Abstract
Purpose:
To investigate the hepatoprotective and antioxidant effeicacies of Silybum marianum’s (silymarin, S) on University of Wisconsin (UW) and histidinetryptophan-ketoglutarate (HTK) preservation solutions.
Methods:
Thirty two Wistar albino adult male rats were used. Group 1: UW group, Group 2: UW + Silymarin group(S), Group 3: HTK group, Group 4: HTK + silymarin group (S), respectively. Silymarin was enforced intraperitoneally before the surgery. Biopsies were enforced in 0, 6 and 12.hours to investigate.
Results:
Biochemical parameters examined in alanine aminotransferase (ALT), furthermore superoxide dismutase (SOD), catalase (CAT), and malondialdehyde (MDA) in rats were also evaluated. Detected histopathological changings were substantially declining in the groups that received silymarin, cellular damage was decreased significantly in HTK + Silymarin group, according to other groups. It has been identified as the most effective group was HTK + silymarin group in evaluation of ALT, electron microscopic results, also decreased MDA and elevated in SOD, and CAT activity. Caspase 3 analysis showed a substantial lower apoptosis ratio in the silymarin groups than in the non-performed groups (p<0.05).
Conclusion:
Histidinetryptophan-ketoglutarate+silymarin group provides better hepatoprotection than other groups, by decreasing the hepatic pathologic damage, delayed changes that arise under cold ischemic terms.
Key words:
Liver; Histidine; Tryptophan; Antioxidants; Milk Thistle; Rats.
Introduction
Protecting the hepatic functions after the cross clamping and harvesting the liver is so significant and vital for donor tissues, otherwise it may cause rejection in the tissue. Organ preservation is needed for inhibiting the possible injuries of free radicals, provide hypotermia, and minimizing the functions and to provide vitality. Its injury absolutely connected with primary nonfunction (PNF) in liver transplantation. Although various molecules have been tried in recent decades. The University of Wisconsin (UW) and histidine-tryptophan-ketoglutarate (HTK) are common used solutions in clinical practice. They also include some substance for preventing cellular disorganization, stabilize cell membrane structure, provide intracellular electrolyte balance, and cytoprorective shield.
The UW solution contains adenosine (for ATP requirement), Hydroxy-ethyl-starch (Interstitial edema inhibitor), glutathione, phosphate for pH stabilization and allopurinol (duty for antioxidant and preserving cell structure) agents whereas the HTK solution uses mannitol, histidine (antioxidant and osmotic, effect), tryptophan (membrano, protective effect), ketoglutarate (membrano protective effect, duty in crebs cyclus), and mannitol.
A novel approach in the transplant society is to increase cytoprotective effects of solutions. Silybum Marinaum(Silymarin) is a plant which contains flavonolignan molecule, also protect liver parenchyma from the free radicals exerts membrane stabilizing and provide the detoxification of liver. Different from other studied molecules silybum marinaum has glutathion which supply cytoprotective effect reduces the inflammatory reaction and inhibit the fibrogenesis of liver. The aim of this research was to evaluate the applying of Silybum Marinaum (silymarin, S) in the aspect of improving the antioxidant and cellular preserving efficacies of HTK and UW solutions11 Mangus RS, Fridell JA, Vianna RM, Milgrom MA, Chestovich P, Chihara RK, Comparison of histidine-tryptophan- ketoglutarate solution and University of Wisconsin solution in extended criter a liver donors. Liver Transpl. 2008;14:365-73. PMID: 18306380.
2 Feng L, Zhao N, Yao X, Sun X, Du L, Diao X. Histidine-tryptophan-ketoglutarate solution vs. University of Wisconsin solution for liver transplantation: a systematic review. Liver Transpl. 2007;13:1125-36. PMID: 17665493.
3 Wilson CH, Brook NR, Talbot D. Preservation solutions for solid organ transplantation. Mini Rev Med Chem. 2006;6:1081-90. PMID: 17073708.-44 D'Alessandro AM, Southard JH, Love RB, Belzer FO. Organ preservation. Surg Clin North Am. 1994;74:1083-95. PMID: 7940062..
Methods
Surgical procedures
The tertiary center local ethics committe for experimental animal research confirmated this study. Research group obeyed the rules of rat procedures for experimental guidelines founded by the National Institutes of Health by the “Guide for the Care and Use of Laboratory Animals” (2013/3-28D).
Thirty two wistar albino rats (average weight: 250-300g) were utilized for this experimental study. Of the 32 rats were separated randomisedly into four groups by equally. These groups are named as follows:
-
Group-I: UW group
-
Group-II: UW+Silymarin group ( UW+S)
-
Group- III: HTK group(HTK)
-
Group-IV: HTK + Silymarin group (HTK+S)
Rats were anesthesiad by applying 30 mg/kg hydrochloridic ketamine (Ketalar, Eczacibasi, Turkey) and 10 mg/kg hydrochloridic xylazine (Rompon, Bayer, Turkey) before the conventional surgery. Wistar rats in groups II and IV were performed silymarin 100 mg/kg (S0292-10G, Sigma aldrich laboratories, Netherlands) via intraperitoneal performing half an hour ago before the surgery. After the verifying, rats were equally and balanced anesthetized, The surgical procedure was perfomed in a closed environment under controlled temperature, conventional laparotomy was applied by the incision midline.
The portal pedicle ligation performed after the cannula settled in portal vein lumen. Group-I rats were perfusated by solution of UW(Viaspan; DuPont Merck Pharmaceutical Company, Wilmington, DE, United States) which reserved at 4 oC Via the catheter inside the portal vein. Group-II rats were medicated silymarin prior to laparotomy later this application, perfusion started with UW, alike in first group (UW+S). Group-III were perfusated by HTK (Custodiol; Odyssey Pharmaceutical Inc, United States) preserved at 4oC. Group-IV rats were firstly perfused silymarin prior to laparotomy, after this perfusion started by HTK (HTK+S)
Pending the applying of perfusion, the suprahepatic part of vena cava was dissected and isolated by cutting whereas discharging of liquid was clear in the liver. The perfusion finished when the liquid becomes clear. Secondly of the perfusion, a regular hepatectomy was applied. The parts of split hepatic tissue were put into bags including UW or HTK solutions and covered in ice slush-contained cases. In follow-up periods at 0, 6, and 12 hour after the hepatectomy (AH), tissue biopsy examples were taken from the dormant hepatic tissues for histopathological and immunohistochemical assessment, and liquid examples were taken for biochemical determination. Hepatic tissue biopsy was also applied 6 hour later the hepatectomy for examination by electron microscopy.
Biochemical analysis
Examples acquired from the stored liquid were frozen at -80°C in nitrogen liquid for consecutive assessments of alanine aminotransferase (ALT) levels. ALT was evaluated with a Ultraviolet (UV) absorbance procedure which is set like a standardized method. Both of the enzyme levels were stated in unit/liter (U/L).
Pathologic evaluation
Liver histopathological examination
After finishing the perfusation of hepatic tissues, weight measurement were enforced and covered at -80oC until use or fixed in 10% buffered formalin and buried in paraffin. Assessment of histopathologic damage was enforced in hematoxylin-eosin staining sections utilized to evaluate four diverse parameters by light microscope. The samples were assessed semi-quantitatively by ranking tissue lesion severity. Range from 0 to 3 depending on the degree and rank of the changings as follows:
Ballooning was evaluated as 0 : ¼ absent, 1 :¼ mild, 2: ¼ moderate, and 3: ¼ severe. Fat deposition was evaluated as a percentage: 0%-30%, 31%-60%, 61%-100%. Pericentral hydropic degeneration and sinusoidal dilation were evaluated as absent, focal, or diffuse FC; absent or present: 1%-30%, 31%-60%, 61%-100%), hepatocyte ballooning degeneration (BD; absent or present: mild, moderate and severe), centrilobular hydropic change (CLHC; absent or present: focal or diffuse), and sinusoidal dilatation (SD; absent or present: focal or diffuse)55 Kanat O, Kurt E, Yalcinkaya U, Evrensel T, Manavoglu O. Comparison of uroprotective efficacy of mesna and amifostine in Cyclophosphamide- induced hemorrhagic cystitis in rats. Indian J Cancer. 2006;43:12-5. PMID: 16763356.,66 Lim AY, Segarra I, Chakravarthi S, Akram S, Judson JP. Histopathology and biochemistry analysis of the interaction between sunitinib and paracetamol in mice. BMC Pharmacol. 2010;10:14. PMID: 20950441..
Determination of tissue levels of oxidative stress markers
All biochemical evaluations were applied on the tissue obtained after centrifugation at 14000 rpm using spectrophotometric methods. In order to assess the lipid peroxidation in free radicals (malondialdehyde, MDA), and efficiency of any enzymatic antioxidants (superoxide dismutase, SOD and catalase, CAT)77 Zhou D, Ruan J, Cai Y, Xiong Z, Fu W, Wei A. Antioxidant and hepatoprotective activity of ethanol extract of Arachniodes exilis (Hance) Ching. J Ethnopharmacol. 2010;129:232-7. PMID: 20347029..
All of the hepatic tissues were centrifuged in ice-cold 140 mM KCI at 16,000 rpm for 2 min using a centrifugator (IKA Ultra-Turrax T25 basic homogenizer, Germany). All of the procedures were performed at 4°C. The results were expressed appropriately to a regular graphic which was equipped from a regular solution by commercial kits( Diagnostics, USA).
Determination of SOD activity
Every piece of tisssue centrifuged homogenate was diluted 1:40 with 10 mM phosphate buffer (pH 7.0). Twenty-five microliters of diluted example was mixed with 850 μL of substrate suspension including 0.05 mmol/L xanthine sodium and 0.025 mmol/L INT (2-[4-iodophenyl]-3-[4-nitrophenol]-5-phenyltetrazolium chloride) in a buffer solution including 50 mmol/L CAPS (N-cyclohexyl-3- aminopropane sulfonic acid) and 0.94 mmol/L EDTA disodium dihydrate (pH 10.2). Then, 125 μL of xanthine oxidase (80 U/L) was attached to the mixture and absorbance elevation was pursued at 505 nm for 3 min against air. Standard solutions were prepared from stock solution of SOD from bovine erythrocytes in the solution range of 0.217 and 5.2 kU/L and phosphate buffer was utilized as the blank solution.
Determination of CAT activity
For determination of the CAT activity, the obtained blend was formed of 50 mM phosphate buffer (pH 7.0), 10 mM H2O2, and tissue homogenate sample. The declining rate of H2O2 was pursued at 240 nm for 45s against air at cell temperature (Park and others 2010). CAT activity was expressed as kU/g protein. Phosphate buffer was used as the blank solution and the calibration curve was plotted with standard solutions (1.25 to 15 U/mL) prepared from CAT stock lyophilized powder77 Zhou D, Ruan J, Cai Y, Xiong Z, Fu W, Wei A. Antioxidant and hepatoprotective activity of ethanol extract of Arachniodes exilis (Hance) Ching. J Ethnopharmacol. 2010;129:232-7. PMID: 20347029..
Measurement of MDA level
Level of MDA was determined as a thiobarbituric acid reactive substance (TBARS) in the liver and brain homogenates according to the method described by Jamall and Smith (1985). Tetramethoxypropane solution was used as the standard for plotting the calibration slope and the results in the sample were expressed as nmol/g protein. Briefly, 200 μL of sample or standard solution, 200 μL of sodium dodecyl sulfate (8.1%), 1500 μL of acetic acid solution (20%), and 1500 μL of thiobarbituric acid solution (0.8%) were mixed. The solution was completed to 4000 μL with distilled water. The tubes were kept at 95°C for 1h. Later, they were refrigerated under tap water and 2000 μL of the mixture was added to 2000 μL of trichloroacetic acid. They were centrifuged at 1017 × g for 10 min. The rate of supernatant absorbance was measured at 532 nm.
Histologic and ultrastructural evaluation
Evaluations were done between groups. All examples were stabled at 2.5% glutaraldehyde solution with phosphate buffer for 3 hours. Later the destabilization process with 1% osmium tetraoxide, they were dried in scaled alcohol baths. Examples passed by propylene oxide were immersed in araldite CY 212, 2-dodecenyl succinic anhydride, benzyldimethylamine and dibutylphthalate.
Thin slices spotted with toluidine blue were evaluated below microscopy. Subsequently, thin slices cut and covered with uranyl acetate and lead citrate were assesses by Zeiss Libra 120 transmission electron microscopy. Also Changes in nucleus, endothelial cells, cell membrane, mitochondrium, and endoplasmic reticulum composition were evaluated. The outcomes were assessed by an specialised histologist.
Assessment of apoptosis in hepatocytes
Three micron transvers-parts from paraffin-coated samples were detected by caspase - 3 method evaluated samples were examined in at least 10 Various sites of view under × 400 magnification, with at least 1000 hepatocytes evaluated. The number of cells with caspase 3 -positive were saved88 Ma ZY, Qian JM, Rui XH, Wang FR, Wang QW, Cui YY. Inhibition of matrix metalloproteinase-9 attenuates acute small-for-size liver graft injury in rats. Am J Transplant. 2010;10:784-95. PMID: 20121733..
Statistical analysis
The outcomes of statistical analysis was enforced by the SPSS 17.0 (IBM, Chicago, IL, United States) software program. Chi-square and Fisher exact tests were used to analyze histopathologic disperancies between the groups. A result of P < 0.05 was accepted to be statistically significant. Intergroup bencmarks of biochemical outcomes were applied utilizing mann whitney u test. Suitable, results submitted in tables are displayed as the mean ± SD.
Results
Evaluation of liver samples histopathology
Hepatic tissue biopsies fixed with hematoxylin and eosin were applied to detect the injury parameters as FC, BD, CLHC, or SD. A substantial difference was detected among groups UW and UW+S in evaluation of SD and CLHC (both of parameters at 6, 12 hours, P < 0.05). Also, this difference detected between HTK and HTK+S groups in SD and CLHC outcomes (p< 0.05 at both time points and parameters) (Figure 1, Table 1).
Comparison of apoptosis rates observed in all groups accordingtoperiod; HTK histidine tryptophan ketoglutarate;UW,University of Wisconsin; Silymarine, Significant declined apoptosis rates obtained at HTK+S group (p<0.05).
Furthermore, the examination of Silymarin applied groups, HTK+S group had the best outcomes and also significant difference detected than UW+S group. More pathological changings occur for SD, CLHC in UW+S group. FC and BD histology was not altered between the groups.
Evaluation of apoptosis
According to Caspase -3 results used to assess apoptosis, in the postoperatively baseline to 6 hours no significant differences detected in apoptosis for any groups. On the other hand significant decline was detected in groups which applied Silymarin than non applied groups. UW group had a significantly higher rate for apoptosis (p<0.05), also secondly higher rate was detected in HTK and respectively lower rates in UW+S and HTK+S Groups. There was no significant difference detected in HTK+S group than UW+S group for apoptosis (p>0.05) (Table 2, Figure 2).
A. Immunohistochemical staining of caspase 3 of HTK 12th hour group (arrows indicate apoptotic cells) x400 magnification. B. UW 12th hour group of caspase 3 immunohistochemical staining x400 magnification. C. UW+S 12th hour group of caspase 3 immunohistochemical staining x400 magnification. D. HTK+S 12th hour group of caspase 3 immunohistochemical staining x400 magnification.
Evaluation of SOD, CAT and MDA levels
SOD, CAT and MDA levels from perfusated liquids were evaluated at 0, 6, and 12 hours after the procedure. The correlations of groups showed that HTK+S group had the highest in SOD and CAT, lowest in MDA scores when has compared to other groups (Figures 3 to 5).
MDA level of liver tissues of rats perfusated by Solutions and/or silymarine, MDA level is expressed as nmol/g protein. Significant differences and decreasing detected between 0 - 6, 0-12 and 6-12 hours in all groups, especially in HTK+S group (p<0.05).
MDA level of liver tissues of rats perfusated by Solutions and/or silymarine, MDA level is expressed as nmol/g protein. Significant differences and decreasing detected between 0 - 6, 0-12 and 6-12 hours in all groups, especially in HTK+S group (p<0.05).
SOD enzyme activity in liver tissues of rats perfusated by solutions, SOD activity is expressed as U/mg protein. Significant differences and increasing detected between 0 - 6, 0-12 and 6-12 hours in all groups, especially in HTK +S group (p<0.05).
Outcomes of ALT levels
Comparisons in ALT hepatic function tests were enforced in groups at specific hours as shown in Table 1. The elevated ALT levels were significantly higher in HTK and UW group versus to other groups (P < 0.05). However ALT levels were significantly lower in groups of HTK+S and UW+S (P < 0.05) ( UW vs UW + S: P< 0.05; HTK vs HTK + S: P < 0.05). The record low ALT levels were detected in the HTK + S group whereas the most elevated levels were observed in the HTK group (Table 3).
Evaluation of outcomes with transmission electron microscopy
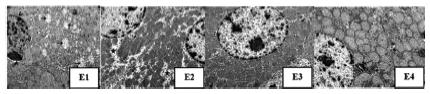
Assessment of the ultrastructure of all groups obtained that the HTK+S group were best preserved, like as standard non pathologic liver example. The UW+S, HTK, and UW groups had a lower preservation result, with some ultrastructural injuries. Furthermore HTK and UW groups were lower preserved. Obtained lipid droplets in liver cells of some groups, make us think about any external nutritient types may effects this sample (Figure 6).
The electron microscopic figures in the liver in groups. E1: Dilation in the perinuclear space (arrows), dilatation and loss of crystals in mitochondria (arrows). E2: Minimal levels of intracellular edema in hepatocytes (stars). E3: Hepatocyte in normal ultrastructural structure. E4: Lipid vacuoles in the hepatocyte cytoplasm (arrows).
Discussion
Transplantation is the life saving and also one of the rare procedure that give a curative result especially in hepatic end stage diseases. Inspite of the novel researchs in surgical procedures, increase in intensive care quality and immunosuppressive drugs, rejection of organs nevertheless arise in early or late time interval of transplantation. In this case situations transplant performed patients have losing time, and lose their chance for a lively life temporarily99 Müller SA, Mehrabi A, Schmied BM, Welsch T, Fonouni H, Engelmann G, Schemmer P, Weitz J, Schmidt J. Partial liver transplantation-living donor liver transplantation and split liver transplantation. Nephrol Dial Transplant. 2007;22 Suppl 8:viii13-viii22. PMID: 17890257..
The term “Primary non-function” (PNF) was utilized to explain this event diagnosed with postoperative blood hepatic function enzyme increasing, decline in gall secretion, also serious coagulopathy. Furthermore some causes like as equipmentless team, extended warm ischemia period, ischemia-reperfusion damage, and poor preservation solutions related with PNF after the transplant process. After this, some negative cyclic occurs such as thrombocyte adhesion, which lead to endothelial damage, reducing in cold presevation period and drop the materials cause to trombosis1010 Cywes R, Mullen JB, Stratis MA, Greig PD, Levy GA, Harvey PR, Strasberg SM. Prediction of the outcome of transplantation in man by platelet adherence in donor liver allografts. Evidence of the importance of prepreservation injury. Transplantation. 1993;56:316-23. PMID: 7689257..
Protecting the tissue and organ, starts when the donor patient is diagnosed as death of brain and also has living healthy, useful organs, and period ends after the organ is starts to work totally functional. At this moment, preservation solutions come to the scene, aim to provide the organ after cross clamping and harvesting for functioning by keeping hypothermic and diminishing the free radical induced damage1111 Bazzoni G, Dejana E, Del Maschio A. Platelet-neutrophil interactions: possible relevance of the pathogenesis of thrombosis and inflamation. Haematologia. 1991;76:e491-9. PMID: 1820986.,1212 Koo A, Komatsu H, Tao G, Inoue M, Guth PH, Kaplowitz N. Controbution of noreflow phenomenon to hepatic injury after ischemia-reperfusion. Evidence for a role for superoxide anion. Hematology. 1992;15:507-14. PMID: 1312056..
The utilizing of preservation liquors has help for conveyance of organs in a easy and feasible way. Nowadays, UW solution is the common used for conveyed abdominal organs and especially, liver. Furthermore, HTK solution, firstly used for heart donors with its low potassium ingredient, has demonstrated effective in organ transplantations. However UW solution includes, electrolytes especially potassium in elevated concentration, also UW solution is increase the vessel efficacies1313 Wilson CH, Brook NR, Talbot D. Preservation solutions for solid organ transplantation. Mini Rev Med Chem. 2006;6:1081-90. PMID: 17073708.,1414 Maathuis MH, Leuvenink HG, Ploeg RJ. Perspectives in organ preservation. Transplantation. 2007;83:1289-98. PMID: 17519776..
Many solutions used for transplantation processess however the best are UW and HTK solutions with minimal side effects and ensure osmotic balance by erasing the free radical effects1515 Jain A, Mohanka R, Orloff M, Abt P, Kashyap R, Cullen J. University of Wisconsin versus histidine-tryptophan-ketoglutarate for tissue preservation in live-donor liver transplantation. Exp Clin Transplant. 2006;4:451-7. PMID: 16827642.. UW solution was found and improved by Belzer et al.1616 Mangus RS, Fridell JA, Vianna RM, Milgrom MA, Chestovich P, Chihara RK. Comparison of histidine-tryptophan- ketoglutarate solution and University of Wisconsin solution in extended criteria liver donors. Liver Transpl. 2008 Mar;14(3):365-73. PMID: 18306380., this solution includes glutathion for protected than free radical effects, hydroxyethyl starch for decreasing interstitial edema and phospate for ph equalization. However it has some bad sides such as increased viscosity, more gall complication proportion, elevated artery capacitance, lead to aggregation.
On the other hand HTK is an another solution for transplantation, firstly founded for cardioplegia later noticed that may use in transplants. It ıncludes mannitol histidin combination for antioxidant effects, ketoglutarat and tryptophan for cell protection also have some plus force like decreased viscosity, quick cooling time, lower capillary permeability, elevated tissue oxygenation. Moray et al.1717 Moray G, Sevmis S, Karakayali FY, Gorur SK, Haberal M. Comparison of histidine-tryptophan-ketoglutarate and University of Wisconsin in living-donor liver transplantation. Transplant Proc. 2006;38:3572-5. PMID: 17175334. found that, statistically un significant differences detected among the UW and HTK groups related after the transplantation hepatic biochemistry, complication ratios, acute rejection possibility, and mortality outcomes1818 Tyagi A, Agarwal C, Harrison G, Glode LM, Agarwal R. Silibinin causes cell cycle arrest and apoptosis in human bladder transitional cell carcinoma cells by regulating CDKI-CDK-cyclin cascade, and caspase 3 and PARP cleavages. Carcinogenesis. 2004;25:1711-20. PMID: 15117815..
In present study, HTK+S group have better results than UW+S and non applied drugs groups. Histopathologic outcomes were obtained preferable in HTK+S than others. Silymarin (the extract of Silybum marianum) is an antioxidant, anti-inflammatory, antifibrotic and hepatoprotective agent which has power of diminishing free oxygen radicals. Due to silymarin’s detoxifying effect, it is used as an anti-hepatotoxic molecule in some poisonings such as acetaminophen, arsenic, carbon tetrachloride, and phenothiazines. In hypercholesterolemia, silymarin diminishes the HMG-CoA reductase (3-hydroxy-3-methylglutaryl coenzyme A) , and degrades formation of cholesterol1919 Jain A, Yadav A, Bozhkov AI, Padalko VI, Flora SJ. Therapeutic efficacy of sylimarin and naringenin in reducing arsenic-induced hepatic damage in young rats. Ecotoxicol Environ Saf. 2011;74:607-14. PMID: 20719385.,2020 Abdel Salam OM, Sleem AA, Shafee N. Hepatoprotective effects of the nitric oxide donor isosorbide-5-mononitrate alone and in combination with the natural hepatoprotectant, silymarin on carbon tetrachloride-induced hepatic injury in rats. Inflammopharmacology. 2010;18:87-94. doi: 10.1007/s10787-009-0027-7.
https://doi.org/10.1007/s10787-009-0027-...
.
The mitochondrial dysfunction effects the variation of lipid metabolism, also cause some cycles that allows the progression of chronic liver damage, such secondary biliary cirrhosis. Furthermore mitochondrial dysfunction leads to increase in ROS production, leading to a advanced stage of cell damage. In this respect, silymarin trigger mitochondrial functions by encouraging an recovery in mitochondrial citrate transport and, triggering ROS (reactive oxygen species) eradication2121 Arduini A, Serviddio G, Tormos AM, Monsalve M, Sastre J. Mitochondrial dysfunction in cholestatic liver diseases. Front Biosci. 2012;4:2233-52. PMID: 22202034.,2222 Serviddio G, Bellanti F, Stanca E, Lunetti P, Blonda M, Tamborra R, Siculella L, Vendemiale G, Capobianco L, Giudetti AM. Silybin exerts antioxidant effects and induces mitochondrial biogenesis in liver of rat with secondary biliary cirrhosis. Free Radic Biol Med. 2014;73:117-26. PMID: 24819445..
Oxidative stress induced by ROS plays a substantial role in hepatotoxicity. Furthermore Reactive oxygen species induces the oxidative stress and this cycle leads to liver injury by lipid peroxidation, and destroying the enzyme activity. MDA is the result of lipid peroxida¬tion, and the elevation of MDA in plasma is an triggering factor for hepatic damage. On the other hand SOD and CAT were the significant pieces for antioxidant sys¬tem, and the declined levels shows the hepatic damage2323 Venukumar M, Latha M. Antioxidant activi¬ty ofcurculigo orchioides in carbon tetrachlo¬ride-induced hepatopathy in rats. Indian J Clin Biochem. 2002;17:80-7. PMID: 23105355.,2424 Yu H, Zheng L, Yin L, Xu L, Qi Y, Han X, Xu Y, Liu K, Peng J. Protective effects of the total saponins from dioscorea nipponica makino against carbon tetrachloride-induced liver in¬jury in mice through suppression of apoptosis and inflammation. Int Immunopharmacol. 2014;19:233-44. PMID: 24491258..
In present study, Silymarin drug caused significant decline in MDA and major increase of SOD, and CAT after the treatment of silymarin (P<0.05). These outcomes showed that silymarin may be feasible hepatoprotective molecule for possible injury. The action of defence may cover the elevating in antioxidant enzyme level and the diminishing in lipid peroxidation procedure2525 Jia XY, Zhang QA, Zhang ZQ, Wang Y, Yuan JF, Wang HY. Hepatoprotective ef-fects of almond oil against carbon tetrachlo¬ride induced liver injury in rats. Food Chem. 2011;125:673-8..
Silymarin’s hepatopreservative effect has been showed by different researchers in experimental partial hepatectomy models. Ligeret et al.2626 Ligeret H, Brault A, Vallerand D, Haddad Y, Haddad PS. Antioxidant and mitochondrial protective effects of silibinin in cold preservation-warm reperfusion liver injury. J Ethnopharm. 2008;115:507-14. PMID: 18061382. studied with silibinin which is the component of Silymarin. They researched the efficacy of silibinin on rat liver at cold preservation-warm reperfusion damage. At the end they obtained that silibinin diminish the findings of oxidative stress in the hepatic cells and elevate the mitochondrial ATP amount than non applied livers. In an experimental renal damage model on rats, found the protective effect on ischemia/reperfusion damages. Mourelle et al.2727 Mourelle M, Muriel P, Favari L, Franco T. Prevention of CCL4-induced liver cirrhosis by silymarin. Fund Clin Pharm. 2009;3:183-91. PMID: 2548940. also found that silymarin cotreatment absolutely protect the liver from the CCL4 effects in cirrhotic rats.
In present research, two silymarin performed group were compared with non performed groups. Biochemical outcomes find out that drug performed groups have lower ALT levels, less vacuolization and epithelial degeneration in the hepatocytes. Also according to immunohistochemical results lower hepatocellular damage detected after the silymarin applying, Ghobadi Pour et al.28 studied the effects of lactulose andsilymarin on hepatic enzymes in cirrhotic rats and found that alone silymarin or combined drugs have decreased the levels of hepatic enzymes as a companion to our work.
Caspase proteins shows a serious act in apoptosis and are liable for many of biochemical and also morphological situations. For this reason, an increased level of activated caspase proteins is one of the most common apoptosis markers that has been used to indicate apoptosis phenotype. Sang et al.2929 Sang JF, Shi XL, Han B, Huang T, Huang X, Ren HZ, Ding YT. Intraportal mesenchymal stem cell transplantation prevents acute liver failure through promoting cell proliferation and inhibiting apoptosis. Hepatobiliary Pancreat Dis Int. 2016 Dec;15(6):602-11. PMID: 27919849. already used caspase-3 for evaluating apoptosis by applying Intraportal mesenchymal stem cell. Consistent with this study, our data showed that the treatment of hepatic cells with silymarin for 6 and 12 hours can elevate the proportion of caspase-3 with an declined number of apoptotic cells. Present study, applying of silymarin obtained significantly declined apoptosis ratios compared to non applied silymarin groups. However, the molecular mechanisms underlying apoptosis in the partial hepatectomy experimental model need more research.
Conclusions
The demand for donor livers is in high proportion furthermore the need for modified preservation solutions is increasing for donor organs. The main aim for research in this field is to diminish cold ischemic injury before the transplantation. We seek a remedy for curing the influences of preservation solutions recently utilized in the surgical process through applying of hepatoprotective agent silymarin.
References
-
1Mangus RS, Fridell JA, Vianna RM, Milgrom MA, Chestovich P, Chihara RK, Comparison of histidine-tryptophan- ketoglutarate solution and University of Wisconsin solution in extended criter a liver donors. Liver Transpl. 2008;14:365-73. PMID: 18306380.
-
2Feng L, Zhao N, Yao X, Sun X, Du L, Diao X. Histidine-tryptophan-ketoglutarate solution vs. University of Wisconsin solution for liver transplantation: a systematic review. Liver Transpl. 2007;13:1125-36. PMID: 17665493.
-
3Wilson CH, Brook NR, Talbot D. Preservation solutions for solid organ transplantation. Mini Rev Med Chem. 2006;6:1081-90. PMID: 17073708.
-
4D'Alessandro AM, Southard JH, Love RB, Belzer FO. Organ preservation. Surg Clin North Am. 1994;74:1083-95. PMID: 7940062.
-
5Kanat O, Kurt E, Yalcinkaya U, Evrensel T, Manavoglu O. Comparison of uroprotective efficacy of mesna and amifostine in Cyclophosphamide- induced hemorrhagic cystitis in rats. Indian J Cancer. 2006;43:12-5. PMID: 16763356.
-
6Lim AY, Segarra I, Chakravarthi S, Akram S, Judson JP. Histopathology and biochemistry analysis of the interaction between sunitinib and paracetamol in mice. BMC Pharmacol. 2010;10:14. PMID: 20950441.
-
7Zhou D, Ruan J, Cai Y, Xiong Z, Fu W, Wei A. Antioxidant and hepatoprotective activity of ethanol extract of Arachniodes exilis (Hance) Ching. J Ethnopharmacol. 2010;129:232-7. PMID: 20347029.
-
8Ma ZY, Qian JM, Rui XH, Wang FR, Wang QW, Cui YY. Inhibition of matrix metalloproteinase-9 attenuates acute small-for-size liver graft injury in rats. Am J Transplant. 2010;10:784-95. PMID: 20121733.
-
9Müller SA, Mehrabi A, Schmied BM, Welsch T, Fonouni H, Engelmann G, Schemmer P, Weitz J, Schmidt J. Partial liver transplantation-living donor liver transplantation and split liver transplantation. Nephrol Dial Transplant. 2007;22 Suppl 8:viii13-viii22. PMID: 17890257.
-
10Cywes R, Mullen JB, Stratis MA, Greig PD, Levy GA, Harvey PR, Strasberg SM. Prediction of the outcome of transplantation in man by platelet adherence in donor liver allografts. Evidence of the importance of prepreservation injury. Transplantation. 1993;56:316-23. PMID: 7689257.
-
11Bazzoni G, Dejana E, Del Maschio A. Platelet-neutrophil interactions: possible relevance of the pathogenesis of thrombosis and inflamation. Haematologia. 1991;76:e491-9. PMID: 1820986.
-
12Koo A, Komatsu H, Tao G, Inoue M, Guth PH, Kaplowitz N. Controbution of noreflow phenomenon to hepatic injury after ischemia-reperfusion. Evidence for a role for superoxide anion. Hematology. 1992;15:507-14. PMID: 1312056.
-
13Wilson CH, Brook NR, Talbot D. Preservation solutions for solid organ transplantation. Mini Rev Med Chem. 2006;6:1081-90. PMID: 17073708.
-
14Maathuis MH, Leuvenink HG, Ploeg RJ. Perspectives in organ preservation. Transplantation. 2007;83:1289-98. PMID: 17519776.
-
15Jain A, Mohanka R, Orloff M, Abt P, Kashyap R, Cullen J. University of Wisconsin versus histidine-tryptophan-ketoglutarate for tissue preservation in live-donor liver transplantation. Exp Clin Transplant. 2006;4:451-7. PMID: 16827642.
-
16Mangus RS, Fridell JA, Vianna RM, Milgrom MA, Chestovich P, Chihara RK. Comparison of histidine-tryptophan- ketoglutarate solution and University of Wisconsin solution in extended criteria liver donors. Liver Transpl. 2008 Mar;14(3):365-73. PMID: 18306380.
-
17Moray G, Sevmis S, Karakayali FY, Gorur SK, Haberal M. Comparison of histidine-tryptophan-ketoglutarate and University of Wisconsin in living-donor liver transplantation. Transplant Proc. 2006;38:3572-5. PMID: 17175334.
-
18Tyagi A, Agarwal C, Harrison G, Glode LM, Agarwal R. Silibinin causes cell cycle arrest and apoptosis in human bladder transitional cell carcinoma cells by regulating CDKI-CDK-cyclin cascade, and caspase 3 and PARP cleavages. Carcinogenesis. 2004;25:1711-20. PMID: 15117815.
-
19Jain A, Yadav A, Bozhkov AI, Padalko VI, Flora SJ. Therapeutic efficacy of sylimarin and naringenin in reducing arsenic-induced hepatic damage in young rats. Ecotoxicol Environ Saf. 2011;74:607-14. PMID: 20719385.
-
20Abdel Salam OM, Sleem AA, Shafee N. Hepatoprotective effects of the nitric oxide donor isosorbide-5-mononitrate alone and in combination with the natural hepatoprotectant, silymarin on carbon tetrachloride-induced hepatic injury in rats. Inflammopharmacology. 2010;18:87-94. doi: 10.1007/s10787-009-0027-7.
» https://doi.org/10.1007/s10787-009-0027-7 -
21Arduini A, Serviddio G, Tormos AM, Monsalve M, Sastre J. Mitochondrial dysfunction in cholestatic liver diseases. Front Biosci. 2012;4:2233-52. PMID: 22202034.
-
22Serviddio G, Bellanti F, Stanca E, Lunetti P, Blonda M, Tamborra R, Siculella L, Vendemiale G, Capobianco L, Giudetti AM. Silybin exerts antioxidant effects and induces mitochondrial biogenesis in liver of rat with secondary biliary cirrhosis. Free Radic Biol Med. 2014;73:117-26. PMID: 24819445.
-
23Venukumar M, Latha M. Antioxidant activi¬ty ofcurculigo orchioides in carbon tetrachlo¬ride-induced hepatopathy in rats. Indian J Clin Biochem. 2002;17:80-7. PMID: 23105355.
-
24Yu H, Zheng L, Yin L, Xu L, Qi Y, Han X, Xu Y, Liu K, Peng J. Protective effects of the total saponins from dioscorea nipponica makino against carbon tetrachloride-induced liver in¬jury in mice through suppression of apoptosis and inflammation. Int Immunopharmacol. 2014;19:233-44. PMID: 24491258.
-
25Jia XY, Zhang QA, Zhang ZQ, Wang Y, Yuan JF, Wang HY. Hepatoprotective ef-fects of almond oil against carbon tetrachlo¬ride induced liver injury in rats. Food Chem. 2011;125:673-8.
-
26Ligeret H, Brault A, Vallerand D, Haddad Y, Haddad PS. Antioxidant and mitochondrial protective effects of silibinin in cold preservation-warm reperfusion liver injury. J Ethnopharm. 2008;115:507-14. PMID: 18061382.
-
27Mourelle M, Muriel P, Favari L, Franco T. Prevention of CCL4-induced liver cirrhosis by silymarin. Fund Clin Pharm. 2009;3:183-91. PMID: 2548940.
-
28Ghobadi Pour M, Mirazi N, Alaei H, Moradkhani S, Rajaei Z, Monsef Esfahani A. Effects of lactulose and silymarin on liver enzymes in cirrhotic rats. Can J Physiol Pharmacol. 2017 May;95(5):522-529. doi: 10.1139/cjpp-2016-0454.
» https://doi.org/10.1139/cjpp-2016-0454 -
29Sang JF, Shi XL, Han B, Huang T, Huang X, Ren HZ, Ding YT. Intraportal mesenchymal stem cell transplantation prevents acute liver failure through promoting cell proliferation and inhibiting apoptosis. Hepatobiliary Pancreat Dis Int. 2016 Dec;15(6):602-11. PMID: 27919849.
-
Financial source:
none
-
1
Research performed at Laboratory of Faculty of Medicine, Kahramanmaras Sütçü Imam University, Kahramanmaras, Turkey.
Publication Dates
-
Publication in this collection
June 2017
History
-
Received
16 Feb 2017 -
Reviewed
14 Apr 2017 -
Accepted
18 May 2017