Abstract
Purpose:
To compare platelet rich plasma (PRP) and fibrin glue about the effect of anastomotic healing.
Methods:
Thirty six Wistar-Albino male rats diveded into 3 groups according to control(Group1), PRP (Group 2) and fibrin glue(Tisseel VH) (Group 3). The colon was transected with scissor and subsequently an end to end anastomosis was performed using continuous one layer 6/0 vicryl sutures. Postoperative 7th day effect of anastomotic healing measuring with tissue hydroxyproline(TH) level and anastomotic bursting pressure(ABP); moreover comparison of cytokine (IL-6 and IL-10) and procalcitonin levels on 1st,3rd and 7th days.
Results:
There was no statistically significant difference of the ABP and hydroxyproline levels between PRP and fibrin glue on the 7th day. There was no statistically significant difference between levels of proinflammatory cytokine (IL-6) (P=0.41), anti-inflammatory cytokine (IL-10) (P=0.35), and procalcitonin levels (P=0.63) on 1, 3 and 7 days.
Conclusion:
Fibrin glue and platelet rich plasma are shown to be effective in healing intestinal anastomoses without superior to each other.
Key words:
Anastomosis, Surgical; Fibrin Tissue Adhesive; Platelet-Rich Plasma; Rats.
Introductıon
Gastrointestinal anastomotic leaks continue to be the nightmare of surgeons with increasing morbidity and mortality, leading to an increase in the length of hospital stay and increased hospital costs. Although technological developments and production of advance devices (laparoscopic and robotic surgery) have become widespread throughout the world however intestinal leaks still remain between 5-10%11 Walker KG, Bell SW, Rickard MJ. Mehanna D, Dent OF, Chapuis PH, Bokey EL.Anastomotic leakage is predictive of diminished survival after potentially curative resection for colorectal cancer. Ann Surg. 2004;240(2):255-9. PMID: 15273549.,22 Law WL, Choi HK, Lee YM, Ho JW, Seto CL. Anastomotic leakage is associated with poor long-term outcome in patients after curative colorectal resection for malignancy. J Gastrointest Surg. 2007;11(1):8-15. PMID:17390180..
The causes of intestinal anastomotic leaks are examined in two groups as local and systemic factors. Hypoalbuminemia, hypovolemia, severe anemia, acidosis, sepsis, immunosuppression, diabetes mellitus, malignancy, cachexia due to malnutrition are systemic factors; surgical technique and suture materials, tension and inadequate blood flow in anastomosis, presence of healty tissue tips, bacterial contamination, distal obstruction, hyperthermia, radiation damage, mechanical trauma and antibiotic use are local factors33 Kingham TP, Pachter HL. Colonic anastomotic leak: risk factors, diagnosis, and treatment. J Am Coll Surg. 2009;208(2):269-78. PMID: 19228539.,44 Buchs NC, Gervaz P, Secic M, Bucher P, Mugnier-Konrad B, Moerl P. Incidence, consequences, and risk factors for anastomotic dehiscence after colorectal surgery: a prospective monocentric study. Int J Colorectal Dis. 2008;23(3):265-70. PMID: 18034250.. Most local factors are associated with surgical factors, associated with surgeon, his/her technique and suture materials with or without local applications which facilitate anastomotic strength.
Developing leaks from anastomosis one of the most important causes of surgical morbidity and mortality. However many materials have been used for the potency of anastomotisis, no ideal therapy has yet been described. PRP (Platelet-rich plasma) and fibrin glue are frequently used in studies because they are easily accessible in clinical practice, simple to administer and easily available everywhere. However, the comparison of these two materials on colon anastomoses has not yet been made.
Platelet-rich plasma (PRP) is known as an autologous platelet concentrate overhead in plasma growth factors55 Yamaguchi R, Terashima H, Yoneyama S, Tadano S, Ohkohchi N. Effects of plateletrichplasma on intestinal anastomotic healing in rats: PRP concentration is a key factor. J SurgRes. 2012;173(2):258-66. PMID: 21074782.. These growth factors are released and accelerate wound healing process. Fibrin glue is natural hemostatic agents which contain thrombin, fibrinogen, calcium, aprotinin and fibrin stabilizing factor66 Bonanomi G, Prince JM, McSteen F, Schauer PR, Hamad GG. Sealing effect of fibrin glue on the healing of gastrointestinal anastomoses: implications for the endoscopic treatment of leaks. Surg Endosc. 2004 Nov;18(11):1620-4. PMID: 15931477.. It is indicated that fibrin glue is useful in maintaining anastomosis safety by support for intestinal anastomosis.
The aim of this experimental study in the rats was to compare PRP an fibrin glue about the effect of anastomotic healing by measuring microcirculatory parameters with tissue hydroxyproline(TH) level and quantifying anastomotic bursting pressure(ABP); moreover comparison of cytokine (IL-6 and IL-10) and procalcitonin levels.
Methods
This study was established at the Experimental Research Center after obtaining the ethical committee approval of Cukurova University, Faculty of Medicine (Approval number: 2016/12).
Thirty six Wistar-Albino male rats, weighting 250-300 g were used in the present study. The animals were maintained at 2°C, humidity at 40-60% with a 12 hr light/dark cycle and allowed free access to water and standard chow during the study. All animals were observed closely and weighed on days 7 after surgery. This research was carried out in accordance with the Guide for the Care and Use of Laboratory Animals.
Preperation of anastomosis of colon and follow-up
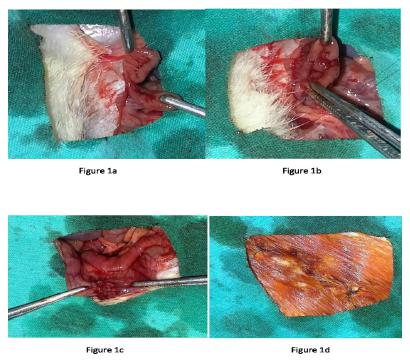
Rats were anesthetized by intramuscular injection of ketamine 40 mg/kg (Ketalar;Parke Davis, Eczacibasi, Istanbul,Turkey) and %2 xylazinebio 8 mg/kg (Rometar; Bioveta ,Chech). A heating lamp was used to maintain the body temperature at 37.2°C. Every efforts was made by the same surgeon with surgical technique due to ensure the standardization. After asepsi a 3 cm midline incision was made and the descending colon was mobilized. The colon was transected with scissor and subsequently an end to end anastomosis was performed using continuous one layer 6/0 vicryl sutures (Figure 1). In order to compare the effects; of PRP and fibrin glue applied on anastomotic line and rats diveded into 3 groups( 12 rats in each group) according to control(Group1), PRP (Group 2) and fibrin glue(Tisseel VH) (Group 3). The median incision was closed with a 3/0 polypropilen sutures. In the post-operative period, no antibiotics were given to the rats. Oral food was started on the post-operative first day. On postoperative day 7 all animals were euthanized by intraperitoneal overdose of 2 ml pentobarbital sodium (175mg/ml, KU Life, Copenhagen, Denmark). After the sacrification, the rat fascia was opened and the anastomosis was achieved by carefully dissecting the adhesions.
a. The descending colon was transected with scissor. b. End to end anastomosis was performed using continuous one layer sutures. c. Final of colonic anastomosis. d. Median incision was closed with a 3/0 polypropilen sutures.
PRP preparation
Blood taken from each rat was used to prepare their own PRP during 3 consecutive days. No morbidity and mortality was seen in any rats. The tail of each animal in the PRP group was shaved and cleaned with antiseptic solution. The tail veins were placed in the water at 37°C for 5 minutes to allow them to more appearance. Friction with alcohol was performed and 2.5 ml blood was taken from each rat with 21 G cannulae. Blood samples taken from each rats buffered with citrate phosphate dextrose (0.5 ml citrate phosphate dextrose buffer (CPD)) was added in the ratio of 0.5 ml of CPD buffer to 2.5 ml of blood. The blood was then centrifuged at 1000 rpm for 5 min using the manufacturer’s recommendation (Joan Lab Medical, China). The material containing PRP was removed and recentrifuged at 3000 rpm for 10 minutes. The PRP was applied anastomotic area. Each 0.5ml of PRP was administered locally.
Anastomotic bursting pressure measurement
The anastomosis was resected with a margin of at least 4 cm on each side with regard to tissue hydroxyproline level followed by measuring the anastomotic bursting pressure (ABP). A 16-gauage silicon catheter was inserted from both sides and feces in the resected column segment were cleared with 0.9% NaCl, then the both part of colon segment was ocluded with 3/0 silk. Isotonic saline was administered at 5ml/min rate with an infusion pump (perfusor secura FT-Braun) while the pressure within the lumen was monitored via the transducer of a pressure monitoring system connected to the other catheter. ABP was defined as the pressure at which saline leak was observed and corresponded with the maximum pressure attained just before rupture of the anastomosis.
Tissue hydroxyproline level measurement
After measuring ABP, 1 cm of the anastomosis including 0.5 cm proximal and distal from the anastomosis were excised and blunt dissection was performed to clear the anastomotic line from adherent tissues. The tissues were cooled in the room heat and neutralized with potassium hydroxide in the phenolphthalein solution indicator. The volumes of all of them were equalized and centrifuged. Alanine and potassium borate buffer were added for saturation oxidation after saturation with a certain volume of potassium chloride. Chloramine-T solution, thiosulfate and toluene were added sequentially after standing for twenty minutes in the room temperature. Since the oxyde form of hydroxyproline was not dissolved with toluene, the samples were heated to the soluble form and the hydroxypyrrole organic phase was removed by addition of toluene again. P-dimethylaminobenzaldehyde was added to provide coloring. The resulting color and intensity were evaluated spectrophotometrically at 560 nm.
Measurement of cytokines and procalcitonin
Blood samples were collected at the 24th postoperative hour, and on the 3rd and 7th( just before sacrifice) postoperative days. We performed enzyme-linked immunosorbent assays (ELISAs) (Eclectica, Germany) to measure levels of IL-6 ( reference range : < 17.4 pg/mL), IL-10( reference range : < 2 pg/mL) and procalcitonin levels( reference range : < 0.15 ng/mL) in collected blood samples of the rats.
Histopathological analysis
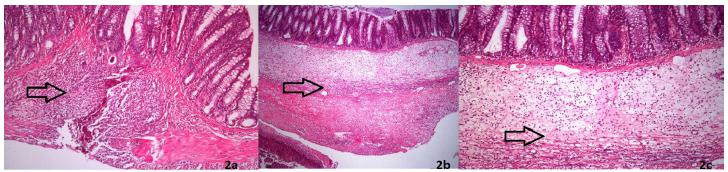
The sample was taken containing the whole anastomosis line and fixed in 10% formaldehyde then embedded in paraffin. Tissues were sectioned at 4 μm and stained with hematoxylin-eosin for evaluating inflammation parameters and Masson trichrome for collagen. Healing parameters fibroblastic infiltration, capillary vascularisation and inflammatory infiltrations in each specimen were assessed with a score of 1-5 for each parameter. Masson’s trichrome stain was assessed for the presence or absence of regular collagen fibers. The degree of collagen deposition was assessed by pathologists.
Statistical analysis
Statistical analyzes were done using (SPSS version 20.0 for Windows; SPSS Inc, Chicago, IL) Kruskal Wallis test was used in the comparison of the groups. Chi-square test was used to compare the three groups in the parameters where significant differences were found. P<0.05 was accepted as statistically significant.
Results
One rat died in each Group 2 and Group 3 and 2 rats died in each Group 1 during the follow-up. These cases were excluded from the study. There is no significant difference in baseline weight values between the three groups. Although the mean body weights were reduced in all groups during the study period, there was no statistical difference among the groups in terms of body weight changes. No local or systemic complications related to PRP and fibrin glue application were observed. There was no statistically significant difference of the ABP between Group 2 and Group 3 (Group1:110.1±35.65mmHg, Group 2: 146.3±44.55 mmHg, Group 3:149.1±72.29 mmHg) of the rats after sacrification on the 7th day (Table 1). There was no statistically significant difference of hydroxyproline levels between Group 2 and Group 3 (Group 1: 96.2±29.22μg/mg, Group 2: 120.1±51.50 μg/mg, Group3:118.71±42.18 μg/mg) on the 7th day of rats (Table 1). There was no statistically significant difference between levels of proinflammatory cytokine (IL-6) (P=0.41), anti-inflammatory cytokine (IL-10) (P=0.35), and procalcitonin levels (P=0.63) on 1, 3 and 7 days (Table 2). There was no statistically significant difference indicators of wound healing parameters (inflammatory infiltrations, capillary vascularisation, fibroblastic infiltration) between Group 2 and Group 3 (p=0.316, Figure 2). Additionally, there was no statistically significant difference in terms of collagen formation between the groups. No significant differences were observed in the comparisons for inflammatory cell (P > 0.05). Fibroblast density and neovascularization showed no differences in the PRP compared to fibrin glue group.
a. Control (Group1): moderate mononuclear cell infiltration with fibroblastic activity. b. PRP (Group 2): moderate infiltration of inflammatory cells with moderate collagen synthesis. c. Fibrin glue (Group 3):moderate inflammatory cell infiltration with moderate fibrosis (H&E ×40).
Dıscussıon
Leakage of anastomoses is a challenging problem causing morbidity and mortality in colorectal surgery in several clinical studies. Many local and systemic factors play a role in the healing of colon anastomoses77 Schrock TR, Deveney CW, Dunphy JE. Factor contributing to leakage of colonic anastomoses. Ann Surg. 1973;177(5):513-8. PMID: 4540874.,88 Strunden MS, Heckel K, Goetz AE, Reuter DA. Perioperative fluid and volume management:physiological basis, tools and strategies.Ann Intensive Care. 2011;1(1):2. PMID: 21906324.. These factors are directly or indirectly influential in the healing process. Numerous procedure and methods are being used to applied intestinal anastomoses that all have the same aim to constitute the safest anastomosis99 Zhou B, Ren J, Ding C, Wu Y, Chen J, Wang G, Gu G, Li J.Protection of colonic anastomosis with platelet-rich plasma gel in the open abdomen. Injury. 2014;45(5):864-8. PMID: 24552769.,1010 Kanellos D, Mantzoros L, Goulirnaris, Zacharakis E, Zavitsanakis A, Betsis D. Effects of the use of fibrin glue around the colonic anastomosis of the rat. Tech Coloproctol. 2003;7(2):82-4. PMID: 14605925.. Therefore, local tissue materials have been used for years and the most important examples of these materials are fibrin glue and PRP.
Fibrin glue was initially intended for hemostasis and control bleeding during surgical procedures1111 Martinowitz U, Schulman S. Fibrin sealent in surgery of patients with hemorrhagic diathesis. Tromb Haemost. 1995;74:486-92. PMID: 8578511.. Due to the biological adhesive material made from concentrated fibrinogen, it started to be used in wound healing. It is a water-resistant cover and can thus constitute a physical barrier around the anastomosis. Clinical reports indicate that it might prevent anastomotic leakage after colonic operations. Kanellos et al.1010 Kanellos D, Mantzoros L, Goulirnaris, Zacharakis E, Zavitsanakis A, Betsis D. Effects of the use of fibrin glue around the colonic anastomosis of the rat. Tech Coloproctol. 2003;7(2):82-4. PMID: 14605925. reported that the application of fibrin glue around a sutured anastomosis reduced the rate of anastomotic leaks and strengthened the anastomosis. In a study by Akgün et al.1212 Akgün A, Kuru S, Uraldi C, Tekin O, Karip B, Tug T, Ongören AU. Early effects of fibrin sealant on colonic anastomosis in rats: an experimental and case-control study. Tech Coloproctol. 2006;10(3):208-14. PMID: 16969615., sutured colocolic anastomosis has been compared to the application of fibrin glue over the sutures. When subjects were compared in terms of anastomosis safety on the 72nd hour postoperatively, they have detected higher bursting pressures of the group that was applied with fibrin glue and have defended safer anastomosis1212 Akgün A, Kuru S, Uraldi C, Tekin O, Karip B, Tug T, Ongören AU. Early effects of fibrin sealant on colonic anastomosis in rats: an experimental and case-control study. Tech Coloproctol. 2006;10(3):208-14. PMID: 16969615..
PRP is generraly applied locally in clinical and experimental studies but systemic use is rare1313 Anitua E, Andia I, Ardanza B, Nurden P, Nurden AT.Autologous platelets as a source of proteins for healing and tissue regeneration.Thromb Haemost. 2004;91(1):4-15. PMID: 14691563.,1414 Yamaguchi R, Terashima H, Yoneyama S, Tadano S, Ohkohchi N. Effects of platelet-rich plasma on intestinal anastomotic healing in rats: PRP concentration is a key factor. J Surg Res. 2012;173(2):258-66. PMID: 21074782.. PRP application to gastrointestinal anastomosis is one of the most useful methods to deliver concentrated amounts of growth factors throughout the surgical site, and expected to accelerate1313 Anitua E, Andia I, Ardanza B, Nurden P, Nurden AT.Autologous platelets as a source of proteins for healing and tissue regeneration.Thromb Haemost. 2004;91(1):4-15. PMID: 14691563.,1414 Yamaguchi R, Terashima H, Yoneyama S, Tadano S, Ohkohchi N. Effects of platelet-rich plasma on intestinal anastomotic healing in rats: PRP concentration is a key factor. J Surg Res. 2012;173(2):258-66. PMID: 21074782.. Many studies showed positive effects of PRP on intestinal anastomoses. In a study conducted by Zhou Bo et al.1515 Zhou B, Ren J, Ding C, Wu Y, Chen J, Wang G, Gu G, Li J. Protection of colonic anastomosis with platelet-rich plasma gel in the open abdomen. Injury. 2014;45(5):864-8. PMID: 24552769., 10 control groups and 10 open-abdominal rat groups compaired that PRP was effective in intestinal anastomoses in open abdomen cases. In another study conducted by Serdar et al.1616 Yol S, Tekin A, YilmazH, Küçükkartallar T, Esen H, Caglayan O, Tatkan Y. Effects of platelet rich plasma on colonic anastomosis. J Surg Res. 2008;146(2):190-4. PMID: 18028949. compared PRP and bioglue and they found that PRP is superior to control and bioglue group in the recovery of intestinal anastomosis. Another experimental study by Alper et al.1717 Sozutek A, Colak T, Cetinkunar S, Reyhan E, Irkorucu O, Polat G, Cennet A. The effect of platelet-rich-plasma on the healing of left colonic anastomosis in a rat model of intra-abdominal sepsis. J Invest Surg. 2016;29(5):294-301. PMID: 26822265. demonstrated that PRP has a positive recovery on intestinal anastomosis in the sespis model.
The most important structure in the healing of the anastomosis is obtaining the tensile strength in the submucosal connective tissue and the collagen contained. In the study of Irvin et al.1818 Irvin TT.Collagen metabolism in infected colonic anastamoses. Surg Gynecol Obstet. 1976;143(2):220-4. PMID: 941076. on rats, level of collagen tissue decreased after the 3rd day and their value getting to normal approximately on the seventh day. In the study of Croin et al.1919 Croinin K, Jackson DS, Dunphy JE. Specific activity of hidroxyproline tritium in the healing colon. Surg Gynecol Obstet. 1968;126(5):1061-5. PMID: 5652652. anastomotic bursting pressure gradually increased from the third day after the anastomosis and reaching maximum in 7th day. At the same time they found 40% of decrease the concentration of hydroxyproline levels in the first 3 days on the anastomotic region and normalized 5th day. Thus, although the debate continues about the postoperative day for measuring the ABP, we evaluated the bursting pressure on postoperative day 7 since anastomotic leakage is often diagnosed in the first postoperative week in clinical practice. In this study, there were no difference in ABP and hydroxyproline levels between the groups of PRP and fibrin glue on the 7th day .
Cytokine levels are frequently used to assess intestinal anastomoses as well as indicators of inflammatory parameters2020 Herwig R, Glodny B, Kuhle C, Schluter B, Brinkmann OA, Strasser H, Senninger N, Winde G. Early identification of peritonitis by peritoneal cytokine measurement. Dis Colon Rectum. 2002;45(4):514-21. PMID: 12006934.
21 Marques e Silva S, Jerônimo MS, Silva-Pereira Id, Tavares AH, Bocca AL, Sousa JB. Effects of metoclopramide on the expression of metalloproteinases and interleukins in left colonic anastomoses. An experimental study. Acta Cir Bras. 2015;30(11):762-9. PMID: 26647796.
22 Ishimura K, Moroguchi A, Okano K, Maeba T, Maeta H. Local expression of tumor necrosis factor-alpha and interleukin-10 on wound healing of intestinal anastomosis during endotoxemia in mice. J Surg Res. 2002;108(1):91-7. PMID: 12443720.-2323 Mateo RB, Reichner JS, Albina JE. Interleukin-6 activity in wounds. Am J Physiol. 1994;266(6):1840-4. PMID: 8024036.. In many studies in the literature, cytokine levels have important role for assessing tissue healing degree with numerical data. The cytokine levels of proinflammatory (IL-1, IL-6, TNF-α) and anti-inflammatory (IL-4, IL-10) cytokines are in continuous relationship with each other during the tissue regeneration process2424 Sousa JB, Soares EG, Aprilli F. Effects of diclofenac sodium on intestinal anastomotic healing. Experimental study on the small intestine of rabbits. Dis Colon Rectum.1991;34(7):613-7. PMID: 2055147..Studies conducted with proinflammatory (especially IL-6 and TNF-α) cytokine levels have been shown to be important predictors of early postoperative complications and these parameters related to anastomosis evaluation in literature is often done on similar days as our study2525 DeCherney AH, diZerega GS. Clinical problem of intraperitoneal postsurgical adhesion formation following general surgery and the use of adhesion prevention barriers. Surg Clin North Am. 1997;77(3):671-88. PMID: 9194886. IL-10 is the most important of the anti-inflammatory cytokines and is responsible for suppressing the pro-inflammatory cytokines2222 Ishimura K, Moroguchi A, Okano K, Maeba T, Maeta H. Local expression of tumor necrosis factor-alpha and interleukin-10 on wound healing of intestinal anastomosis during endotoxemia in mice. J Surg Res. 2002;108(1):91-7. PMID: 12443720.. In a study conducted by Herwig et al.2020 Herwig R, Glodny B, Kuhle C, Schluter B, Brinkmann OA, Strasser H, Senninger N, Winde G. Early identification of peritonitis by peritoneal cytokine measurement. Dis Colon Rectum. 2002;45(4):514-21. PMID: 12006934., early elevation of IL-6 and TNF-α levels and higher levels of IL-1 on 3rd day were shown as a marker of intestinal anastomosis leaks. Also procalcitonin levels are considered to be an important indicator of acute inflammation (especially in bacterial infection). It has important role in predicting intestinal anastomotic leaks in clinical practice2626 Rongione AJ, Kusske AM, Ashley SW, Reber HA, McFadden DW. Interleukin-10 prevents early cytokine release in severe intraabdominal infection and sepsis. J Surg Res. 1997;70(2):107-12. PMID: 9237883.,2727 Novotny AR, Emmanuel K, Hueser N, Knebel C, Kriner M, Ulm K, Bartels H, Siewert JR, Holzmann B. Procalcitonin ratio indicates successful surgical treatment of abdominal sepsis. Surgery. 2009;145:20-6. PMID: 19081471.. In our study we found no difference between groups of the cytokine (IL-6 and IL-10) and procalcitonin levels at 1,3 and 7 days. Although the anastomotic power and hydroxyproline levels are more effective than Group 1, they are not reflected in the inflammatory parameters. We interpreted that local application of the anastomosis healing systematically did not affect the laboratory values.
Conclusıon
Fibrin glue and platelet rich plasma are shown to be effective in healing colon anastomoses we found no differences between fibrin glue and PRP effects of colonic anastomosis significantly superior to each other.
References
-
1Walker KG, Bell SW, Rickard MJ. Mehanna D, Dent OF, Chapuis PH, Bokey EL.Anastomotic leakage is predictive of diminished survival after potentially curative resection for colorectal cancer. Ann Surg. 2004;240(2):255-9. PMID: 15273549.
-
2Law WL, Choi HK, Lee YM, Ho JW, Seto CL. Anastomotic leakage is associated with poor long-term outcome in patients after curative colorectal resection for malignancy. J Gastrointest Surg. 2007;11(1):8-15. PMID:17390180.
-
3Kingham TP, Pachter HL. Colonic anastomotic leak: risk factors, diagnosis, and treatment. J Am Coll Surg. 2009;208(2):269-78. PMID: 19228539.
-
4Buchs NC, Gervaz P, Secic M, Bucher P, Mugnier-Konrad B, Moerl P. Incidence, consequences, and risk factors for anastomotic dehiscence after colorectal surgery: a prospective monocentric study. Int J Colorectal Dis. 2008;23(3):265-70. PMID: 18034250.
-
5Yamaguchi R, Terashima H, Yoneyama S, Tadano S, Ohkohchi N. Effects of plateletrichplasma on intestinal anastomotic healing in rats: PRP concentration is a key factor. J SurgRes. 2012;173(2):258-66. PMID: 21074782.
-
6Bonanomi G, Prince JM, McSteen F, Schauer PR, Hamad GG. Sealing effect of fibrin glue on the healing of gastrointestinal anastomoses: implications for the endoscopic treatment of leaks. Surg Endosc. 2004 Nov;18(11):1620-4. PMID: 15931477.
-
7Schrock TR, Deveney CW, Dunphy JE. Factor contributing to leakage of colonic anastomoses. Ann Surg. 1973;177(5):513-8. PMID: 4540874.
-
8Strunden MS, Heckel K, Goetz AE, Reuter DA. Perioperative fluid and volume management:physiological basis, tools and strategies.Ann Intensive Care. 2011;1(1):2. PMID: 21906324.
-
9Zhou B, Ren J, Ding C, Wu Y, Chen J, Wang G, Gu G, Li J.Protection of colonic anastomosis with platelet-rich plasma gel in the open abdomen. Injury. 2014;45(5):864-8. PMID: 24552769.
-
10Kanellos D, Mantzoros L, Goulirnaris, Zacharakis E, Zavitsanakis A, Betsis D. Effects of the use of fibrin glue around the colonic anastomosis of the rat. Tech Coloproctol. 2003;7(2):82-4. PMID: 14605925.
-
11Martinowitz U, Schulman S. Fibrin sealent in surgery of patients with hemorrhagic diathesis. Tromb Haemost. 1995;74:486-92. PMID: 8578511.
-
12Akgün A, Kuru S, Uraldi C, Tekin O, Karip B, Tug T, Ongören AU. Early effects of fibrin sealant on colonic anastomosis in rats: an experimental and case-control study. Tech Coloproctol. 2006;10(3):208-14. PMID: 16969615.
-
13Anitua E, Andia I, Ardanza B, Nurden P, Nurden AT.Autologous platelets as a source of proteins for healing and tissue regeneration.Thromb Haemost. 2004;91(1):4-15. PMID: 14691563.
-
14Yamaguchi R, Terashima H, Yoneyama S, Tadano S, Ohkohchi N. Effects of platelet-rich plasma on intestinal anastomotic healing in rats: PRP concentration is a key factor. J Surg Res. 2012;173(2):258-66. PMID: 21074782.
-
15Zhou B, Ren J, Ding C, Wu Y, Chen J, Wang G, Gu G, Li J. Protection of colonic anastomosis with platelet-rich plasma gel in the open abdomen. Injury. 2014;45(5):864-8. PMID: 24552769.
-
16Yol S, Tekin A, YilmazH, Küçükkartallar T, Esen H, Caglayan O, Tatkan Y. Effects of platelet rich plasma on colonic anastomosis. J Surg Res. 2008;146(2):190-4. PMID: 18028949.
-
17Sozutek A, Colak T, Cetinkunar S, Reyhan E, Irkorucu O, Polat G, Cennet A. The effect of platelet-rich-plasma on the healing of left colonic anastomosis in a rat model of intra-abdominal sepsis. J Invest Surg. 2016;29(5):294-301. PMID: 26822265.
-
18Irvin TT.Collagen metabolism in infected colonic anastamoses. Surg Gynecol Obstet. 1976;143(2):220-4. PMID: 941076.
-
19Croinin K, Jackson DS, Dunphy JE. Specific activity of hidroxyproline tritium in the healing colon. Surg Gynecol Obstet. 1968;126(5):1061-5. PMID: 5652652.
-
20Herwig R, Glodny B, Kuhle C, Schluter B, Brinkmann OA, Strasser H, Senninger N, Winde G. Early identification of peritonitis by peritoneal cytokine measurement. Dis Colon Rectum. 2002;45(4):514-21. PMID: 12006934.
-
21Marques e Silva S, Jerônimo MS, Silva-Pereira Id, Tavares AH, Bocca AL, Sousa JB. Effects of metoclopramide on the expression of metalloproteinases and interleukins in left colonic anastomoses. An experimental study. Acta Cir Bras. 2015;30(11):762-9. PMID: 26647796.
-
22Ishimura K, Moroguchi A, Okano K, Maeba T, Maeta H. Local expression of tumor necrosis factor-alpha and interleukin-10 on wound healing of intestinal anastomosis during endotoxemia in mice. J Surg Res. 2002;108(1):91-7. PMID: 12443720.
-
23Mateo RB, Reichner JS, Albina JE. Interleukin-6 activity in wounds. Am J Physiol. 1994;266(6):1840-4. PMID: 8024036.
-
24Sousa JB, Soares EG, Aprilli F. Effects of diclofenac sodium on intestinal anastomotic healing. Experimental study on the small intestine of rabbits. Dis Colon Rectum.1991;34(7):613-7. PMID: 2055147.
-
25DeCherney AH, diZerega GS. Clinical problem of intraperitoneal postsurgical adhesion formation following general surgery and the use of adhesion prevention barriers. Surg Clin North Am. 1997;77(3):671-88. PMID: 9194886
-
26Rongione AJ, Kusske AM, Ashley SW, Reber HA, McFadden DW. Interleukin-10 prevents early cytokine release in severe intraabdominal infection and sepsis. J Surg Res. 1997;70(2):107-12. PMID: 9237883.
-
27Novotny AR, Emmanuel K, Hueser N, Knebel C, Kriner M, Ulm K, Bartels H, Siewert JR, Holzmann B. Procalcitonin ratio indicates successful surgical treatment of abdominal sepsis. Surgery. 2009;145:20-6. PMID: 19081471.
-
Financial source:
none
-
1
Research performed at Experimental Medicine Research and Application Center, Cukurova University, Adana, Turkey.
Publication Dates
-
Publication in this collection
Apr 2018
History
-
Received
26 Dec 2017 -
Reviewed
22 Feb 2018 -
Accepted
23 Mar 2018