were maintained at 23°C ± 2°C, under a cycle of 12h light/12h darkness and were allowed access to food and water ad libitum before the experimental procedure. The rats were anesthetized in a chamber with 5% isoflurane, intubated and ventilated with a rodent ventilator (Harvard Apparatus, model 683, USA) with tidal volume of 10 mL/kg and frequency of 70 breaths/min. The anesthesia was maintained with continuous inhalation of 2% isoflurane. The carotid artery was cannulated for continuous blood pressure monitoring and blood sampling. The jugular vein was cannulated for infusion of saline solution (2 mL/h).
Brain death model
The brain death model was based on the model used by Simas et. al.11. Simas R, Kogiso DH, Correia Cde J, Silva LF, Silva IA, Cruz JW, Sannomiya P, Moreira LF. Influence of brain death and associated trauma on solid organ histological characteristics. Acta Cir Bras. 2012 Jul;27(7):465-70. PMID: 22760831.,33. Simas R, Sannomiya P, Cruz JW, Correia Cde J, Zanoni FL, Kase M, Menegat L, Silva IA, Moreira LF. Paradoxical effects of brain death and associated trauma on rat mesenteric microcirculation: an intravital microscopic study. Clinics (Sao Paulo). 2012 Jan; 67(1):69-75. PMID: 22249483. Through a drilled parietal burr hole, a catheter Fogarty(r)4F (Baxter Health Care Co., USA) was inserted in the intracranial space. BD was induced by rapid inflation of the baloon with 400-500μL of saline solution. After BD induction, the isoflurane inhalation was interrupted. BD was confirmed by maximal pupil dilatation, apnea, absence of reflex, and drop of mean arterial pressure (MAP). All animals were monitored for 6 hours after BD induction. At the end of the experiments, the animals were exsanguinated from the abdominal aorta and lungs, heart, liver and kidneys were removed. Blood samples were collected for cellular inflammatory proteins and hormonal quantification.
Ovariectomy
Ten days before BD induction, ovariectomized female rats were anesthetized with an intraperitoneal injection containing ketamine (100 mg/kg) and xylazine (20 mg/kg). A midline laparotomy was performed and the ovaries were identified, their pedicles were ligated and the ovaries removed. The abdominal wall was closed with a 4.0 nylon thread in two layers. After the procedure, Tramadol (5 mg/kg, intramuscular) and Penthabiotic (540 mg/kg, intramuscular) were administered. After the period of 10 days, the ovariectomy was confirmed by vaginal smears compatible with the diestrus phase.
Histopathological analyses
The collected lungs, heart, liver and kidneys were removed, fixed in formalin, and paraffin embedded. Samples were cut into 4 mm sections, and stained with hematoxylin and eosin (H&E). The parameters investigated were leukocyte infiltration (in the lungs, heart, liver and kidneys), edema (interstitial edema in the lungs and heart, intracellular edema in the liver and tubular edema in the kidneys), and hemorrhage (in the lungs and liver). Analyses were performed by two researchers and the score used to measure the intensity of tissue alterations was 1, 2, 3 or 4 (absent, slight, moderate and intense, respectively).
Determination of cellular inflammatory proteins
Cytokine-induced neutrophil chemoattractant 1 (CINC-1) and macrophage-inflammatory protein 2 (MIP-2) were quantified in serum aliquots collected 6 hours after BD induction using a Milliplex(r) MAP kit (Merk Millipore, Billerica, USA).
Determination of plasmatic hormonal levels
Following BD, blood samples were collected and centrifuged (700 g, 25 minutes, 25ºC) for determination of plasmatic estradiol and progesterone levels using radioimmunoassay kits (Coat-A-Count, Siemens, Germany). Corticosterone levels were determined using enzyme immunoassay kit, following manufacturer's recommended protocol (Cayman Chemical, Ann Arbor, USA).
Statistical analyses
Histopathological and cellular inflammatory proteins data are presented as median and upper/lower limits. These overall group differences were compared using a Kruskal-Wallis test followed by a post hoc Dunn multiple comparison test. Hemodynamic and hormonal data are presented as mean ± standard error of the mean. Comparisons were made by one-way analysis of variance followed by Tukey's multiple comparisons test or paired student's t test. Each p-value was adjusted to account for multiple comparisons. A p-value of less than 0.05 was considered significant. All statistical analyses were performed using GraphPad Prism(r) version 6.10 for Windows (GraphPad Software, La Jolla California, USA).
Results
Hemodynamic parameters
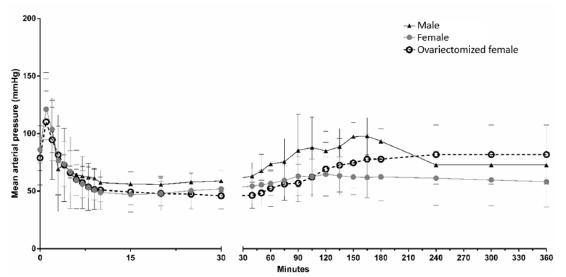
All rats showed a sudden increase in the MAP in the first minute after the induction of BD, which was followed by a decrease in the MAP below the baseline level, and a subsequent increase to a normal level after approximately one hour. As illustrated in Figure 1, there were no significant differences in the MAP over time among the groups. Furthermore, no differences were observed in arterial blood gases, lactate, electrolytes and hematocrit values at baseline and 6 h after the surgical procedures (data not shown).
- Mean arterial blood pressure of brain dead male (n=5), female (n=10) and ovariectomized female (n=5) rats. All animals were monitored over time before the balloon catheter inflation (0h) until the end of the experimental period (6h). Data are presented as mean ± standard error of the mean. There were no significant differences among groups.
Histopathological evaluation
Results, summarized in Table 1, showed that female rats presented an increased number of leukocytes in the lungs when compared to male rats. The lungs of ovariectomized female rats showed to be more hemorrhagic than the lungs of female or male rats. The interstitial edema did not differ among the groups. Representative photomicrographs are shown in Figure 2. In the heart, female rats presented increased leukocyte infiltration and tissue edema compared with male rats (Table 1, Figure 2). In the liver, all groups presented slight to moderate leukocyte infiltration, absent to moderate edema, and absent to slight hemorrhage, with no statistical differences among groups (Table 1, Figure 2). In the kidneys, the leukocyte infiltration had a wide distribution in the rats of the female group, mainly absent to a moderate level. In the male group there was a slight leukocyte infiltration, and in the ovariectomized female group infiltration varied from absent to slight, with no statistical differences among groups (Table 1, Figure 2). The tubular edema varied from absent to slight in male and ovariectomized female groups, and from absent to moderate in the female group, with no statistical significant differences among groups (Table 1, Figure 2).
- Photomicrographs of heart, lung, liver and kidney from brain dead female, male and ovariectomized female rats. 1: Female rats; 2: Male rats; 3: Ovariectomized female rats. A: Heart; B: Lung; C: Liver; D: Kidney. Arrows indicate intercellular edema in heart (Female and Ovariectomized female) and arrowheads show inflammatory cells in heart and lungs (Female). Heart and lungs from Female group presented bigger leukocyte infiltration than the same organs from Male group.
Cellular inflammatory proteins levels
Plasmatic levels of cellular inflammatory proteins obtained 6 hours after BD induction are showed in Figure 3. CINC-1 levels were significantly higher in female rats, when compared to male animals. Similar behavior was also observed regarding MIP-2 determinations, but without statistical significance.
- Plasmatic levels of cellular inflammatory proteins (CINC-1 and MIP-2), 6 hours after BD induction. Values are presented as median and upper/lower limits for 5-10 rats in each group.
Plasmatic hormonal levels
As illustrated in Figure 4, levels of plasmatic estradiol and progesterone in the female group were sharply reduced 6 hours after the induction of BD. Initial levels of both hormones were reduced in ovariectomized female rats compared to the initial levels in the female group. Similar levels were observed in male rats. Furthermore, values attained 6 hours after BD did not differ between female and ovariectomized female groups. In male rats, values of estradiol and progesterone were almost undetectable (Figure 4). In addition, there were no differences in plasma corticosterone levels among groups 6 hours after BD. Values were (mean ± standard error of the mean): 623.3 ± 48.5 pg/ml in female rats; 527.0 ± 64.2 pg/ml in ovariectomized female rats, and 759.4 ± 246.1 pg/ml in male rats (p=0.4734).
- Plasmatic estradiol and progesterone levels before (initial) and after brain death (6h) of female (n=10), ovariectomized female (Female Ovx, n=5), and male (n=5) rats. Data are presented as mean ± standard error of the mean. *p<0.001 compared to initial values; Өp<0.01 and Φp<0.05 compared to initial values in female group.
Discussion
Data presented strengthen the idea that genders may have an important influence in the histopathological changes that occur after BD. Despite transplantation of brain dead organs depends on many criteria55. Chaney J, Suzuki Y, Cantu E, van Berkel V. Lung donor selection criteria. J Thorac Dis. 2014 Aug;6(8):1032-8. PMID: 25132970. and donor management strategies are still evolving1717. Zuber K, Howard T, Davis J. Transplant in the 21st century. JAAPA. 2014 Nov;27(11):26-34. PMID: 25299652., the injury that occurs in the brain dead donor, mainly in the lungs and heart11. Simas R, Kogiso DH, Correia Cde J, Silva LF, Silva IA, Cruz JW, Sannomiya P, Moreira LF. Influence of brain death and associated trauma on solid organ histological characteristics. Acta Cir Bras. 2012 Jul;27(7):465-70. PMID: 22760831., is a serious factor that can determine a reduced graft function.
The results of the present study indicate that the balloon inflation resulted in a transient hypertensive peak followed by sustained hypotension. This pattern was observed in all the studied groups. In a rodent model of BD it was demonstrated that the hemodynamic instability due to hypovolemia or neurogenic hypotension is critical for the consequences of BD observed in the lungs1818. Rostron AJ, Avlonitis VS, Cork DM, Grenade DS, Kirby JA, Dark JH. Hemodynamic resuscitation with arginine vasopressin reduces lung injury after brain death in the transplant donor. Transplantation. 2008 Feb;85(4):597-606. PMID: 18347540.. In this regard, it is important to reiterate that there were no hemodynamic differences between male and female rats.
Analyzing the histopathological findings, it is clear that male and female rats presented many statistically significant differences. The alterations were observed mainly at the lungs and heart of the studied animals, while liver and kidneys showed no differences between groups. These alterations arise together with hormonal changes; in female rats, estradiol and progesterone serum levels were sharply reduced after BD to values observed in male rats.
Data presented herein indicate that lungs and heart are the organs most affected by BD. These conclusions are consistent with data from a previously published study11. Simas R, Kogiso DH, Correia Cde J, Silva LF, Silva IA, Cruz JW, Sannomiya P, Moreira LF. Influence of brain death and associated trauma on solid organ histological characteristics. Acta Cir Bras. 2012 Jul;27(7):465-70. PMID: 22760831.. Indeed, lungs are the organs most often considered unsuitable, with only 10-20% of lungs from multiple organ donors used for transplantation1919. Venkateswaran RV, Patchell VB, Wilson IC, Mascaro JG, Thompson RD, Quinn DW, Stockley RA, Coote JH, Bonser RS. Early donor management increases the retrieval rate of lungs for transplantation. Ann Thorac Surg. 2008 Jan;85(1):278-86. PMID: 18154823.. In addition, gender-related hormonal differences may play a role in the acute lung lesion due to intestinal ischemia/reperfusion injury1313. Breithaupt-Faloppa AC, Fantozzi ET, de Assis Ramos MM, Vitoretti LB, Couto GK, Lino-dos-Santos-Franco A, Rossoni LV, Oliveira-Filho RM, Vargaftig BB, Tavares-de-Lima W. Protective effect of estradiol on acute lung inflammation induced by an intestinal ischemic insult is dependent on nitric oxide. Shock. 2013 Sep;40(3):203-9. PMID: 23846411..
In animal models, a previous study has demonstrated that lungs from brain dead male rats have an increased number of polymorphonuclear cells and macrophages2020. Zweers N, Petersen AH, van der Hoeven JA, de Haan A, Ploeg RJ, de Leij LF, Prop J. Donor brain death aggravates chronic rejection after lung transplantation in rats. Transplantation. 2004 Nov;78(9):1251-8. PMID: 15548960.. In this study, we corroborate this evidence and notice that lungs from brain dead female rats presented an elevated number of leukocytes when compared to brain dead male rat lungs. This difference indicates a higher inflammatory level in female lungs. The higher hemorrhage incidence in the ovariectomized female group could be due to the reduction of estradiol levels that down-regulate vascular endothelial growth factor (VEGF) production1515. Xu J, Xiang Q, Lin G, Fu X, Zhou K, Jiang P, Zheng S, Wang T. Estrogen improved metabolic syndrome through down-regulation of VEGF and HIF-1alpha to inhibit hypoxia of periaortic and intra-abdominal fat in ovariectomized female rats. Mol Biol Rep. 2012 Aug;39(8):8177-85. PMID: 22570111..
In regard to the damages found in the heart, it is known that BD produces severe derangements in cardiac function2121. Apostolakis E, Parissis H, Dougenis D. Brain death and donor heart dysfunction: implications in cardiac transplantation. J Card Surg. 2010 Jan-Feb;25(1):98-106. PMID: 19549049. and there is a clear association between BD time and failure of the graft arising from brain dead donors2222. Marasco S, Kras A, Schulberg E, Vale M, Chan P, Lee GA, Bailey M. Donor brain death time and impact on outcomes in heart transplantation. Transplant Proc. 2013 Jan-Feb;45(1):33-7. PMID: 23375272.. Yeh et al.2323. Yeh T Jr, Wechsler AS, Graham L, Loesser KE, Sica DA, Wolfe L, Jakoi ER. Central sympathetic blockade ameliorates brain death-induced cardiotoxicity and associated changes in myocardial gene expression. J Thorac Cardiovasc Surg. 2002 Dec;124(6):1087-98. PMID: 12447173. using a similar model of BD, but with rabbits, found many histological manifestations in the heart, such as cytoplasmic clearing, contraction banding and loss of myofibrilar striations. Another study, performed by Wilhelm et al.2424. Wilhelm MJ, Pratschke J, Beato F, Taal M, Kusaka M, Hancock WW, Tilney NL. Activation of the heart by donor brain death accelerates acute rejection after transplantation. Circulation. 2000 Nov;102(19):2426-33. PMID: 11067799. and using a model of gradual onset of BD in rats, found that hearts from brain dead animals were rejected earlier by their recipients because of an accelerated inflammatory response and leukocyte infiltration. Indeed, it has been shown that overexpression of inflammatory markers, including cytokines and endothelial adhesion molecules, occurs in hearts from male brain dead rats2525. Segel LD, vonHaag DW, Zhang J, Follette DM. Selective overexpression of inflammatory molecules in hearts from brain-dead rats. J Heart Lung Transplant. 2002 Jul;21(7):804-11. PMID: 12100907.. As demonstrated herein, brain dead female rats exhibited greater leukocyte infiltration and intracellular edema when compared to brain dead male rats, which suggest that some degree of discrepancies may have occurred due to species particularities and methodological distinctions.
The differences between the groups in the liver and kidneys are less evident than the ones occurred in the other organs analyzed. It does not mean that liver and kidneys are not affected by BD. A clinical study have attested that liver suffer important impairments after BD2626. Dziodzio T, Biebl M, Pratschke J. Impact of brain death on ischemia/reperfusion injury in liver transplantation. Curr Opin Organ Transplant. 2014 Apr;19(2):108-14. PMID: 24565958., due to hemodynamic instability and an activated inflammatory status of the donor liver. Furthermore, it is well determined that kidneys are also damaged by BD, event that triggers an ischemic injury that reduces graft function of kidneys from brain dead donors2727. Kosieradzki M, Rowinski W. Ischemia/reperfusion injury in kidney transplantation: mechanisms and prevention. Transplant Proc. 2008 Dec;40(10):3279-88. PMID: 19100373.. However, in the present study, all groups of rats were injured in similar degrees, which demonstrate that the protective action conferred by female hormones were not so pronounced in the liver and kidneys as it was in the heart and lungs.
Plasmatic levels of CINC-1, much higher in female rats, show that animals from this group were more inflamed than male animals at the end of the procedures. Although without statistical significance, MIP-2 plasmatic levels showed the same behavior. These proteins, as markers of inflammation, influence the mechanism of inflammatory cells migration, which determine a higher inflammation degree in the female group. When analyzed together with hormonal and histological findings, this result strengthen the hypothesis that female sex hormones have an important influence on inflammation after BD.
BD leads to inflammatory, circulatory, and hormonal alterations11. Simas R, Kogiso DH, Correia Cde J, Silva LF, Silva IA, Cruz JW, Sannomiya P, Moreira LF. Influence of brain death and associated trauma on solid organ histological characteristics. Acta Cir Bras. 2012 Jul;27(7):465-70. PMID: 22760831.
2. Smith M. Physiologic changes during brain stem death--lessons for management of the organ donor. J Heart Lung Transplant. 2004 Sep;23(9 Suppl):S217-22. PMID: 15381167.
3. Simas R, Sannomiya P, Cruz JW, Correia Cde J, Zanoni FL, Kase M, Menegat L, Silva IA, Moreira LF. Paradoxical effects of brain death and associated trauma on rat mesenteric microcirculation: an intravital microscopic study. Clinics (Sao Paulo). 2012 Jan; 67(1):69-75. PMID: 22249483.-44. Salim A, Martin M, Brown C, Belzberg H, Rhee P, Demetriades D. Complications of brain death: frequency and impact on organ retrieval. Am Surg. 2006 May;72(5):377-81. PMID: 16719188.. Due to pituitary failure, there is a significant decrease of all hormones, including the female ones measured in this study. There are also important immunological alterations, such as cytokine release and upregulation of adhesion molecules33. Simas R, Sannomiya P, Cruz JW, Correia Cde J, Zanoni FL, Kase M, Menegat L, Silva IA, Moreira LF. Paradoxical effects of brain death and associated trauma on rat mesenteric microcirculation: an intravital microscopic study. Clinics (Sao Paulo). 2012 Jan; 67(1):69-75. PMID: 22249483.,2727. Kosieradzki M, Rowinski W. Ischemia/reperfusion injury in kidney transplantation: mechanisms and prevention. Transplant Proc. 2008 Dec;40(10):3279-88. PMID: 19100373., microcirculatory hypoperfusion, increased inflammation and organ dysfunction33. Simas R, Sannomiya P, Cruz JW, Correia Cde J, Zanoni FL, Kase M, Menegat L, Silva IA, Moreira LF. Paradoxical effects of brain death and associated trauma on rat mesenteric microcirculation: an intravital microscopic study. Clinics (Sao Paulo). 2012 Jan; 67(1):69-75. PMID: 22249483..
We observed that female rats presented a worse response than male rats to the harm caused by BD. The explanation of this fact starts from the observed drop in estradiol and progesterone levels after BD in female rats. The suggestion is that the abrupt change in female hormones suddenly withdraw the protection that these hormones used to confer to females. Once these females are not accustomed to the absence of female hormones (mainly estradiol), they suffer the effects of BD more than the other rats. Gender has already been proposed as a factor that may influence the outcome of transplantations77. Roberts DH, Wain JC, Chang Y, Ginns LC. Donor-recipient gender mismatch in lung transplantation: impact on obliterative bronchiolitis and survival. J Heart Lung Transplant. 2004 Nov;23(11):1252-9. PMID: 15539123.,88. Sato M, Gutierrez C, Kaneda H, Liu M, Waddell TK, Keshavjee S. The effect of gender combinations on outcome in human lung transplantation: the International Society of Heart and Lung Transplantation Registry experience. J Heart Lung Transplant. 2006 Jun;25(6):634-7. PMID: 16730568.. Averring the findings of this study, it is also suggested that female-to-male transplantation is worse than male-to-male one88. Sato M, Gutierrez C, Kaneda H, Liu M, Waddell TK, Keshavjee S. The effect of gender combinations on outcome in human lung transplantation: the International Society of Heart and Lung Transplantation Registry experience. J Heart Lung Transplant. 2006 Jun;25(6):634-7. PMID: 16730568.,99. Zeier M, Döhler B, Opelz G, Ritz E. The effect of donor gender on graft survival. J Am Soc Nephrol. 2002 Oct;13(10):2570-6. PMID: 12239247.,1111. Vereerstraeten P, Wissing M, De Pauw L, Abramowicz D, Kinnaert P. Male recipients of kidneys from female donors are at increased risk of graft loss from both rejection and technical failure. Clin Transplant. 1999 Apr;13(2):181-6. PMID: 10202615., which is probably due to the absence of protective effect of female hormones found at male subjects, but not at female ones1313. Breithaupt-Faloppa AC, Fantozzi ET, de Assis Ramos MM, Vitoretti LB, Couto GK, Lino-dos-Santos-Franco A, Rossoni LV, Oliveira-Filho RM, Vargaftig BB, Tavares-de-Lima W. Protective effect of estradiol on acute lung inflammation induced by an intestinal ischemic insult is dependent on nitric oxide. Shock. 2013 Sep;40(3):203-9. PMID: 23846411.,1616. Cutolo M, Sulli A, Seriolo B, Accardo S, Masi AT. Estrogens, the immune response and autoimmunity. Clin Exp Rheumatol. 1995 Mar-Apr;13(2):217-26. PMID: 7656468..
Ovariectomized female rats have not presented damage in the same level of female rats because, unlike the female ones, they have had some time between the decrease of female hormonal levels and the BD. Thus, ovariectomized female and male rats proved to be similar to each other; rats from both groups have not presented significant histopathological differences. This stems from the fact that, some days after the ovariectomy, female rats do not have high serum concentrations of female hormones and, therefore, resemble to male rats. The present study demonstrated that female subjects do not tolerate well the acute absence of estradiol. Its lack is known to be a deleterious factor to female organs1212. Müller V, Szabó A, Viklicky O, Gaul I, Pörtl S, Philipp T, Heemann UW. Sex hormones and gender-related differences: their influence on chronic renal allograft rejection. Kidney Int. 1999 May;55(5):2011-20. PMID: 10231466., and that is why female rats present damages more relevant than the other groups of animals since this hormone has an important role as a modulator of the immune response1313. Breithaupt-Faloppa AC, Fantozzi ET, de Assis Ramos MM, Vitoretti LB, Couto GK, Lino-dos-Santos-Franco A, Rossoni LV, Oliveira-Filho RM, Vargaftig BB, Tavares-de-Lima W. Protective effect of estradiol on acute lung inflammation induced by an intestinal ischemic insult is dependent on nitric oxide. Shock. 2013 Sep;40(3):203-9. PMID: 23846411.,1616. Cutolo M, Sulli A, Seriolo B, Accardo S, Masi AT. Estrogens, the immune response and autoimmunity. Clin Exp Rheumatol. 1995 Mar-Apr;13(2):217-26. PMID: 7656468..
Hormonal replacement is already included in some protocols of brain dead organs transplantation2828. Novitzky D, Cooper DK, Rosendale JD, Kauffman HM. Hormonal therapy of the brain-dead organ donor: experimental and clinical studies. Transplantation. 2006 Dec;82(11):1396-401. PMID: 17164704.. Thyroid hormone (T3) replacement is proven to be beneficial both to donor and recipient of brain dead organs2929. Cooper DK, Novitzky D, Wicomb WN, Basker M, Rosendale JD, Myron Kauffman H. A review of studies relating to thyroid hormone therapy in brain-dead organ donors. Front Biosci (Landmark Ed). 2009 Jan;14:3750-70. PMID: 19273308.. Corticosteroid replacement has some evidence of being beneficial, but it is not universally accepted3030. Ranasinghe AM, Bonser RS. Endocrine changes in brain death and transplantation. Best Pract Res Clin Endocrinol Metab. 2011 Oct;25(5):799-812. PMID: 21925079.. Female hormones (estradiol and progesterone) may modulate inflammatory events triggered by BD, which establishes perspectives for a possible therapeutic use of these hormones on female brain dead donors. Nevertheless, further studies are needed to better understand the dynamic of this protective effect of estradiol and to assess current outcome indicators.
Further studies are also necessary to evaluate the effects of estradiol specifically as a treatment. Thus, the magnitude of the reduction of the inflammation degree that a treatment with estradiol could provide after BD can be stated more emphatically. The effects that a big amount of female hormones could have in male rats after BD is another point that need further investigation.
Conclusions
Sex hormones influence the development of inflammatory events triggered by brain death and the status of the organs that may be used for transplantation. The increased inflammation in lungs and heart of female rats might be a result of the acute reduction in female hormones triggered by BD. Therefore, the idea of introducing a therapeutic use of female sex hormones on female brain dead donors could be considered.
References
-
1Simas R, Kogiso DH, Correia Cde J, Silva LF, Silva IA, Cruz JW, Sannomiya P, Moreira LF. Influence of brain death and associated trauma on solid organ histological characteristics. Acta Cir Bras. 2012 Jul;27(7):465-70. PMID: 22760831.
-
2Smith M. Physiologic changes during brain stem death--lessons for management of the organ donor. J Heart Lung Transplant. 2004 Sep;23(9 Suppl):S217-22. PMID: 15381167.
-
3Simas R, Sannomiya P, Cruz JW, Correia Cde J, Zanoni FL, Kase M, Menegat L, Silva IA, Moreira LF. Paradoxical effects of brain death and associated trauma on rat mesenteric microcirculation: an intravital microscopic study. Clinics (Sao Paulo). 2012 Jan; 67(1):69-75. PMID: 22249483.
-
4Salim A, Martin M, Brown C, Belzberg H, Rhee P, Demetriades D. Complications of brain death: frequency and impact on organ retrieval. Am Surg. 2006 May;72(5):377-81. PMID: 16719188.
-
5Chaney J, Suzuki Y, Cantu E, van Berkel V. Lung donor selection criteria. J Thorac Dis. 2014 Aug;6(8):1032-8. PMID: 25132970.
-
6Fessart D, Dromer C, Thumerel M, Jougon J, Delom F. Influence of gender donor-recipient combinations on survival after human lung transplantation. Transplant Proc. 2011 Dec;43(10):3899-902. PMID: 22172868.
-
7Roberts DH, Wain JC, Chang Y, Ginns LC. Donor-recipient gender mismatch in lung transplantation: impact on obliterative bronchiolitis and survival. J Heart Lung Transplant. 2004 Nov;23(11):1252-9. PMID: 15539123.
-
8Sato M, Gutierrez C, Kaneda H, Liu M, Waddell TK, Keshavjee S. The effect of gender combinations on outcome in human lung transplantation: the International Society of Heart and Lung Transplantation Registry experience. J Heart Lung Transplant. 2006 Jun;25(6):634-7. PMID: 16730568.
-
9Zeier M, Döhler B, Opelz G, Ritz E. The effect of donor gender on graft survival. J Am Soc Nephrol. 2002 Oct;13(10):2570-6. PMID: 12239247.
-
10Prendergast TW, Furukawa S, Beyer AJ, Browne BJ, Eisen HJ, Jeevanandam V. The role of gender in heart transplantation. Ann Thorac Surg. 1998 Jan;65(1):88-94. PMID: 9456101.
-
11Vereerstraeten P, Wissing M, De Pauw L, Abramowicz D, Kinnaert P. Male recipients of kidneys from female donors are at increased risk of graft loss from both rejection and technical failure. Clin Transplant. 1999 Apr;13(2):181-6. PMID: 10202615.
-
12Müller V, Szabó A, Viklicky O, Gaul I, Pörtl S, Philipp T, Heemann UW. Sex hormones and gender-related differences: their influence on chronic renal allograft rejection. Kidney Int. 1999 May;55(5):2011-20. PMID: 10231466.
-
13Breithaupt-Faloppa AC, Fantozzi ET, de Assis Ramos MM, Vitoretti LB, Couto GK, Lino-dos-Santos-Franco A, Rossoni LV, Oliveira-Filho RM, Vargaftig BB, Tavares-de-Lima W. Protective effect of estradiol on acute lung inflammation induced by an intestinal ischemic insult is dependent on nitric oxide. Shock. 2013 Sep;40(3):203-9. PMID: 23846411.
-
14Oberholzer A, Keel M, Zellweger R, Steckholzer U, Trentz O, Ertel W. Incidence of septic complications and multiple organ failure in severely injured patients is sex specific. J Trauma. 2000 May;48(5):932-7. PMID: 10823539.
-
15Xu J, Xiang Q, Lin G, Fu X, Zhou K, Jiang P, Zheng S, Wang T. Estrogen improved metabolic syndrome through down-regulation of VEGF and HIF-1alpha to inhibit hypoxia of periaortic and intra-abdominal fat in ovariectomized female rats. Mol Biol Rep. 2012 Aug;39(8):8177-85. PMID: 22570111.
-
16Cutolo M, Sulli A, Seriolo B, Accardo S, Masi AT. Estrogens, the immune response and autoimmunity. Clin Exp Rheumatol. 1995 Mar-Apr;13(2):217-26. PMID: 7656468.
-
17Zuber K, Howard T, Davis J. Transplant in the 21st century. JAAPA. 2014 Nov;27(11):26-34. PMID: 25299652.
-
18Rostron AJ, Avlonitis VS, Cork DM, Grenade DS, Kirby JA, Dark JH. Hemodynamic resuscitation with arginine vasopressin reduces lung injury after brain death in the transplant donor. Transplantation. 2008 Feb;85(4):597-606. PMID: 18347540.
-
19Venkateswaran RV, Patchell VB, Wilson IC, Mascaro JG, Thompson RD, Quinn DW, Stockley RA, Coote JH, Bonser RS. Early donor management increases the retrieval rate of lungs for transplantation. Ann Thorac Surg. 2008 Jan;85(1):278-86. PMID: 18154823.
-
20Zweers N, Petersen AH, van der Hoeven JA, de Haan A, Ploeg RJ, de Leij LF, Prop J. Donor brain death aggravates chronic rejection after lung transplantation in rats. Transplantation. 2004 Nov;78(9):1251-8. PMID: 15548960.
-
21Apostolakis E, Parissis H, Dougenis D. Brain death and donor heart dysfunction: implications in cardiac transplantation. J Card Surg. 2010 Jan-Feb;25(1):98-106. PMID: 19549049.
-
22Marasco S, Kras A, Schulberg E, Vale M, Chan P, Lee GA, Bailey M. Donor brain death time and impact on outcomes in heart transplantation. Transplant Proc. 2013 Jan-Feb;45(1):33-7. PMID: 23375272.
-
23Yeh T Jr, Wechsler AS, Graham L, Loesser KE, Sica DA, Wolfe L, Jakoi ER. Central sympathetic blockade ameliorates brain death-induced cardiotoxicity and associated changes in myocardial gene expression. J Thorac Cardiovasc Surg. 2002 Dec;124(6):1087-98. PMID: 12447173.
-
24Wilhelm MJ, Pratschke J, Beato F, Taal M, Kusaka M, Hancock WW, Tilney NL. Activation of the heart by donor brain death accelerates acute rejection after transplantation. Circulation. 2000 Nov;102(19):2426-33. PMID: 11067799.
-
25Segel LD, vonHaag DW, Zhang J, Follette DM. Selective overexpression of inflammatory molecules in hearts from brain-dead rats. J Heart Lung Transplant. 2002 Jul;21(7):804-11. PMID: 12100907.
-
26Dziodzio T, Biebl M, Pratschke J. Impact of brain death on ischemia/reperfusion injury in liver transplantation. Curr Opin Organ Transplant. 2014 Apr;19(2):108-14. PMID: 24565958.
-
27Kosieradzki M, Rowinski W. Ischemia/reperfusion injury in kidney transplantation: mechanisms and prevention. Transplant Proc. 2008 Dec;40(10):3279-88. PMID: 19100373.
-
28Novitzky D, Cooper DK, Rosendale JD, Kauffman HM. Hormonal therapy of the brain-dead organ donor: experimental and clinical studies. Transplantation. 2006 Dec;82(11):1396-401. PMID: 17164704.
-
29Cooper DK, Novitzky D, Wicomb WN, Basker M, Rosendale JD, Myron Kauffman H. A review of studies relating to thyroid hormone therapy in brain-dead organ donors. Front Biosci (Landmark Ed). 2009 Jan;14:3750-70. PMID: 19273308.
-
30Ranasinghe AM, Bonser RS. Endocrine changes in brain death and transplantation. Best Pract Res Clin Endocrinol Metab. 2011 Oct;25(5):799-812. PMID: 21925079.
-
Financial source: FAPESP (Grant nº 2013/20282-0)
-
1
Research performed at Laboratory of Cardiovascular Surgery and Circulation Pathophysiology (LIM-11), Instituto do Coração (InCor), School of Medicine, Universidade de São Paulo (USP), Brazil.
Publication Dates
-
Publication in this collection
Apr 2016
History
-
Received
02 Dec 2015 -
Reviewed
10 Feb 2016 -
Accepted
08 Mar 2016