Abstracts
The authors report five cases of cystic dilatation of the common bile duct Type I (Todani’s classification) in adults patients, in Division of General Surgery of a University Hospital, treated over a- 25-year- period from 1974 to 1999, among 16.057 operations, and not previously published. Diagnosis was obtained by operative cholangiogram (OC) in the first case, percutaneous transhepatic cholangiogram on the second one (PTHC) and by ultrasonography (US), endoscopic retrograde cholangiopancreatography (ERCP), and operative cholangiogram (OC), respectively, on the last three cases. The second patient had an adenocarcinoma arising in the cystic wall associated with peritoneal metastasis. The first two cases were treated by internal drainage and the last three by excision of the cysts and bilioenteric anastomoses. Classification, incidence, etiology, diagnosis, malignization and surgical treatment of biliary cystic disease (BCD) were revised, with the conclusion that resection must be the preferable method of treatment, when possible, especially due to the concern of malignization.
Common bile duct; Adult; Classification; Diagnosis
Os autores apresentam cinco casos de dilatação cística do ducto biliar comum do Tipo I (classificação de Todani) em adultos, anteriormente não relatados, num período de 25 anos no Servico de Cirurgia Geral de um Hospital Universitário, entre 16.057 operacões, no período de 1974 e 1999. O diagnóstico dos cistos foi realizado através de colangiografia operatória (CO) no primeiro, por colangiografia transparietohepática no segundo (CTPH) e por ultra-sonografia (US), colangiopancreatografia endoscópica retrógrada (CPER) and colangiografia operatória (CO), respectivamente, nos três últimos casos. Em um dos pacientes, foi detectado adenocarcinoma localizado na parede posterior do cisto, associado à metástases peritoniais. Os dois primeiros casos foram tratados através de derivação cistoentérica, sendo nos três últimos realizada a excisão do cisto, seguida de hepaticojejunostomia em Y de Roux. Foram revisados classificação, incidência, etiologia, diagnóstico, malignização e tratamento cirúrgico da doença cistica biliar (DCB), concluindo-se que a terapêutica cirúrgica de escolha deve ser a ressecção, quando possível, sobretudo devido ao risco significativo de malignização.
Ducto biliar comum; Adulto; Classificação; Diagnóstico
9 - CASE REPORT
CYSTIC DILATATION OF THE COMMON BILE DUCT IN ADULTS: REPORT OF FIVE CASES AND REVIEW OF LITERATURE11. From the Division of General Surgery, Universitary Hospital, Federal University of Pernambuco Brazil.. From the Division of General Surgery, Universitary Hospital, Federal University of Pernambuco Brazil.2. Associated Professor, Master, Department of Surgery, University Hospital , Federal University of Pernambuco Brazil.3. Associated Professor, Ph.D., Department of Surgery, University Hospital, Federal University of Pernambuco Brazil.4. Professor of Post-Graduation Courses, Ph.D., Department of Surgery, University Hospital, Federal University of Pernambuco Brazil.5. Full Professor, Ph.D., Department of Surgery, University Hospital, Federal University of Pernambuco Brazil.
Pedro Carlos Loureiro de Arruda21. From the Division of General Surgery, Universitary Hospital, Federal University of Pernambuco Brazil.. From the Division of General Surgery, Universitary Hospital, Federal University of Pernambuco Brazil.2. Associated Professor, Master, Department of Surgery, University Hospital , Federal University of Pernambuco Brazil.3. Associated Professor, Ph.D., Department of Surgery, University Hospital, Federal University of Pernambuco Brazil.4. Professor of Post-Graduation Courses, Ph.D., Department of Surgery, University Hospital, Federal University of Pernambuco Brazil.5. Full Professor, Ph.D., Department of Surgery, University Hospital, Federal University of Pernambuco Brazil.
Antonio Roberto de Barros Coelho31. From the Division of General Surgery, Universitary Hospital, Federal University of Pernambuco Brazil.. From the Division of General Surgery, Universitary Hospital, Federal University of Pernambuco Brazil.2. Associated Professor, Master, Department of Surgery, University Hospital , Federal University of Pernambuco Brazil.3. Associated Professor, Ph.D., Department of Surgery, University Hospital, Federal University of Pernambuco Brazil.4. Professor of Post-Graduation Courses, Ph.D., Department of Surgery, University Hospital, Federal University of Pernambuco Brazil.5. Full Professor, Ph.D., Department of Surgery, University Hospital, Federal University of Pernambuco Brazil.
José Falcão Corrêa Lima Filho41. From the Division of General Surgery, Universitary Hospital, Federal University of Pernambuco Brazil.. From the Division of General Surgery, Universitary Hospital, Federal University of Pernambuco Brazil.2. Associated Professor, Master, Department of Surgery, University Hospital , Federal University of Pernambuco Brazil.3. Associated Professor, Ph.D., Department of Surgery, University Hospital, Federal University of Pernambuco Brazil.4. Professor of Post-Graduation Courses, Ph.D., Department of Surgery, University Hospital, Federal University of Pernambuco Brazil.5. Full Professor, Ph.D., Department of Surgery, University Hospital, Federal University of Pernambuco Brazil.
Ricardo José Caldas Machado31. From the Division of General Surgery, Universitary Hospital, Federal University of Pernambuco Brazil.. From the Division of General Surgery, Universitary Hospital, Federal University of Pernambuco Brazil.2. Associated Professor, Master, Department of Surgery, University Hospital , Federal University of Pernambuco Brazil.3. Associated Professor, Ph.D., Department of Surgery, University Hospital, Federal University of Pernambuco Brazil.4. Professor of Post-Graduation Courses, Ph.D., Department of Surgery, University Hospital, Federal University of Pernambuco Brazil.5. Full Professor, Ph.D., Department of Surgery, University Hospital, Federal University of Pernambuco Brazil.
Ayrton Ponce de Souza41. From the Division of General Surgery, Universitary Hospital, Federal University of Pernambuco Brazil.. From the Division of General Surgery, Universitary Hospital, Federal University of Pernambuco Brazil.2. Associated Professor, Master, Department of Surgery, University Hospital , Federal University of Pernambuco Brazil.3. Associated Professor, Ph.D., Department of Surgery, University Hospital, Federal University of Pernambuco Brazil.4. Professor of Post-Graduation Courses, Ph.D., Department of Surgery, University Hospital, Federal University of Pernambuco Brazil.5. Full Professor, Ph.D., Department of Surgery, University Hospital, Federal University of Pernambuco Brazil.
Carlos Augusto de Carvalho Mathias31. From the Division of General Surgery, Universitary Hospital, Federal University of Pernambuco Brazil.. From the Division of General Surgery, Universitary Hospital, Federal University of Pernambuco Brazil.2. Associated Professor, Master, Department of Surgery, University Hospital , Federal University of Pernambuco Brazil.3. Associated Professor, Ph.D., Department of Surgery, University Hospital, Federal University of Pernambuco Brazil.4. Professor of Post-Graduation Courses, Ph.D., Department of Surgery, University Hospital, Federal University of Pernambuco Brazil.5. Full Professor, Ph.D., Department of Surgery, University Hospital, Federal University of Pernambuco Brazil.
Álvaro Antônio Bandeira Ferraz31. From the Division of General Surgery, Universitary Hospital, Federal University of Pernambuco Brazil.. From the Division of General Surgery, Universitary Hospital, Federal University of Pernambuco Brazil.2. Associated Professor, Master, Department of Surgery, University Hospital , Federal University of Pernambuco Brazil.3. Associated Professor, Ph.D., Department of Surgery, University Hospital, Federal University of Pernambuco Brazil.4. Professor of Post-Graduation Courses, Ph.D., Department of Surgery, University Hospital, Federal University of Pernambuco Brazil.5. Full Professor, Ph.D., Department of Surgery, University Hospital, Federal University of Pernambuco Brazil.
Edmundo Machado Ferraz51. From the Division of General Surgery, Universitary Hospital, Federal University of Pernambuco Brazil.. From the Division of General Surgery, Universitary Hospital, Federal University of Pernambuco Brazil.2. Associated Professor, Master, Department of Surgery, University Hospital , Federal University of Pernambuco Brazil.3. Associated Professor, Ph.D., Department of Surgery, University Hospital, Federal University of Pernambuco Brazil.4. Professor of Post-Graduation Courses, Ph.D., Department of Surgery, University Hospital, Federal University of Pernambuco Brazil.5. Full Professor, Ph.D., Department of Surgery, University Hospital, Federal University of Pernambuco Brazil.
Arruda PCL, Coelho ARB, Lima Filho JFC, Machado RJC, Souza AP, Mathias CAC, Ferraz AAB, Ferraz EM. Cystic dilatation of the common bile duct in adults: report of five cases and review of literature. Acta Cir Bras [serial online] 2000 Oct-Dec;15(4). Available from: URL: http://www.scielo.br/acb.
ABSTRACT: The authors report five cases of cystic dilatation of the common bile duct Type I (Todanis classification) in adults patients, in Division of General Surgery of a University Hospital, treated over a- 25-year- period from 1974 to 1999, among 16.057 operations, and not previously published. Diagnosis was obtained by operative cholangiogram (OC) in the first case, percutaneous transhepatic cholangiogram on the second one (PTHC) and by ultrasonography (US), endoscopic retrograde cholangiopancreatography (ERCP), and operative cholangiogram (OC), respectively, on the last three cases. The second patient had an adenocarcinoma arising in the cystic wall associated with peritoneal metastasis. The first two cases were treated by internal drainage and the last three by excision of the cysts and bilioenteric anastomoses. Classification, incidence, etiology, diagnosis, malignization and surgical treatment of biliary cystic disease (BCD) were revised, with the conclusion that resection must be the preferable method of treatment, when possible, especially due to the concern of malignization.
SUBJECT HEADINGS: Common bile duct. Adult. Classification. Diagnosis.
INTRODUCTION
Herein, among 16.057 operations performed in the General Surgery Division at a University Hospital, between 1974 and 1999, five cases of common bile duct cystic dilatation are presented, having an adenocarcinoma been detected in one of them.
Although relatively rare in Western countries, biliary cystic disease (BCD) is more often found in Eastern populations, mainly among Japanese. Vater, quoted by Flanigan1, addressed on the first reported case in 1723. In the world literature Alonso - Lej et alli2 issued a review on 403 cases in 1959 and Flanigan reviewed 955 cases in 19751,3. Yamaguchi analyzed 1433 cases in the Japanese literature in 19804. In these three reviews a prevalence of female gender was noted, while the most affected groups were represented by children and young adults. In 1997 it was estimated that more than 3.000 cases of BCD have been reported5. The most important complication of BCD is association of malignant neoplasm4. In Brazil, incidence of BCD is unknown. However several short series have been published6,7,8,9,10,11,12,13,14,15,16. Probably, the national incidence of BCD may be compared to the Western populations. Etiology is uncertain, being considered embriogenic acquired or combined mechanisms. In adults, BCD must be included as a differential diagnosis among the most common biliary diseases. Imaging methods of diagnosis currently used in disease of biliary tree are accurate for diagnosis of BCD. New methods as MRCP are promising for diagnosis of anatomical and functional features of BCD without potential complication of other procedures. Excisional surgery of cysts is aimed mainly to prevent cancer arising in the biliary dilatation. Classification, incidence, etiologic concepts, imaging diagnostic approaches, and surgical management were revised.
CASES REPORTS
Case no 1 A-19- year old white female patient complained when admitted of intermittent abdominal pain, jaundice and mass in the right upper quadrant over the last three years. Physical examination, on admission, revealed no jaundice and showed a smooth, mobile cystic mass in the upper superior quadrant, measuring 11 cm from the right costal margin. An intravenous pyelography and a barium swallow showed extrinsic compression. By September 1974 an exploratory laparotomy was undertaken during which an infrahepatic cystic mass was confirmed, and an operative cholangiogram (OC) was consistent with a choledochal cyst Type I according to Todanis classification (Table1-Figure1).A Roux-en-Y choledochocystojejunostomy was performed and specimen examination from cyst wall revealed fibrous tissue without epithelial lining, chronic inflammatory changes and no evidence of malignancy. Liver biopsy showed features of biliary cirrhosis. The patient was discharged on the 12th post-operative day after an uneventful recovery and was found assymptomatic after a-5-y-follow up.
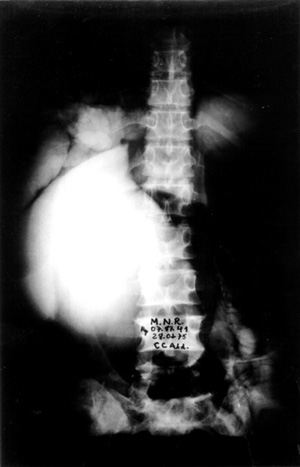
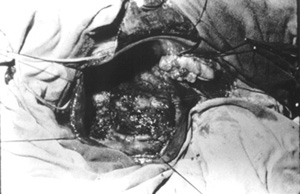
Case no 2 A- 46 year old white woman was seen at the outpatient clinic with a-five-month lasting history of colicky epigastric and upper right quadrant abdominal pain, bilious vomits, abdominal mass, progressive increasing of abdominal girth and weight loss of about 15 kg. Episode of jaundice, pain, dark urine and increased volume of right upper quadrant was observed at the age of 2 years old. Physical examination showed jaundice and a smooth, regular cystic and mobile epigastric and upper right quadrant (URQ) growth, measuring 17 cm from the costal margin. Blood tests revealed moderate anemia and increased bilirrubins, ALT, AST and alkaline fosfatase, Upper Gl barium study and coeliac axis arteriography showed displacement of the surrounding structures due toto compression. An ERCP failed to demonstrate the distal end of biliary duct and the cyst, showing the duct of Wirsung displaced downward and to the left side. A percutaneous transhepatic cholangiography (PTC) demonstrated a large cystic dilatation of the common bile duct Type I (Table 1 - Figure 2). Operated on February 1975, a common bile duct cyst was confirmed, containing biliary stones and a vegetating tumor at the posterior wall (Figure 3), as well as diffuse carcinomatosis.Surgical exploration of papilla of Vater disclosed obstruction of common bile duct.A latero-lateral choledochocystoduodenostomy was performed and histology of cystic wall and omentum showed adenocarcinoma. The patient died four months later.
- Percutaneous transhepatic cholangiography (PTC) showing a large cystic dilatation of common bile duct (Todanis Type I) ,and contrast in the small intestine from a previous ERCP(Case no 2).
- Vegetating tumor (adenocarcinoma) found at the posterior wall of biliary duct dilatation (BCD) (Case no 2).
Case no 3 A-24- year old black male was admitted with history of acute severe right upper quadrant pain, which subsided with antispasmodics, and vomits. Four other similar episodes were noticed over the last four years. Physical examination was normal, except for slight jaundice. An abdominal ultrasound demonstrated a gallbladder containing stones and a cystic dilatation corresponding to the middle and distal thirds of the common bile duct also housing echogenic images with acoustic shadowing. An endoscopic retrograde cholagiopancreatography (ERCP) showed complete filling of the intra and extrahepatic biliary system, outlining an 8 cm,0 in diameter cystic dilatation of the distal two thirds of the common bile duct, clearing sparing the proximal third, and containing luscent images (Figure 4). A laparotomy performed on July 1992, revealed a 10 cm thin walled Type I cystic lesion (Table 1) involving the middle and distal thirds of the common bile duct, not allowing the visualization of the cystic duct common bile duct junction. Except for the presence of stones the gallbladder looked quite normal. A resection of the cystic lesion down to a point below the upper edge of the duodenum and a cholecystectomy were undertaken followed by a Roux-en-Y termino-lateral hepaticojejunostomy. The specimen examination demonstrated chronic cholecystitis and choledochal cyst without epithelial lining and presence of granulation tissue. Malignancy features were not apparent. The postoperative recovery was uneventful and the patient was discharged by the 6th post-operative day, being assymptomatic 30 months after de operation.
- Endoscopic retrograde cholangiopancreato- graphy (ERCP) showing choledochal cyst Type I (Todanis classification), dilatation of extrahepatic and intrahepatic biliary system, as well as lucent images inside the cyst (Case no 3).
Case no 4 A-42-year old white lady complained of severe upper right quadrant colicky pain since her childhood, worsening her symptoms over the last five years. When seen by the first time she was assymptomatic and the physical examination was normal. With the diagnosis of cholelithiasis she was submitted to an ultrasonography which showed a 3.8 x 3.7 cm extrahepatic bile duct dilatation and acoustic shadowing into the gallbladder (Figure 5). An ERCP confirmed this finding, demonstrating a proximal third of the common bile duct .The Wirsung duct was normal and ecogenic images were visualized inside the cyst. Liver tests were within the normal range. By January 1994 a laparotomy was performed, revealing a Type I (Table 1) choledochal cyst involving the distal two thirds of the common bile duct and a gallbladder containing stones. A cholecystectomy and resection of the cyst well below the upper edge of the duodenum were undertaken followed by Roux - en - Y terminolateral hepaticojejunostomy. An external biliary fistula followed, which settled down after two weeks with conservative treatment. After 15 months of operation the patient is doing quite well.
- Ultrasonography (US) showing an extrahepatic duct dilatation (38x37mm) and ecogenic images with acoustic shadow into the gallbladder (Case no4).
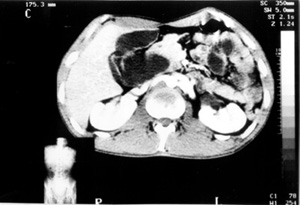
Case no 5 A-27-year old white man was admitted elsewhere with a history of chronic alcoholism, as well as abdominal pain, jaundice, and weight loss of 15 kg during the last three months. At admission, mild to severe jaundice was seen. Liver tests showed evidence of cholestasis and hepatic necrosis. By the time, a diagnosis of alcoholic hepatitis was formulated, and since an ultrasonography showed a cystic image compatible with hepatic cyst, the patient was referred to Federal University Hospital of Pernambuco. On 05.16.95, another US disclosed an intrahepatic biliary dilatation and a 8,7x5,7cm cystic formation in contact with the head of pancreas. A CT scan delineated an extrahepatic dilatation, separated from gallbladder, suggesting a BCD Type I. The head of pancreas and uncinate process were in close relation with the cystic dilatation (Figure 6). Hypothesis of choledochal cyst and pseudocyst of pancreas were then considered. On 06-07-95, an ERCP revealed a choledochal cyst, without visualization of common bile duct and intrahepatic biliary tree. In the same day, during surgery, an operative cholangiogram (OC) confirmed a Type I biliary cystic dilatation, that was completely ressected together with gallbladder, followed by a Roux-en-Y end-to-side hepaticojejunostomy reconstruction. The pathologic examination showed choledochal cyst, chronic cholecistitis, hepatic changes secondary to biliary obstruction, and no evidence of malignancy. Two years afterwards, the patient was doing quite well.
CT scan showing an extrahepatic cystic formation, separated from the gallbladder.Head of pancreas and uncinate process were closely related to the cystic dilatation (Case nº5).
COMMENTS
Classification
Based upon morphologic features and anatomic segments involved, Alonso-Lej et al.2classified the choledochal cysts in three types: Type I - congenital fusiform or sacular cystic dilatation of the common hepatic and/or common bile duct; Type II - supraduodenal congenital diverticulum of the common hepatic or common bile duct; and Type III - congenital choledochocele. Following the same principle, as additional anomalies of the extra and intrahepatic biliary tree have been disclosed by modern image techniques, other classifications have been proposed3,17,18,19, with particular relevancy to Todanis classification20 (Table 1),being these dilatations known as biliary cystic diseases(BCD).
Incidence and malignization
The incidence in the Western countries is 1:2.000.000 of births, as opposed to the incidenceof 1:13.000 of births in Japan, therefore up to 150 times more frequent in the latter21. Biliary cystic diseases are more common in women than in males (4:1)11. Being a problem of childhood (75-85%), up to 20% of the diagnosis are made in adult life22,23. Until 1997, Fieber et al. estimated that more than 3.000 cases of BCD have been reported5. The BCD Type I is more frequent, having occurred in 77.7% of 878 cases reviewed by Yamaguchi4, and in 92% of 359 patients studied by Fonkalsrud24. With a fusiform or cilindric shape it may extend up to the common hepatic duct, involving all the extrahepatic biliary ducts, usually sparing the intrahepatic ducts. One of the most important complications of BCD is the malignization of the cysts25. The incidence of cancer associated with BCD ranges from 0.74% to 28%2,3,4,5,24,26,27,28, occurring more frequently in Type I and Type IV cysts23. The large incidence variation is probably due to sample size reported and occidental or oriental cases procedence. It is more frequent in adult females and adenocarcinoma is the most usual histologic variety reported1
Etiology
The etiology of the choledochal cyst is controversial. Yotsuyanagi29 and others2,4,28,30, assumed that an excessive proliferation of epithelial cells in the primitive choledochus, during the embriogenic phase, when the biliary ducts have a solid structure, would take place. This proliferation, much more active in the proximal segment of common bile duct, rather than in the distal one, would be followed by hipervacuolization which would take to abnormal proximal dilatation and stenosis of the distal end of the biliary ducts.
Babbit31 supported by others18,27,32,33considered that an anomalous biliopancreatic junction (ABPJ) , consisting of a right angle entering of the common bile duct in the duct of Wirsung, at a longer than normal distance from the ampulla of Vater, has been found to be associated with choledochal cysts. This congenital anomaly precludes the development of a sphincter at the junction of common bile duct and pancreatic duct that permits free flow of pancreatic juice into the choledochus, by pressure gradient, producing recurrent bouts of cholangitis. They postulate that as a consequence of reflux of the pancreatic secretion to the choledochus, inflammation, fibrosis, obstruction, dilatation and finally cyst formation took place. As a matter of fact, It is not unusual to find high concentrations of amylase into these cysts. In vivo, through magnetic resonance cholangiopacreatography (MRCP) and secretin estimulation , patients with ABPJ presented reflux to the biliary tract and enlargement of gallbladder, while normal patients had normal duodenal filling, supporting Babbitt,s theory34. Moreover, there are a variety of pancreaticobiliary diseases that has been found to be associated with ABPJ, as BCD, cancer arising into the cyst, gallbladder cancer, common bile duct cancer, gallbladder adenomyomatosis, pancreatitits, cholelithiasis, hilar cholangiocarcinoma and pancreatic cancer. However a common channel, longer than normal, is not seen in all patients with BDC or biliary cancer, and its presence in normal individuals is not exceptional21,35. The role of ABPJ in these conditions remains uncertain, and needs to be further delineated25. As many cases of BCD have been diagnosed during fetal life, when secretory response of human pancreas to secretagogus is minimal or absent, a lesion of common bile duct owing to amylase during pre-natal period is doubtful because this enzyme is not present before birth.
Although there is no convincing theory that explains it, there is one which should be taken into account: 1) the dilatation of the biliary ducts and the anomalous biliopancreatic junction(ABPJ) may occur, simultaneously, during the embrionary development; 2) the dilatation of the biliary ducts may be a secondary abnormality, due to reflux of pancreatic secretion into the biliary tract, consequent to the abnormal biliopancreatic junction.; 3) the biliary dilatation is acquired, secondary to distal stenosis of the biliary tract, which is an integral part of the anomalous junction of the biliopancreatic channels; 4) there are BCD without anomalous biliopancreatic junction, as well ABPJ may exists in absence of BCD; 5) ABPJ may be associated to a variety of other bilio-pancreatic diseases than BCD; 6) the role of ABPJ in these all conditions remains uncertain.
Diagnosis and preoperative studies
The classical clinical triad, represented by pain, jaundice and abdominal mass1,2,5,11,37 , is found in just 13 to 38%38. More usual are the findings of episodic abdominal pain, occurring during months or even years, sometimes associated with mild jaundice that may even be unnoticed. In the present report the prevailing symptoms were abdominal pain and jaundice, claimed by three patients. Severe loss of weight was found in two patients. In one of them it was associated with cancer into the cyst, and in the other it was related to alcoholism and malnutrition. Two patients claimed for episodes of the same actual symptoms and signs since childhood. Abdominal mass was detected in two patients. The laboratory findings are not specific and alterations indicating biliary obstruction with variable levels of hepatic dysfunction may be found, as in the cases herein reported.
The preoperative diagnosis may be accurately established by the modern methods used in patients with suspected biliary tract diseases, like ultrasonography (US),computed tomography(CT), endoscopic retrograde colangiopancreatography (ERCP) and fine needle percutaneous transhepatic colangiography (PTC)38. Ultrasonography, as a non-invasive procedure, is the initial method of choice39,40, being able to make the diagnosis even during the intra-uterine period38. US shows the characteristic of the cyst, separated from gallbladder, communication with dilated common hepatic or intrahepatic ducts, but may underestimate the extent of disease40. Computed tomography (CT) is used to confirm the diagnosis and evaluate the extent of disease40. It has the drawback of using radiation, undesirable in the pediatric age group.
The hepatic nuclear scan employing 99TC binded to iminodiacetic acid derivated, especially the 99TC - DISIDA, allows satisfactory images of the biliary tract with low irradiation to the patient41. Although its results are limited to functional informations rather than anatomical,it may be a complementary method to the US, CT and even inconclusive ERCP. Presence of bile stagnation in a dilated bile duct, despite free flow across the ampulla is considered diagnostic of choledochal cyst21. 99TC-HIDA scintigraphy may be useful in evaluating bilioenteric anastomoses in the follow-up42. More recently, endoscopic ultrasound (EUS), and especially magnetic resonance cholangiopancreatography (MRCP) have drawn an attention since these methods are less invasive than those ever being used43. MRCP has been shown straight correlation with direct cholangiography (ERCP, PTC) in defining characteristic of the cyst, intrahepatic involvement and presence of ABPJ34,42.As a non-invasive and less meticulous technique, MRCP could become the imaging method of choice, while avoiding potential hazards of ERCP34 ,or even PTC.
An accurate diagnosis through the complete radiographic demonstration of the biliary system may be obtained by currently means of ERCP and PTC 11,40. In case no 2, distal end of common bile duct was obstructed as suggested by ERCP and confirmed by surgical exploration, limiting the usefulness of this technique. ERCP or PTC is also useful to evaluate the anatomy of biliary tract and pancreaticobiliary junction40. PTC is particularly advantageous in cases in which previous Roux-en-Y cystoenterostomy was done or when intrahepatic cysts are suspected , but it may fail to define ABPJ44. When unavailable, an operative cholangiogram (OC) must be performed, as it helps in defining the limits and anatomic landmarks of the lesion. The biliary cystic disease of Type II and Type III are usually diagnosed by these methods11. ERCP,PTC and OC were decisive for the diagnosis of the most of cases above reported. No additional effort was done to search for ABPJ in the present series.
Surgical management
Surgery is the best treatment for BCD. A report by Trout et al.37 showed that conservative treatment was followed by 96.6% mortality due to complications as biliary cirrhosis, hepatic abscess, cyst rupture, pancreatitis, gastrointestinal hemorrhage, portal vein thrombosis and malignancy. In Type I cyst resection is the method of choice2,3,4,17,20,22,24,27,28,33,37,45,46,47,48 Flanigan et al.3 reporting on 235 patients followed for a mean period of 5.2 years, found out that the morbidity was more significant among those submitted to internal drainage compared to those who undergone to cyst excision, althought no difference was seen in operative mortality. After excisional surgery of cysts Tipe I, cancer arising in the intrahepatic ducts rarely occurs, due to long standing biliary stricture49. Cancer also occurs in the biliopancreatic remnant duct, and excision of the distal duct in the pancreas has been recommended49. However, since occurrency of cancer in the intrahepatic bile ducts and in the distal remnant of biliary duct after incomplete excision of the cyst is also said to be rare, excision of the bulk of cyst, performance of hepatico-jejunostomy, and interruption of anomalous biliopancreatic junction, should be considered as a surgical strategy to preclude morbidity related to extensive dissection in porta hepatis or in the area of pancreaticobiliary junction50.
Despite the use of internal drainage in the first two patients, surgical resection of the cyst, as done in the last three cases, whenever possible, is a better option, as its persistence, favoring bile stasis, allows the occurrence of cholangitis, stone formation, hepatic cirrhosis and, above all, malignancy. In the second case the bilioenteric anastomose was adopted as palliation.
In cysts Type II, the treatment of choise is surgical excision and in Type III a surgical transduodenal excision or endoscopic sphinterotomy have been proposed 3,11,20,40,46,51,52
In Type IV-A, extrahepatic dilations are also treated by ressection40,53. The intrahepatic cysts present complications, as hilar or intrahepatic obstructions, intrahepatic stones, cholangitis, and above all malignancy53. The controversy arises over what to do with intrahepatic cystic formations, because leaving these cysts behind may increase the occurrence of cancer40. When the intrahepatic dilatations are confined to one lobe of liver, lobectomy can be performed25,53. When they are multiple and diffuse into the liver, theoretically, the only form of treatment is liver transplantation, although some have advised medical treatment for recurrent infection or even drainage of the cysts, with close surveillance for tumor development40. However there are little data concerning the most effective way to follow patients with extrahepatic resected cysts for development of cancer, especially those in whom intrahepatic cysts were left in situ40. Strictures after hepaticojejunostomy, as well as extraction of intrahepatic stones may be managed by percutaneous transhepatic balloon instrumentation42 or by endoscopy through an access loop44, being the last useful in direct inspection and biopsy of suspected lesions.
Type IV-B cysts, resection associated with hepaticojejunostomy is the best treatment53.
In Type V (Carolis disease), treatment is controversial. Localized forms could be treated satisfactorily by partial hepatectomy, whereas in diffuse forms, management is difficult because frequent infectious complication due to stones, having a poor prognosis19,40,53. Since diffuse Carolis disease is prone to develop cirrhosis, portal hipertension, chronic cholangitis, hepatic failure and malignization, orthotopic liver transplantation could offer a chance of success 40,54,55.
CONCLUSION
Based on the literature review and on their own experience the authors had observed that: 1. BCD is a relative rare disease; 2. The main diagnostic tools are US, TC, ERCP and PTC, as well as OC, when other methods are unavailable; 3. The main principle of treatment is total excision of extrahepatic dilatation to prevent occurrence of cancer, cholangitis and jaundice; 4. For localized intrahepatic dilatation, lobectomy may be performed; and 5. Management of diffuse forms of intrahepatic cystic disease may include medical treatment for cholangitis, and close surveillance for tumor development. In extreme cases orthotopic liver transplantation may be considered.
Arruda PCL, Coelho ARB, Lima Filho JFC, Machado RJC, Souza AP, Mathias CAC, Ferraz AAB, Ferraz EM. Dilatação cística do ducto biliar comum em adultos: relato de cinco casos e revisão de literatura Acta Cir Bras [serial online] 2000 Oct-Dec;15(4). Available from: URL: http://www.scielo.br/acb.
RESUMO: Os autores apresentam cinco casos de dilatação cística do ducto biliar comum do Tipo I (classificação de Todani) em adultos, anteriormente não relatados, num período de 25 anos no Servico de Cirurgia Geral de um Hospital Universitário, entre 16.057 operacões, no período de 1974 e 1999. O diagnóstico dos cistos foi realizado através de colangiografia operatória (CO) no primeiro, por colangiografia transparietohepática no segundo (CTPH) e por ultra-sonografia (US), colangiopancreatografia endoscópica retrógrada (CPER) and colangiografia operatória (CO), respectivamente, nos três últimos casos. Em um dos pacientes, foi detectado adenocarcinoma localizado na parede posterior do cisto, associado à metástases peritoniais. Os dois primeiros casos foram tratados através de derivação cistoentérica, sendo nos três últimos realizada a excisão do cisto, seguida de hepaticojejunostomia em Y de Roux. Foram revisados classificação, incidência, etiologia, diagnóstico, malignização e tratamento cirúrgico da doença cistica biliar (DCB), concluindo-se que a terapêutica cirúrgica de escolha deve ser a ressecção, quando possível, sobretudo devido ao risco significativo de malignização.
DESCRITORES: Ducto biliar comum. Adulto. Classificação. Diagnóstico.
Endereço para correspondência:
Dr. Edmundo Machado Ferraz
Av. Rosa e Silva, 2063
Recife PE - Brazil
52050-020
Tel/Fax: (81)271-1526 / 441-4460
Data do recebimento: 17/08/2000
Data da revisão: 22/09/2000
Data da aprovação: 11/10/2000
- 1 . Flanigan DP. Biliary cysts. Ann Surg 1975;182:635-43.
- 2 . Alonso-Lej F, Rever Jr. WB, Pessagno DJ. Congenital choledochal cyst, with a report of 2, and analysis of 94 cases. Int Abst Surg1959;108(1):1-30.
- 3 . Flanigan DP. Biliary carcinoma associated with biliary cysts. Cancer 1977;40:880-3.
- 4 . Yamaguchi M. Congenital choledochal cyst: analysis of 1433 patients in the japanese literature. Am J Surg 1980;140:653-7.
- 5 . Fieber SS, Nnance FC. Choledochal cyst and neoplasm: a comprehensive review of 106 cases and presentation of two original cases. Am Surg 1997;63(11):982-7.
- 6 . Alves Jr R., Jaber BA. Cisto de colédoco em adulto. Rev Col Bras Cir.1997;24(3):197-8.
- 7 . Andrade MAC, Passos E, Corręa MM. Cisto gigante de colédoco tipo Ia. GED 1990;9(2):39-44.
- 8 . Castro MAM, Henrique PRF, Bonfante B. Cisto de colédoco. Vittale 1986;2: 53-6.
- 9 . Dias JLF, Jano S, Nogueira PC. Cisto de colédoco: uma forma incomum de apresentaçăo. In: CONGRESSO DA SOCIEDADE BRASILEIRA DE CIRURGIA PEDIÁTRICA. 9. 1982. Curitiba. Anais do IX Congresso da Sociedade Brasileira de Cirurgia Pediátrica. Curitiba: 1982. p 17-19.
- 10 . Pereira-Lima JC, Raymondi RP, De Carli LA, Franciosi LE, Ferreira CT, Zim MCA. Dilataçăo cística difusa da via biliar (cisto de colédoco do tipo Ic e junçăo pancreatobiliar anômala: relato de um caso. GED 1999;18(1):38-40.
- 11 . Pohl F, Mestrinho JLD, Barbosa JÁ, Cavalcanti AM, Jesus SP, Carvalho MB. Dilataçőes císticas do conduto biliar. HFA Publ Téc Cient 1987;2(1):9-15.
- 12 . Rena CL, Tostes AVT, Pinheiro DMN, Freguglia WN. Cisto do colédoco. Rev Col Bra. Ci. 1986;13(2):69-72.
- 13. Sabbaga CC, Schuls C, Avilla SG, Garbers JC, Schulz ES. Complicaçőes no tratamento cirúrgico do cisto de colédoco: revisăo de 18 anos. Rev Col Bras Cir1995;22(4):213-6.
- 14 . Salem MZ, Jukemura J, Cunha JEM, Buchpiguel CA, Kato M, Calvo IPL, Cerri GG, Machado MCC. Cisto de colédoco: diagnóstico por métodos năo invasivos - relato de caso. Rev Imagem 1991; 3(1):33-5.
- 15 . Sales JLP, Antunes CRH, Almeida HC, Souza JCK, Carbonera MR, Mello Jussara S, Bassols JV, Peterson CAH. Cisto de colédoco causando obstruçăo pilórica no recém-nascido. In: CONGRESSO DA SOCIEDADE BRASILEIRA DE CIRURGIA PEDIÁTRICA. 9. 1982. Curitiba. Anais do IX Congresso da Sociedade Brasileira de Cirurgia Pediátrica. Curitiba: 1982. p 14-17.
- 16 . Tinoco RC, Tinoco LA, Cavichini QN, Brum AV, Anderson PAV. Dilataçăo cística do colédoco: relato de quatro casos. GED 1987;6(3):73-7.
- 17 . Deziel DJ, Rossi RL, Munson JL, Braasch JJ, Silverman ML. Management of bile ducts cysts in adults. Arch Surg 1986;121:410-5.
- 18. Komi N, Udaka H, Ikeda N, Kashiwagi Y. Congenital dilatation of the biliary tract: new classification and study with particular reference to anomalous arrangement of the pancreatobiliary ducts. Gastroenterol Japan 1977;12:293-304.
- 19. Mercadier M, Chigot JP, Clot JP, Langlois P, Lansiaux P. Carolis disease. World J Surg 1984;8(1):22-9.
- 20. Todani T, Watanabe Y, Narusue N, Tabuchi K, Okajima K. Congenital bile duct cysts: classification, operative procedures, and review of thirty-seven cases including cancer arising from choledochal cyst. Am J Surg 1977;134:262-9.
- 21. Jesudadson SRB, Govil S, Mathai V, Kuruvilla R, Muthusami JC. Choledocahl cysts in adults. Ann R Coll Surg Engl 1997;79(6):410-3.
- 22. Gertler JP, Cahow CE. Choledochal cyst in the adult. J Clin Gastroenterol 1988;10(3):315-9.
- 23. Vercruysse R,Van Den Bossche MR. Choledochal cyst in adults. Acta Chir Belg 1998;98(5):220-2.
- 24 . Fonkalsrud EW. Choledochal cysts. Surg Clin North Am 1973;53(5):1275-81.
- 25 . Wang WP, Wu MS, Lin CC, Chang LY, Kao AW, Wang HH, Lin JT. Pancreaticobiliary diseases associated with anomalous pancreaticobiliary ductal union. Gastrointest Endosc 1998;48(2):84-9 (Abstract).
- 26. Chaudhuri PK, Chaudhuri B, Schuler JJ, Nyhus LM. Carcinoma associated with congenital cystic dilatation of bile ducts. Arch Surg 1982;117:1349-51.
- 27. Ono J, Sakoda K, Akita H. Surgical aspect of cystic dilatation of the bile duct. Ann Surg 1982;195(2):203-8.
- 28. Powell CS, Sawyers JL, Reunoldas VH. Management of adult choledochal cysts. Ann Surg 1981;193(5):666-76.
- 29 . Yotuyanagi, S. Contributions to the aetiology and pathogeny of the idiopathic cystic dilatation of the common bile duct with report of three cases. Gann 1936;30:601-6.
- 30.Ito T, Ando H, Nagaya M, Sugito T. Congenital dilatation of the common bile duct in children - the etiologic significance of the narrow segment distal to the dilated common bile duct. Zeit Kinderchir 1984;39:40-4.
- 31. Babbitt DP, Starshak RJ, Clemett AR. Choledochal cyst: a concept of etiology. Am J Roentgenol 1973;119(1):57-62.
- 32. Miyano T, Suruga K, Chen SC. A clinicopathologic study of choledochal cyst. World J Surg 1980;4(2):231-8.
- 33. Rattner DW, Shapiro RH, Warshaw AL. Abnormalities of the pancreatic and biliary ducts in adult patients with choledochal cysts. Arch Surg 1983;118:1068-73.
- 34. Matos C, Nicaise N, Deviere J, Metens T, Struyven J, Cremer M. Choledochal cysts: comparition of findings at MR cholangiopancreatography and retrogradecholangiopancreatography in eight patients. Radilology 1998;209(2):443-8
- 35. Mori K, Akimoto R, Kanno M, Kamata T, Hirono Y, Matsumura A. Anomalous union of the pancreaticobiliary ductal system without dilation of the common bile duct or tumor: case reports and literature review. Hepatogastroenterology 1999;46(25):142-7 (Abstract).
- 36. Benhidjeb T, Said S, Rudolph B, Siegmund E. Anomalous pancreatico-biliary junction: report of a new experimental model and review of literature. J Pediatr Surg 1996;31(12):1670-74.
- 37. Trout HH, Longmire Jr WP. Long-term follow-up study of patients with congenital cystic dilatation of the common bile duct. Am J Surg 1971;121:68-86.
- 38 . Karanikas ID, Koundourakis SS, Macheras AN, Panagiotidis HCH, Liakakos TD, Dendrinos SS. Long-term results of management of Type I choledochal cysts in adults. Acta Cir Belg 1997;97(1):13-8.
- 39 . Reid MH, Philips HE. O papel da tomografia computadorizada e do ultrassom na doença do trato biliar. Clin Cir Am Norte 1981;61(4):809-48.
- 40. Weyant MJ, Maluccio, MA, Daly JM. Choledochal cyst in adults: a repport of two cases and review of literature. Am J Gastroenterol 1998;93(12):2580-3.
- 41 Stadalnik RC, Matolo NM. Cintilografia da árvore biliar. Clin Cir Am Norte 1981;61(4):849-64.
- 42. Belli G, Rotondano G, Dagostino A, Iannelli A, Marano I, Santangelo, ML. Cyst dilation of extrahepatic bile ducts in adulthood: diagnosis, surgical treatment and long term results. HPB Surg 1998;10(6):379-85.
- 43 . Funabiki T, Matsubara T, Ochiai M. Symptoms, diagnosis and treatment of pancreaticobiliary mal junction associated with congenital cystic dilatation of bile duct. Nippon Geka Gakkai Zasshi 1996;97(8):582-8 (Abstract).
- 44. Dun SM, Williamson RCN. Cyst dilation of extrahepatic bile ducts in adulthood: diagnosis, surgical treatment and long term results. HPB Surg 1998;10(6):379-85.
- 45. Chijiiwa K, Koga A. Surgical management and long term follow-up of patients with choledochal cysts. Am J Surg 1993;165 238-42.
- 46 . Cuschieri A, Byrne D. Cystic disease of the biliary tract. Ann Chir Gynaecol 1989;78(4):259-66.
- 47. Kimura K, Tsugawa C, Ogawa K. Choledochal cyst: etiological considerations and surgical management in 22 cases. Arch Surg 1978;113:159.
- 48. Moreno Gonzalez E, Garcia Garcia I, Hidalgo Pascual M, Calleja Kempin J, Garcia Blanch G, Gomez Gutierrez M, Arias Diaz J, Calle Santiuste A. Choledochal cyst ressection and reconstruction by biliary-jejuno-duodenal diversion. World J Surg 1989;13(2):232-7.
- 49. Todani T, Toki A. Cancer arising in choledochal cyst and management. Nippon Geka Gakkai Zasshi 1996;97(8):594-8 (Abstract).
- 50. Ishibashi T, Kasahara K, Yasuda Y, Nagai H, Makino S, Kanazawa K. Malignant change in the biliary tract after excision of choledochal cyst. Br J Surg 1997;84(12):1687-91.
- 51. Kato T. Etiological relationship between choledochal cyst and anomalous junction of the pancreatobiliary ductal system. Keio J Med 1989;38(2):136-51.
- 52. Mouzas A, Neuhaus H. Choledochocele and its endoscopic management. Hepato-Gastroenterol 1992;39(2):109-1.
- 53. Nagorney DM. Choledochal cysts in adult life. In: Blumgart LH. Surgery of liver and biliary tract. New York: Churchill Livingstone; 1988. p 1003-12.
- 54. Joseph VT. Surgical techniques and long-term results in the treatment of choledochal cyst. J Pediatric Surg 1990;75(7):782-7.
- 55. Oneill Jr, JA. Choledochal cyst. Curr Probl Surg 1992;29(6):361-410.
Publication Dates
-
Publication in this collection
08 Nov 2000 -
Date of issue
Dec 2000
History
-
Received
17 Aug 2000 -
Reviewed
22 Sept 2000 -
Accepted
11 Oct 2000