Abstract
Trauma and neurodegenerative diseases commit the nervous system. After an axotomy or nerve injury in the peripheral nervous system the regeneration of the nerve fibers and reinervation of the target are seen. In central nervous system these events are restrictive, however their occurrence are related to the state of glial reaction and the synthesis of neurotrophic factors. Basic fibroblast growth factor (bFGF) has been considered an important trophic factor for neurons and astrocytes of many central nervous system regions. In this study rats were submitted to one of following neurosurgery procedures: callosotomy, pyramidectomy or complete transection of hypoglossal nerve (XII). Sham operations were made in control animals. Seven days later animals were sacrificed and their brains processed for immunohistochemistry. Coronal sections were taken from the central nervous system and incubated with antisera against the glial fibrillary acidic protein (GFAP) or neurofilament (NF), markers for astrocyte and neuronal cell body and fibers, respectively, as well as with the antiserum against the bFGF. The degree of the labelling was quantified with computer assisted stereological methods. The analysis of the NF immunoreactivity revealed a disappearance of fibers in the white matter distal to the pyramidectomy and callosotomy, however no disappearance of NF immunoreactive neurons was found in the XII nucleus following axotomy. These changes was accompanied by a massive astrocytic reaction. The reactive astrocytes synthesized increased amounts of bFGF. These findings suggest that glial reaction synthesizing neurotrophic factors may influence the wound and repair after mechanical lesions of central nervous and subsequent neuronal trophism and plasticity which may be relevant to the regenerative process of the nervous tissue
Nervous system; Regeneration; Lesion; Plasticity
EXPERIMETAL MICRONEUROSURGERY OF THE CENTRAL AND PERIPHERAL NERVOUS SYSTEM IN THE STUDY OF THE NEURONAL AND GLIAL TROPHISM AND PLASTICITY1 1 Article from the Laboratory of Neurotrophic Factor and Neuronal Plasticity, Departament of Anatomy, University of São Paulo, São Paulo.
Gerson Chadi 2 2 MD, Associate Professor, Department of Anatomy, ICB/USP
Patrícia Castelucci3 3 Graduate Students from ICB/USP
Vânia C. Gomide 3 3 Graduate Students from ICB/USP
Chadi, G; Castelucci, P.; GOMIDE, V.C. - Experimetal microneurosurgery of the central and peripheral nervous system in the study of the neuronal and glial trophism and plasticity. Acta Cir. Bras, 13(1):08-17, 1998.
SUMMARY:Trauma and neurodegenerative diseases commit the nervous system. After an axotomy or nerve injury in the peripheral nervous system the regeneration of the nerve fibers and reinervation of the target are seen. In central nervous system these events are restrictive, however their occurrence are related to the state of glial reaction and the synthesis of neurotrophic factors. Basic fibroblast growth factor (bFGF) has been considered an important trophic factor for neurons and astrocytes of many central nervous system regions.
In this study rats were submitted to one of following neurosurgery procedures: callosotomy, pyramidectomy or complete transection of hypoglossal nerve (XII). Sham operations were made in control animals. Seven days later animals were sacrificed and their brains processed for immunohistochemistry. Coronal sections were taken from the central nervous system and incubated with antisera against the glial fibrillary acidic protein (GFAP) or neurofilament (NF), markers for astrocyte and neuronal cell body and fibers, respectively, as well as with the antiserum against the bFGF. The degree of the labelling was quantified with computer assisted stereological methods.
The analysis of the NF immunoreactivity revealed a disappearance of fibers in the white matter distal to the pyramidectomy and callosotomy, however no disappearance of NF immunoreactive neurons was found in the XII nucleus following axotomy. These changes was accompanied by a massive astrocytic reaction. The reactive astrocytes synthesized increased amounts of bFGF.
These findings suggest that glial reaction synthesizing neurotrophic factors may influence the wound and repair after mechanical lesions of central nervous and subsequent neuronal trophism and plasticity which may be relevant to the regenerative process of the nervous tissue.
Subject Headings: Nervous system. Regeneration. Lesion. Plasticity
INTRODUCTION
Many neurodegenerative diseases and trauma commit the nervous system. In the peripheral nervous system, the axotomy or other nerve injury can be followed by the regeneration of the nerve fiber and the reinervation of the target when appropriate treatment is applied8,24,32,33. In the central nervous system, when a tract of fibers is lesioned, a more restrictive phenomenon is observed48, however, in many case, regeneration of the lesioned fibers may take place7,27,36,40,42.
It has been observed in the last ten years the potential capability of the regeneration of the central nervous system15,29. Thus, many recent works have been performed to better understand the phenomenon related to neural degeneration, as well as, the circumstances regarding the regenerative events and plasticity of both central and peripheral nervous system3,5,15,21,34,42,47.
It has been described that the events regarding the trophic state and regeneration of the peripheral and central nervous system are related to the state of reactive glial cells and the trophic factor synthesized by the neuroglia and neurons1,3,5,8,14,35,48. Basic fibroblast growth factor (bFGF) is a trophic factor for neurons from many central nervous system regions5,11,52. bFGF is also a potent angiogenic and gliogenic factor, thus, being important in the repair process following a nervous tissue lesion3,13.
The present work has been performed to analyse the neuronal and glial plasticity as well as the trophic responses observed by synthesis of neurotrophic factor following lesions placed in either peripheral or central nervous system. Experimental microneurosurgeries were done in rats to perform a stereotaxical lesion of the corpus callosum, a complete transection of the bulbar pyramid or an axotomy of the hypoglossal (XII) nerve. Immunohistochemistry of the intermediate filament of the cytoskeletum of neurons (neurofilament, NF) and of astrocytes (glial fibrillary acidic protein, GFAP) combined to quantitative computer assisted stereological analysis was used.
METHOD
Adult male Wistar rats from the Institute of Biomedical Sciences (São Paulo, Brazil) with body weight (b.w.) of 250 g were used in the experiments. The animals were kept under controlled temperature and humidity conditions with a standardized light and dark cycle (lights on at 7:00 a.m. and off at 7:00 p.m.) with free access to food pallets and tap water. Under chloral hydrate (7%, Merck, Germany, 350 mg/Kg/b.w., i.p) anaesthesia, animals were submitted to one of the following mechanical lesions of the nervous system: transection of the corpus callosum, transection of bulbar pyramid or axotomy of the hypoglossal (XII) nerve. The procedures are described in the following.
Microneurosurgery procedures
Stereotaxical mechanical lesion of the corpus callosum. In these group 10 rats were used. The rats were placed in a stereotaxical apparatus (Kopf, figure 1A, B) and by means of an adjustable wire knife (Kopf) the corpus callosum was lesioned in the midline (n=5). Firstly, the skull was opened with a drill, the cannula was placed in an inclined plane (118.5° to the rat skull) in a point of 4.0 mm anterior to the bregma and it was introduced 5.9mm deep. In this way the tip of the cannula reached a position of +1.2mm cranio-caudal (cc), 5.2mm antero-posterior (ap) and 0.0mm latero-lateral (ll) according to the atlas of Paxinos and Watson39. After that, the blade, that was previously turned to the caudal of the animal, was extended 3.0mm. The cannula was then lifted 4.0mm to cut the corpus callosum (Figures 2A, 3A). Following, the blade was withdrawn and the cannula taken out of the skull. The skin was sutured. The sham operation was made with the introduction of the cannula only (n=5).
Cervical mechanical lesion of the bulbar pyramid. Ten rats were used in this experiment. The microneurosurgery was made by means of an incision in the skin. The superficial cervical fascia on the midline of the neck and the anatomical structures of the right side were dissected. The tendon of digastric muscle was sectioned and its posterior belly was deflected. The great horn of the hyoid bone was cut, so that the stylohyoid muscle could be deflected. The sternocleidomastoid and the sternohyoid muscles were separated and the omohyoid muscle sectioned. Following, the anterior scalenus muscle was divulsioned between the carotid sheath and the trachea, so that, the occipital bone and condyle could be identified. The bone was opened with a drill in a point located 2 mm medial and 3 mm cranial to the condyle. The dura mater was opened and the right pyramid was identified and sectioned (n=5) with delicate tweezers (Figures 1C, 2B). The same procedure was done in the sham operated rats (n=5) without lesioning the dura mater and the pyramid. After that, the superficial cervical fascia and the skin were sutured.
Axotomy of the hypoglossal nerve. This group of rats had their right XII nerve transected at its first bifurcation (Figure 3C). The cervical skin was opened in the midline and the XII nerve was isolated close to the tendon of digastric muscle. The XII nerve was completely transected (n=5) with delicate tweezers and the distal and proximal nerve branches were inverted and tied. The contralateral (unlesioned) side was used as a control (control side).
Immunohistochemical procedures. Seven days after the lesions the rats were deeply anaesthetized with 10% chloral hydrate (420 mg/kg, b. w., i.p.) and killed by a perfusion through a cannula inserted in the ascending aorta with 50 ml of isotonic saline at room temperature followed by 350 ml of fixation fluid (4°C) during 6 min. The fixative (Zamboni and De Martino, 1967) consisted of 4% (w/v) paraformaldehyde and 0.2% (v/v) picric acid in 0.1M phosphate buffer (pH 6.9). The brains and spinal cords were dissected out and kept in the fixative solution for 90 min. The fixed regions of central nervous system were washed in 10% to 20% sucrose dissolved in 0.1M phosphate buffered saline (PBS) for 2 days, frozen in dry ice and stored at -70°C freezer. Coronal brain sections were made through the XII nucleus from bregma - 14.08mm to - 13.24mm (experiments of axotomy of XII nerve), throught the forebrain from bregma 1.00mm to -1.30mm (experiment of callosotomy) and through the brain stem from bregma -4.30mm to -14.60mm as well as the entire cervical spinal cord (experiment of pyramidectomy), according to the atlas of Paxinos and Watson39, using a Leica cryostat (CM 3000, Germany). The sections were sampled systematically and series in a rostrocaudal order including every fifth section were used for immunohistochemical analysis.
The sections were incubated overnight at 4°C with one of the following antisera: a rabbit polyclonal bFGF antiserum12 (diluted 1:800), a rabbit polyclonal antiserum against the glial fibrilary acidic protein (GFAP, 1:1500, Dakoppats, Denmark) or a mouse monoclonal antiserum against the NF of molecular weight 200 kD (1:1000) (Sigma, USA). The antibodies were diluted in PBS containing 0.3%-0,5% Triton X-100 (Sigma) and 0.5% bovine serum albumin (Sigma). The detection of the antibodies was achieved by the indirect immunoperoxidase method (ABC)22 using the avidin-biotin peroxidase technique as previously described5,6. After washing in PBS (3x10 min), the sections were incubated with a biotinylated goat anti-rabbit or biotinylated horse anti-mouse antibodies (both diluted 1:200, Vector, USA) for one hour. In a third step the sections were washed in PBS and incubated with avidin-biotin peroxidase complex (both diluted 1:100, Vectastain, Vector, USA) during 45 min. The staining was performed using 0.03% of 3, 3' diaminobenzidine tetrahydrochloride (DAB, Sigma) as a chromogen and 0.05% (v/v) of H2O2 (Sigma) during 6 - 8 min, which gave a brownish colour to the immunoreaction. One series of sections were stained for cresyl violet (CV). For standardization of the immunohistochemical procedures we have used a dilution of the primary antibody and a concentration of DAB far from saturation and an incubation time adjusted, so that the darkest elements in the brain sections were below saturation54. The bFGF antiserum used is a well characterized polyclonal antiserum raised against the n terminal (residues 1-24) of the synthetic peptide of bovine bFGF (1-146)12. This antiserum does not recognize acidic FGF (cross reactivity less than 1%)12. As control, sections were incubated overnight at 4°C with the bFGF antiserum (diluted 1:800) pre-incubated with human recombinant bFGF (50 mg/ml, for 24h at 4°C). To further analyse the specificity of the immunostaining sections were also incubated with the solvent of the primary and secondary antibodies solutions as well as the solvent of the avidin-biotin solution and processed simultaneously in the experimental sections. These immunohistochemical procedures have labelled the intermediate filaments of neurons and astrocytes with the NF and GFAP immunoreactivities, respectively. The trophic responses of the lesioned nervous system was analysed by means of the bFGF immunoreactivity.
Two colour immunoperoxidase procedures. The two-colour immunoperoxidase method was employed in a series of sections for the simultaneous detection of the bFGF and GFAP immunoreactivities. The bFGF immunoreactivity was firstly demonstrated as described above. Following the DAB reaction, the sections were rinsed several times in PBS and were incubated during 48 h with the rabbit polyclonal antiserum against GFAP (1:500). After several rinses in PBS, the sections were incubated with biotinylated horse anti-mouse immunoglobulins (1:200, Vector) for 1 hour at room temperature and with an avidin and biotin peroxidase solution (both diluted, 1:100; Vectastain, Vector) for 45 minutes at room temperature. The staining was performed using 4-chloro naphthol 0.05% (Sigma) as a chromogen and 0.05% (v/v) of H2O2 (Sigma) during 10 min. This procedure gave a brownish colour to bFGF immunoreactivity and a bluish a colour to GFAP immunoreactivity. The qualitative analyses were performed under light microscopy.
Procedures for the stereological analysis of the Areal Fraction of the GFAP and the Intensity of the bFGF immunoreactivities. The Areal Fraction (AA) of the astroglial GFAP immunoreactive cytoplasm was calculated employing stereological methods in the studied regions. Sampled fields was applied on the white and gray matters, close to the area of callosotomy and pyramidectomy, respectively, of the lesioned and sham operated rats as well as on the ipsilateral and contralateral entire XII nucleus of the axotomized rats (Figures 2B, C; 3A). The AA was obtained using the CAST-system (Computer Assisted Stereological Toolbox, Olympus, Denmark) which created a set of regularly spaced points in fields systematically sampled3,5,17. Briefly, a microscope (BX50 Olympus, Denmark) was interfaced with a computer (IBM 330-P75) and a colour video camera (JAL 2040; Protec, Japan), both linked to a colour video monitor (G70, IBM). GRID software package (Interactivision, Denmark) generated sampling and point-grid frames as an overlay image to the microscopic image on the monitor3,5,17 as well as to control the motorized X-Y stage (Lang, Germany). The border of each region was outlined using a 4 x objective and the delineated area was obtained. Step rates were entered (80-120 µm, depending on the experiments), after which the system generated a randomized and systematic sampling within the outlined region. For counting the profiles a 100 x objective was used. The points hitting all immunoreactive profiles found in the counting frames were counted (SPstruture). The points hitting the sampled tissue were also counted (SPtissue). Thus the Areal Fraction AA(structure/section) could be calculated AA = SPstructure/ SPtissue. The intensity of the nuclear bFGF immunoreactivity of the sampled glial cells was semiquantitatively analyzed by rating the intensity of the colour reaction of each counted profile. The profiles were sampled with the above described system employing a counting frame in fields systematically sampled in the studied regions, as an overlay image to the microscope image on the monitor. The same 100 x objective described above was used. The light, the aperture of the camera and the colour conditions of the monitor were rigorously kept constant throughout the procedure. The scores ranged from low (1+, yellow), moderate (2+, brown) and high (3+, dark brown). Thus, the values grouped in 3 classes of immunoreaction indicated the glial profiles showing weak, moderate and strong nuclear bFGF immunoreactivity. The mean of the scores was obtained for each animal. Following, the mean ± S.E.M. of the scores was obtained in each group of experiments.
Statistical analysis. Statistical analysis was performed according to the non-parametric two-tailed Main-Whitney U-test20. The injured side of the central nervous system regions of the lesioned and sham operated rats were compared in the experiments of callosotomy and pyramidectomy. In the experiment of XII axotomy the lesioned side was compared to the contralateral side.
RESULTS
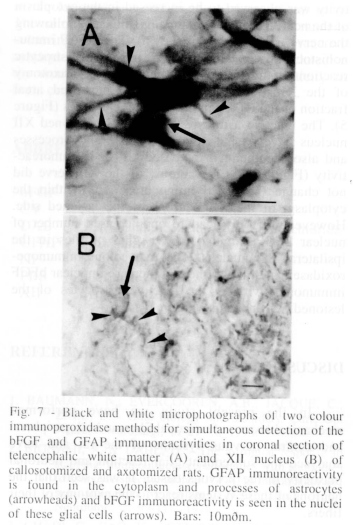
Analysis of animal submitted to callosotomy. After callosotomy a degeneration of the NF immunoreactive fibers was observed in the white matter 7 days after the surgery. The changes were seen in the white matter (Figure 3A) close to the area 1 of the frontal cortex (Fr1 white matter-medial) and in the region of the external capsule close to the area 2 of the parietal cortex (white matter-lateral). The GFAP immunohistochemistry revealed an astrocytic reaction in the white matter-medial and lateral as seen by the increases in the areal fraction of the GFAP immunoreactive profiles (Figure 5). The changes are illustrated in figure 6A, B. The astrocytic reaction was observed by the increases in the size of the cytoplasm and processes of the astrocytes as well as by the increased GFAP immunoreactivity per glial cells (Figure 6A, B). The callosotomy also promoted increases in the bFGF immunoreactivity in the nuclei of glial cells in the entire white matter. However, a massive increase of bFGF immunoreactivity was found in the white matter close to the injury (Table I). The two colour immunoperoxidase studies for the simultaneous detection of bFGF and GFAP immunoreativities demonstrated increases of the bFGF immunoreactivity in the nuclei of the reactive astrocytes labelled with GFAP immunoreactivity in the white matter after the callosotomy (Figure 7).
Analysis of animal submitted to pyramidectomy. In the present analysis we have also studied the effects of the transection of the corticospinal tract at the level of the pyramid of the medulla oblongata 7 days after the surgery. We have observed a massive disappearance of NF immunoreactive fibers in the lesioned pyramid (Figure 4C, D) and dorsal corticospinal tract of the spinal cord (data not shown) 7 days after the surgery. In coronal sections proximal to the lesion, no disappearance of NF immunoreactive fibers was seen (not shown). The GFAP immunohistochemistry revealed an astrocytic reaction in the entire level of the pyramidectomy, however, a massive reaction was found on the lesioned site and in the region surrounding the lesioned pyramid as seen by the increases in the areal fraction of the GFAP immunoreactive profiles (Figure 5). The reactive astrocytes had increased size and thick processes and also accumulated increased amount of GFAP immunoreactivity in the same fashion seen after callosotomy (Figure 6C, D). Reactive astrocytes were also seen in the entire lesioned dorsal corticospinal tract of the spinal cord (data not shown). The two colour immunoperoxidase study for the simultaneous detection of bFGF and GFAP immunoreativities revealed the in the nuclei of reactive astrocytes increased amount of the nuclear bFGF immunoreactivity as observed after callosotomy (Figure 7).
Specificity of the immunoreactivies. The analysis of the sections from control experiments related to the specificity of the immunoreactions revealed no specific labelling in the immunohistochemical procedures performed in this study which indicated the specificity of the findings.
Analysis of animal submitted to axotomy of XII nerve. It was not found any disappearance of XII nerve cell bodies stained by either cresyl violet or NF immunohistochemistry in the lesioned of the XII nucleus after the transection of the XII nerve (Figure 4E, F) 7 days after axotomy. The NF immunoreactivity was observed to be increased in the cytoplasm of the neurons of the ipsilateral XII nucleus following the nerve transection (not shown). The GFAP immunohistochemistry demonstrated a massive astrocytic reaction in the lesioned XII nucleus after axotomy of the XII nerve as seen by the increased areal fraction of the GFAP immunoreactive profiles (Figure 5). The reactive astrocytes found in the lesioned XII nucleus have an increased cytoplasm and processes and also accumulated increased GFAP immunoreactivity (Figure 6E, F). Axotomy of the XII nerve did not change the bFGF immunoreactivity within the cytoplasm of the XII neurons of the lesioned side. However, axotomy lead to an increased number of nuclear bFGF immunoreactive glial profiles in the ipsilateral XII nucleus. The two colour immunoperoxidase experiments showed increased nuclear bFGF immunoreactivity in the reactive astrocytes of the lesioned XII nucleus (Figure 7).
DISCUSSION
In the present study we have performed mechanical lesions of neuronal fibers in both peripheral and central nervous systems of the rat and have analysed the subsequent glial reaction and neuronal plasticity in the nervous tissue. It was seen that the neurons of these systems react to the axotomy of their fibers.
The microneurosurgery procedures performed here were appropriate for the analysis since complete transection of the tracts (callosotomy and pyramidectomy) and XII nerve were achieved. Experimental transection of the pathways in the central nervous system7,11,28,29,48 and of nerves in the periphery8,16,19,32,33,46,47 has been performed. However we have not found a description of callosotomy performed with the device employed in our study. In the present study we have transected the XII nerve and the proximal and distal nerve branches were inverted and tied to ensure that findings would be related to axotomy instead of regeneration of the fibers.
A distal degeneration of the nerve fibers was observed within the telencephalic white matter and dorsal corticospinal tract 7 days after the transection of the corpus callosum and the bulbar pyramid, respectively. The degeneration of the pathways distal to the lesions was accompanied by an astrocytic reaction and an increased synthesis of astroglial bFGF. Astrocytic reaction was not seen only distal to the lesion of the central pathways but a remarkable astrocytic activation was also found on the lesioned site and in the neighbourhood in a variable distance from the injuries.
Following axotomy of the XII nerve, a massive astrocytic reaction with astrocytes synthesizing increased amount of bFGF was also detected in the lesioned XII nucleus.
The reaction of glial cells, i.e. the astrocytic response, has commonly been described following an injury of the central nervous system3,23,25,45. The reactive astrocytes have been found close to the lesion site2 as well as accompanying the lesioned pathway4,45. Reactive astrocytes are also observed in pathways not primarily related to injury, which indicates that the signal triggering the glial response runs through the synapses41,43.
Following a mechanical lesion of the central nervous system the astrocytic reaction in the lesioned site may be related to the wound and repair2. However, that can not explain by itself the presence of reactive astrocytes in the regions not primarily lesioned as well as in the neuronal pathways far from injury41.
It is known that in the adult central nervous system the gliotic scar may impairs the growth of the regenerated fibers through the wound, which makes difficult the occurrence of regeneration of the central nervous system26,34. However, recent observations have demonstrated that the sprouts from cut corticospinal axons can persist in the presence of astrocytic sacaring in long term lesion of the adult rat spinal cord30.
In spite of the fact that the gliotic scar following the lesion of the central nervous system may impair the outgrowth of the lesioned fibers, it has been postulated that the reactive glial cells in the lesioned brain may favour the neuronal plasticity and trophism1,35,37.
In the present analysis we have observed that the reactive astrocytes found in the lesioned site of callosotomy and pyramidectomy and in the areas distal to the lesions can produce increased amount of glial bFGF. Our results are in full agreement with many publications which have demonstrated that the reactive astrocytes following an injury of the central nervous system can synthesize increased amount of bFGF 5,9,10,11,23.
It has been demonstrated that bFGF is an important survival factor to neurons from many central nervous system regions50,54. Furthermore, bFGF is also an important mitogenic factor to endothelial and glial cells, actions that underline its additional role in the wound and repair18,51.
Thus, the upregulation of the glial bFGF found after callosotomy and pyramidectomy may be related to the paracrine trophic actions of this neurotrophic factor to the lesioned central nervous system, which may stimulate the repair and the maintenance of the neurons close to the wound3,5,7,9,11,23,31.
The present work has also found, an intense astrocytic reaction and an increased synthesis of glial bFGF in the XII nucleus following axotomy of their fibers. These phenomena were accompanied by the maintenance of the XII cell bodies, since we have not observed a neuronal disappearance of the XII nerve cells following the lesion. Our results are also in accordance with the findings that the axotomy of a motor nerve does not trigger the degeneration of perikarya located within the central nervous system of adult animals46. The regeneration of the motor nerve after the axotomy and adequate repair has been consistently demonstrated in the literature8,16,33. The findings that the axotomy of XII nerve induced an increased synthesis of bFGF by the reactive astrocytes in the lesioned motors nucleus indicate that the astrocytes may also promote a paracrine role in the survival of peripheral motor neurons. In spite of the fact that many studies have demonstrated the role of the target derived neurotrophic factors in the maintenance of the peripheral sensory and motor neurons19,49, a paracrine support mediated by astroglial neurotrophic factor to these neurons has not been mentioned.
Thus, the present analysis speculates that astroglial bFGF within the lesioned XII nucleus may help to maintain the XII neurons which may favour the growth of fibers if adequate repair of the nerve branches is performed.
In view of previous publications28,38,53 it is also possible that the callosotomy and pyramidectomy performed in this paper have not triggered the disappearance of cortical neurons, in spite of the regeneration of the fibers has not seen, at least at 7 days after the lesions. That seems likely, since many experiments have demonstrated the growth of fibers with or without nerve grafts bridging a traumatic gap placed i.e. in the spinal cord7,30,36,44. This is in line with the observation that administration of neurotrophic factors increases the rate of fiber regeneration7.
It seems possible that the increased synthesis of bFGF by reactive astrocytes may favour the maintenance of projecting neurons and the long term regeneration of the lesioned pathway.
Thus, taking together the results obtained in the present study have indicated that following a lesion of the peripheral and central nerve fibers, the reactive astrocytes participate in the wound and also may maintain the trophism and the plasticity of the central nervous system neurons by paracrine actions through the synthesis of neurotrophic factor.
CONCLUSION
At the present work we can conclude that following a short time period (7 days) of axotomy of the central pathways, an astrocytic reaction and an increased synthesis of astroglial bFGF take place in the lesioned site and in the pathway distal to it. The same phenomenon is seen in the XII nucleus following axotomy. It is possible that the reactive astrocytes and the astroglial bFGF may be related to the wound, neuronal maintenance and plasticity in both central and peripheral nervous system.
Acknowledgements: FAPESP (94/3858-3; 95/9060-6; 96/3110-4; 96/4381-1), CNPq (524277/96-6), Sandoz Foundation for Gerontological Research (LA-94208), TWAS (94-448RG/BIO/LA).
ABBREVIATION USED:
bFGF basic fibroblast growth factor
CV cresyl violet
DAB 3,3' diaminobenzidine tetrahidrochloride
GFAP glial fibrillary acidic protein
NF neurofilament
PBS phosphate buffer saline
XII hypoglossal
Address reprint request:
Gerson Chadi, M.D., Ph.D.
Departamento de Anatomia
Universidade de São Paulo
Av. Prof. Lineu Prestes, 2415
5508-900 - São Paulo
Brasil
FAX: 55 11 813 0845
e-mail: gerchadi@usp.br
- 1. Baumann, N.; Evercooren, A.B.; Jacque, C.; Zalc, B. - Glial biology and disorders. Curr. Op. Neurol. Neurosurg., 6: 27-33, 1993.
- 2. Bignami, A. & Dahl, D. - The astroglial response to stabbing. Immunofluorescence studies with antibodies to astrocyte-specific protein (GFA) in mammalian and submammalian vertebrates. Neuropathol. Appl. Neurobiol., 2: 99-111, 1976.
- 3. Chadi, G.; Cao, Y.; Pettersson, R.F.; Fuxe, K. - Temporal and spatial increase of astroglial basic fibroblast growth factor synthesis after 6-hydroxydopamine- induced degeneration of the nigrostriatal dopamine neurons. Neuroscience, 61: 891-910, 1994.
- 4. Chadi, G. & Fuxe, K. - Involviment of astroglial basic fibroblast growth factor and microglia in the 6-OHDA-induced lesion of the nigral dopamine neurons. Submitted, 1998.
- 5. Chadi, G.; Mřller, A.; Rosén, L.; Janson, A.M.; Agnati, L.A.; Goldstein, M.; Ogren, S.O.; Pettersson, R.F.; Fuxe, K. - Protective actions of human recombinant basic fibroblast growth factor on MPTP-lesioned nigrostriatal dopamine neurons after intraventricular infusion. Exp. Brain Res., 97: 145-58, 1993b.
- 6. Chadi, G.; Rosén, L.; Cintra, A.; Tinner, B.; Zoli, M.; Pettersson, R.F.; Fuxe, K. - Corticosterone increases FGF-2 (bFGF) immunoreactivity in the substantia nigra of the rat. Neuroreport, 4: 783-6, 1993a.
- 7. Cheng, H.; Cao, Y.; Olson, L. - Spinal cord repair in adult paraplegic rats: partial restorations of hind limb function. Science, 273: 510-513, 1996.
- 8. Fawcett, J.W. & Keynes, J. - Peripheral nerve regeneration. Annu. Rev. Neurosci., 13: 46-60, 1990.
- 9. Finklestein, S.P.; Apostolides, P.J.; Caday, C.G.; Prosser, J.; Philips, M.F.; Klagsbrun, M. - Increased basic fibroblast growth factor (bFGF) immunoreactivity at the site of focal brain wounds. Brain Res., 460: 253-9, 1988.
- 10. Frautschy, S.A.; Walicke, P.A.; Baird, A. - Localization of basic fibroblast growth factor and its mRNA after CNS injury. Brain Res., 553: 291-299, 1991.
- 11. Gómez-Pinilla, F.; Lee, J.W.; Cotman, C.W. - Basic FGF in adult rat brain: cellular distribution and response to enthorhinal lesion and fimbria-fornix transection. J. Neurosci., 12: 345-355, 1992.
- 12. Gonzalez, A.M.; Buscaglia, M.; Ong, M.; Baird, A. - Distribuiton of basic fibroblast growth factor in the 18-day rat fetus: localization in the basement mebranes of diverse tissues. J. Cell. Biol., 110: 753-765, 1990.
- 13. Gospodarowicz, D.; Neufeld, G.; Schweigerer, L. - Fibroblast growth factor: structural and biological properties. J. Cell Physiol. Suppl., 5: 15-26, 1987.
- 14. Graeber, M.B. & Kreutzberg, G.W. - Astrocytes increase in glial fibrillary acidic protein during retrograde changes of facial motor neurons. J. Neurocytol., 15: 363-373, 1986.
- 15. Grill, R.; Murai, K.; Blesch, A.; Gage, F.H.; Tuszynski, M.H. - Cellular delivery of neurotrophin-3 promotes corticospinal axonal growth and partial functional recovery after spinal cord injury. J. Neurosci., 17: 5560-5572, 1997.
- 16. Grothe, C. & Unsicker, K. - Basic fibroblast growth factor in the hypoglossal system: specific retrograde transport, trophic, and lesion-related responses. J. Neurosci. Res., 32: 317-28, 1992.
- 17. Gundersen, H.J.G.; Bendtsen, T.F.; Korbo, L.; Marcussen, N.; MŘller, A.; Nielsen, K.; Nyengaard, J.R.; Pakkenberg, B.; SŘrensen, F.B.; Vesterby, A.; West, M. - Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. Acta Pathol. Microbiol. Immun. Scand., 96: 379-394, 1988.
- 18. Haynes, L.W. - Fibroblast (heparine-binding) growing factors in neuronal development and repair. Mol. Neurobiol., 2: 263-289, 1988.
- 19. Heumann, R.; Korsching, S.; Bandtlow, C.; Thoenen, H. - Changes of nerve growth factor synthesis in nonneuronal cells in response to sciatic nerve transection. J. Cell Biol., 104: 1623-1631, 1987.
- 20. Hollander, M. & Wolfe, D.A. - Non-parametric Statistical Methods. Wiley, New York. 1973.
- 21. Honmou, O.; Felts, P.A.; Waxman, G.S.; Kocsis, J.D. - Restoration of normal conduction properties in demyelinated spinal cord axons in the adult rat by transplatation of exogenous Schwann cells. J. Neurosci., 16: 3199-3208, 1996.
- 22. Hsu, S.; Raine, L.; Fanger, H. - Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabelad antibody (PAP) procedures. J. Histochem. Cytochem., 29: 577-580, 1981.
- 23. Humpel, C.; Chadi, G.; Lippoldt, A.; Ganten, D.; Fuxe, K.; Olson, L. - Increase of basic fibroblast growth factor (bFGF, FGF-2) menssenger RNA and protein following implantation of a microdialysis probe into rat hippocampus. Exp. Brain Res., 98: 229-37, 1994.
- 24. Ide, G. & Osawa, T. - Nerve regeneration through allogenic nerve grafts, with special reference to the role of schwann cell basal lamina. Prog. Neurobiol., 34: 1-38, 1990.
- 25. Jensen, M.B.; Gonzŕlez, B.; Castellano, B.; Zimmer, J. - Microglial and astroglial reactions to anterograde axonal degeneration: a histochemical and immunocytochemical study of the adult rat fascia dentata after entorhinal perforant path lesions. Exp. Brain Res., 98: 245-260, 1994.
- 26. Keifer, J. & Kalil, K. - Effects of infant versus adult pyramidal tract lesion on locomotor behavior in hamsters. Exp. Neurol., 111: 98-105, 1991.
- 27. Kunkel-Bagden, E.; Hai-Ning, D.; Bregman, B.S. - Methods to assess the development and recovery of locomotor function after spinal cord injury in rats. Exp. Neurol., 119: 153-164, 1993.
- 28. Lent, R. - Neuroanatomical effects of neonatal transection of the corpus callosum in hamsters. J. Comp. Neurol., 223: 548-555, 1984.
- 29. Li, W.W.Y.; Yew, D.T.W.; Chuah, M.I.; Leugg, P.C.; Tsangs, D.S.C. - Axonal sprouting in the hemisected adult rat spinal cord. Neuroscience, 61: 133-139, 1994.
- 30. Li, Y. & Raisman, G. - Sprouts from cut corticospinal axons persist in the presence of astrocytic scarring in long-term lesions of the adult rat spinal cord. Exp. Neurol., 134: 102-11, 1995.
- 31. Logan, A.; Frautschy, S.A.; Gonzalez, A.M.; Baird, A. - A time course for the focal elevation of synthesis of basic fibroblast growth factor and one of its high-affinity receptors (flg) following a localized cortical brain injury. J. Neurosc., 12: 3828-37, 1992.
- 32. Lundborg, G. - Regeneration of peripheral nerves - a biological and surgical problem. Scand. J. Plast. Reconstr. Surg., Suppl. 19: 38-44, 1982.
- 33. Lundborg, G. - Nerve regeneration and repair. Acta Orthop. Scand., 58: 145-69, 1987.
- 34. McKeon, R.J.; Schreiber, R.C.; Rudge, J.S.; Silver, J. - Reduction of neurite outgrowth in a model of glial scarring following CNS injury is correlated with the expression of inhibitory molecules on reactive astrocytes. J. Neurosci., 11: 3398-3411, 1991.
- 35. McMillian, M.K.; Thai, L.; Hong, J.S.; O'Callaghan, J.P.; Pennypacker, K.R. - Brain injury in a dish: a model for reactive gliosis. Trends Neurosci, 17: 138-42, 1994.
- 36. Merline, M. & Kalil, K. - Cell death of corticospinal neurons is induced by axotomy before but not after innervation of spinal tragets. J. Comp. Neurol., 296: 506-516, 1990.
- 37. Moonen, G.; Rogister, B.; Leprince, P.; Rego, J.M.; Delreé, P.; Lefebvre, P.P. - Neuro-glial interactions and neuronal plasticity. Progress in Brain Res, 86: 63-73, 1990.
- 38. Ozaki, H.S. & Shimada, M. - The fibbers which course within the Probst's longitudinal bundle seen in the brain of a congenitally acallosal mouse: a study with the horseradish peroxidase technique. Brain Res., 441: 5-14, 1988.
- 39. Paxinos, G. & Watson, C. - The rat brain: in stereotaxic coordinates San Diego, Hartcourt Brace Jovanovich, 1986. pages p.
- 40. Reh, T. & Kalil, K. - Functional role of regrowing pyramidal tract fibers. J. Comp. Neurol., 211: 276-283, 1982.
- 41. Riva, D.I.; Fardeau, C.; Mariani, J.; Bouchaud, C.; Delhaye, B.N. - Contribution of peripheral macrophages and microglia to the cellular reaction after mechanical or neurotoxin-induced lesions of the rat brain. Exp Neurol, 128: 77-87, 1994.
- 42. Schnell, L. & Schwab, M.E. - Sprouting and regeneration of lesioned corticospinal tract fibers in adult rat spinal cord. Eur. J. Neurosci., 5: 1156-1171, 1993.
- 43. Shao, Y. & McCarthy, K.D. - Plasticity of astrocytes. Glia, 11: 147-155, 1994.
- 44. Smith, G.M.; Miller, R.H.; Silver, J. - Changing role of forebrain astrocytes during development, regenerative failure and induced regeneration upon transplantation. J. Comp. Neurol., 251: 23-43, 1986.
- 45. Strömberg, I.; Bjorklund, H.; Dahl, D.; Jomsson, G.; Sundstrom, E.; Olson, L. - Astrocytes responses to dopaminergic denervations by 6-hydroxydopamine and 1-metil-4-phenil-1,2,3,6-tretrahydropyridine as evidenced by glial fibrillary acidic protein immunohistochemistry. Brain Res. Bull., 17: 225-236, 1986.
- 46. Sumner, B.E.H. & Sutherland, F.I. - Quantitative electron microscopy of the injured hypoglossal nucleus of the rat. J. Neurocytol., 2: 315-328, 1973.
- 47. Tetzlaff, W.; Bisby, M.A.; Kreutzberg, G.W. - Changes in cytoskeletal proteins in the rat facial nucleus following axotomy. J. Neurosci., 8: 3181-3189, 1988.
- 48. Tetzlaff, W.; Alexander, S.W.; Miller, F.D.; Bisby, M.A. - Response of facial and rubrospinal neurons to axotomy: changes in mRNA expression for cytoskeletal proteins and GAP-43. J Neurosci, 11: 2528-44, 1991.
- 49. Thoenen, H.; Bandtlow, C.; Heumann, R. - The physiological function of nerve growth factor in the central nervous system: comparison with the periphery. Rev. Physiol. Biochem. Pharmacol., 109: 145-178, 1987.
- 50. Thomaz, K.A. - Fibroblast growth factor. J. FASEB, 1: 434-440, 1987.
- 51. Vlodavsky, I.; Fuks, Z.; Ishai, M.R.; Bashkin, P.; Levi, E.; Korner, G.; Bar, S.R.; Klagsbrun, M. - Extracellular matrix-resident basic fibroblast growth factor: implication for the control of angiogenesis. J. Cell Biochem., 45: 167-176, 1991.
- 52. Walicke, P.A. - Basic and acidic fibroblast growth factor have trophic effects on neuron from multiple CNS regions. J. Neurosci., 8: 2618-27, 1988.
- 53. Whishaw, I.Q.; Zaborowski, J.; Kolb, B. - Postsurgical enrichment aids adult hemidecorticate rats on a spatial navigation task. Behav. Neural Biol., 42: 183-190, 1984.
- 54. Zoli, M.; Zini, I.; Aganati, L.F.; Guidolin, D.; Ferraguti, F.; Fuxe, K. - Aspects of neuronal plasticity in the central nervous system. I. Computer-assisted image analysis methods. Neurochem. Int., 16: 383-418, 1990.
Publication Dates
-
Publication in this collection
18 Nov 1998 -
Date of issue
Jan 1998